G protein-coupled receptors (GPCRs) play an inordinately large role in human health. They are the targets of many clinically relevant medications and are also candidate therapeutic targets under current investigation for a wide spectrum of diseases. Drugs targeting GPCRs are used as antihypertensives, anti-allergenic/asthmatics, antipsychotics, and to treat numerous other disorders across a broad spectrum of diseases. One recent study focusing on the DrugBank database found that 19% of all human drug targets are GPCRs and ~36% of all drugs currently approved for market target GPCRs.1 Accordingly, GPCRs are by far the single largest protein family for drug targets; currently, 63 of 200 drugs with the highest sales volume in the United States target GPCRs. Further, this overrepresentation of GPCRs has been steady since the early 1980s and continues to this day.1,2
Trace amine associated receptor 1 (TAAR1) is a GPCR that was first identified in 2001.3,4 It is a member of the rhodopsin-type superfamily that has a predicted seven transmembrane spanning domain structure with short N- and C-terminal domains. It is one of a family of receptors that were initially discovered as a result of a search for novel serotonin receptors,3 where 15 mammalian orthologues were identified. Further analysis of chromosomal localizations, phylogenetic relationships, and ligand pockets of TAAR family members revealed three distinct receptor subfamilies.5 The gene name was initially abbreviated to TA and TAR, but only two of the subfamily members (TAAR1 and TAAR4) were found to have high affinity for trace amines. Also, as discussed below, TAAR1 has a wide agonist spectrum, and although it has apparent affinity for trace amines in the low nanomolar range, it remains to be elucidated whether trace amines achieve nanomolar concentrations in the vicinity of TAAR1 receptors in vivo, making it impetuous to refer to the receptor as TA1. Collectively, these observations led to the adoption of the nomenclature of ‘trace amine associated receptor 1’, or TAAR1, but this nomenclature is still not used universally.
Because this receptor was the first discovered to be potently activated by trace amines, which have long been implicated in psychiatric disorders6,7 and the rewarding effects of psychostimulants,8 interest in this receptor as a potential therapeutic target was immediate and has persisted ever since. To date, the data reporting specific functional consequences of activation of this receptor are largely limited to investigations in brain monoaminergic systems, though it is recognized that the receptor has a broader distribution in the brain and in the periphery that has yet to be systematically investigated. Nonetheless, the specific functional effects of TAAR1 activation that are presently recognized suggest that targeting this receptor can have a therapeutic relevance and provide trajectories for realizing such potential. Accordingly, this Perspective focuses specifically on recent evidence directly related to the potential of TAAR1 as a novel therapeutic target from the point of view of what has been learned to date about how the receptor functions. A synopsis of recent findings specifically related to TAAR1 function are presented in the context of the potential therapeutic efficacy of novel TAAR1-targeted compounds.
Upon its discovery and until recently, no specific agonists or antagonists existed that targeted TAAR1. Furthermore, it was evident early on that TAAR1 is expressed largely on intracellular membranes in transfected cell lines in vitro.4, 9,10 These two aspects were significant obstacles that slowed the early pharmacological characterization of the receptor, but also inspired some creative approaches to assessing pharmacology and later, function. Different laboratories took divergent approaches to establishing model cell systems for pharmacological characterization, including modifying intracellular loops, developing human-rat chimeras,11,12 co-expressing human TAAR1 with rat Gαs signaling protein,13 co-expressing human TAAR1 with promiscuous G(q), Gα16 to couple receptor activation to the mobilization of internal calcium,14 and alteration of the N terminus of TAAR1 to stabilize its expression at the plasma membrane so that a bioluminescence resonance energy transfer cAMP biosensor assay could be used to pharmacologically characterize the chimeric receptor.15 Our group utilized a highly sensitive CRE-luciferase assay that involved a prolonged period of test drug exposure to pharmacologically characterize rhesus monkey TAAR1.9,16 Pharmacological and functional analyses of TAAR1 that were initiated in cell systems were facilitated by the development of TAAR1 knockout mice17,18 which provided a resource for addressing the phenotypic consequences of TAAR1 transgenic ablation in the animal as well as a valuable tissue resource for assessing functional consequences at neurochemical, cellular and systems levels.
TAAR1 is expressed in the brain and the periphery,3 and is activated by a wide spectrum of agonists that include, but are not limited to, “trace” amines, common biogenic amines, amphetamine-like psychostimulants and thyroid hormone derivatives such as 3-iodothyronamine (thyronamine), among others.4,19 Actually, the discovery that TAAR1 is potently activated by thyronamines spurred the discovery of the first rationally designed “superagonist” and antagonist compounds that target TAAR1.20 Upon activation, the receptor is now known to signal through both the cAMP/PKA/CREB and the PKC/Ca++/NFAT pathways, as evidenced by elevations in cAMP levels following receptor stimulation,3,4 direct measures of phosphorylation of Protein Kinase A (PKA) and Protein Kinase C (PKC) in TAAR1-transfected cells and activated primate lymphocytes,21 TAAR1-mediated, PKA- and PKC-dependent effects on monoamine transporter kinetic activity in co-transfected cells in vitro,22,23,24,25 and in mouse and primate striatal and thalamic synaptosomes,23,24,25 expression of CRE-luciferase in transfected cells,9,16 and expression of CREB-luciferase and NFAT-luciferase reporter constructs in lenti-transduced cells that stably express rhesus monkey TAAR1.21 Accordingly, specific drugs that target TAAR1 will likely result in alterations in the PKA and PKC signaling pathways in brain monoaminergic terminals and in activated peripheral lymphocytes, among other potential actions that are as of yet unrecognized.
Although a detailed and rigorous description of the regional distribution of TAAR1 protein and mRNA expression has yet to be accomplished, it is established that TAAR1 protein and mRNA are expressed in monoaminergic brain regions.3,16,26 In our laboratory, we exploited our prowess with assessing changes in kinetic activity of the dopamine transporter (DAT), norepinephrine transporter (NET) and serotonin transporter (SERT) to assess TAAR1 functionality. Because many of the identified ligands that activate TAAR1 are also substrates at the monoamine transporters and TAAR1 localization in transfected cells was shown to remain largely intracellular,4 we had reasoned early on that monoamine transporters could serve as conduits for TAAR1 agonists to enter cells, thereby providing access of the agonist to an intracellular pool of TAAR1 receptors. We speculated that if this were the case there could be a special relationship between TAAR1 and monoamine transporters because they share some of the same ligands. Indeed, we observed substantially enhanced CRE-luciferase signaling in transfected cells that co-expressed both TAAR1 and a monoamine transporter (DAT, NET or SERT).9,16 Because monoamine transporter kinetic regulation and internalization is a consequence of upregulated cellular phosphorylation cascades,27,28,29 we reasoned that the enhanced TAAR1 signaling could in turn trigger changes in the kinetic activity of the monoamine transporters under conditions of co-localization. This too was born out in a series of studies from our laboratory which demonstrated that TAAR1 activation drives the PKA and PKC cellular signaling cascades that result in inhibition of monoamine uptake and transporter reversal (efflux) in DAT/TAAR1, NET/TAAR1 and SERT/TAAR1 co-transfected cells in vitro, as well as in mouse and primate striatal (DAT, SERT) and thalamic (NET) synaptosomes ex vivo.22,23,24,25,30 TAAR1 specificity for mediation of the observed kinetic effects on the monoamine transporters was further confirmed in these studies by demonstrating the absence of noncompetitive effects of the trace amine beta-phenylethylamine (β-PEA),23 the common biogenic amines,24 and methamphetamine25 on uptake inhibition and substrate-induced monoamine efflux in synaptosomes generated from TAAR1 knockout mice. Accordingly, specific drugs that target TAAR1 will likely result in alterations in monoamine kinetic function and brain monoamine levels via this mechanism.
As noted above, TAAR1 responds to a wide spectrum of agonists and some of these agonists are monoamine transporter substrates (e.g., common and trace biogenic amine, amphetamines3,4,16), but others are not (e.g., thyronamines31,32). It is also the case that some of these agonists activate other monoamine receptors whereas others do not. For example, the common biogenic amines activate a variety of dopamine, adrenergic and serotonergic receptors and generally have considerably higher affinity for these receptors than for TAAR1,3,4 whereas the trace amine β-PEA has very high affinity for TAAR13,13 but very low affinity for and does not activate other monoamine receptors.23 As noted above, TAAR1 activation results in phosphorylation cascades that in turn alter monoamine transporter kinetic function, and such is also the case for other GPCRs that co-express with monoamine transporters when they are activated, including monoamine autoreceptors.24 In this regard, our laboratory has demonstrated that TAAR1 activation augments, whereas monoamine autoreceptor activation attenuates, CRE-luciferase expression (indicative of cAMP accumulation) via receptor association with stimulatory and inhibitory G protein coupling, respectively.24 When both TAAR1 and a monoamine autoreceptor were co-expressed together in the same cell, signaling at one receptor attenuated signaling by the other. In cell culture systems, this cross-attenuation of signaling differentiated TAAR1 agonists on the basis of their ability to bind to monoamine autoreceptors that inhibit the cAMP/PKA pathway. For example, dopamine induced CRE-luciferase expression in TAAR1-transfected cells via its interaction with TAAR1, whereas it failed to do so when the D2 dopamine receptor was co-transfected with TAAR1, because both receptors signal oppositely to one another. However, the psychostimulant methamphetamine, or the trace amine β-phenylethylamine (both are potent TAAR1 agonists), which have very low affinity for D2, each produced similar and robust activation of CRE-luciferase in both TAAR1 and TAAR1/D2 cells. Similar interactions between TAAR1 and the adrenerigic receptors alpha2A and alpha2B, or the serotonergic receptors 5HT1A and 5HT1B also were observed in response to norepinephrine and serotonin, respectively, in analogous experiments, reviewed elsewhere.33 Therefore, the status of monoamine autoreceptor activation and whether a TAAR1 agonist can also bind to monoamine autoreceptors (or other receptors that co-express with TAAR1) can play a role in the responsiveness of the TAAR1 agonists. Additionally, TAAR1 antagonists are expected to block both endogenous common biogenic amine and trace amine signaling at TAAR1 and thereby alter the balance of signaling by the endogenous common biogenic amines at monoamine autoreceptors. This, therefore, represents another mechanism by which drugs that specifically target TAAR1 could affect brain monoaminergic function. It may also be the case that current clinically relevant drugs that target monoamine autoreceptors may have efficacy, in part, via their ability to modify TAAR1 signaling in response to its endogenous or exogenous ligands.
Additional reports in the literature further reveal the inter-relationship of TAAR1 and monoamine autoreceptors. Wolinsky et al. (2007) first reported that TAAR1 knockout mice show an upregulation of dopamine D2 high affinity receptors.17 It was subsequently shown that the potency of dopamine at D2 receptors in dopamine neurons is greater in TAAR knockout mice as compared with wild type mice, and that a specific TAAR1 antagonist, N-(3-ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide 1 (EPPTB; to be further discussed below) increases the potency of dopamine at D2 receptors in dopamine neurons.34 There is also recent evidence that TAAR1 and D2 receptors form heterodimers when co-expressed in HEK293 cells35, that haloperidol can disrupt this interaction35, and that TAAR1 activation alters the desensitization rate and agonist potency at 5-HT1A receptors in the dorsal raphe,35,36 consistent with the previous findings from our laboratory16,24. Accordingly, specific drugs that target TAAR1 are likely to result in alterations in monoamine autoreceptor signaling and monoaminergic tone, and potentially, the clinical responsivity to drugs that target monoamine autoreceptors.
It is now recognized that in addition to effects on monoamine transporter function, TAAR1 activation also regulates the firing rate of dopamine neurons. In normal mice, TAAR1 activation was shown to decrease the spike frequency of dopamine neurons as well as tonically activate inwardly rectifying K+ channels resulting in a reduction of the basal firing frequency of dopamine neurons in the ventral tegmental area (VTA).18 It was also observed that TAAR1 knockout mice have an elevated spontaneous firing rate of dopaminergic neurons in the VTA.18 It was in this context that Bradaia et al. (2009) reported the discovery of a TAAR1 antagonist, 1, that could increase the firing frequency of VTA dopamine neurons in normal mice.34 1 was also shown to prevent the reduction in the firing frequency of VTA dopamine neurons induced by activation of TAAR1 with the trace amine p-tyramine, and it could also block the TAAR1-mediated activation of an inwardly rectifying K+ current. In a subsequent report, Revel et al. (2011) reported the discovery of the specific TAAR1 agonist [(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine] 2 (RO5166017), and showed that it inhibited the firing frequency of dopaminergic and serotonergic neurons in the VTA and dorsal raphe nucleus, respectively.36 Accordingly, drugs that specifically target TAAR1 will likely modulate monoaminergic neuronal firing rates. This then, represents another mechanism by which TAAR1 activation can affect brain monoamine levels.
It was first observed by Bunzow et al. (2001)4 and then further expanded by Reese et al. (2007)12 and Lewin et al. (2011)37 that amphetamine and amphetamine-like drugs were pharmacological agonists at TAAR1. Wolinsky et al. (2007)17 and Lindemann et al. (2008)18 reported that deletion of the TAAR1 gene in mice results in the pharmacogenic phenotype of an enhanced locomotive response to amphetamine, coincident with an amphetamine-induced increase in the release of biogenic amines. We recently extended these studies by comparing the psychomotor stimulating effects of amphetamine and methamphetamine and the conditioned rewarding effects of methamphetamine and morphine between wild-type and TAAR1 knockout mice.38 Both single and repeated exposure to amphetamine or methamphetamine generated significantly higher levels of total distance travelled in the TAAR1 knockout mice. In conditioned place preference (CPP) studies, TAAR1 knockout mice acquired methamphetamine-induced CPP earlier than wild-type mice and retained CPP longer during extinction training. In comparison, both genotypes displayed similar levels of morphine-induced CPP. These data suggest that TAAR1 has a modulatory role in the behavioral sensitization to amphetamine-based psychostimulants, and a selective role in the conditioned reinforcing effects of methamphetamine versus morphine. Accordingly, specific drugs that target TAAR1 may influence amphetamine-like psychostimulant responsiveness, addictive processes and reward salience.
It has also been observed that TAAR1 activation results in an inhibition of hyperactivity in dopamine transporter knockout mice, which have a hyperactive phenotype, and that double-knockout mice lacking both the dopamine transporter and TAAR1 display a significantly enhanced level of spontaneous activity in comparison to the heightened level of activity present in dopamine transporter knockout mice.39,40 Accordingly, these data suggest that specific TAAR1-targeted drugs might affect dopamine-dependent movement or hyperactivity.
Although most research to date on Trace Amine Associated Receptor 1 (TAAR1) function has focused on its role in the brain, it has been recognized since its discovery in 2001 that TAAR1 mRNA is expressed in peripheral tissues as well, suggesting that this receptor may play a role in non-neurological pathways. Our lab cloned rhesus monkey TAAR19 and in the process of screening different cell lines for endogenous TAAR1 mRNA expression, we had observed strong expression of TAAR1 mRNA in immortalized rhesus monkey T cell lines T444 and T44541 and also had detected TAAR1 protein in rhesus monkey macrophages and dendritic cells using immunocytochemistry (unpublished observations). Nelson et al. (2007) reported that mouse B cells and NK cells isolated from spleen express TAAR1, as well as several other TAAR family receptor subtypes, but no TAAR expression was detected in mouse macrophages or dendritic cells.42 Also, TAAR1 mRNA expression was upregulated in human peripheral blood mononuclear cells (PBMC) following in vitro stimulation with phytohaemagglutinin (PHA). 42 We have recently reported that TAAR1 is robustly expressed in rhesus monkey immortalized B cells, and is upregulated in rhesus monkey PBMC that are stimulated with PHA.21 We showed that rhesus monkey B cells and activated PBMC respond to the TAAR1 agonist methamphetamine with upregulation of phosphorylated PKA and phosphorylated PKC, and that this response is blocked in the presence of the TAAR1 antagonist 1.21 Accordingly, specific TAAR1-targetted drugs will likely have direct effects on activated lymphocytes and on methamphetamine-induced PKA and PKC phosphorylation cascades in activated lymphocytes.
Our studies in transfected cells and in mouse and rhesus monkey brain synaptosomes demonstrated that monoamine autoreceptor signaling is highly attenuated when TAAR1 is present and signaling.24 To draw a parallel to the immune system, it is possible that TAAR1 signaling decreases the signaling of other GPCRs, in particular chemokine receptors and receptors for lipid mediators of inflammation, which share inhibitory signaling properties (e.g., inhibition of cAMP) with monoamine autoreceptors. Ultimately, upregulation of TAAR1 and subsequent TAAR1 signaling in immune cells may be a mechanism for limiting the cell’s responsiveness to chemokines or lipid mediators of inflammation, autocrine responses, or run-away immune system activation that can give rise to autoimmunity, an abundance of non-specifically activated T cells, or whole body systemic inflammation. Accordingly, specific TAAR1-targeted drugs may alter the functioning of the immune system.
As noted above, thyronamine is a TAAR1 agonist which, unlike the biogenic amines and amphetamines, is not a substrate at monoamine transporters but rather a blocker of monoamine uptake through the transporters.19,31,32 Thyronamine produces a profound hypothermic response when administered in vivo.19,32 Amphetamines also cause thermoregulatory responses, and because these drugs are agonists at TAAR1 it was thought that the receptor could be mediating thermoregulatory responses. This notion was further supported by Doyle et al. (2007),43 who reported that treatment with thyronamine produced a robust hypothermia that reduced infarct volume by about one third in a middle cerebral artery occlusion mouse model of focal ischemia. As thermoregulatory effects are highly relevant with regard to the biological actions of potential therapeutics that target TAAR1, our laboratory took advantage of the availability of TAAR1 knockout mice to address whether TAAR1 is involved in thermoregulatory responses. We demonstrated time-dependent thermoregulatory effects of various TAAR1 agonists, including thyronamine, MDMA, methamphetamine, tyramine and β-PEA in normal mice, and strikingly similar thermoregulatory effects of these drugs in TAAR1 knockout mice.32 In a more thorough investigation of thermoregulatory responses in wild type and TAAR1 knockout mice in response to varied doses of MDMA, Di Cara et al. (2011) reported a more complex thermoregulation profile in which MDMA elicited a time-, dose-, and ambient temperature-dependent hypothermia and hyperthermia in wild-type mice, whereas TAAR1 knockout mice displayed hyperthermia only, with genotypic differences occurring most predominantly at high doses of MDMA.44 These authors also observed that MDMA elevated extracellular 5-HT levels to a greater extent in TAAR1 knockout mice as compared to wild-type mice. The genotypic differences observed could be due to TAAR1 involvement, but could also reflect the action of MDMA to imbalance monoaminergic systems which are likely to have many compensatory changes in TAAR1 knockout mice. As noted previously, Wolinsky et al. (2007) observed that TAAR1 knockout mice show an upregulation of dopamine D2 high affinity receptors.17 Though it has yet to be explored, it stands to reason that TAAR1 knockout mice may also show a dysregulation of other monoaminergic receptor systems, particularly those that have functional relationships with TAAR1 (such as 5-HT1A, as noted previously), and these compensatory changes could influence the pharmacologically-induced aberrant thermoregulatory responses in the TAAR1 knockout mice. For example, Rusyniak et al. (2007) reported that pretreating rats with a 0.5 mg/kg i.p. dose of the 5-HT1A antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY 100635) not only prevented MDMA-induced hypothermia, but resulted in the development of hyperthermia,45 and so adaptations in the serotonergic system in the TAAR1 knockout mice may play a role in the genotypic differences in response to MDMA. Taken together, then, it is conceivable that the pharmacologically-induced thermoregulatory effects caused by MDMA and other compounds that are TAAR1 agonists may occur in a TAAR1-independent fashion, and that TAAR1-directed compounds will likely not affect thermoregulation per se under normal physiological conditions in humans, nor are they likely to be therapeutic cryogens. Lastly, it has been observed that the TAAR1 agonist 2 prevented stress-induced hyperthermia in normal but not TAAR1 knockout mice.36 This may indicate a role for TAAR1 in the stress response, which has yet to be explored but is quite easily rationalized given the prominent role of monoamines in regulating the hypothalamic pituitary-adrenal axis. But we need to assess whether this genotypic difference is the result of compensatory changes in monoamine receptors such as 5-HT1A, which are involved in stress-induced hyperthermia.46
Collectively, these first functional studies on TAAR1 discussed above establish new avenues for advancing the development of TAAR1-targeted compounds as potential therapeutic agents. It is now recognized that TAAR1 signals through the cAMP/PKA/CREB and the PKC/Ca++/NFAT pathways. With regard to its function in brain monoaminergic systems, TAAR1 activation affects monoamine transporter kinetic activity and monoamine autoreceptor signaling, modulates the firing rate of dopamine neurons and mediates some of the effects of amphetamine-like psychostimulants. In the immune system, TAAR1 activation results in PKA and PKC signaling in activated lymphocytes. While there are likely to many more biological roles for this receptor that are revealed by further research on TAAR1, these first functional studies discussed above provide an initial framework to encourage new avenues for the development of this receptor as a novel therapeutic target.
Figure 1.
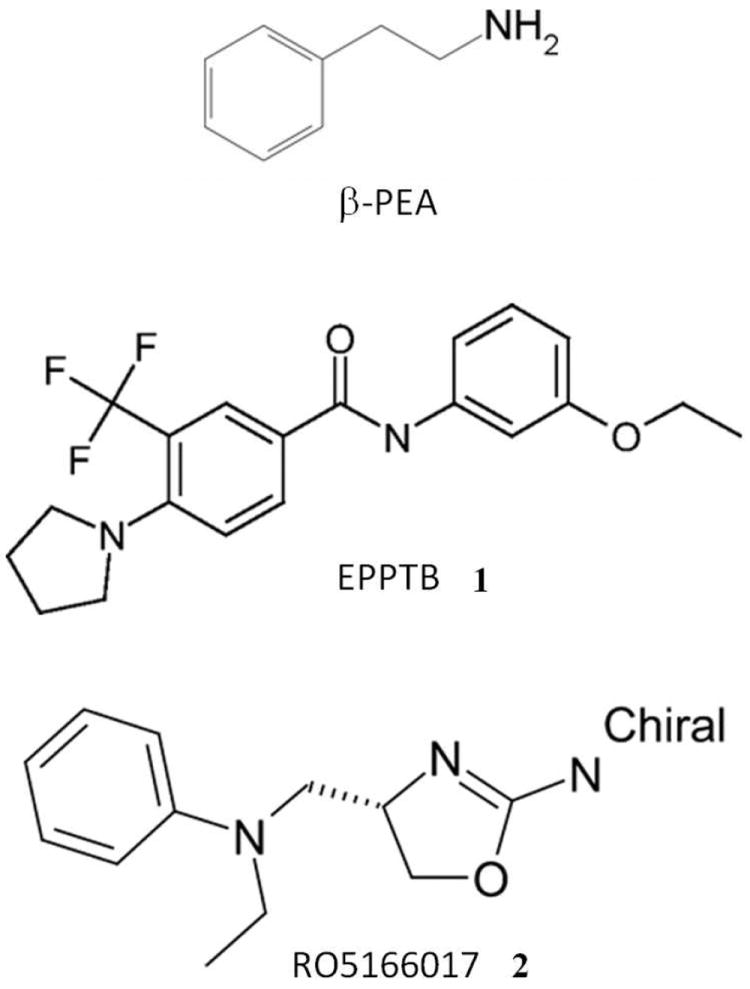
Chemical structure of Beta-phenylethylamine (β-PEA), the selective TAAR1 antagonist N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide 1 (EPPTB), and the selective TAAR1 agonist [(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine] 2 (RO5166017).
Acknowledgments
Support: DA025697 (GMM), RR00168 (NEPRC)
Abbreviation list
- GPCR
G protein-coupled receptor
- TAAR1
Trace amine associated receptor 1
- DAT
dopamine transporter
- NET
norepinephrine transporter
- SERT
serotonin transporter
- CPP
conditioned place preference
- VTA
ventral tegmental area
- PBMC
peripheral blood mononuclear cells
- PHA
phytohaemagglutinin
- PKA
protein kinase A
- PKC
protein kinase C
- MDMA
3,4-Methylenedioxymethamphetamine
Biography
Dr. Gregory Miller is an Assistant Professor in the Department of Psychiatry, Harvard Medical School. His research program at The New England Primate Research Center in Southborough, Massachusetts focuses on the neurobiology and genetics of drug addiction and stress, and the advancement of the nonhuman primate model as a preclinical platform for pharmacogenomic medications development.
References
- 1.Rask-Andersen M, Almén MS, Schiöth HB. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10(8):579–590. doi: 10.1038/nrd3478. [DOI] [PubMed] [Google Scholar]
- 2.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 3.Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60(6):1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 5.Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85(3):372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AA. Trace amines and mental disorders. Can J Neurol Sci. 1980;7(3):261–263. doi: 10.1017/s0317167100023313. [DOI] [PubMed] [Google Scholar]
- 7.Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2(1):3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- 8.Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90(2):257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller GM, Verrico CD, Jassen A, Konar M, Yang H, Panas H, Bahn M, Johnson R, Madras BK. Primate trace amine receptor 1 modulation by the dopamine transporter. J Pharmacol Exp Ther. 2005;313(3):983–994. doi: 10.1124/jpet.105.084459. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z, Vallender EJ, Yu N, Kirstein S, Yang H, Bahn M, Westmoreland SV, Miller GM. Cloning, expression and functional analysis of rhesus monkey trace amine-associated receptor 6: Evidence for lack of monoaminergic association. J Neurosci Res. 2008;86(15):3435–3446. doi: 10.1002/jnr.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci. 2005;26(5):274–281. doi: 10.1016/j.tips.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Reese EA, Bunzow JR, Arttamangkul S, Sonders MS, Grandy DK. Trace amine-associated receptor 1 displays species-dependent stereoselectivity for isomers of methamphetamine, amphetamine, and para-hydroxyamphetamine. J Pharmacol Exp Ther. 2007;321(1):178–186. doi: 10.1124/jpet.106.115402. [DOI] [PubMed] [Google Scholar]
- 13.Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL. Pharmacologic characterization of the cloned human trace amine-associated receptor1(TAAR1) and evidence for species differences with the rat TAAR1. J Pharmacol Exp Ther. 2007;320(1):475–485. doi: 10.1124/jpet.106.112532. [DOI] [PubMed] [Google Scholar]
- 14.Navarro HA, Gilmour BP, Lewin AH. A rapid functional assay for the human trace amine-associated receptor 1 based on the mobilization of internal calcium. J Biomol Screen. 2006;11(6):688–693. doi: 10.1177/1087057106289891. [DOI] [PubMed] [Google Scholar]
- 15.Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, Gainetdinov RR. Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1(TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Mol Pharmacol. 2008;74(3):585–94. doi: 10.1124/mol.108.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Z, Westmoreland S, Bahn ME, Chen G-L, Yang H, Vallender EV, Yao WD, Madras BK, Miller GM. Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther. 2007;321(1):116–127. doi: 10.1124/jpet.106.116863. [DOI] [PubMed] [Google Scholar]
- 17.Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6(7):628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324(3):948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- 19.Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10(6):638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 20.Tan ES, Groban ES, Jacobson MP, Scanlan TS. Toward deciphering the code to aminergic G protein-coupled receptor drug design. Chem Biol. 2008;15(4):343–353. doi: 10.1016/j.chembiol.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panas MW, Xie Z, Panas HN, Hoener MC, Vallender EJ, Miller GM. Trace Amine Associated Receptor 1 Signaling in Activated Lymphocytes. J Neuroimmune Pharmacol. 2011 Oct 29; doi: 10.1007/s11481-011-9321-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Z, Miller GM. Trace Amine-Associated Receptor 1 is a Modulator of the Dopamine Transporter. J Pharmacol Exp Ther. 2007;321(1):128–136. doi: 10.1124/jpet.106.117382. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Miller GM. β-Phenylethylamine Alters Monoamine Transporter Function via Trace Amine-Associated Receptor 1: Implication for Modulatory Roles of Trace Amines in Brain. J Pharmacol Exp Ther. 2008;325(2):617–628. doi: 10.1124/jpet.107.134247. [DOI] [PubMed] [Google Scholar]
- 24.Xie Z, Westmoreland SV, Miller GM. Modulation of Monoamine Transporters by Common Biogenic Amines via Trace Amine-Associated Receptor 1 and Monoamine Autoreceptors in HEK293 Cells and Brain Synaptosomes. J Pharmacol Exp Ther. 2008;325(2):629–640. doi: 10.1124/jpet.107.135079. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Miller GM. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharmacol Exp Ther. 2009;330(1):316–325. doi: 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Z, Miller GM. Trace amine-associated receptor 1 as a monoaminergic modulator in brain. Biochem Pharmacol. 2009;78(9):1095–1104. doi: 10.1016/j.bcp.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitayama S, Dohi T, Uhl GR. Phorbol esters alter functions of the expressed dopamine transporter. Eur J Pharmacol. 1994;268(2):115–119. doi: 10.1016/0922-4106(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 28.Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285(5428):763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 29.Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279(18):19315–19326. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- 30.Vallender EJ, Xie Z, Westmoreland SV, Miller GM. Functional evolution of the trace amine associated receptors in mammals and the loss of TAAR1 in dogs. BMC Evol Biol. 2010;10:51–60. doi: 10.1186/1471-2148-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snead AN, Santos MS, Seal RP, Miyakawa M, Edwards RH, Scanlan TS. Thyronamines inhibit plasma membrane and vesicular monoamine transport. ACS Chem Biol. 2007;2(6):390–398. doi: 10.1021/cb700057b. [DOI] [PubMed] [Google Scholar]
- 32.Panas HN, Lynch LJ, Vallender EJ, Xie Z, Chen GL, Lynn SK, Scanlan TS, Miller GM. Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res. 2010;88(9):1962–1969. doi: 10.1002/jnr.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116(2):164–176. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, Bettler B. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci U S A. 2009;106(47):20081–20086. doi: 10.1073/pnas.0906522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, Caron MG, Gainetdinov RR. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol. 2011;80(3):416–425. doi: 10.1124/mol.111.073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A. 2011;108(20):8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044–7048. doi: 10.1016/j.bmc.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achat-Mendes C, Lynch LJ, Sullivan KA, Vallender EJ, Miller GM. Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol Biochem Behav. 2011 Nov 4; doi: 10.1016/j.pbb.2011.10.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sotnikova TD, Budygin EA, Jones SR, Dykstra LA, Caron MG, Gainetdinov RR. Dopamine transporter-dependent and -independent actions of trace amine beta-phenylethylamine. J Neurochem. 2004;91:362–373. doi: 10.1111/j.1471-4159.2004.02721.x. [DOI] [PubMed] [Google Scholar]
- 40.Sotnikova TD, Zorina OI, Ghisi V, Caron MG, Gainetdinov RR. Trace amine associated receptor 1 and movement control. Parkinsonism Relat Disord. 2008;14(Suppl. 2):S99–S102. doi: 10.1016/j.parkreldis.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein S, Brown CR, Dehghani H, Lifson JD, Hirsch VM. Intrinsic susceptibility of rhesus macaque peripheral CD4(+) T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J Virol. 2000;74(20):9388–9395. doi: 10.1128/jvi.74.20.9388-9395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson DA, Tolbert MD, Singh SJ, Bost K. Expression of neuronal trace amine-associated receptor (Taar) mRNAs in leukocytes. J Neuroimmunol. 2007;192(1-2):21–30. doi: 10.1016/j.jneuroim.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyle KP, Suchland KL, Ciesielski TM, Lessov NS, Grandy DK, Scanlan TS, Stenzel-Poore MP. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke. 2007;38(9):2569–2576. doi: 10.1161/STROKEAHA.106.480277. [DOI] [PubMed] [Google Scholar]
- 44.Di Cara B, Maggio R, Aloisi G, Rivet JM, Lundius EG, Yoshitake T, Svenningsson P, Brocco M, Gobert A, De Groote L, Cistarelli L, Veiga S, De Montrion C, Rodriguez M, Galizzi JP, Lockhart BP, Cogé F, Boutin JA, Vayer P, Verdouw PM, Groenink L, Millan MJ. Genetic Deletion of Trace Amine 1 Receptors Reveals Their Role in Auto-Inhibiting the Actions of Ecstasy (MDMA) J Neurosci. 2011;31(47):16928–16940. doi: 10.1523/JNEUROSCI.2502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther. 2007;323(2):477–487. doi: 10.1124/jpet.107.126169. [DOI] [PubMed] [Google Scholar]
- 46.Olivier JD, Cools AR, Olivier B, Homberg JR, Cuppen E, Ellenbroek BA. Stress-induced hyperthermia and basal body temperature are mediated by different 5-HT(1A) receptor populations: a study in SERT knockout rats. Eur J Pharmacol. 2008;590(1-3):190–197. doi: 10.1016/j.ejphar.2008.06.008. [DOI] [PubMed] [Google Scholar]


