Abstract
Objective
Non diabetic African American (AA) youth have an up regulated insulin secretion relative to insulin sensitivity compared with their American White (AW) peers. We investigated if similar racial differences exist in youth with T2DM.
Research Design and Methods
14AA and 14AW T2DM adolescents underwent evaluation of insulin sensitivity (IS) and clearance [hyperinsulinemic-euglycemic clamp], 1st and 2nd phase insulin and C-peptide secretion [hyperglycemic clamp]; body composition (DEXA), and abdominal adiposity (CT).
Results
AA and AW T2DM had similar HbA1c, diabetes duration, BMI and % body fat, with lower visceral fat in AAs (p=0.013). While insulin-stimulated glucose disposal was similar in AA and AW (7.5±1.0 vs. 7.3±0.9 mg/kgFFM/min), insulin sensitivity trended to be lower (2.5±0.4 vs. 3.8±0.6 mg/kgFFM/min per µu/ml, p=0.081). 1st (175.7±52.9 vs 66.6±10.8 µu/ml, p=0.01) and 2nd phase insulin (236.2±40.7 vs 105.1±17.9µu/ml, p=0.008), and 1st (8.2±1.2 vs 5.0±0.3 ng/ml, p=0.02) and 2nd phase C-peptide (10.8±0.9 vs 7.6±0.6 ng/ml, p=0.012) were higher in AA. β-cell function relative to insulin sensitivity was higher in AA vs. AW (259.5±35.3 vs 168.8±25.1 mg/kgFFM/min, p= 0.043).
Conclusions
Racial differences in insulin secretion can be demonstrated with the clamp technique in obese adolescents with T2DM. Similar to non diabetic youth, AA adolescents with T2DM compared with their AW counterparts have an upregulated β-cell function relative to insulin sensitivity, the reasons for which remain to be investigated.
Keywords: Youth type 2 diabetes, Insulin secretion, insulin clearance, racial differences
INTRODUCTION
Type 2 diabetes and its complications are more prevalent in minority ethnic groups, particularly AA compared with AW adults (1–3). Similar observations are made in childhood T2DM (4, 5), with incidence rates reported to be four fold higher in AA compared with AW youth (6). Moreover, there are racial disparities in glycemic control in adolescents with T2DM manifested in higher HBA1c levels and higher rates of hospitalization in black youth (7). The mechanism(s) underlying this increased risk for T2DM and the higher prevalence rates in AA children are not well understood.
We had reported that healthy AA youth have ~ 75% higher insulin secretion relative to insulin sensitivity, which is above the compensatory β-cell response to insulin resistance (8). Even when normal weight AA youths are matched for insulin sensitivity to their AW peers, there is evidence of an upregulated β-cell function in AA adolescents (9). It remains unanswered if these physiological differences between AA and AW youth exist under the pathopysiological condition of T2DM, which is characterized by severe insulin resistance combined with β-cell failure (10,11). Therefore, the aim of the present study was to investigate if racial differences in insulin secretion relative to insulin sensitivity are present in adolescents with T2DM.
RESEARCH DESIGN AND METHODS
Study Population
Twenty-eight obese adolescents, 14 African American (AA) and 14 American White (AW) with T2DM were studied as part of our ongoing studies of youth T2DM. Racial group was determined according to participants’ report of ethnicity in 3 generations. All subjects reported in the present manuscript have been previously reported in the context of other hypotheses (10, 11). The current analysis aimed to investigate the hypothesis that the up-regulated β-cell function observed in non diabetic AA vs. AW adolescents persists in T2DM, despite the pathophysiological impairment consequent to diabetes. The adolescents, 12.0 to less than 19.0 years old, were clinically diagnosed with T2DM (12) and were negative for glutamic acid decarboxylase (GAD) and insulinoma associated protein-2 autoantibody (IA2 Ab) with the DK harmonization assay (13). They were on treatment with lifestyle alone (n=5), metformin alone (n=10), metformin and insulin (n=12) or insulin alone (n=1). The modality of treatment did not differ by ethnic group. Metformin and long acting insulin were discontinued 48 hrs before the clamp studies as before (10). Prior to enrollment, participants were required to have HbA1c ≤ 8.0% to avoid the potential confounding effect of chronic hyperglycemia on insulin sensitivity and secretion. All studies were approved by the Institutional Review Board of the University of Pittsburgh. Informed consent and assent were obtained. Clinical characteristics of the study subjects are summarized in Table 1.
Table 1.
Clinical, phenotypic and metabolic characteristics of AA and AW obese adolescents with T2DM.
| AA (n=14) |
AW (n=14) |
t-test or MWU p-value |
|
|---|---|---|---|
| Age (years) | 14.3±0.5 | 15.8±0.4 | 0.03 |
| Sex* (M/F) | 5M / 9F | 6M /8F | ns |
| Tanner stage* II–III IV–V |
1 13 |
1 13 |
ns |
| Duration of diabetes (months) | 9.8±2.5 | 4.8±1.4 | ns |
| Treatment Modality* n (%): Lifestyle Metformin Metformin+Insulin Insulin |
3 (21%) 5 (36%) 5 (36%) 1 (7%) |
2 (14%) 5 (36%) 7 (50%) 0 (0%) |
ns |
| BMI (kg/m2) | 35.4±1.3 | 36.3±1.3 | ns |
| Waist circumference (cm) | 104.8±3.6 | 108.6±2.9 | ns |
| % Body Fat | 41.2±1.3 | 43.0±1.9 | ns |
| SAT (cm2) | 509.3±36.4 | 557.7±39.9 | ns |
| VAT (cm2) | 67.3±3.9 | 94.1±8.9 | 0.013 |
| HbA1c (%) | 6.3±0.2 | 6.6±0.2 | ns |
| Fasting glucose (mg/dl) | 109.3±4.4 | 124.7±6.1 | 0.051 |
| Fasting insulin (µu/ml) | 50.0±7.1 | 48.8±8.6 | ns |
| Fasting C-peptide (ng/ml) | 4.4±0.5 | 3.9±0.3 | ns |
| Cholesterol (mg/dl) | 149.9±8.0 | 161.6± 8.0 | ns |
| HDL (mg/dl) | 36.6±1.5 | 38.4± 2.0 | ns |
| LDL (mg/dl) | 94.8±7.8 | 92.9±6.9 | ns |
| Triglycerides (mg/dl) | 95.7±9.1 | 152.1±20.7 | 0.02 |
| TG/HDL ratio | 2.7±0.3 | 4.0±0.5 | 0.04 |
| Postabsorptive hepatic glucose production (mg/kg/min) | 2.7±0.2 | 2.5±0.2 | ns |
| Postabsorptive hepatic insulin sensitivity (mg/kg/min per µu/ml)−1 | 12.3±3.8 | 12.8±2.9 | ns |
The x2 analyses revealed no significant differences between the 2 groups with respect to sex distribution, Tanner stage or modality of treatment.
SAT: subcutaneous abdominal adipose tissue; VAT: visceral abdominal adipose tissue.
Clamp Studies
Participants were admitted twice within 4 weeks to the Pediatric Clinical and Translational Research Center (PCTRC) the day before the clamp studies. In random order, subjects were admitted once for a hyperinsulinemic-euglycemic clamp and the other time for a hyperglycemic clamp. One subject underwent a hyperglycemic clamp only and was not included in the comparison of insulin sensitivity. One female had very elevated insulin levels related to non specific binding. Therefore, only her C-peptide secretion data were included.
In-vivo insulin sensitivity
A fasting blood sample was obtained for determination of lipid profile and HbA1c. Fasting endogenous glucose production was measured with a primed constant-rate infusion of [6,6-2H2] glucose (0.306±0.009 µmol/kg/min) (Isotech, Miamisburg, OH) (11). Blood was sampled at the start of the 2-hr stable isotope infusion and every 10 min from −30 to 0 time (basal period) for determination of plasma glucose, insulin, and isotopic enrichment of glucose. Fasting turnover calculations were made during the last 30 min of the basal period. Insulin-stimulated glucose metabolism and insulin sensitivity were evaluated during a 3-h hyperinsulinemic-euglycemic clamp (11). Intravenous crystalline regular insulin (Humulin; Lilly Indianapolis, IN) was infused at a constant rate of 80 mu/m2/min as before (11). We have not conducted any clamps using humalog or novolog. Plasma glucose was clamped at 100 mg/dl with a variable rate infusion of 20% dextrose, based on arterialized plasma glucose determinations every 5 minutes. Continuous indirect calorimetry by a ventilated hood (Deltatrac Metabolic Monitor, Sensormedics, Anaheim, CA) was used to measure CO2 production, O2 consumption and respiratory quotient (RQ). Measurements were made for 30 minutes at baseline and at the end of the euglycemic clamp (11).
In-vivo insulin secretion
First and second phase insulin secretion and C-peptide were evaluated during a 2-h hyperglycemic clamp (225 mg/dl) as before (10–11).
Body Composition
Body composition was determined by DEXA, and subcutaneous abdominal adipose tissue (SAT) and visceral adipose tissue (VAT) by a single slice CT scan at L4–L5 (11,12). One AA and 1 AW adolescents did not have VAT data (technical error in CT acquisition).
Biochemical Measurements
Plasma glucose was measured with a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, Ohio), insulin and C-peptide by radioimmunoassay (RIA) as before (11). The insulin assay (Millipore Inc.) is 100% specific for Human Insulin with <0.2% cross reactivity to human proinsulin. In our lab, the assay has an inter-assay CV of 7.4% and intra-assay CV of 6.3%. The same kit from the same supplier was used over time. Samples are run in duplicates with no differences in the distribution of samples from AA and AW participants over time in between assays. HbA1c was measured by high performance liquid chromatography (Tosoh Medics, Inc. 1998) and lipids using the standards of the Centers for Disease Control and Prevention (11). Deuterium enrichment of glucose in the plasma was determined on a Hewlett-Packard Co. 5973 mass spectrometer (Palo Alto, CA) coupled to a 6890 gas chromatograph (11). Pancreatic autoantibodies were determined using the NIDDK-sponsored standardization assay in the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington (Seattle, WA) as reported by us (13).
Calculations
Fasting hepatic glucose production (HGP) was calculated during the last 30 min of the 2-hr isotope infusion according to steady-state tracer dilution equations (11,14). In the fasting state, hepatic insulin sensitivity was calculated as 1000/HGP X fasting insulin levels (14). Insulin stimulated glucose disposal rate (Rd) was calculated during the last 30 minutes of the euglycemic clamp to be equal to the rate of exogenous glucose infusion. Peripheral insulin sensitivity was calculated by dividing the Rd by the delta increase in insulin concentration from baseline to steady-state over the last 30 min of the clamp, and expressed per fat free mass (mg/kgFFM/min per µu/ml). Insulin-stimulated carbohydrate oxidation rates were calculated according to the formulas of Frayn from the indirect calorimetry data (8). Non-oxidative glucose disposal was estimated by subtracting the rate of glucose oxidation from the total insulin-stimulated glucose disposal.
During the hyperglycemic clamp, the first and second phase insulin and C-peptide concentrations were calculated as described previously (11, 14). Disposition index (DI) was calculated as the product of IS × 1st phase insulin and expressed per Kg (mg/kg/min) and per FFM (mg/kgFFM/min).
Statistics
Two group comparisons were performed using independent t-test in the case of normally distributed continuous variables, or the Mann-Whitney test for non-normally distributed continuous variables. Chi-square test was used for comparison of categorical variables. Pearson’s correlation coefficient for normally distributed variables or Spearman’s rho test for non normally distributed variables, were used to evaluate bivariate relationships, respectively. Data are presented as mean±SEM. Two-tailed p ≤ 0.05 was considered statistically significant.
RESULTS
Study subjects and fasting metabolic profile (Table 1)
The AW adolescents were slightly older than the AA T2DM; otherwise they didn’t differ in Tanner stage, diabetes duration, therapeutic modality, metabolic control, gender distribution, BMI, % body fat or subcutaneous abdominal fat. AA adolescents had lower VAT consistent with our past observations of black/white differences in body composition and VAT (15). The rate of [6,6-2H2] glucose infusion in blacks was 0.059 ± 0.01 mg/kg/min and 0.051 ± 0.02 mg/kg/min in whites, p=0.1. In the last 30 minutes of the 2 hours baseline isotope infusion, the mean % isotopic enrichment was 2.3 ± 0.1 with a coefficient of variation (CV) of 5.0% in AA and % isotopic enrichment was 2.2 ± 0.1 with CV 4.8 % in AW indicating steady state. There were no differences in fasting insulin, C-peptide, endogenous glucose production (HGP), and postabsorptive hepatic insulin sensitivity between AA and AW with T2DM; however fasting glucose tended to be lower, and triglycerides and TG/HDL ratio were lower in AA youth (Table 1).
Insulin stimulated glucose disposal, Insulin Sensitivity and Clearance (Figure 1)
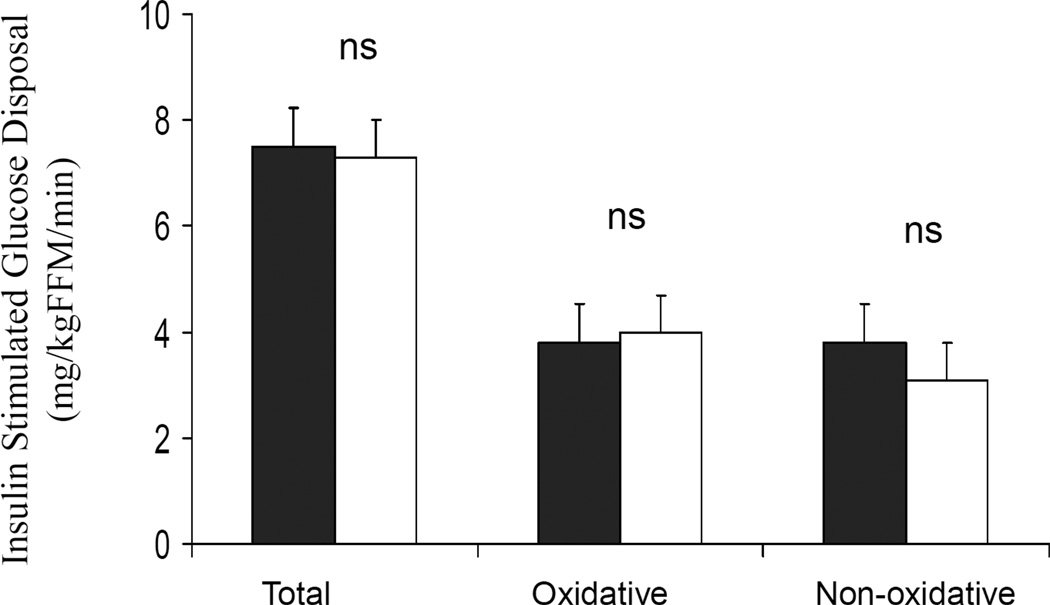
Figure 1.
A) Insulin-stimulated total, oxidative and non oxidative glucose disposal in AA (dark bar) vs. AW (white bar) youth with T2DM.
Steady-state plasma glucose levels during the hyperinsulinemic-euglycemic clamp were not different between AA and AW (101.1 ±0.7 and 101.4 ± 0.7 mg/dl), however steady-state plasma insulin concentrations were higher in AAs (367.4±30.1 vs. 277.6±33.6 µu/ml, p=0.005). The delta increase in insulin from baseline to clamp steady –state was higher in AAs (313.7±26.5 vs. 222.1±25.9 µu/ml, p=0.02). Metabolic clearance rate of insulin was lower in AA compared with AW youth (10.7±0.8 vs. 15.8±1.3 ml/KgFFM/min, p= 0.003). Insulin stimulated glucose disposal, total (7.5±1.0 vs. 7.3±0.9 mg/kgFFM/min), oxidative (3.8±0.4 vs. 4.0±0.4 mg/kgFFM/min) and non oxidative (3.8±0.8 vs. 3.1±0.9 mg/kgFFM/min) were comparable between AA and AW respectively (Figure 1). Insulin sensitivity trended to be lower in AA vs. AW youth using two tailed statistics (2.5±0.4 vs. 3.8±0.6 mg/kgFFM/min per µu/ml, p=0.081), and p=0.04 with one tailed statistics applied based on observations in non diabetic youth (8).
Insulin and C-peptide Secretion and Disposition Index (Figure 2)
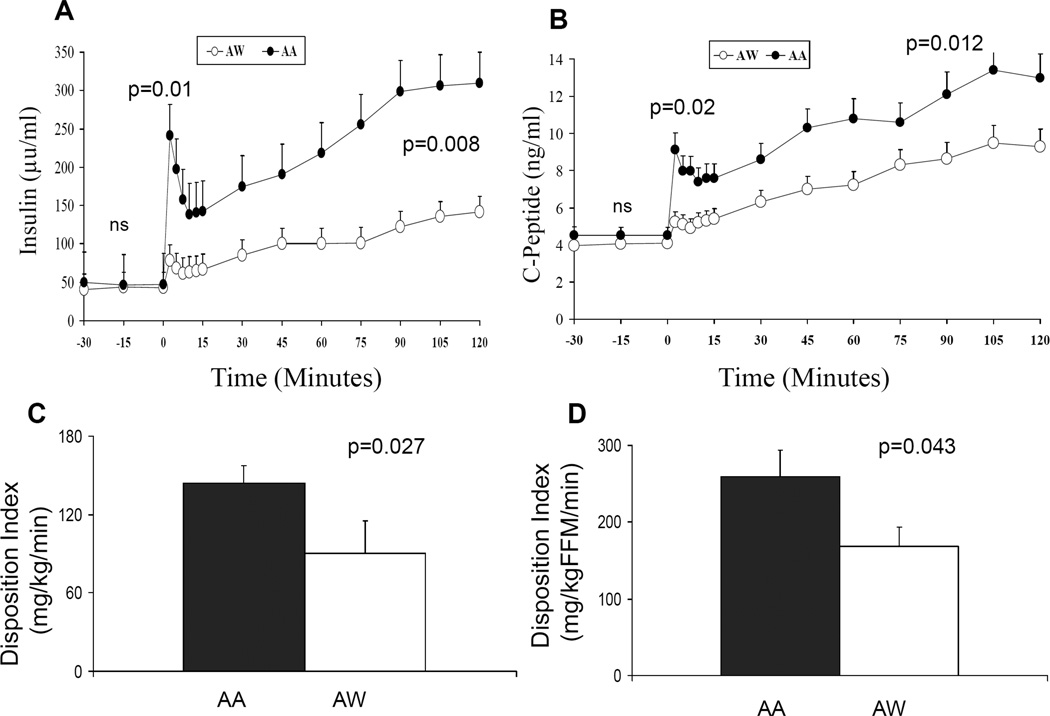
Figure 2.
A) Insulin levels and B) C-peptide levels in AA (dark circles) and AW (empty circles) youth with T2DM during the hyperglycemic clamp; C) Disposition index (DI) per Kg (mg/kg/min) in AA (dark bar) vs. AW (white bar); D) Disposition index (DI) per FFM (mg/kgFFM/min) in AA (dark bar) vs. AW (white bar).
First phase glucose (226.5±2.9 mg/dl in AA and 228.4±2.4 mg/dl in AW) and second phase glucose (228.0±0.9 mg/dl in AA and 229.7±1.0 mg/dl in AW) levels were not different between the 2 groups. Insulin and C-peptide concentrations during the hyperglycemic clamp were significantly higher in AA vs. AW youth with T2DM (Figure 2 A & B). First phase insulin concentration was 175.7± 52.9 µu/ml in AA and 66.6±10.8 µu/ml in AW, p=0.01; and second phase insulin concentration was 236.2±40.7 µu/ml in AA and 105.1 ±17.9 µu/ml in AW, p= 0.008. Similarly, first and second phase C-peptide concentrations were higher in AA vs. AW (8.2±1.2 ng/ml in AA vs. 5.0±0.3 ng/ml in AW, p=0.02; and 10.8±1.0 vs. 7.6±0.6 ng/ml, p=0.012, respectively). The disposition index, i.e. insulin secretion relative to insulin sensitivity was higher in AA vs AW adolescents with T2DM (Figures 2C and 2D).
Determinants of HbA1c in AA and AW
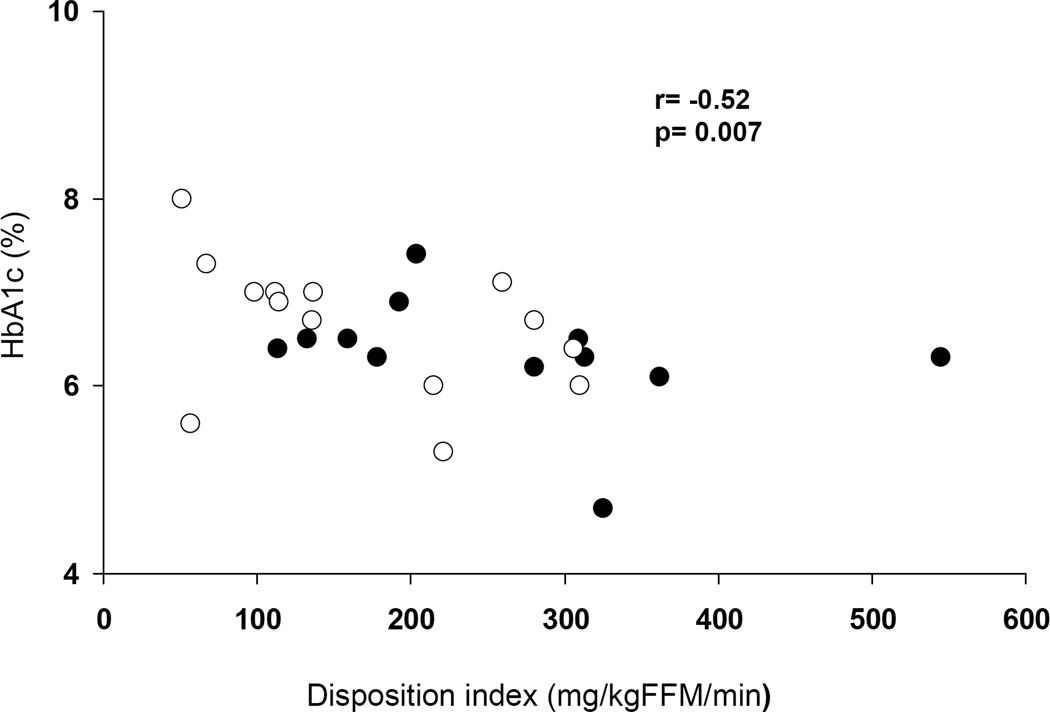
HbA1C correlated with DI in the total group of patients with T2DM (r= −0.52, p=0.007) (Figure 3). HbA1C did not correlate with insulin sensitivity in the total group (r=−0.1, p=0.6) or in either group separately (data not shown), but it correlated with first phase C-peptide (r= −0.4, p=0.04) and second phase C-peptide (r= −0.4, p=0.03). Duration of diabetes did not correlate with HbA1c nor with insulin secretion or sensitivity.
Figure 3.
Relationship between Disposition index and HbA1c in AA (dark circles) and AW (empty circles) adolescents with T2DM.
DISCUSSION
The increased risk for T2DM in African Americans has been attributed to societal, environmental (16) as well as genetic factors (17). Herein, we provide evidence of metabolic differences between the 2 racial groups with T2DM, indicating a race differential in insulin secretion between AA and AW youth with T2DM. Consistent with our previous observations of higher insulin secretion in AA vs. AW non diabetic adolescents, with similar insulin sensitivity (9), the present study in T2DM adolescents demonstrates similar findings.
In the current study, despite 30% lower visceral adipose tissue in AA adolescents with T2DM, their insulin sensitivity is not better than their AW peers, rather it is ~ 35% lower. This is in line with our prior findings in non diabetic obese AA vs. AW adolescents (18). Similar findings of no racial differences in insulin sensitivity were reported in pubertal youth evaluated using the frequently sampled intravenous glucose tolerance test (19) and in obese youth with normal and impaired glucose tolerance using OGTT-derived estimates of insulin sensitivity (20). This is in contrast to the lower insulin sensitivity reported in prepubertal normal weight (8, 21) and overweight AA compared with AW children (21, 22). This may be related to the effect of obesity-related insulin resistance which overshadows the race- related differences in insulin sensitivity (23). On the other hand, many studies evaluating insulin sensitivity in children do not take into consideration abdominal fat distribution (19,20). The absence of a higher insulin sensitivity in AA vs. AW adolescents with T2DM despite 30% lower VAT in AA could be interpreted as being reflective of an inherently lower insulin sensitivity in blacks, since visceral adiposity is inversely related to insulin sensitivity (24). This would be consistent with the findings in AA adults (25, 26) compared with whites whereby lower insulin sensitivity in blacks persists after accounting for differences in body composition (25, 26). This inherent insulin resistance in blacks can not be explained by higher intramuscular (27) or intrahepatic (28) fat content as these ectopic fat depots are not increased in blacks. Genetic admixture studies point towards a genetic cause for the insulin resistance as well as the higher insulin secretion in AAs (23).
The hyperinsulinemia characteristic of normoglycemic normal weight (8, 29) and overweight AA youth (20, 21) and adults (25,26) compared with AWs is also detectable in the setting of T2DM. This appears to be related to both increased secretion, as evidenced by higher C-peptide levels in AA in accordance with the findings in non diabetic children (8,9), and ~30% decrease in the metabolic clearance rate of insulin in AA compared with AW youth with T2DM. There is also the hypothetical possibility of less suppression of endogenous insulin secretion during the euglycemic clamp in AA youth resulting in higher steady-state insulin concentrations; however C-peptide was not measured during the euglycemic clamp. The observed lower insulin clearance in AA youth with T2DM is consistent with reports of decreased insulin clearance in non diabetic AA children (8, 21, 29–31) and adults (17) in comparison with AW individuals. Besides decreased insulin clearance, the increased insulin levels are thought to be a β-cell compensatory response to the lower insulin sensitivity in AAs (8, 30). However, the significantly higher disposition index, which is a measure of β–cell function relative to insulin sensitivity, in AA youth compared with their AW peers (8) would suggest that this hyperinsulinemia is not merely a compensatory response (8,9), rather an added effect of an up regulated β-cell function in AAs, both in non diabetic (8–9) and diabetic youth. This hyperinsulinemia could be related to greater β-cell sensitivity to glucose in AA compared with Caucasian children (29). This ethnic difference in disposition index has been observed in obese AA youth with normal and impaired glucose tolerance compared with their white peers utilizing OGTT-derived measures (20). In that study, insulin clearance was lower, and early insulin secretion higher in AA compared with AW for any given insulin sensitivity quartile (20). Our current findings that these racial differences persist in the setting of type 2 diabetes, support the notion that in AAs, the relationship between insulin sensitivity and secretion is shifted upward. Hence, their β-cells appear to be under increased demand (related to relative insulin resistance and possibly increased responsiveness to hyperglycemia) and fail at a higher set point compared with whites. It remains unknown however if the higher insulin levels in blacks are of lower bioactivity than in whites.
In conclusion, there appears to be a race differential in the pathophysiological mechanisms responsible for youth T2DM, wherein a greater impairment in insulin sensitivity in blacks and a greater impairment in insulin secretion in whites might be operative.
A possible drawback of this study is the relatively small sample size. Despite this however, significant differences were detected in insulin secretion and disposition index between the 2 racial groups. Some of the T2DM youth were receiving metformin which was discontinued 48 hours before the clamp studies. However, there was no significant difference in the treatment modality between the 2 groups and therefore the racial differences observed are unlikely to be related to metformin use. Our study is limited by its cross-sectional nature, and longitudinal studies are needed to assess the natural history of T2DM and the changes in insulin sensitivity and secretion in AA vs. AW youth.
However, in the absence of any such data in youth with T2DM, our observations may provide important insight into the need for potentially different approaches in the prevention and treatment of youth T2DM in the different racial groups. Based on the higher insulin secretion in AA T2DM youth, one might ponder if prevention strategies targeting insulin sensitization may prove more fruitful, while in AW youth early interventions targeting beta cell function may be more needed.
ACKNOWLEDGEMENTS
These studies would not have been possible without the nursing staff of the Pediatric Clinical and Translational Research Center, the research team (Lori Bednarz, RN, CDE, and Nancy Guerra, CRNP, Kristin Porter RN, and Sally Foster RN, CDE); the laboratory expertise of Theresa Stauffer MS, and Katie McDowell BS; the secretarial assistance of Pat Antonio, and most importantly the commitment of the study volunteers and their parents.
This work was supported by United States Public Health Service grant K24 HD01357 (SA), Richard L Day endowed Chair (SA), Department of Defense (SA, FB and SJL), Thrasher Research Fund (FB, NG), MO1 RR00084 (GCRC) and UL1 RR024153 (CTSA).
Footnotes
Disclosure Summary: The Authors have nothing to disclose.
REFERENCES
- 1.Harris MI. Non-insulin-dependent diabetes mellitus in black and white Americans. Diabetes Metab Rev. 1990;6:71–90. doi: 10.1002/dmr.5610060202. [DOI] [PubMed] [Google Scholar]
- 2.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: the Third National Health and Nutrition Examination Survey 1988–4. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Bowman BA, Ford ES, Marks VF, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;10:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 5.Dabelea D, Pettitt DJ, Jones KL, Arslanian SA. Type 2 diabetes mellitus in minority children and adolescents. An emerging problem. Endocrinol and Metab Clinics North Am. 1999;28(4):709–729. doi: 10.1016/s0889-8529(05)70098-0. [DOI] [PubMed] [Google Scholar]
- 6.The Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2176–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 7.Rothman R, MD, Mulvaney S, Elasy T, VanderWoude A, Gebretsadik T, Shintani A, Potter A, Russell W, Schlundt D. Self-management behaviors, racial disparities, and glycemic control among adolescents with type 2 Diabetes. Pediatrics. 2008;121:e912–e919. doi: 10.1542/peds.2007-1484. [DOI] [PubMed] [Google Scholar]
- 8.Arslanian S, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American Children. Diabetes. 2002;51:3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 9.Hannon T, Bacha F, Lin Y, Arslanian S. Hyperinsulinemia in African American (AA) Adolescents Compared with Their American White (AW) Peers despite Similar Insulin Sensitivity: A Reflection of Up-Regulated β-Cell Function? Diabetes Care. 2008;31:1445–1447. doi: 10.2337/dc08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth Type 2 Diabetes Mellitus: Insulin resistance, beta-cell failure or both? Diabetes Care. 2005;28:638–644. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacha F, Gungor N, Lee S, Arslanian S. In Vivo Insulin Sensitivity and Secretion in Obese Youth: What are the Differences between NGT, IGT and Type 2 Diabetes? Diabetes Care. 2009;32:100–105. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. [Google Scholar]
- 13.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus - positive patients. Diabetes. 2009;58:738–744. doi: 10.2337/db08-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacha F, Saad R, Gungor N, Arslanian S. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity. 2008;16:1066–1071. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 16.LaVeist TA. Disentangling Race and Socioeconomic Status: A Key to Understanding Health Inequalities. J Urban Hlth. 2005;82:26–34. doi: 10.1093/jurban/jti061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwame O, Schuster D, Samuel K, Albert GBA. Race and ethnicity determine serum insulin and c-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African Ancestry and White Americans. Metabolism. 1997;46:53–58. doi: 10.1016/s0026-0495(97)90167-0. [DOI] [PubMed] [Google Scholar]
- 18.Bacha F, Saad R, Gungor N, Janosky J, Arslanian S. Obesity, regional fat distribution and syndrome X in obese black vs white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 19.Duck MM, Hoffman RP. Impaired endothelial function in healthy African-American adolescents compared with Caucasians. J Pediatr. 2007;150:400–406. doi: 10.1016/j.jpeds.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss R, Dziura J, Burgert S, Taksali S, Tamborlane W, Caprio S. Ethnic Differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49:571–579. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 21.Uwaifo G, Nguyen T, Keil M, Russel D, Nicholson J, Bonat S, McDuffie J, Yanovski J. Differences in insulin secretion and sensitivity of Caucasian and African American prepubertal children. J Pediatr. 2002;140:673–680. doi: 10.1067/mpd.2002.124312. [DOI] [PubMed] [Google Scholar]
- 22.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr. 2000;71:725–732. doi: 10.1093/ajcn/71.3.725. [DOI] [PubMed] [Google Scholar]
- 23.Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes. 2003;52:1047–1051. doi: 10.2337/diabetes.52.4.1047. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29:51–56. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 25.Haffner SM, D'Agostino R, Jr, Saad MF. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 26.Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? J Clin Endocrinol Metab. 2010;95:2426–2432. doi: 10.1210/jc.2009-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liska D, Dufour S, Zern TL, Taksali S, Calí AM, Dziura J, Shulman GI, Pierpont BM, Caprio S. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;27;2(6):e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in african-american and caucasian children. J Clin Endocrinol Metab. 2002;87:2218–2224. doi: 10.1210/jcem.87.5.8498. [DOI] [PubMed] [Google Scholar]
- 30.Goran M, Bergman R, Cruz M, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Sathanur SR, Radhakrishnamurthy B, Dalferes ER, Berenson GS. Racial (black-white) differences in insulin secretion and clearance in adolescents: the Bogalusa Heart Study. Pediatrics. 1996;97:357–360. [PubMed] [Google Scholar]