Abstract
Ageing is a continuum of biological processes characterized by progressive adaptations which can be influenced by both genetic and physiological factors. In terms of human maturation, physically and cognitively functional centenarians certainly represent an impressive example of successful healthy ageing. However, even in these unique individuals, with the passage of time, declining lung function and sarcopenia lead to a progressive fall in maximal strength, maximal oxygen uptake, and therefore reduced exercise capacity. The subsequent mobility limitation can initiate a viscous downward spiral of reduced physical function and health. Emerging literature has shed some light on this multi-factorial decline in function associated with aging and the positive role that exercise and physical capacity can play in the elderly. Recognizing the multiple factors that influence ageing, the aim of this review is to highlight the recently elucidated limitations to physical function of the extremely old and therefore evaluate the role of exercise capacity in the health and longevity of centenarians.
Keywords: Mechanical efficiency, VO2max, Independence, Lifestyle, Mobility
1. Introduction
Unveiling the ‘secret’ of human longevity is undoubtedly one of the most intriguing challenges for the scientific community. Certainly, genetic factors are amongst the determinants of successful ageing, however, an active lifestyle, especially regular exercise, is also a positive contributor [1–4] and has been recognized as such for quite some time. Indeed, in 44 BC Marcus Tullius Cicero, a Roman philosopher, reported in the XXXIV paragraph of the Cato Maior de Senectute, “Potest igitur exercitatio et temperantia etiam in senectute conservare aliquid pristini roboris” which translates to, rather profoundly for this early period in human history, ‘Even in old age exercise and moderation can preserve something of young vigour’ [5]. It is now clearly apparent that a decline in specific physical characteristics such as maximal strength, maximal oxygen uptake, and reduced exercise capacity can initiate a viscous downward spiral of reduced physical function and health.
In terms of human maturation, physically and cognitively functional centenarians represent an impressive example of successful healthy ageing. In fact, some would argue, and perhaps correctly so, that people of 100 years of age and beyond represent the best example of successful human aging as they live ~50% longer than the world average. Although human functional independence and health undoubtedly decline progressively with advancing age, a significant percentage of centenarians maintain some level of independence and are able to perform the basic activities of daily of life [6]. To our knowledge, the first document that identifies the determinants of longevity in centenarians is a manuscript published in 1899 and authored by T.E. Young. It was aptly entitled: ‘On centenarians and the duration of the human race’. Interestingly, more than 100 years ago factors such as moderation, genetics, and physical activity were already being methodically documented as contributors to the longevity of centenarians: ‘The majority of centenarians were moderate or small eaters … took but little animal food or alcohol … had experienced few illnesses during their life-time … and as a rule the records showed that outdoor exercise and early rising constituted important factors’ [7].
While living to an age of 100 years is not a new phenomenon, it was far less frequent in the relatively recent past. Indeed, in western countries, the number of centenarians is growing at the rapid rate of approximately 8% per year, while, to put this in perspective, the worldwide population is only growing at a rate of 1% per year [8]. Significant improvements in the quality of life and advances in medicine in the last half century have, at least in part, influenced this growth in the centenarian population. Such medical advances are particularly important because centenarians die as a consequence of disease and not because of ‘old age’, as commonly assumed, with the majority of centenarians presenting with chronic co-morbidities even if they are considered otherwise healthy [9]. However, centenarians, despite great variability in terms of health, cognitive function and independence [10], have a better pathological profile than the majority of their elderly, but still much younger, counterparts who are likely not to survive to the age of 100 years. Although epidemiological studies agree that regular exercise appears to positively enhance health and independence in centenarians, it was not until recently that direct assessments were made of what limits their physical function, facilitating an examination of the role of exercise capacity in the health and longevity of this population. Therefore, considering the multiple factors influencing successful ageing, the aim of this review is to describe the physical capacity of extremely old individuals and to evaluate how exercise impacts the health and longevity of centenarians.
2. Inspirational examples of human longevity and more typical experiences with extreme ageing
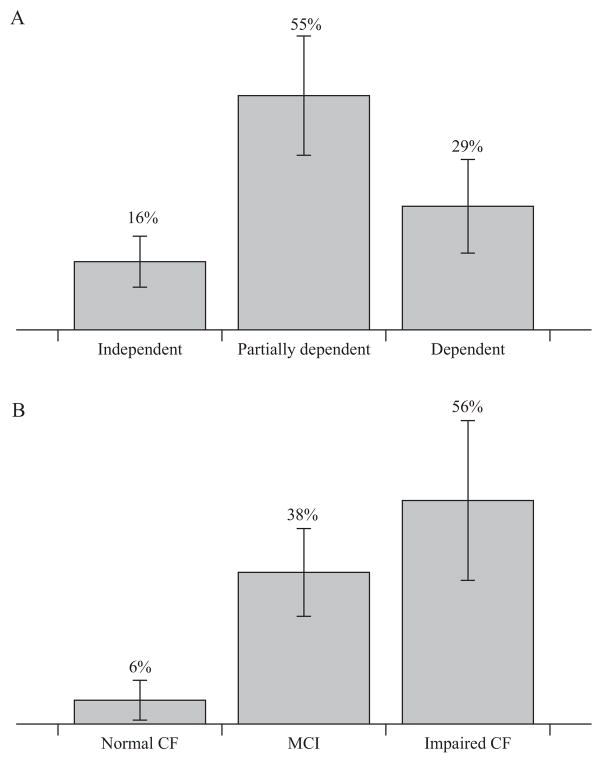
Although certainly in the minority, some centenarians maintain a standard of physical capacity commensurate with continued professional activities, and therefore it is now possible to find some centenarians that continue to work as managers, artists, scientists, and politicians. An example of the latter being the Nobel Prize winner Dr. Rita Levi-Montalcini who, at the age of 103, in 2012, is a senator for life in the Italian Parliament [11]. There are also longevous individuals whose functional capacity allows them to continue to participate in challenging sporting events such as Mr. Fauja Singh, who, in 2011, as a centenarian completed a marathon in Toronto, Canada, in 8 h and 25 min [12]. However, despite these rare and rather inspiring examples of successful ageing, the reality is that several epidemiological studies [6,10,13–17] have revealed that only ~16% of centenarians have the physical capacity to independently perform the activities of daily living, with the majority being partially or totally dependent (Fig. 1; Panel A). Moreover, age-related cognitive dysfunction also plays a significant role in centenarians, with a combination of data from 3 epidemiological studies [10,14,18], indicating that only 6% of centenarians display completely preserved cognitive function (Fig. 1; Panel B). This diminished cognitive function in the very old is clearly an important and widespread issue that likely interacts significantly with physical function and the potential role that exercise can play in the health of this population, but is beyond the scope of this review.
Fig 1.
Independence and cognitive function (CF) of centenarians. Data are presented as mean ± SD. The average level of independence (Panel A) was calculated by taking into account the ADL scale from 6 epidemiological studies (3341 centenarians). The mean level of cognitive function (Panel B) was calculated from the cognitive scores reported in 3 epidemiological studies (1150 subjects). MCI, mild cognitive impairment.
3. Physical capacity, ageing, and longevity
Many factors including genetics and quality of health care combine to yield longevity, however, the important components of a healthy life-style, such as maintaining exercise capacity, is certainly important [1]. Indeed, the capacity to limit age-related diseases has been proposed as one of the mechanisms responsible for successful aging in extremely old subjects [19], and maintaining exercise capacity and subsequently physical function likely plays a significant role in this process. Exercise capacity, defined by maximal oxygen consumption (VO2max) in response to a graded exercise test, is a strong predictor of health and independence in older adults [20] and has been documented to decline by 10–15% per decade between the ages of 50 and 75 years [21]. Indeed, there is a strong association between ageing, independence, and maximal aerobic capacity; thus, understanding the physiological basis for the decline in VO2 with age has clear practical significance in terms of identifying the means by which the capacity for an independent lifestyle can be maintained [22].
The potential physiological limitations to maximal exercise are many, with deep rooted arguments about the role of oxygen supply and demand [23,24], however all agree that the crucial journey that oxygen makes from air to muscle cells commences in the lung. With progressive age, there is significant decline in lung function, due predominantly to a loss of elastic recoil [25]. This increase in lung compliance with age results in reduced maximal expiratory flow rates and an increase in resting functional residual capacity [26]. During exercise this translates into marked mechanical ventilatory constraints and increased ventilatory requirement in the 9th and 10th decades of life [27]. Thus, due to these mechanical challenges, the strategy used to achieve an exercise-induced increase in ventilation differs between the young and the old with the very elderly being likened to patients with chronic obstructive pulmonary disease (COPD) [27].
Certainly the decline in maximal exercise capacity with ageing is caused by many factors and not all factors contribute equally over the lifespan, however there is considerable evidence that blood flow and progressively attenuated vascular function play a significant role. Indeed, studies suggest that declining muscle blood flow [28], decreased maximal cardiac output [29], and potentially a maldistribution of that cardiac output, contribute significantly to the of age-related decline in VO2 between the 5th and 8th decades of human life. The interface between the vasculature and skeletal muscle, the capillary bed, the site for the diffusion of oxygen from the red blood cells to muscle mitochondria, is also a major factor in determining VO2 [30]. Thus, a declining muscle-diffusing capacity likely contributes to the reduction in VO2 with ageing. However, a definitive conclusion must be tempered by some reports that indicate that the number of capillaries around a fiber does not decline with ageing [31,32].
Skeletal muscle itself, toward the end of the oxygen cascade, determines oxygen demand and therefore has the potential to tremendously impact exercise capacity. Aging is associated with sarcopenia, the etiology of which is not well understood, but is likely related to age-related alterations in the nervous system, the hormonal milieu, nutrition, and physical activity which speed the loss of muscle mass [33]. Indeed, thigh muscle volume is typically reduced 24–27% between the second and seventh decade [34] and this decline in muscle mass and lower limb power correlates well with quality of life and activities of daily living (ADL) in the elderly [35]. Interestingly, there is evidence that sarcopenia is muscle fiber type specific, with type II fibers being more susceptible to atrophy than type I fibers [36]. Specifically, Lexell et al. [36] revealed that the type II fibers of 80-year-old subjects were ~26% smaller than 20-year-old controls, while type I fibers were not different in size. This age-related loss of fast motor units (type IIA and IIX fibers) causes a progressive shift toward a slower phenotype [37], which, although, as of yet, not definitively associated with the aging process, could conceivably result in improved mechanical efficiency [38]. Additionally, although studies have documented an overall reduced mitochondrial volume with ageing [39], there are contradictory results in the literature on the effects of ageing and mitochondrial oxidative capacity [40,41].
4. Physical function and centenarians
Insight into the effect of ageing on maximal exercise capacity can be achieved by studying a successful model of human ageing, such as centenarians. Indeed, when the factors that appear to have been beneficial to centenarians are evaluated, regular physical activity seems to be the most important lifestyle behavior that positively influences chronic disease risk factors. For example, a recent cross-sectional epidemiological study by Ozaki et al. [13] found that the level of independence, lifestyle, and health of Japanese centenarians (566 men, 1341 women) were significantly correlated with the level of physical activity. Specifically, the capacity to escape or delay cardiovascular impairment associated with ageing has been proposed as one of the mechanisms that may help to explain successful ageing in centenarians [19].
In the Georgia Centenarian Study [16,18], maximal knee extension and handgrip strength, indicators of physical capacity, were measured in a cohort of 153 centenarians. Interestingly, using these measures, the effect of extreme longevity was documented to reverse the standard ratio of maximal force between the lower and upper limbs. Specifically, in a prior study in young healthy subjects, Samson et al. [42] revealed that maximal quadriceps strength was 43% higher than the maximal strength of the forearm muscles and that the age-related rate of decrease in maximal quadriceps strength was more pronounced than in the upper limbs, such that at 80 years old this difference was reduced to 27%. Following this trend, but suggestive of a much greater trajectory with increased age, additional data reported by Davey et al. [18] indicated that maximal quadriceps strength was actually 33% less than maximal handgrip strength in 27 male centenarians and 22% less in 126 of their female counterparts. Therefore, it seems that the rate of decline in maximal quadriceps strength decreases dramatically from 80 to 100 years old, while, in contrast, the tendency for forearm strength to decline is less remarkable. In agreement with this interpretation, previous studies have documented that the age-related decrease in limb mass is greater in the lower limbs (~15%) compared to the upper limbs (~10%) [34]. Also likely contributing this phenomenon, is the recognized greater loss of motor units in the lower compared to the upper limbs with age [43]. Therefore, although the reasons for this difference are currently unclear, an additional undeniable factor is that, even in completely independent older subjects, locomotor based physical activity is significantly reduced with old age and the greater decrease in muscle mass in the lower limbs might simply be the result of reduced use.
Of significant importance in terms of exercise capacity and centenarians, in the only study to directly assess graded whole body (cycle) exercise in Centenarians, our group [44] revealed that Centenarians have a significantly attenuated oxygen cost for a given absolute work rate. As expected, young controls had a typical slope of oxygen utilization of 9–11 ml/O2/W, but, remarkably, used the same amount of oxygen at 15–20 W as the centenarians did at their maximum work rate of 35 W (Fig. 2A). It was speculated that this increased skeletal muscle work efficiency exhibited by healthy centenarians is primarily due to the loss of fast motor units in the lower limbs. Specifically, selective sarcopenia might contribute to a positive effect on mechanical efficiency, and therefore on the oxygen cost of performing work, by resulting in a slower muscle fiber phenotype [38]. Such an improvement, or selection process, which results in improved mechanical efficiency with extreme age has important implications for functional capacity as this means that the amount of work that centenarians can achieve within their much-attenuated scope of aerobic capacity is greatly enhanced.
Fig 2.
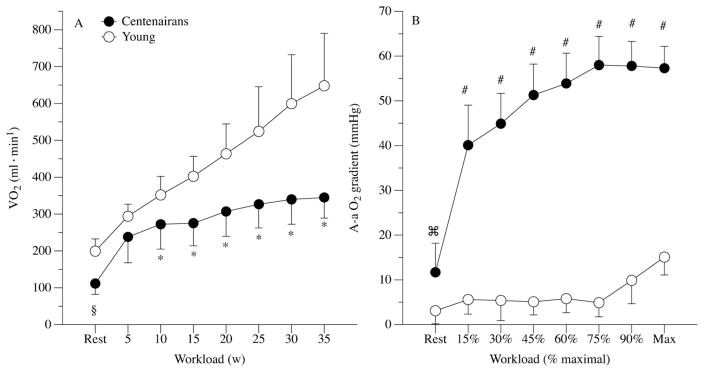
Oxygen uptake at rest and during incremental cycle exercise to maximal effort in centenarians and sub-maximal effort in young controls (Panel A). Alveolar to arterial oxygen partial pressure gradient at rest and during incremental exercise to maximal effort in both centenarians and young controls (Panel B). Data expressed as mean ± SD. §, significantly reduced in the centenarians at rest. *, significantly reduced in the centenarians during exercise. ⌘, significantly elevated in the centenarians at rest. #, significantly elevated in the centenarians during exercise.
In this same study [44], we assessed pulmonary function both during rest and exercise in these apparently healthy centenarians. As the first organ that facilitates the oxygen cascade from air to cell, the lungs play an important role in the essential physiological process of oxygen transport and therefore physical capacity. At rest, although ventilation itself was not different between the young controls and the centenarians, upon closer inspection of the open circuit calorimetry data, even in this state of relatively low metabolic demand, there was already a very important distinction between these two groups. Specifically, the dead-space to tidal volume ratio was significantly elevated in the centenarians, likely explained by an age-induced increase in lung compliance. Such a conclusion is supported by the spirometric assessments that revealed a significant reduction in FEV1, FVC, and the ratio of these two variables in the centenarians compared to the young controls. The practical consequence of this pulmonary dysfunction was that even at rest there was a clear alveolar to arterial (A-a) O2 gradient in the centenarians that was not evident in the young controls.
Upon the commencement of exercise, the A-a gradient in the Centenarians was dramatically increased and grew with exercise intensity (Fig. 2B). Based upon both the exercise-induced hypoxemia literature [45] and evidence that direct hypoxia itself [46] can severely impact exercise performance, although admittedly not performed in people of this age, there is little doubt that this large A-a gradient had a negative impact on the maximum work rate achieved during cycle exercise in the centenarians. Although of greater magnitude, which may be explained by the ~25 year age difference between the centenarians studied in the current research and the subjects who were considered old in previous work, these findings are in agreement with recognized decrements in lung function during exercise with advancing age [47].
It is also interesting to note that, in this same maximal exercise study in healthy centenarians by our group [44], VO2 peak and maximal handgrip strength, both recognized as strong predictors of health and independence in older adults [20,48], were well correlated. In agreement with these observations, a significant relationship between maximal handgrip strength, cognitive function, and independence was recorded in a European study of 476 centenarians [49]. These correlations underline the potential use of both handgrip strength and whole body maximal testing as good predictors of successful ageing in this longevous population, with the former approach being less complex and with reduced risk.
5. Multi-factorial influences on physical function and human longevity
Ageing is a gradual biological process from the cellular to the systemic level and is generally characterized by continuous, progressive modifications over one’s lifespan. This process can be partially considered a series of inevitable, regressive physiological phenomenon [1,3], like reduced lung function, decreased maximal oxygen uptake and maximal strength, increased body mass and body mass index, intensified sarcopenia and the alteration of muscle phenotype. However, other age-related factors such as orthopedic issues and cognitive deterioration affect human physical function and therefore contribute to the longevity of centenarians (Fig. 3). All of these phenomenon can worsen the frailty threshold of the older population with the consequential loss of adaptability, which is essential for successful ageing [8]. Therefore, ageing can be considered a ‘network’ characterized by genetic and physiological factors. In Fig. 3 these contributing factors to successful or unsuccessful aging are acknowledged and the possible role of altered skeletal muscle efficiency, and subsequent capacity to maintain a reasonable level of physical activity, recognized recently by our group [44], is highlighted.
Fig 3.
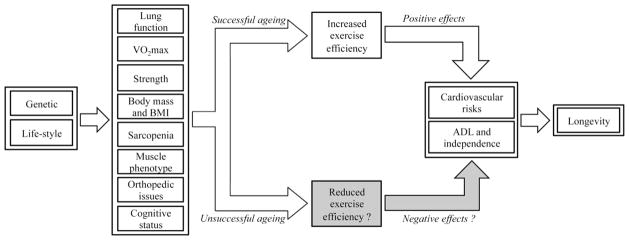
Schematic pathway of the multi-factorial influences on longevity, and the potential role of altered exercise efficiency in this process.
6. Conclusions
Progressive reductions in lung function, maximal oxygen uptake, maximal strength, and sarcopenia, are just some examples of the multi-factorial decline in physical function that is typically associated with ageing. Although other age-related factors that may be more difficult to target with exercise such orthopedic issues and cognitive deterioration do play a significant role, the scientific literature underlines the positive effects of exercise on the physical capacity and longevity of extremely old people such as centenarians. Recognizing the strong relationship between ageing, exercise capacity, and independence, understanding the physiological basis of this multi-factorial decline has important practical significance in terms of identifying methods by which an independent lifestyle can be maintained.
Acknowledgments
Funding
This work was financially supported in part by the NIH (PO1 HL, 09830), the VA (Merit Grant E6910R), and Mons Mazzali Foundation.
Footnotes
Contributors
The authors declare that they contributed to writing and review this manuscript.
Competing interest
The authors have no conflicts of interests.
Provenance and peer review
Commissioned and externally peer reviewed.
References
- 1.Perls T, Terry D. Understanding the determinants of exceptional longevity. Annals of Internal Medicine. 2003;139(Septmber 5 Pt 2):445–9. doi: 10.7326/0003-4819-139-5_part_2-200309021-00013. [DOI] [PubMed] [Google Scholar]
- 2.Schoenhofen EA, Wyszynski DF, Andersen S, et al. Characteristics of 32 super-centenarians. Journal of the American Geriatrics Society. 2006;54(8):1237–40. doi: 10.1111/j.1532-5415.2006.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastiani P, Solovieff N, Dewan AT, et al. Genetic signatures of exceptional longevity in humans. PloS one. 2012;7(1):e29848. doi: 10.1371/journal.pone.0029848. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terry DF, Sebastiani P, Andersen SL, Perls TT. Disentangling the roles of disability and morbidity in survival to exceptional old age. Archives of Internal Medicine. 2008;168(February 3):277–83. doi: 10.1001/archinternmed.2007.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cicero MT, Powell JGF. Cato Maior de senectute. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- 6.Antonini FM, Magnolfi SU, Petruzzi E, et al. Physical performance and creative activities of centenarians. Archives of Gerontology and Geriatrics. 2008;46(March–April 2):253–61. doi: 10.1016/j.archger.2007.04.005. [Review] [DOI] [PubMed] [Google Scholar]
- 7.Young TE. On Centenarians; and the duration of the Human Race. S.l: Charles and Edwin Layton; 1899. [Google Scholar]
- 8.Perls T, Levenson R, Regan M, Puca A. What does it take to live to 100? Mechanisms of Ageing and Development. 2002;123(January 2–3):231–42. doi: 10.1016/s0047-6374(01)00348-7. [Research Support, Non-U.S. Gov’t Research Support U.S. Gov’t, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 9.Berzlanovich AM, Keil W, Waldhoer T, Sim E, Fasching P, Fazeny-Dorner B. Do centenarians die healthy? An autopsy study. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2005;60(July 7):862–5. doi: 10.1093/gerona/60.7.862. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 10.Motta M, Bennati E, Ferlito L, Malaguarnera M, Motta L. Successful aging in centenarians: myths and reality. Archives of Gerontology and Geriatrics. 2005;40(May–June 3):241–51. doi: 10.1016/j.archger.2004.09.002. [Multicenter Study] [DOI] [PubMed] [Google Scholar]
- 11.Abbott A. Neuroscience: One hundred years of Rita. Nature. 2009;458(April 7238):564–7. doi: 10.1038/458564a. [Biography Historical Article News] [DOI] [PubMed] [Google Scholar]
- 12.100-year-old British man completes Toronto marathon. The Telegraph. 2011 [17.10.11] [Google Scholar]
- 13.Ozaki A, Uchiyama M, Tagaya H, Ohida T, Ogihara R. The Japanese Centenarian Study: autonomy was associated with health practices as well as physical status. Journal of the American Geriatrics Society. 2007;55(January 1):95–101. doi: 10.1111/j.1532-5415.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- 14.Gondo Y, Hirose N, Arai Y, et al. Functional status of centenarians in Tokyo, Japan: developing better phenotypes of exceptional longevity. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61(March 3):305–10. doi: 10.1093/gerona/61.3.305. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 15.Bauco C, Golosio F, Cinti AM, et al. Functional status and well-being of centenarians. Archives of Gerontology and Geriatrics. 1996;22(Suppl 1):363–6. doi: 10.1016/0167-4943(96)86962-7. [DOI] [PubMed] [Google Scholar]
- 16.Cress ME, Gondo Y, Davey A, Anderson S, Kim SH, Poon LW. Assessing physical performance in centenarians: norms and an extended scale from the georgia centenarian study. Current Gerontology and Geriatrics Research. 2010 doi: 10.1155/2010/310610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terry DF, Wilcox M, McCormick MA, Lawler E, Perls TT. Cardiovascular advantages among the offspring of centenarians. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2003;58(May 5):M425–31. doi: 10.1093/gerona/58.5.m425. [DOI] [PubMed] [Google Scholar]
- 18.Davey A, Elias MF, Siegler IC, et al. Cognitive function, physical performance, health, and disease: norms from the georgia centenarian study. Experimental Aging Research. 2010;36(October 4):394–425. doi: 10.1080/0361073X.2010.509010. [Comparative Study Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galioto A, Dominguez LJ, Pineo A, et al. Cardiovascular risk factors in centenarians. Experimental Gerontology. 2008;43(February 2):106–13. doi: 10.1016/j.exger.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Paterson DH, Govindasamy D, Vidmar M, Cunningham DA, Koval JJ. Longitudinal study of determinants of dependence in an elderly population. Journal of the American Geriatrics Society. 2004;52(October 10):1632–8. doi: 10.1111/j.1532-5415.2004.52454.x. [DOI] [PubMed] [Google Scholar]
- 21.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(August 5):674–82. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Archiv: European Journal of Physiology. 2003;446(May 2):270–8. doi: 10.1007/s00424-003-1022-2. [DOI] [PubMed] [Google Scholar]
- 23.Samaja M, Rovida E, Motterlini R, Tarantola M, Rubinacci A, di Prampero PE. Human red cell age, oxygen affinity and oxygen transport. Respiration Physiology. 1990;79(January 1):69–79. doi: 10.1016/0034-5687(90)90061-3. [DOI] [PubMed] [Google Scholar]
- 24.Johnson BD, Dempsey JA. Demand vs. capacity in the aging pulmonary system. Exercise and Sport Sciences Reviews. 1991;19:171–210. [PubMed] [Google Scholar]
- 25.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. American Review of Respiratory Disease. 1983;127(June 6):725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 26.DeLorey DS, Babb TG. Progressive mechanical ventilatory constraints with aging. American Journal of Respiratory and Critical Care Medicine. 1999;160(July 1):169–77. doi: 10.1164/ajrccm.160.1.9807045. [DOI] [PubMed] [Google Scholar]
- 27.McClaran SR, Babcock MA, Pegelow DF, Reddan WG, Dempsey JA. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. Journal of Applied Physiology. 1995;78(May 5):1957–68. doi: 10.1152/jappl.1995.78.5.1957. [DOI] [PubMed] [Google Scholar]
- 28.Poole D, Behnke B, Musch T. Capillary hemodynamics and oxygen pressures in the aging microcirculation. Microcirculation. 2006;13(June 4):289–99. doi: 10.1080/10739680600618793. [DOI] [PubMed] [Google Scholar]
- 29.Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometry. Journal of Applied Physiology. 1998;84(February 2):599–605. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- 30.Richardson RS, Grassi B, Gavin TP, et al. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. Journal of Applied Physiology. 1999;86(March 3):1048–53. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- 31.Mathieu-Costello O, Ju Y, Trejo-Morales M, Cui L. Greater capillary-fiber interface per fiber mitochondrial volume in skeletal muscles of old rats. Journal of Applied Physiology. 2005;99(July 1):281–9. doi: 10.1152/japplphysiol.00750.2004. [DOI] [PubMed] [Google Scholar]
- 32.Ryan NA, Zwetsloot KA, Westerkamp LM, Hickner RC, Pofahl WE, Gavin TP. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. Journal of Applied Physiology. 2006;100(January 1):178–85. doi: 10.1152/japplphysiol.00827.2005. [DOI] [PubMed] [Google Scholar]
- 33.Guillet C, Auguste P, Mayo W, Kreher P, Gascan H. Ciliary neurotrophic factor is a regulator of muscular strength in aging. Journal of Neuroscience. 1999;19(February 4):1257–62. doi: 10.1523/JNEUROSCI.19-04-01257.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Journal of Applied Physiology. 2000;89(July 1):81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 35.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. British Medical Bulletin. 2010;95:139–59. doi: 10.1093/bmb/ldq008. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- 36.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the Neurological Sciences. 1988;84(April 2–3):275–94. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 37.Lexell J. Human aging, muscle mass, and fiber type composition. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 1995;50(November):11–6. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 38.Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle and Nerve. 2001;24(May 5):654–61. doi: 10.1002/mus.1051. [DOI] [PubMed] [Google Scholar]
- 39.Wanagat J, Wolff MR, Aiken JM. Age-associated changes in function, structure and mitochondrial genetic and enzymatic abnormalities in the Fischer 344 × Brown Norway F(1) hybrid rat heart. Journal of Molecular and Cellular Cardiology. 2002;34(January 1):17–28. doi: 10.1006/jmcc.2001.1483. [DOI] [PubMed] [Google Scholar]
- 40.Hepple RT, Baker DJ, McConkey M, Murynka T, Norris R. Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation Research. 2006;9(Summer 2):219–22. doi: 10.1089/rej.2006.9.219. [DOI] [PubMed] [Google Scholar]
- 41.Mortensen SP, Dawson EA, Yoshiga CC, et al. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. Journal of Physiology. 2005;566(July Pt 1):273–85. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age and Ageing. 2000;29(May 3):235–42. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- 43.Galea V. Changes in motor unit estimates with aging. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society. 1996;13(May 3):253–60. doi: 10.1097/00004691-199605000-00010. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 44.Venturelli M, Schena F, Scarsini R, Muti E, Richardson RS. Limitations to exercise in female centenarians: evidence that muscular efficiency tempers the impact of failing lungs. Age (Dordrecht) 2012 Jan; doi: 10.1007/s11357-011-9379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dempsey JA, McKenzie DC, Haverkamp HC, Eldridge MW. Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest. 2008;134(September 3):613–22. doi: 10.1378/chest.07-2730. [DOI] [PubMed] [Google Scholar]
- 46.Roca J, Agusti AG, Alonso A, et al. Effects of training on muscle O2 transport at VO2max. Journal of Applied Physiology. 1992;73(September 3):1067–76. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- 47.Johnson BD, Badr MS, Dempsey JA. Impact of the aging pulmonary system on the response to exercise. Clinics in Chest Medicine. 1994;15(Junuary 2):229–46. [PubMed] [Google Scholar]
- 48.Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clinical Science (London) 1993;84(March 3):331–7. doi: 10.1042/cs0840331. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 49.Jeune B, Skytthe A, Cournil A, et al. Handgrip strength among nonagenarians and centenarians in three European regions. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61(July 7):707–12. doi: 10.1093/gerona/61.7.707. [DOI] [PubMed] [Google Scholar]