Abstract
Aging is associated with a gradual decline in skeletal muscle mass and strength leading to increased risk for functional impairments. Although basal rates of protein synthesis and degradation are largely unaffected with age, the sensitivity of older muscle cells to the anabolic actions of essential amino acids appears to decline. The major pathway through which essential amino acids induce anabolic responses involves the mammalian target of rapamycin (mTOR) Complex 1, a signaling pathway that is especially sensitive to regulation by the branched chain amino acid leucine. Recent evidence suggests that muscle of older individuals require increasing concentrations of leucine to maintain robust anabolic responses through the mTOR pathway. While the exact mechanisms for the age related alterations in nutritional signaling through the mTOR pathway remain elusive, there is increasing evidence that decreased sensitivity to insulin action, reductions in endothelial function, and increased oxidative stress may be underlying factors in this decrease in anabolic sensitivity. Ensuring adequate nutrition, including sources of high quality protein, and promoting regular physical activity will remain among the frontline defenses against the onset of sarcopenia in older individuals.
Keywords: Skeletal muscle, protein synthesis, anabolic resistance
Introduction
The world population is steadily aging with the proportion of adults aged 65 and older expected to exceed the number of children aged 5 years or younger within the decade (WHO 2011). As life expectancy continuous to increase, so does the need to better understand the aging process in order to promote long-term health, physical independence, and quality of life in our aging population. Not only is adequate strength required to perform activities of daily living, skeletal muscle is a key metabolic tissue in the regulation of energy homeostasis, and functions as the largest pool of amino acids for utilization by peripheral tissues (Wolfe 2006). Maintaining adequate skeletal muscle mass and strength is essential for life, and aging is associated with a gradual decline in skeletal muscle mass and strength. This decline is generally referred to as sarcopenia, although there is increasing consensus that this term should be reserved for measurable losses that result in lean muscle mass of at least 2 standard deviations below that of healthy young individuals matched for gender and ethnicity (Cruz-Jentoft et al. 2010; Morley 2008; Thomas et al. 2000).
Sarcopenia is evident in approximately 5% of people at age 65 and nears 50% of people aged 80 and older (Baumgartner et al. 1998; Morley 2008, 2012). While there are many possible causes for the age related decline in skeletal muscle mass, at a fundamental level it is thought that age-related changes in the regulation of skeletal muscle protein metabolism lead to a condition of negative protein balance, where the average rate of protein anabolism no longer keeps up with that of protein catabolism, resulting in a gradual net loss of skeletal muscle protein (Katsanos et al. 2005; Morley 2012). Protein balance is regulated by many factors that are each susceptible to change during the aging process, including hormone status (i.e. insulin, growth hormone, testosterone, and IGF-1), mechanical forces (i.e. physical activity and exercise), and nutrition (i.e. AA intake and metabolism) (Abbatecola et al. 2011; Dillon et al. 2010; Horstman et al. 2012; Kimball et al. 2002; Walker et al. 2011; Wall et al. 2012).
Besides serving as substrates in energy metabolism, AAs are important among the macronutrients as being both the building blocks of proteins as well as potent signaling molecules in the regulation of protein metabolism (Kimball and Jefferson 2006a; Wu 2009). AAs stimulate muscle protein synthesis (Crozier et al. 2005; Liu and Barrett 2002; Paddon-Jones et al. 2004; Watt et al. 1992) and the anabolic effects of AAs on protein synthesis are beyond that which can be explained by the simple increase in their presence as substrate alone (Rennie et al. 2006). Oral AAs acutely induce a stimulatory response in muscle protein anabolism of older individuals similar to that observed in young (Paddon-Jones et al. 2004) and increases in muscle mass (Borsheim et al. 2008; Dillon et al. 2009), or function (Tieland et al. 2012) have been demonstrated in older individuals following chronic AA supplementation. Among the 21 AA necessary for protein synthesis in mammals, 9 are considered nutritionally EAA as they cannot be synthesized in adult humans. In addition, some NEAA can be regarded as conditionally essential in circumstances where rates of de novo synthesis are unable to keep up with physiological demands (Wu 2009). The majority of the anabolic effects during AA feeding can be attributed to the presence of EAAs (Freudenberg et al. 2012; Tipton et al. 1999; Volpi et al. 2003). Among the EAA, the BCAA leucine is a key regulator of protein synthesis (Atherton et al. 2009; Buse and Reid 1975; Kimball and Jefferson 2006b).
Because of the complexity of anabolic signals involved in protein metabolism, much of the research into the regulation of skeletal muscle anabolism is therefore understandably focused on not only the individual contributions of the major regulators (i.e. hormonal (Sattler et al. 2009; Sheffield-Moore et al. 2011) vs. nutritional (Paddon-Jones et al. 2004) vs. mechanical (Fujita et al. 2007; Sheffield-Moore et al. 2004)) but also on the interactions between combinations of these stimuli (i.e hormones + AA: (Dennis et al. 2011; Sheffield-Moore 2000; Volpi et al. 2000), AA + physical activity: (Dreyer et al. 2008; Drummond et al. 2008; Durham et al. 2010; Ferrando et al. 2009), physical activity + insulin: (Biolo et al. 1999; Fujita et al. 2007)). Elucidating the age-related differences in the mechanisms of muscle protein synthesis is of great clinical importance. Halting or slowing the gradual loss of lean body mass that occurs during sarcopenia likely involves long term combinatorial measures aimed at improving both postabsorptive and postprandial rates of muscle protein synthesis in elderly. While the main focus of this review will be on the contributions of AA nutrition in the regulation of protein metabolism during aging, it is necessary to appreciate the complexity of the many other factors that are necessary to maximize the anabolic effect of these nutrients and signaling regulators.
Postabsorptive protein synthesis in aging
In the absence of anabolic stimuli, such as at rest in the postabsorptive (fasted) state, the rate of protein synthesis is lower than that of protein degradation resulting in a net loss of skeletal muscle protein during these periods (Figure 1). Fasting rates of protein degradation are similar between old and young adults but there has been a fair amount of discourse regarding whether basal rates of skeletal muscle protein synthesis are further reduced with aging. Some studies have reported lower rates of protein synthesis of older individuals when compared to young (Guillet et al. 2004; Rooyackers et al. 1996; Welle et al. 1993; Yarasheski et al. 1993), while others have not (Cuthbertson et al. 2005; Dillon et al. 2011; Katsanos et al. 2005, 2006; Paddon-Jones et al. 2004; Symons et al. 2009; Volpi et al. 2000; Volpi et al. 1999; Volpi et al. 2001).
Figure 1. Skeletal Muscle Protein Turnover.
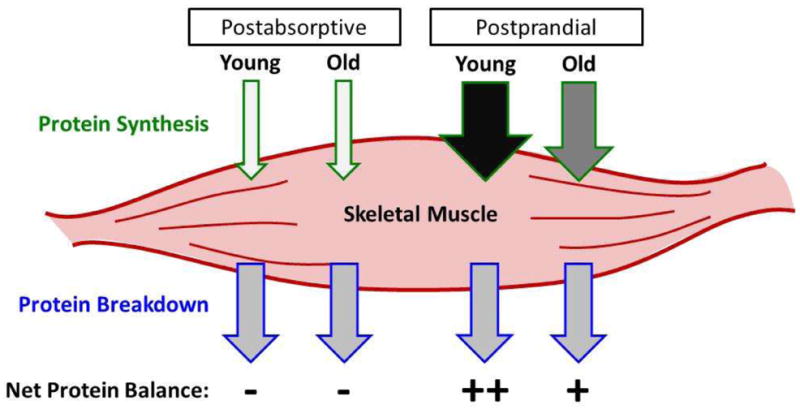
Balance between protein synthesis and breakdown in skeletal muscle switches from negative (−) net balance in the fasted (postabsorptive) state to positive (+) following AA intake (postprandial), primarily due to an upregulation of protein synthesis. The anabolic sensitivity to AA availability is blunted with age, which can result in a diminished acute synthetic response following meal ingestion in Old (+) when compared to Young (++) muscle.
Chronic dietary supplementation with EAAs has been shown to increase fasting rates of muscle protein synthesis in older individuals by some investigators (Casperson et al. 2012; Dillon et al. 2009) while others have shown no such effects (Verhoeven et al. 2009; Walrand et al. 2008; Yarasheski et al. 2011). The possible reasons for the disparities between these reports regarding fasting rates of muscle protein synthesis during aging and the influence of AA availability remain elusive but may at least in part be due to differences in study methodologies, timing of measurements, subject characteristics, habitual dietary intakes, and supplement dosing regimens (Table 1). For instance, the high protein diets utilized by Walrand et al. 2008 and Tieland et al 2012, as well as the leucine supplement used by Verhoeven et al. 2009 (2.5 g leucine compared to 4 g leucine used by Casperson et al. 2012) may have been insufficient to fortify the meals with the amount of leucine necessary to stimulate chronic changes in baseline anabolic activity (Table 1). Secondly, a possible explanation for the observed differences between studies is the dietary protein intakes of the subjects before supplementation. It is possible that habitual intakes close to the current recommendations for adults of 0.8 g protein·kg body weight−1·d−1 do not maximally stimulate protein synthesis in all older adults (Paddon-Jones and Rasmussen 2009). Findings by Walrand et al. 2008 that a high protein diet (3.0g·kg fat free mass−1·d−1) did not further stimulate protein synthesis in young or old when compared to a control protein diet containing 1.5g·kg fat free mass−1·d−1 may have reflected adequate AA intake during the control period whereas the free-living diets of the subjects in the study by Dillon et al. 2009 were not controlled and were possibly lower in protein content. Similarly, the habitual diets of 0.99 g protein·kg body weight−1·d−1 reported by Verhoeven et al. 2009 and 1.0 g reported by Tieland et al. 2012 (vs. 0.8 reported by Casperson et al. 2012) may have been adequate before supplementation. Finally, regardless of total daily dietary intake, differences in the acute distribution of daily amino acid intake may be a crucial determinant in the observed chronic effects. The effectiveness of a chronic with-meal supplementation approach vs. a between-meal approach likely depends in part on how close dietary AA intake at each meal approximates the threshold necessary to acutely reach circulating leucine concentrations necessary to elicit a maximum anabolic response in the individual. A with-meal approach may be more beneficial if dietary protein intake is not sufficient to reach this threshold but would have no further benefit on net balance across the day if this acute threshold is already met in the absence of supplementation. A between-meal approach may be more beneficial in such a case in order to increase the number of anabolic responses, and amount of time spent in positive net balance, across the day.
Table 1.
Comparison of leucine contents in supplements and concluded anabolic responses in older adults.
| Age (yrs) | Habitual protein intake (g protein/kg/day) | Supplement | Acute dose [Leu] (g) | Peak Arterial [Leu] (nmol/ml) | Concluded Acutely Anabolic in Older Adults? | Chronic Dosing | Concluded Chronically Anabolic in Older Adults? | |
|---|---|---|---|---|---|---|---|---|
| Symons et al. 2007, 2009 | 68 ± 2 | NR | 340 g Beef (90 g protein, 30 g EAA) | 5.9 | NR | Yes | - | - |
| 113 g Beef (30 g protein, 10 g EAA) | 2.0 | 330 | Yes | |||||
| Yang et al. 2012 | 70 ± 4 | 1.03 | 40 g Whey (12% Leu) | 4.8 c | 300 e,v | Yes | - | - |
| 70 ± 5 | 1.04 | 40 g Soy (8% Leu) | 3.2 c | 225 e,v | No | |||
| 72 ± 5 | 1.04 | 20 g Whey (12% Leu) | 2.4 c | 250 e,v | Yes | |||
| 72 ± 6 | 1.03 | 20 g Soy (8% Leu) | 1.6 c | 220 e,v | No | |||
| Pennings et al. 2012 | 73 ± 1 | NR | 35 g Whey | NR | 575 e,v | Yes | - | - |
| 73 ± 2 | 20 g Whey | NR | 475 e,v | Intermediate | ||||
| 73 ± 2 | 10 g Whey | NR | 375 e,v | No | ||||
| Borsheim et al. 2008 | 67 ± 6 | 0.99 ± 0.21 | EAA + Arg (22 g/day) | 4.0 | NR | - | between meals | Yes (16 weeks) |
| Casperson et al. | 68 ± 2 | 0.75–0.85 (range) | Leu (12 g/day) | 4 | NR | - | with meal | Yes (2 weeks) |
| 7 g EAA + glucose | 1.7 | NR | Yes | - | - | |||
| Rieu et al. 2006 | 70 ± 1 | 0.8 e | BCAA (0.052 g Leu/kg BW) | 3.8 c | 350 e | Yes | - | - |
| Tieland et al. 2012 | 78 ± 1 | 1.0 ± 0.1 | Protein (30 g/day) | NR | NR | - | with meal | No (24 weeks) |
| Koopman et al. 2009 | 64 ± 1 | NR | 35 g Casein | 3 c | 250 e,v | Yes | - | - |
| Paddon-Jones et al. 2004 | 67 ± 2 | NR | 15 g EAA | 2.8 | NR | Yes/Blunted vs. Young | - | - |
| Katsanos et al. 2005, 2006 | 67 ± 2 | NR | 6.7 g EAA (41% Leu) | 2.8 | 700 e | Yes | - | - |
| 67 ± 2 | 6.7 g EAA (26% Leu) | 1.7 | 459 | No/Blunted | ||||
| Verhoeven et al. 2009 | 71 ± 4 | 0.99 ± 0.07 | Leu (7.5 g/day) | 2.5 | NR | - | with meal | No (3 months) |
| Cuthbertson et al. 2005 | 70 ± 6 | NR | 10 g EAA | NR | 600 e,v | Blunted vs. Young | - | - |
| 20 g EAA | NR | 800 e,v | Blunted vs. Young | |||||
| Dillon et al. 2009 | 68 ± 2 | NR | EAA (15 g/day) | 1.4 | NR | - | between meal | Yes (3 months) |
| 7.5 g EAA | 1.4 | NR | Yes | - | - | |||
| Volpi et al. 1998 | 71 ± 2 | NR | 10% AA (Intravenous) | NR | 352 ± 18 | Yes | - | - |
| Volpi et al. 2000 | 72 ± 1 | NR | 40 g AA + 40 g Glucose (2.2 g AA every 10 minutes for 3 hours) | NR | 347 | Blunted vs. Young | - | - |
| Guillet et al. 2004 | 72 ± 2 | 1.1 | 10% AA (intravenous) | NR | 351 h | Blunted vs. Young | - | - |
| Yarasheski et al. 2011 | 70 ± 4 | 0.9–1.1 (range) | Habitual Diets were adjusted ± 0.2 g protein/kg BW/day | NR | NR | - | with meal | No (3 days) |
Values are Mean ± SEM unless specified otherwise.
NR = Not reported
NS = Not significant
Calculated estimate from dose description provided in the reference.
Estimated from graphs in the reference.
Arterialized blood from heated hand vein.
Venous blood concentrations are given when arterial concentrations were not provided in the reference.
It is unclear how improving AA intake may chronically alter baseline rates of skeletal muscle protein synthesis in older individuals. However, increases in baseline protein synthesis do not effect changes in the maximum attainable acute anabolic response. For instance, the maximum acute synthetic response to an EAA load did not further improve following 3 months of EAA supplementation despite chronic improvements in basal FSR (Dillon et al, 2009). This is similar to observations in adult rats where 10 days of leucine-enhanced feeding resulted in maximally induced FSR in the postabsorptive state without further induction following acute meal feeding (Rieu et al. 2003). In contrast to adult rats, old rats receiving leucine enhanced diets in that study did not display these chronic elevations of basal FSR but did have improved anabolic sensitivity to acute meal ingestion. Observations of changes in basal protein synthesis with unchanged acute responses to AA are similar to those following chronic androgen administration in older adults and possibly reflect improvements in synthetic efficiency and increased reutilization of proteolysis-derived AA for protein synthesis. (Ferrando et al. 2003; Sheffield-Moore et al. 2000). In short, these studies may collectively point to the importance of chronically maintaining proper meal distributions and adequate acute AA intakes during aging to prevent declines in baseline muscle protein synthesis. In addition to maintaining adequate baseline rates of protein synthesis, optimization of the acute anabolic response to AA feeding will add towards protection against the onset and progression of sarcopenia.
Postprandial protein synthesis in aging
Increased AA availability enhances skeletal muscle protein synthesis in healthy older adults (Paddon-Jones et al. 2004; Pennings et al. 2012; Rasmussen et al. 2002; Symons et al. 2007; Symons et al. 2009; Volpi et al. 1998; Volpi et al. 1999; Yang et al. 2012). Some (Boirie et al. 1997; Volpi et al. 1999), but not all (Koopman et al. 2009), have shown age related increases in splanchnic extraction of oral AAs, possibly reducing AA availability for skeletal muscle protein synthesis. However, despite increased first-pass splanchnic AA uptake, skeletal muscle protein synthesis was induced similarly in older and young individuals following ingestion of 40 g of mixed AAs (Volpi et al. 1999). Besides the possibility that age related changes in AA availability may play a role in some individuals, there is a general consensus that there are changes in anabolic sensitivity to AAs at the cellular level with increased age, resulting in subpar anabolic responses under conditions of low AA availability (Breen and Phillips 2011; Cuthbertson et al. 2005; Dardevet et al. 2000).
The term anabolic resistance is generally used to describe reduced anabolic responses to stimuli. Links between reduced anabolic sensitivity to AAs and impaired insulin action have been shown in older individuals (Volpi et al. 2000) and a diminished sensitivity in anabolic signaling through the mTOR pathway in response to leucine with increased age has clearly been demonstrated in rat muscle (Dardevet et al. 2000). Insulin increases capillary recruitment and skeletal muscle perfusion (Coggins et al. 2001; Zhang et al. 2004) and is required for the optimum stimulation of mTOR signaling by AAs (Dennis et al. 2011; Prod’homme et al. 2005). The permissive action of insulin during the anabolic response to protein ingestion in healthy older persons becomes even more apparent when dietary leucine content is below the threshold to reach its maximum anabolic capacity (Katsanos et al. 2008). Furthermore, while maintaining insulin sensitivity during aging is important in order to retain robust anabolic responses through mTOR signaling following meals (Chevalier et al.), some healthy older adults with normal glucose turnover may still have blunted anabolic response to small boluses of EAAs when compared to younger individuals, even under hyperinsulinemic conditions (Volpi et al. 2000).
It has become increasingly evident that a minimum threshold concentration of AAs needs to be reached to exert a robust anabolic response in skeletal muscle protein synthesis (Kobayashi et al. 2003) and that this threshold is increased in aged muscle, requiring higher concentrations of amino acids like leucine to elicit maximum anabolic responses in old muscle comparable to those observed in young muscle (Dardevet et al. 2000; Breen and Phillips 2011). The anabolic resistance may therefore be overcome by ensuring adequate acute intakes of high quality sources of AAs, or leucine, above a minimum threshold necessary to stimulate protein synthesis (Cuthbertson et al. 2005; El-Kadi et al. 2012; Gazzaneo et al. 2011; Katsanos et al. 2006; Pennings et al. 2012; Rieu et al. 2006; Symons et al. 2007; Symons et al. 2009; Yang et al. 2012) (Table 1). It has been suggested that promoting a general minimum daily protein recommendation of 0.8 g·kg−1·d−1 for adults may not be sufficient to protect older individuals with anabolic resistance by not ensuring that the minimum threshold is met at each meal (Paddon-Jones and Rasmussen 2009). Katsanos et al. 2006 showed that the provision of 6.7 g of EAA increased muscle protein synthesis in older subjects only after the leucine content was raised from ~26% to 41% (Katsanos et al. 2006). In general, higher acute doses of AA supplements yield more robust anabolic responses in older individuals. For instance, the supplement used by Volpi et al. 2000 was administered as small boluses containing 2.2 g AA every 10 minutes for 3 hours (i.e. totaling 40 g AA), and resulted in a blunted anabolic response in the older vs. younger subjects (Volpi et al. 2000). In contrast, acute ingestion of a single 35 g protein bolus yields skeletal muscle protein synthetic responses that are similar between young and old (Koopman et al. 2009), and such dose responses to oral protein loads have been confirmed in older adults (Pennings et al. 2012). Likewise, Symons et al. 2009 showed that older and younger men had similar increases in FSR in response to either 113 g or 340 g of lean beef (containing ~30 and 90 g protein, and ~10 and 30 g EAA respectively) suggesting that protein synthesis was maximally induced in both age groups with either dose (Symons et al. 2009). Besides absolute EAA content, protein quality may have a large influence on the observed anabolic response to a meal. Yang et al. 2012 recently reported that 40 g soy protein isolate is less effective in stimulating muscle protein synthesis in older men than equal quantities of whey protein, likely due to differences in digestibility/absorption and relative leucine content (~8 and 12% for soy and whey respectively) (Yang et al. 2012). Collectively, these studies show that while the maximum rate of protein synthesis that can be induced is similar between old and young, the quantity and quality of AAs necessary to reach this maximum rate can vary. However, it remains unclear exactly which changes in cellular machinery are responsible for the age related declines in anabolic sensitivity.
EAAs and mechanisms of protein synthesis stimulation
The most widely recognized mechanism of AA induced protein synthesis involves the mTOR Complex 1 pathway (Dodd and Tee 2012), although mTOR-independent mechanisms exist (Haegens et al. 2012). Activation of mTOR Complex 1 requires interaction between mTOR and several regulatory proteins including Rheb, Rag, raptor, GβL, and LRS which leads to downstream signaling through S6K1 and 4E-BP1 and translation initiation (Han et al. 2012; Kimball and Jefferson 2006b; Roccio et al. 2006) Figure 2. Insulin facilitates AA induced stimulation of the mTOR pathway through PI3K/Akt, involving the inhibition of TSC2 (Kimball and Jefferson 2006b). TSC2, when complexed with TSC1, functions as a GTPase activating protein and results in reduction of Rheb-bound GTP to GDP and the dissociation of Rheb from the mTOR Complex 1. While the presence of insulin is required, signals provided by EAAs are necessary for full activation of protein synthesis (Anthony et al. 2002; Balage et al. 2001; Campbell et al. 1999; Hafen 2004). Association of Rheb to the mTOR Complex 1 appears to require AA, and GTP loading of Rheb has been shown to be blocked by AA depletion in vitro (Long et al. 2005; Roccio et al. 2006). Alterations in regulation of Rheb may be contributing to age related impairments in AA induced signaling through mTOR, as the upregulation of Rheb expression following resistive exercise plus ingestion of essential amino acids appears to be blunted in older adults (Drummond et al. 2009).
Figure 2. Regulation of skeletal muscle protein synthesis by essential amino acids.
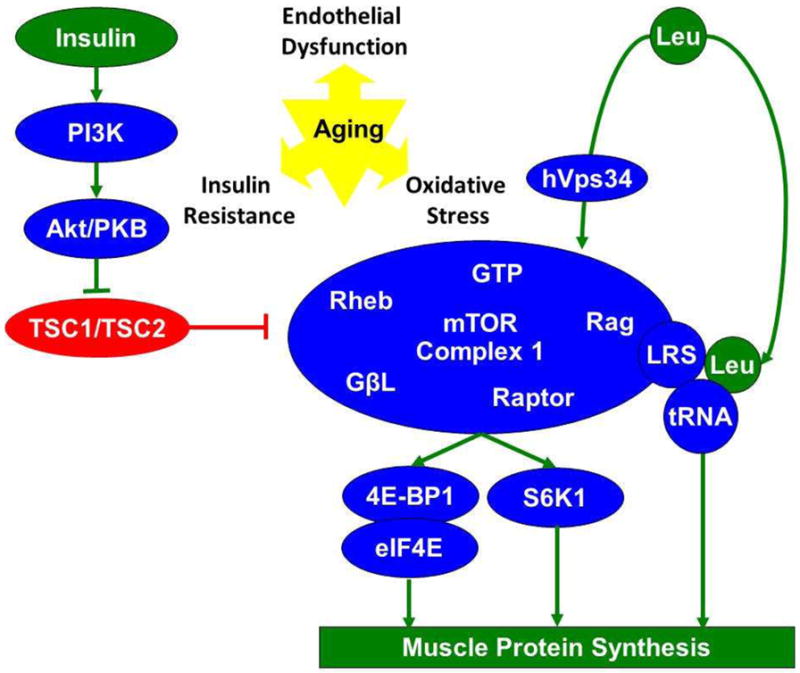
This simplified schematic illustrates some of the key known and hypothesized pathways of mTORC1 regulation by leucine and insulin discussed in this review. Although the exact mechanisms behind the age related anabolic resistance to leucine induced skeletal muscle protein synthesis remain unclear, insulin resistance, endothelial dysfunction, and oxidative stress are thought to be among the potential contributors.
The BCAA leucine is of particular interest in the discussion of AA induced anabolism because of its unique potency among AAs in stimulating protein synthesis through the mTOR pathway in adults (Atherton et al. 2009). Interestingly, the BCAAs valine and isoleucine do not appear to share this anabolic potency despite their close structural relationship to leucine (Atherton et al. 2009). However, other AAs such as glutamine and arginine, which are conditionally EAAs for infants (Wu 1998; Wu et al. 2004), appear to play important roles in the regulation of mTOR activity in earlier stages of mammalian development (Gonzalez et al. 2011; Wang et al. 2012; Xi et al. 2011; Yao et al. 2008).
The stimulatory effects of AAs, and leucine in particular, on the initiation of protein synthesis have been described in several tissues including muscle, heart, liver, pancreas, jejunum, and ovary (Dennis et al. 2011; Glynn et al. 2010; Suryawan et al. 2012a; Suryawan et al. 2012b; Wang et al. 1998; Xu et al. 1998). The regulatory role of EAAs on protein expression enters at the level of initiation of mRNA translation leading to increased synthesis of ribosomal proteins (Deldicque et al. 2005; Kimball and Jefferson 2006a; McKinnell and Rudnicki 2004; Proud 2004a; Tremblay et al. 2005). This stimulatory effect is detected only when EAAs are present and is in particular attributed to the action of the BCAAs (Kimball and Jefferson 2006b). Among these, leucine is the most potent in activating protein synthetic processes in vitro (Buse and Reid 1975) and the stimulatory effect on muscle protein synthesis is also evident in vivo, requiring the presence of either insulin or carbohydrates (Anthony et al. 2002). Single oral loads of leucine, in amounts up to 100% of the daily requirement of this AA, have been shown to increase skeletal muscle protein synthesis in a concentration dependent manner in food deprived rats (Crozier et al. 2005) but the anabolic response to leucine feeding changes with age. Older rats show improved acute anabolic responses to leucine enriched diets meal after 10 days of supplementation (Rieu et al. 2003) and this is sustained through at least 30 days (Rieu et al. 2007). In contrast to old rats, adult rats fed similar leucine-rich diets had maximally stimulated rates of protein synthesis in the postabsorptive state after 10 days (Rieu et al. 2003).
Increasing leucine intakes are positively associated with hyperphosphorylation of 4E-BP1, dissociation of 4E-BP1-eIF4E, phosphorylation of eIF4G Ser1108, association of eIF4G-eIF4E, and phosphorylation of S6K1Thr389 and promotion of translation initiation in perfused rat hindlimb (Balage et al. 2001; Nagasawa et al. 2002). However, phosphorylation of S6K1 is blunted following AA infusion in older adults and is associated with a diminished protein synthetic response when compared to responses in young adults (Guillet et al. 2004). In addition to activating translation initiation, leucine and insulin activate elongation through activation (dephosphorylation) of eEF2 through an mTOR mediated pathway (Browne and Proud 2004; Proud 2004a, b).
The detailed mechanisms through which leucine and other AAs relay signals to the mTOR pathway to regulate protein anabolism remain unclear, and how aging affects these mechanisms is even more elusive. Suggested leucine mediated mechanisms include promotion of Rheb binding to mTOR, stabilization of the activated mTOR-raptor complex, or mTOR/raptor activation through an alternative class 3 PI3K (hVps34) parallel to that controlled through insulin (Kimball and Jefferson 2006b; Long et al. 2005; Nobukuni et al. 2005). Recent studies have implicated hVps34 (Byfield et al. 2005; Gran and Cameron-Smith 2011; Nobukuni et al. 2005) and LRS (Han et al. 2012), as rate limiting leucine sensors for mTOR mediated protein synthesis. Aminoacyl-tRNA synthetases, including LRS, have been shown vulnerable to oxidative damage (Takahashi and Goto 1990) providing a possible age associated mechanism for decreased sensitivity of aging muscle to the anabolic response to leucine.
Physical activity, AAs, and aging
Physical activity provides important direct mechanical anabolic stimuli to skeletal muscle (Dreyer et al. 2010; Goodman et al. 2011) but also induces hemodynamic responses leading to increased AA availability and utilization for skeletal muscle protein synthesis. The delivery and utilization of AA for muscle protein synthesis is facilitated by increases in macrovascular blood flow (Biolo et al. 1995) and by the opening of the capillary beds for nutrient flow within the muscle (Vincent et al. 2006). This increase in nutritive flow allows for an increased exchange of AA and other substrates into, and products of degradation out of, the muscle. Moderate aerobic exercise induces muscle protein synthesis in the old and young (Sheffield-Moore et al. 2004), and while insulin mediated blood flow is impaired with age (Meneilly et al. 1995), aerobic exercise normalizes insulin induced vasodilation and skeletal muscle protein synthesis in older subjects to those found in younger subjects for up to 18 hours following the bout of exercise (Fujita et al. 2007). These changes are associated with reductions in the vasoconstrictor endothelin-1 suggesting improvements in endothelial function and increased vasorelaxation. However, despite similar responses in FSR between old and young, the anabolic efficiency of older muscle in response to AAs following exercise is reduced when compared to young (Durham et al. 2010). While microvascular blood flow was lower before and after exercise in older vs. younger subjects, the age related reduction in anabolic sensitivity to AAs during exercise could not be explained by impairments in AA availability as arterial and interstitial concentrations of several amino acids, including leucine, were higher in old when compared to young. In addition, insulin increased marginally in the old but not in the young in response to exercise plus AA infusion. Insulin mediated vasodilation in skeletal muscle is NO dependent (Steinberg et al. 1994) and age related changes in nitric oxide (NO) signaling may be central to these impairments during exercise.
Production of NO from Arginine and O2 by NO synthase (NOS) is regulated by many constitutive and inducing factors including insulin, micronutrients, and macronutrients such as amino acids (Wu and Meininger 2002). Uncoupling of endothelial NOS (eNOS) in arterioles of sedentary older rat muscle results in decreased NO production and increased production of O2− in response to in vitro stimulated flow when compared to young muscle (Sindler et al. 2009). In support of a role for reduced endothelial function in skeletal muscle anabolism during exercise in older individuals, blunting in skeletal muscle synthetic efficiency is not evident following induction of miscrovascular blood flow with a nitric oxide (NO) donor, sodium nitroprusside (SNP), plus AAs in the absence of exercise (Dillon et al. 2011). Similarly, SNP infusion improves the anabolic response to insulin in absence of exercise in skeletal muscle of older subjects (Timmerman et al. 2010). While these studies suggest that the aging muscle remains responsive to the actions of NO, age related impairments in endogenous NO production may nevertheless be both caused by (Landmesser et al. 2003) and be contributing factor to (Sindler et al. 2009) increased oxidative stress and further contribute to the impaired anabolic responses to changes in blood flow during exercise. Whether age related changes in insulin sensitivity, sarcolemmal integrity, oxidative stress, endothelial function, and/or responsiveness to exercise play significant roles in the decreased anabolic sensitivity to AAs remains to be determined.
Conclusion and perspectives
There is overwhelming evidence suggesting age related changes in amino acid metabolism contribute to the development of sarcopenia. The basic machinery necessary for anabolic responses appear to be in place with age as fasting rates of protein synthesis and degradation do not change per se and robust skeletal muscle protein anabolism can be induced when certain minimum threshold levels of anabolic stimulation are met. However, several points are emerging from the research regarding the anabolic effectiveness of AA supplementation in older adults: 1) provision of adequate amounts of leucine can acutely stimulate skeletal muscle protein synthesis in older adults equal to that in young, 2) with-meal AA supplementation may have chronic benefit if habitual dietary AA intake is inadequate, 3) between-meal AA supplementation may have chronic benefits by maximizing the frequency of anabolic stimuli, and 4) optimizing the quality of the AA source may reduce the quantity of AAs required to reach the anabolic threshold.
While aging skeletal muscle protein synthesis can be induced to levels found in young, the sensitivity of aging tissues to the presence of the anabolic stimuli decreases due to yet largely unidentified mechanisms. There appear to be clear links between this phenomenon of anabolic resistance and other age related risks such as decreased nutritional intake, decreased physical activity, decreased endothelial function, increased insulin resistance, increased oxidative stress, and increased inflammation. Successful prevention of the onset of sarcopenia will likely involve a multifactorial approach and ensuring optimum nutritional intake of quality sources of amino acids is paramount.
In the meantime, further research is needed to elucidate 1) how alterations in AA nutrition can chronically improve postabsorptive and postprandial protein synthetic efficiency in older adults, 2) how leucine directly interacts with regulatory factors of the mTOR pathway, 3) whether the anabolic machinery of muscle cells is altered with increased age in a way that explains the reductions in anabolic sensitivity to leucine, and 4) how age related changes in other anabolic factors including insulin action and responses to exercise affect the anabolic sensitivity to AA,
Acknowledgments
This work was supported, in part, through NIH/NCI RO1 CA127971 (M. Sheffield-Moore, Ph.D.). I thank William J. Durham, Ph.D. and Kathleen M. Randolph, B.S. for their input during the preparation of this manuscript.
Abbreviations
- AA
amino acid
- Akt/PKB
protein kinase B
- BCAA
branched chain AA
- EAA
essential AA
- 4E-BP1
eIF4E binding protein
- eEF
eukaryotic elongation factor
- eIF
eukaryotic initiation factor
- FSR
fractional synthetic rate
- GβL
G-protein-β-subunit-like protein
- GDP
guanosine diphosphate
- GTP
guanosine triphosphate
- hVps34
human vacuolar protein sorting 34
- IGF-1
insulin-like growth factor 1
- LRS
Leucyl-tRNA synthetase
- mTOR
mammalian target of rapamycin
- NEAA
nonessential amino acids
- NO
nitric oxide
- PI3K
phosphatidylinositol 3-kinase
- Rag
Ras-related GTPase
- Raptor
Regulatory associated protein of mTOR
- Rheb
Ras homologue enhanced in brain
- S6K1
p70 ribosomal protein S6 kinase 1
- SNP
sodium nitroprusside
- TSC
tuberous sclerosis complex
References
- Abbatecola AM, Paolisso G, Fattoretti P, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15 (10):890–895. doi: 10.1007/s12603-011-0366-0. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Lang CH, Crozier SJ, et al. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282 (5):E1092–1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Smith K, Etheridge T, et al. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2009;38 (5):1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- Balage M, Sinaud S, Prod’homme M, et al. Amino acids and insulin are both required to regulate assembly of the eIF4E. eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281 (3):E565–574. doi: 10.1152/ajpendo.2001.281.3.E565. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147 (8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268 (3 Pt 1):E514–520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Biolo G, Williams BD, Fleming RY, et al. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48 (5):949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65 (2):489–495. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- Borsheim E, Bui QU, Tissier S, et al. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008;27 (2):189–195. doi: 10.1016/j.clnu.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond) 2011;8:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24 (7):2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56 (5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280 (38):33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Wang X, Proud CG. Nutrients differentially regulate multiple translation factors and their control by insulin. Biochem J. 1999;344(Pt 2):433–441. [PMC free article] [PubMed] [Google Scholar]
- Casperson SL, Sheffield-Moore M, Hewlings SJ, et al. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012 doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S, Goulet ED, Burgos SA, et al. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci. 66(6):681–688. doi: 10.1093/gerona/glr036. [DOI] [PubMed] [Google Scholar]
- Coggins M, Lindner J, Rattigan S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50 (12):2682–2690. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- Crozier SJ, Kimball SR, Emmert SW, et al. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135 (3):376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39 (4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19 (3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, et al. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130 (11):2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Deldicque L, Theisen D, Francaux M. Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur J Appl Physiol. 2005;94 (1–2):1–10. doi: 10.1007/s00421-004-1255-6. [DOI] [PubMed] [Google Scholar]
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286 (10):8287–8296. doi: 10.1074/jbc.M110.209171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon EL, Casperson SL, Durham WJ, et al. Muscle protein metabolism responds similarly to exogenous amino acids in healthy younger and older adults during NO-induced hyperemia. Am J Physiol Regul Integr Comp Physiol. 2011;301 (5):R1408–1417. doi: 10.1152/ajpregu.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon EL, Durham WJ, Urban RJ, et al. Hormone treatment and muscle anabolism during aging: androgens. Clin Nutr. 2010;29 (6):697–700. doi: 10.1016/j.clnu.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon EL, Sheffield-Moore M, Paddon-Jones D, et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94 (5):1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd KM, Tee AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab. 2012;302 (11):E1329–1342. doi: 10.1152/ajpendo.00525.2011. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, Pennings B, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294 (2):E392–400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Glynn EL, et al. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010;199 (1):71–81. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104 (5):1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Miyazaki M, Dreyer HC, et al. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol. 2009;106 (4):1403–1411. doi: 10.1152/japplphysiol.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham WJ, Casperson SL, Dillon EL, et al. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010;24 (10):4117–4127. doi: 10.1096/fj.09-150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kadi SW, Suryawan A, Gazzaneo MC, et al. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab. 2012;302 (6):E674–686. doi: 10.1152/ajpendo.00516.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando AA, Paddon-Jones D, Hays NP, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2009;29 (1):18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Paddon-Jones D, et al. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88 (1):358–362. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- Freudenberg A, Petzke KJ, Klaus S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, Cadenas JG, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56 (6):1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaneo MC, Suryawan A, Orellana RA, et al. Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr. 2011;141 (12):2152–2158. doi: 10.3945/jn.111.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn EL, Fry CS, Drummond MJ, et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140 (11):1970–1976. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez IM, Martin PM, Burdsal C, et al. Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev Biol. 2011;361 (2):286–300. doi: 10.1016/j.ydbio.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Frey JW, Mabrey DM, et al. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol. 2011;589 (Pt 22):5485–5501. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran P, Cameron-Smith D. The actions of exogenous leucine on mTOR signalling and amino acid transporters in human myotubes. BMC Physiol. 2011;11:10. doi: 10.1186/1472-6793-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Prod’homme M, Balage M, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18 (13):1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- Haegens A, Schols AM, van Essen AL, et al. Leucine induces myofibrillar protein accretion in cultured skeletal muscle through mTOR dependent and -independent control of myosin heavy chain mRNA levels. Mol Nutr Food Res. 2012;56 (5):741–752. doi: 10.1002/mnfr.201100695. [DOI] [PubMed] [Google Scholar]
- Hafen E. Interplay between growth factor and nutrient signaling: lessons from Drosophila TOR. Curr Top Microbiol Immunol. 2004;279:153–167. doi: 10.1007/978-3-642-18930-2_10. [DOI] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149 (2):410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Horstman AM, Dillon EL, Urban RJ, et al. The Role of Androgens and Estrogens on Healthy Aging and Longevity. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos CS, Chinkes DL, Paddon-Jones D, et al. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res. 2008;28 (10):651–658. doi: 10.1016/j.nutres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82 (5):1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291 (2):E381–387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Invited Review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93 (3):1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006a;83 (2):500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006b;136 (1 Suppl):227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Borsheim E, Anthony TG, et al. Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B. Am J Physiol Endocrinol Metab. 2003;284 (3):E488–498. doi: 10.1152/ajpendo.00094.2002. [DOI] [PubMed] [Google Scholar]
- Koopman R, Walrand S, Beelen M, et al. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr. 2009;139 (9):1707–1713. doi: 10.3945/jn.109.109173. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111 (8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Barrett EJ. Human protein metabolism: its measurement and regulation. Am J Physiol Endocrinol Metab. 2002;283 (6):E1105–1112. doi: 10.1152/ajpendo.00337.2002. [DOI] [PubMed] [Google Scholar]
- Long X, Ortiz-Vega S, Lin Y, et al. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280 (25):23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004;119 (7):907–910. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Meneilly GS, Elliot T, Bryer-Ash M, et al. Insulin-mediated increase in blood flow is impaired in the elderly. J Clin Endocrinol Metab. 1995;80 (6):1899–1903. doi: 10.1210/jcem.80.6.7775638. [DOI] [PubMed] [Google Scholar]
- Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12 (7):452–456. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29(Suppl 1):i44–i48. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Kido T, Yoshizawa F, et al. Rapid suppression of protein degradation in skeletal muscle after oral feeding of leucine in rats. J Nutr Biochem. 2002;13 (2):121–127. doi: 10.1016/s0955-2863(01)00209-1. [DOI] [PubMed] [Google Scholar]
- Nobukuni T, Joaquin M, Roccio M, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102 (40):14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12 (1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286 (3):E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Pennings B, Groen B, de Lange A, et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302 (8):E992–999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- Prod’homme M, Balage M, Debras E, et al. Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol. 2005;563 (Pt 1):235–248. doi: 10.1113/jphysiol.2004.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 2004a;313 (2):429–436. doi: 10.1016/j.bbrc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004b;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6 (6):358–362. [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Bohe J, Smith K, et al. Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr. 2006;136 (1 Suppl):264S–268S. doi: 10.1093/jn/136.1.264S. [DOI] [PubMed] [Google Scholar]
- Rieu I, Balage M, Sornet C, et al. Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in aging rats. Nutrition. 2007;23 (4):323–331. doi: 10.1016/j.nut.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Rieu I, Balage M, Sornet C, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575 (Pt 1):305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Sornet C, Bayle G, et al. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133 (4):1198–1205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- Roccio M, Bos JL, Zwartkruis FJ. Regulation of the small GTPase Rheb by amino acids. Oncogene. 2006;25 (5):657–664. doi: 10.1038/sj.onc.1209106. [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93 (26):15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94 (6):1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000;32 (3):181–186. doi: 10.3109/07853890008998825. [DOI] [PubMed] [Google Scholar]
- Sheffield-Moore M, Dillon EL, Casperson SL, et al. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab. 2011;96 (11):E1831–1837. doi: 10.1210/jc.2011-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield-Moore M, Wolfe RR, Gore DC, et al. Combined effects of hyperaminoacidemia and oxandrolone on skeletal muscle protein synthesis. Am J Physiol Endocrinol Metab. 2000;278 (2):E273–279. doi: 10.1152/ajpendo.2000.278.2.E273. [DOI] [PubMed] [Google Scholar]
- Sheffield-Moore M, Yeckel CW, Volpi E, et al. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287 (3):E513–522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, et al. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587 (Pt 15):3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, et al. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94 (3):1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Nguyen HV, Almonaci RD, et al. Differential regulation of protein synthesis in skeletal muscle and liver of neonatal pigs by leucine through an mTORC1-dependent pathway. J Anim Sci Biotechnol. 2012a;3(3) doi: 10.1186/2049-1891-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Torrazza RM, Gazzaneo MC, et al. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res. 2012b;71 (4 Pt 1):324–331. doi: 10.1038/pr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons TB, Schutzler SE, Cocke TL, et al. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86 (2):451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- Symons TB, Sheffield-Moore M, Wolfe RR, et al. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109 (9):1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Goto S. Alteration of aminoacyl-tRNA synthetase with age: heat-labilization of the enzyme by oxidative damage. Arch Biochem Biophys. 1990;277 (2):228–233. doi: 10.1016/0003-9861(90)90573-h. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Ashmen W, Morley JE, et al. Nutritional management in long-term care: development of a clinical guideline. Council for Nutritional Strategies in Long-Term Care. J Gerontol A Biol Sci Med Sci. 2000;55 (12):M725–734. doi: 10.1093/gerona/55.12.m725. [DOI] [PubMed] [Google Scholar]
- Tieland M, van de Rest O, Dirks ML, et al. Protein Supplementation Improves Physical Performance in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Med Dir Assoc. 2012 doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, Lee JL, Fujita S, et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59 (11):2764–2771. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton KD, Gurkin BE, Matin S, et al. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. 1999;10 (2):89–95. doi: 10.1016/s0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Jacques H, Marette A. Modulation of insulin action by dietary proteins and amino acids: role of the mammalian target of rapamycin nutrient sensing pathway. Curr Opin Clin Nutr Metab Care. 2005;8 (4):457–462. doi: 10.1097/01.mco.0000172589.55434.03. [DOI] [PubMed] [Google Scholar]
- Verhoeven S, Vanschoonbeek K, Verdijk LB, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89 (5):1468–1475. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290 (6):E1191–1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, et al. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101 (9):2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78 (2):250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Rasmussen BB, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85 (12):4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE, et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277 (3 Pt 1):E513–520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, et al. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286 (10):1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DK, Dickinson JM, Timmerman KL, et al. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc. 2011;43 (12):2249–2258. doi: 10.1249/MSS.0b013e318223b037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall BT, Dirks ML, Verdijk LB, et al. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly, type 2 diabetic men. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00138.2012. [DOI] [PubMed] [Google Scholar]
- Walrand S, Short KR, Bigelow ML, et al. Functional impact of high protein intake on healthy elderly people. Am J Physiol Endocrinol Metab. 2008;295 (4):E921–928. doi: 10.1152/ajpendo.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Campbell LE, Miller CM, et al. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334 (Pt 1):261–267. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Zhou G, et al. Dietary l-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br J Nutr. 2012:1–11. doi: 10.1017/S0007114511006763. [DOI] [PubMed] [Google Scholar]
- Watt PW, Corbett ME, Rennie MJ. Stimulation of protein synthesis in pig skeletal muscle by infusion of amino acids during constant insulin availability. Am J Physiol. 1992;263 (3 Pt 1):E453–460. doi: 10.1152/ajpendo.1992.263.3.E453. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Jozefowicz R, et al. Myofibrillar protein synthesis in young and old men. Am J Physiol. 1993;264 (5 Pt 1):E693–698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- WHO. Global Health and Aging. 2011 http://www.who.int/ageing/publications/global_health.pdf.
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84 (3):475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128 (8):1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37 (1):1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Wu G, Jaeger LA, Bazer FW, et al. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem. 2004;15 (8):442–451. doi: 10.1016/j.jnutbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002;22:61–86. doi: 10.1146/annurev.nutr.22.110901.145329. [DOI] [PubMed] [Google Scholar]
- Xi P, Jiang Z, Dai Z, et al. Regulation of protein turnover by l-glutamine in porcine intestinal epithelial cells. J Nutr Biochem. 2011;23 (8):1012–1017. doi: 10.1016/j.jnutbio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Xu G, Kwon G, Marshall CA, et al. Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic beta-cells. A possible role in protein translation and mitogenic signaling. J Biol Chem. 1998;273 (43):28178–28184. doi: 10.1074/jbc.273.43.28178. [DOI] [PubMed] [Google Scholar]
- Yang Y, Churchward-Venne TA, Burd NA, et al. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 2012;9 (1):57. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Yin YL, Chu W, et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008;138 (5):867–872. doi: 10.1093/jn/138.5.867. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Castaneda-Sceppa C, He J, et al. Whole-body and muscle protein metabolism are not affected by acute deviations from habitual protein intake in older men: the Hormonal Regulators of Muscle and Metabolism in Aging (HORMA) Study. Am J Clin Nutr. 2011;94 (1):172–181. doi: 10.3945/ajcn.110.010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265 (2 Pt 1):E210–214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- Zhang L, Vincent MA, Richards SM, et al. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes. 2004;53 (2):447–453. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]


