Abstract
Objective
We hypothesized that an oscillatory abnormality that is consistently observed across various testing paradigms may index an elementary neuronal abnormality marking schizophrenia risk.
Methods
Compared neural oscillations in resting EEG and sensory gating conditions in schizophrenia patients (n=128), their first-degree relatives (n=80), and controls (n=110) and calculated phenotypic and/or genetic correlation of the abnormal measure across these conditions.
Results
Using a uniform, single trial analytical approach, we identified two prominent oscillatory characteristics in schizophrenia: 1) augmented neural oscillatory power was pervasive in medicated schizophrenia patients in most frequencies, most prominent in the theta-alpha range (4-11 Hz) across the two paradigms (all p<0.007); and, 2) their first-degree relatives shared significantly augmented oscillatory energy in theta-alpha frequency in resting (p=0.002) and insufficient suppression of theta-alpha in sensory gating (p=0.01) compared with normal controls. Heritability estimates for theta-alpha related measures for resting and gating conditions ranged from 0.44 to 0.49 (p < 0.03). The theta-alpha measures were correlated genetically with each other (RhoG = 0.82 ± 0.43; p < 0.05).
Conclusions
Augmented theta-alpha rhythm may be an elementary neurophysiological problem associated with genetic liability of schizophrenia.
Signficance
This finding helps to refine key electrophysiological biomarkers for genetic and clinical studies of schizophrenia.
Keywords: Neural oscillation, evoked potential, theta, gamma, schizophrenia, heritability
Introduction
Neural oscillations are electrical activities of the brain observable at different frequencies. These fundamental neural properties can be measured at various levels ranging from single neuronal firing to synchronized activities in local field potential and scalp electroencephalogram (EEG). A complex electrical signal contains essentially a full spectrum of frequencies. Measuring oscillations in schizophrenia in specific frequencies, particularly gamma band, has gained increasing attention although abnormalities in all frequency bands have been associated with schizophrenia, including gamma (Spencer et al., 2004;Cho et al., 2006), beta (Ford et al., 2008a;Tekell et al., 2005), alpha (Sponheim et al., 1994;Galderisi et al., 1991;Winterer et al., 2004), and theta and delta (Fehr et al., 2003;Winterer et al., 2004). Findings are varied with studies reporting increases or decreases in each frequency in schizophrenia. Gamma band, for example, is often found reduced in schizophrenia (Kwon et al., 1999;Brenner et al., 2003;Light et al., 2006;Spencer et al., 2004;Cho et al., 2006) although increased gamma is also found (Flynn et al., 2008;Basar-Eroglu et al., 2007) and associated with psychotic symptoms (Spencer et al., 2004). Studies typically target a specific paradigm/cognitive function and specific frequency band(s), which clearly has merits, but could result in missed opportunities to compare oscillatory anomalies across paradigms/frequency bands.
Indeed, brain electrical signals in schizophrenia patients have been found abnormal under many paradigms that involve different sensory and cognitive processes (Haenschel et al., 2009;Ford et al., 2008b;Winterer et al., 2004;Kwon et al., 1999;Brenner et al., 2003;Light et al., 2006;Spencer et al., 2004;Cho et al., 2006). These pervasive findings suggest that a common underlying oscillatory dysfunction(s) could be present across paradigms. To test this, we used a uniform single trial, full-spectrum analytic approach to measure oscillations across resting EEG and a sensory gating paradigm. Schizophrenia patients often show abnormalities in resting and sensory gating paradigms (Karson et al., 1987;Clementz et al., 1994;Sponheim et al., 1994;Freedman et al., 1996;Javitt et al., 1995). A uniform analytic approach examining the full spectrum of frequencies across paradigms may provide a comprehensive view on the myriad of oscillatory dysfunctions and at the same time test the hypothesis that there are individual oscillatory component(s) that may index the key oscillatory dysfunction in schizophrenia.
Sensory gating is typically measured by the averaged evoked potential P50. Averaging evoked potentials (AEPs) across trials produces signals that are stationary to the stimuli. While averaging helps to remove “random noise”, it can also remove non-stationary but biologically relevant signals. Neural processing of biological signals is unlikely to be restricted only to a time-locked mechanism. Measurement of oscillations that include both stationary and non-stationary components may yield complementary, perhaps even more biologically meaningful, signals. Previous studies have examined heritability on P50 sensory gating (Jansen et al., 2004;Hall et al., 2006;Greenwood et al., 2007;Anokhin et al.,2007). We have previously shown that the theta-alpha oscillation during sensory gating measured in single trials has a significant heritability that is much higher than the AEP based P50 measure (Hong et al., 2008). Similarly, resting EEG is typically analyzed as single trial oscillation rather than with the averaging approach, and oscillations measured in resting EEG represent some of the most heritable characteristics in human neural activities (van Beijsterveldt et al., 1996). In addition, AEP is also thought to be a result of summation of evoked oscillatory response with superimposed gamma, alpha, theta, and delta rhythms (Basar, 1980), and phase resetting of ongoing EEG oscillation (Sauseng et al., 2007). Therefore, single trial oscillatory signals could complement existing AEP in our search for valid, heritable endophenotypes. The term ‘neural oscillation’ does not differentiate between resting and evoked, or between transient and prolonged oscillatory activities in the context of this study. In this study, we sought to elucidate shared neural oscillatory element(s) across resting and sensory gating paradigms, which if present, may index the underlying shared genetic path that is closer to the core genetic pathology of the disease.
Material and Methods
Participants
The sample included 318 subjects: 128 patients with schizophrenia, 80 of their non-schizophrenia, non-medicated, first-degree relatives, and 110 healthy control subjects. All subjects gave written informed consent approved by local Institutional Review Board. The Structured Clinical Interview for DSM-IV (First et al., 1997) and Personality Diagnoses (Pfohl et al., 1997) were used to make Axis I and II diagnoses. Major medical and neurological illnesses, history of head injury with cognitive sequelae, mental retardation, substance dependence within the past 6 months or current substance abuse (except nicotine) were exclusionary. Smokers were required to abstain for 60 minutes before testing. Nine patients were on a first-generation antipsychotic, four were not receiving antipsychotic medication, and the rest were on one or more second-generation antipsychotic agents. Relatives with schizophrenia were excluded from group comparisons, but relatives with Axis II schizophrenia spectrum personality disorders (SSPD) were included. Controls were recruited by matching the age (± 3 years), gender, ethnicity, and zip code of the patients (some patients do not have a matching control). Controls had no DSM-IV psychotic disorders, SSPD, or family history of psychotic illness. All available and eligible first-degree relatives of the patients and controls were recruited. Subjects were between 18 to 58 years of age.
Symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS). Global functions were measured by the Strauss-Carpenter Level of Function (LOF) scale (Strauss and Carpenter, Jr., 1977). Cognition function was assessed by a cognitive battery that included 16 tasks categorized into 4 domains using their z scores: memory, problem solving, processing-speed, and working-memory composite scores (individual tasks available upon request).
The sensory gating recordings from 77% of the subjects were used in a previous report (Hong et al., 2008), although for the purpose of cross-paradigm comparisons, the entire dataset was re-processed (see below). The current data differ from the ones in Hong et al 2008 by 1) including 72 additional subjects; 2) reprocessing the entire sensory gating data using a 250ms epoch and 10 channels rather than a single channel used in the previous report; and 3) including resting EEG in all 318 subjects for testing the new hypothesis proposed here. Patients, relatives, and controls were not significantly different in age (40.0±11.6, 43.8±11.0, 40.9±12.3, respectively, F=2.67, p=0.07). Fewer males were in the relative than the patient or control groups (%male: 35.0, 68.0, 60.0%, respectively, 2=22.47, p<0.001). Patients and controls did not significantly differ in gender ( 2=1.64, p=0.20). The sample included 78 family units ( 2 subjects per family): 54 patient probands and 88 relatives belonging to 32 families of size 2, 14 of size 3, 6 of size 4, and 2 of size 6; and 24 families from community controls (22 of size 2, 1 of size 3, 1 of size 4). They formed 181 informative pairs for heritability estimates.
Laboratory Procedure
Resting EEG and sensory gating were recorded in a fixed order using 1 KHz sampling rate at 0.1-200 Hz. Data were collected using a Neuroscan (Charlotte, NC) SynAmp 32 channel system which includes HEOG and VEOG. Eye blink information from VEOG was used in continuous recordings to mathematically reduce eye movement artifacts. Subjects sat in a semi-reclining chair inside a sound-attenuated chamber. For resting EEG, subjects closed their eyes for 5 minutes. For the paired-click paradigm, subjects listened to 150 pairs of clicks (1-ms, 75 dB, 500-ms interclick interval, 10 second intertrial interval). Data were recorded using a cap containing 28 Silver/Silver-Chloride electrodes arranged in accordance with the international 10/20 system (Quick-Cap, Neuromedical Supplies, El Paso, TX). Linked mastoid electrodes served as reference. Linked mastoid electrodes served as reference. Electrode impedance was kept below 5 kΩ.
AEP Processing
To obtain AEP P50, records were filtered at 3-100 Hz (24 octave), epoched, baseline-corrected, threshold-filtered at ±75 μV, and averaged to obtain the first (S1) and second (S2) stimulus P50 waves. P50 gating was the S2/S1 P50 ratio (Hong et al., 2008). CZ was used for AEP P50 scoring (Nagamoto et al., 1989;Clementz et al., 1998).
Single Trial Oscillation Processing
Single trial analyses were performed on 10 electrodes distributed across the scalp (Figure 1). To maximize comparability, records from both paradigms were epoched for a uniform 250 ms using the original 0.1-200 Hz data: the first 250 ms of every 1 second resting EEG and a 25 to 275 ms post-stimulus period for S1 and S2. The 250-ms duration was based on analysis of 125-ms epochs, which showed maximal signals in 25-275 ms post-stimulus (Hong et al., 2008). An 8-level biorthogonal discrete wavelet transform (DWT) was then applied to each single-trial to decompose activities into 8 “details” (D1 to D8). In DWT, each detail is orthogonal to the others (Daubechie, 1992), permitting separation of EEG oscillatory signals into different elements that are mathematically independent to the others (Hong et al., 2007). Wavelet transform of single trials also has the advantage of extracting both stationary and nonstationary energy. Wavelets can be used as continuous or discrete wavelet transforms, i.e., CWT or DWT. In CWT, the dilation and translation parameters vary continuously; in DWT, the parameters are discretized. CWT and DWT do not provide frequency bands. They provide scales. To communicate WT decompositions in frequency bands, it is common to use a scale-to-pseudo-frequency conversion. From our simulations the formula for pseudo-frequency calculation is not always precise for EEG/ERP data but one can run a simulation to verify the frequency bands (Hong et al 2008). Biorthogonal DWT decompositions have interesting mathematical properties because they are constructed in a way that if the details and the approximation are reversed, one returns the ‘sum’ of decompositions into the original signal. This is one of the mathematical arguments on the merit of DWT for EEG/ERP data. CWT is advantageous in many applications, for example providing a display of the entire frequency spectrum distribution. It does not provide a band. Defining a frequency band on CWT derived data is arbitrary. CWT and Fourier transform provide limitless ways to segregate data into frequency bands and this flexibility has many advantages. DWT details (used as frequency bands) are determined by the type of DWT chosen and the nature of the data. DWT provides a rigid but perhaps disciplined means for frequency band definition, although the validity of such frequency band definition will take time and evidence to support or disapprove.
Figure 1.
Channels used for data analysis
By simulation, we estimated the frequency band of each detail: D3 corresponded to high gamma frequency activities >85 Hz; D4: gamma at 40 – 85 Hz; D5: low gamma at 20 – 40 Hz; D6: beta at 12-20 Hz; D7: theta-alpha at 5-12 Hz, D8: delta at 1-4 Hz (Hong et al., 2008). D1-D2 represented very high frequency noise and was not used. Energy within each DWT decomposition was measured by power spectrum density (PSD) using a nonparametric Welch method (Welch, 1967;Kay, 1988). Single trial data are relatively noisy compared with averaged data. Our goal here was to study the overall characteristics of the oscillations rather than individual single trial events. Therefore, single trials with PSD outside one standard deviation of the means of each frequency/electrode/stimulus single trial series were excluded, which aimed to remove trials that deviated substantially from the means and were thus more likely containing frequency- and channel-specific transient noise. There were no significant diagnosis effect in the percentage of trials removed in any paradigms (all p>0.45); and on grand average, we removed 8.7%±0.6% (mean±s.e.), 8.8%±0.6%, and 7.7%±0.7 single trials in controls, patients, and relatives, respectively. The PSD was then averaged for statistical analyses. Gating of oscillations is S2 PSD / S1 PSD. AEP P50 was scored blindly. Oscillatory measures were scored by algorithms without subjective scoring.
Statistical Methods
To define a common underlying rhythm(s) associated with schizophrenia liability, we carried out a staged analysis by first comparing between patients, relatives, and controls across paradigms (an omnibus test), followed by tests in each paradigm using mixed model for unbalanced repeated measures ANOVA, where 6 frequency bands and 10 channels were within subject factors, 3 groups the between subject factor, household a random effect, and significant age and gender covariates. Greenhouse-Geisser corrections were applied when appropriate. Post-hoc tests examined if patients and controls were significantly different in each paradigm; and if observed, whether relatives were significantly different from controls in the same frequency band and direction as the patients. The above were repeated for each paradigm.
When a frequency band fulfills all of the above, i.e., abnormal in patients and relatives in the same direction, same frequency, and in both paradigms, we would further examine its phenotypic and genetic correlation across paradigms. Pearson’s correlation was performed for phenotypic correlation. For genetic correlation, we first determined the genetic contribution to each trait by estimating the proportion of the variance attributed to additive genetic effects, calculated using variance components analysis implemented in the SOLAR program (Almasy and Blangero, 1998). Significant effects of age and sex were adjusted. If there are indeed heritable traits within each paradigm, we would then examine their genetic correlation using bivariate analyses to estimate the additive genetic correlation rhoG and the shared environmental correlation rhoE in SOLAR. Conceptually, two heritable traits could have shared or independent genes. Quantitative traits with shared genetic contribution could indicate overlapped underlying genetic pathways and help to better understand the genetic relationships between oscillatory dysfunctions. The significance of genetic sharing was evaluated by likelihood ratio test comparing with zero (no genetic sharing) (Almasy and Blangero, 1998).
To control Type I error rates, we only performed post hoc group comparisons that were implicated by significant main effect or interaction terms. Furthermore, significance levels were corrected for the number of dependent variables in a paradigm, e.g., p thresholds for main effect and group x site x frequency band 3-way interaction were corrected for testing three oscillatory responses (S1, S2, and ratio, p<0.017=0.05/3) in the sensory gating paradigm. Significant interactions were followed by channel x group 2-way tests on each frequency, here correcting for 6 frequency band tests (p< 0.0083 =0.05/6). Significant findings were examined by secondary analyses applying corresponding repeated measure ANOVA or comparison of simple effects (Cohen and Cohen, 1983). Heritability was estimated on measures consistent with cross-paradigm, familial oscillatory abnormalities. We also examined correlations with clinical features in symptom, cognition, and function. Relationships between AEP and oscillatory measures were also examined. Significance levels were corrected for the number of correlations performed.
Results
The omnibus test for paradigm x frequency x channel x group showed significant effects of group (F(6, 298)=6.71, p<0.001) and a 4-way interaction (F(178, 8862)=2.43, p<0.001). Subsequent analyses were carried out separately in the two test conditions.
1. Resting state neural oscillations
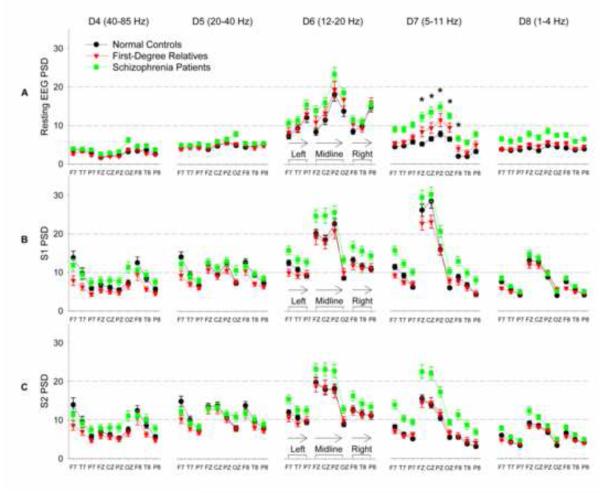
All group mean and variance data are plotted in Figure 2A and statistics are in Table 1A. There were significant group (p=0.001) and frequency x group interaction (p=0.001) effects for resting oscillations. Post hoc tests were performed on Bonferroni corrected significant frequencies in gamma, theta-alpha, and delta bands (in bold, Table 1A). Resting gamma (40-85 Hz) was elevated in patients compared with controls (F(1, 218)=4.09, p=0.04) but no significant control-relatives differences (p=0.25). In the low frequencies, resting theta-alpha band (5-11 Hz) was elevated in patients compared with controls (F(1, 218) = 32.38, p < 0.001), with significant effects seen in all 10 channels (all p<0.001). Relatives also showed elevated energy in resting theta-alpha (p = 0.008) compared with controls in midline (FZ: p=0.002, CZ: p=0.02, PZ: p=0.03, OZ: p=0.02) and right frontal (F8: p=0.01) channels (Figure 3B plots data from FZ). Delta band (1-4 Hz) showed a significant group effect. Patients (F(1,218) =18.85, p<0.001) but not relatives (p=0.17) showed increased delta energy compared with controls. To summarize the findings in resting oscillations, augmented gamma and markedly augmented low frequency activities at rest separated patients and controls; while augmented theta-alpha energy in the midline (FZ, CZ, PZ, OZ) and right frontal sites (F8) were the only resting oscillatory abnormalities that were present in both patients and unmedicated relatives.
Figure 2.
Oscillatory activities across channels, frequencies, and paradigms (mean±s.e.) and their scalp topographic distributions. Single trial oscillatory activities from each channel are plotted here. To help viewing of the graphs, data from left, midline, and right channels are clustered together, and within each cluster plotted from anterior to posterior. D3 (>85 Hz) is not plotted here due to space constrains and the effects are similar to D4 (40-85 Hz) in most cases. Note the highly replicable topographic patterns across the three groups as stimulus conditions change. Statistics of specific analyses are in Table 1. * Significantly different between controls and both patients and relatives in these oscillatory components
Table 1.
Statistical results of group main effects and interactions for resting EEG (A) and sensory gating (B). All mean and s.e. values are presented at Figure 2. “Group” is group main effect among patients, relatives, and controls. All analyses in the table used age and gender as covariates. In 3-way tests, p value threshold for significance were adjusted to 0.05 for resting EEG and 0.017 (0.05/3) for sensory gating to account for 3 tests of S1, S2, and S2/S1 ratio. Only interactions related to frequency and group are presented here given that these are related to the primary aims. In the 2-way tests, threshold p values for main effects and interactions were 0.0083 (0.05/6), to account for testing 6 frequency bands. Note theta-alpha is the only frequency band showing group effect across all conditions. Significant findings after Bonferroni corrections are shown in bold.
|
A: Resting EEG Oscillations Statistics
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
3-way Tests (freq x channel x group) |
2-way Tests (channel x group) |
|||||||||||||||
| Event | Group Effect |
Freq. × Group |
Freq. × Channel × Group |
High gamma (>85 Hz) |
Gamma (40-85 Hz) |
Low gamma (20-40 Hz) |
Beta (12-20 Hz) |
Theta-alpha (5-11 Hz) |
Delta (1-4 Hz) |
|||||||
| Group Effect |
Group × Channel |
Group Effect |
Group × Channel |
Group Effect |
Group × Channel |
Group Effect |
Group × Channel |
Group Effect |
Group × Channel |
Group Effect |
Group × Channel |
|||||
| n/a | F | 7.69 | 4.97 | 1.12 | 3.29 | 1.20 | 4.88 | 2.43 | 1.86 | 1.88 | 2.75 | 1.2 | 16.25 | 1.48 | 12.7 | 0.96 |
| p | [0.001] | [0.001] | 0.20 | 0.04 | 0.28 | [0.008] | 0.006 | 0.16 | 0.03 | 0.07 | 0.28 | [0.000] | 0.15 | [0.000] | 0.48 | |
|
B: Sensory Gating Oscillations Statistics
| ||||||||||||||||
| Ratio | F | 3.05 | 6.29 | 1.45 | 2.12 | 0.73 | 4.24 | 2.57 | 4.97 | 2.47 | 0.95 | 1.62 | 10.9 | 1.62 | 10.72 | 1.06 |
| p | 0.05 | [0.000] | [0.013] | 0.12 | 0.75 | 0.02 | 0.01 | [0.008] | [0.002] | 0.39 | 0.07 | [0.000] | 0.048 | [0.000] | 0.39 | |
| S1 | F | 5.78 | 2.23 | 1.74 | 7.20 | 2.71 | 5.10 | 2.56 | 2.17 | 2.62 | 5.32 | 1.09 | 6.13 | 1.77 | 1.59 | 1.96 |
| p | [0.003] | 0.05 | [0.017] | [0.001] | [0.004] | [0.007] | 0.009 | 0.12 | [0.004] | [0.005] | 0.37 | [0.002] | 0.09 | 0.21 | 0.06 | |
| S2 | F | 7.16 | 3.54 | 2.24 | 6.98 | 2.29 | 4.60 | 2.63 | 2.46 | 2.60 | 4.80 | 0.90 | 13.52 | 2.46 | 7.95 | 2.26 |
| p | [0.001] | [0.005] | [0.001] | [0.001] | 0.02 | 0.01 | [0.006] | 0.09 | [0.004] | 0.009 | 0.57 | [0.000] | 0.01 | [0.001] | 0.03 | |
Figure 3.
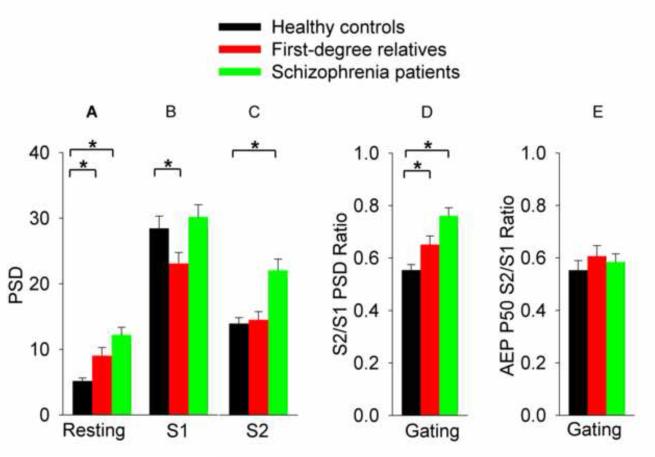
Comparisons of theta-alpha oscillations and AEP across paradigms. Data were from FZ for resting and CZ for sensory gating (S1, S2). Asterisk and bracket indicate those comparisons where patients or relatives were significantly different from healthy controls. A: Power spectrum density (PSD) of theta-alpha from resting EEG. B: PSD of theta-alpha from S1 of the double clicks. C: PSD of theta-alpha from S2 of the double clicks. D: Suppression of theta-alpha oscillatory responses based on S2/S1 PSD ratio. E. Averaged evoked potential (AEP) P50 S2/S1 ratio. Note increased theta-alpha were present in patients in resting EEG and all stimulus types. In resting EEG, theta-alpha power was significantly augmented in both patients and their first-degree relatives compared to controls (A); in sensory gating, significantly reduced suppression of theta-alpha was observed in patients and their first-degree relatives compared to controls (D).
2. Sensory gating of neural oscillations
Key findings were previously reported based on 125-ms epochs analysis at CZ (Hong et al., 2008). The current analysis adds 72 more subjects and uses a 250-ms epoch in 10 channels. There were significant 3-way interactions (p=0.013; detailed statistics see Table 1B). Post-hoc tests showed significant effects at low gamma gating (20-40 Hz), theta-alpha, and delta bands. Low gamma band was significantly different only between patients and relatives but not significantly different between controls and these two groups so no further analysis was performed. Theta-alpha gating showed a significant group effect: patients had reduced gating (F(1,214) =20.32, p<0.001) and a group x channel interaction (p=0.01) compared with controls and the effect was in all channels except OZ [largest effect in CZ (F=28.06, p<0.001) and T8 (F=20.76, p<0.001)]. Relatives also showed an interaction (p=0.02) compared with controls and reduced gating was seen in midline (CZ: p=0.01) (Figure 3C) and right hemisphere (F8: p=0.04, T8: p=0.04). These results are consistent with our previous findings at CZ (Hong et al., 2008) in this expanded sample. Delta band showed reduced suppression of delta oscillations in patients (F=15.68, p<0.001) but not in relatives (p=0.95) so no further analysis was performed. To summarize the findings in the oscillatory gating, reduced suppression of the theta-alpha oscillation at the CZ, F8, and T8 sites were the only oscillatory abnormalities present in both patients and relatives compared with controls.
When analyzing S1 and S2 responses separately, there were significant findings in gamma, beta, theta-alpha, and delta bands (Table 1B), although post hoc tests found no component that was significantly abnormal in both patients and relatives in the same direction as compared with controls (Figure 2B and 2C).
AEP P50 gating was not significantly different among the groups (Figure 3A). Correlations between P50 gating and theta-alpha gating were small for the entire sample (r=0.16) as well as in separate groups (in the range of r=0.03 - 0.29; more details in Table 2), suggesting limited overlap in AEP and single-trial based gating.
Table 2.
Cross-paradigm correlations among theta-alpha oscillations and between theta- alpha oscillations and AEPs. AEP: Averaged evoked potential. r: Pearson’s correlation coefficient. All: Three groups combined. Sample size for each group: see Methods (may vary by a few subjects in some cells due to missing data points).
| Single Trial Based Theta-Alpha |
AEP based | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Single Trial Based Theta- Alpha |
Group | S1 |
S2 |
Gating |
P50 Gating |
||||
| r | p | r | p | r | p | r | p | ||
| Resting EEG | All | .34 | .000** | .41 | .000** | .16 | .005 | − .05 | .451 |
| Controls | .27 | .006 | .30 | .002** | − .04 | .702 | − .01 | .905 | |
| Relatives | .18 | .119 | .13 | .257 | .06 | .617 | .02 | .886 | |
| Patients | .47 | .000** | .46 | .000** | .12 | .176 | − .15 | .157 | |
| S1 | All | .70 | .000** | − .19 | .001** | − .31 | .000** | ||
| Controls | .68 | .000** | − .45 | .000** | − .27 | .018 | |||
| Relatives | .78 | .000** | − .16 | .150 | − .24 | .046 | |||
| Patients | .74 | .000** | − .13 | .154 | − .37 | .000** | |||
| S2 | All | .43 | .000** | − .17 | .006 | ||||
| Controls | .22 | .024 | − .16 | .164 | |||||
| Relatives | .37 | .001** | − .05 | .686 | |||||
| Patients | .45 | .000** | -.29 | .005 | |||||
| Gating | All | .16 | .012 | ||||||
| Controls | .21 | .070 | |||||||
| Relatives | .29 | .014 | |||||||
| Patients | .03 | .772 | |||||||
Correlation was significant after Bonferroni correction for 26 comparisons (p < 0.002 ≈ 0.05/26). Replication of significant correlations in at least two of the three groups are shown in bold. Data for sensory gating (AEP and oscillations) were from CZ. Data for resting EEG were from FZ
Therefore, patients had augmented oscillations across resting and paired-sound paradigms but most of these anomalies were not found in relatives except for the theta-alpha band: its abnormalities were present in both patients and relatives, suggesting a liability rather than secondary medication effects. The abnormalities occurred coherently across the two paradigms such that theta-alpha energy was elevated at rest and did not suppress well during sensory gating.
3. Relationships of neural oscillation measures across paradigms
Resting theta-alpha and theta-alpha gating were not correlated phenotypes (r=−0.04-0.16; Table 2). However, resting theta-alpha activity was associated with S1 and S2 event-related theta-alpha power in the combined group; and the correlation between S2 and resting theta-alpha was significant in two independent groups (patients and controls).
4. Heritability
AEP P50 gating showed no significant heritability as reported earlier (Hong et al., 2008;Greenwood et al., 2007). A priori tests were performed on measures that showed significant effects in both patients and relatives. For theta-alpha resting EEG (at FZ, CZ, PZ, OZ, F8) and gating (at CZ, F8, T8), significant heritability was found in resting theta-alpha at FZ (h2=0.45, p=0.009), CZ (h2=0.44, p=0.01), and F8 (h2=0.44, p=0.02), and in theta-alpha gating at CZ (h2=0.49, p=0.005) and F8 (h2=0.44, p=0.02). Therefore, both resting and gating of theta-alpha were heritable despite the finding that they were minimally correlated traits (all r 0.16), suggesting either distinct genetic contributions or same gene(s) with pleiotropic effects. SOLAR bivariate polygenic analysis was performed on resting theta-alpha at FZ and gating of theta-alpha at CZ, which showed a significant shared gene effect (RhoG = 0.82±0.43; p=0.04). The shared environmental effect measure RhoE was not significant (p=0.51). These results indicate that they are pleiotropic traits with significant gene sharing.
5. Clinical correlates
Correlations were performed between resting theta-alpha power (at FZ) and gating of theta-alpha (at CZ) with level of functioning (LOF), symptom (BPRS total and 6 subscales), and cognition (IQ and 4 composite scores). Based on 26 correlations performed or p<0.002 0.05/26, poorer LOF was associated with higher resting theta-alpha (r=−0.23, p<0.001) and less theta-alpha gating (r=−0.25, p<0.001). Less theta-alpha gating was also associated with reduced processing speed (r=−0.24, p<0.001). No symptoms, IQ, or other cognitive scores were significant after Bonferroni corrections.
Discussion
Applying a uniform, full spectrum analysis of EEG measures obtained from resting EEG and sensory gating, this study found that augmented energy in the theta-alpha band is the only abnormality shared by patients and their relatives. These findings expand on the previously reported abnormality in gating of theta-alpha band power in the paired-click paradigm (Hong et al., 2008), and further demonstrate that the abnormality in this frequency band occurs across resting and a passive task condition in schizophrenia patients and their relatives. Measures related to theta-alpha power were among the most heritable across paradigms, and resting and gating of the theta-alpha activities appear to be pleiotropic traits with significant genetic sharing.
Our findings highlight the importance of examining the full oscillatory spectrum in ERP studies (Ford et al., 2007) and reveal extensive oscillatory abnormalities in schizophrenia, particularly in low frequencies. Low frequency rhythms contribute to normal adaptive behavior by enforcing oscillatory phase alignment to task-relevant high excitability (Lakatos et al., 2008;Schroeder and Lakatos, 2009). Abnormally elevated theta-alpha oscillations and/or abnormal gating of these oscillations could interfere with such information processing. It has been argued that cognitive impairments in complex diseases are not solely due to anomalies in single frequency such as gamma (Basar-Eroglu et al., 2008) and impairments in gamma could be related to alterations in low-frequency activities (Uhlhaas and Singer, 2010), a suggestion that is supported by extensive mechanistic studies showing that coordinated theta and gamma oscillations organize several key brain functions (Jensen and Lisman, 1998;Maurer et al., 2006;Sederberg et al., 2003;Canolty et al., 2006;Shirvalkar et al., 2010;Tort et al., 2009).
The neurobiological basis of the observed theta-alpha augmentation is unclear. The underlying mechanisms of theta-alpha rhythm generation are perhaps best understood in the septo-hippocampal pathway where medial septum provides GABAergic and cholinergic inputs to the hippocampus (Buzsaki, 2002;Stewart and Fox, 1990;Manseau et al., 2008). For example, theta oscillations (4-12 Hz is often called theta in animal literature) depend on fast GABAA receptor mediated mutual inhibition (Wulff et al., 2009). Theta oscillations also originate from hippocampus, and are capable of autonomous self-generation (Goutagny et al., 2009), and important for gating information flow in the hippocampal-prefrontal network (Siapas et al., 2005). However, the single trial based post-stimulus PSD could be a more complex measure because it is likely involved with time-locked evoked oscillations, non-stationary induced oscillations, the underlying ongoing oscillations, and their possible interactions. Whether intracranial theta oscillations observed in experimental animals are the same as the ones observed in the scalp-recorded single trial theta-alpha in humans requires additional studies. It is important to model this heritable, low-frequency abnormality observed in schizophrenia and their relatives in laboratory animals, and examine the underlying molecular mechanisms that can be targeted for drug development. Since abnormal low frequency oscillations are correlated with core cognitive and functional deficits, a novel drug treatment thus developed would be relevant to improving functional outcomes in schizophrenia.
The heritability estimates in our sample were modest compared with estimates in healthy twins (van Beijsterveldt et al., 1996). This may be due to the added noise from disease related factors when estimating heritability within pairs where one set of members has the illness. We should note that heritability calculated by SOLAR in family samples is strictly speaking familiality.
In contrast to the robust low frequency band augmentation finding, there was no clear or robust evidence of gamma band reduction in single trial analyses across the two paradigms. The lack of findings in the gamma band observed in the current study is not consistent with many reports of reduced gamma band in schizophrenia. Differences in the analytic methods (e.g., analyses of single trial vs. averaged data) or task conditions may explain the inconsistencies in findings. It is possible that reduced energy in the gamma band is observed under cognitively more demanding conditions as shown by many studies. Instead, a consistent finding in medicated schizophrenia patients is the high energy across frequencies and conditions. A tally of Figure 2 showed that patients had numerically elevated power compared with controls in 91% (137/150) of the measures. Equally intriguing is the reduced power in the relatives in many frequencies. One likely explanation is that the antipsychotic medications increased power across all frequencies, and unmedicated patients would otherwise have lower energy levels. With only 4 unmedicated patients, we lack the power to examine this directly. Clozapine could enhance gamma synchronization (Hong et al., 2004) and indeed patients taking clozapine (n=23) had high power in some conditions compared with the other patients (data not shown); although patients were put on clozapine often because they failed treatment or had poor functional outcomes so the effect could be either due to the underlying psychopathology or clozapine. Another possibility is that the non-schizophrenia status of the relatives may be associated with their ability to suppress augmented oscillations in conditions besides resting and sensory gating. Testing non-medicated patients and their relatives may resolve this issue. More female relatives could not explain the reduced power because female relatives had insignificantly higher overall PSD compared with male relatives. Ocular artifacts can contaminate both high and low frequency measures. We performed eyeblink correction of eye movement artifacts using a built-in Neuroscan add-on routine, done before any data processing. Amplitude rejection is a necessary additional step because the eye blink reduction routine fails to reject some additional high amplitude artifacts. However, high frequency, low amplitude ocular artifacts from rapid eye movements could still confound records in different frequency bands, although saccade related activities are typically over 20 Hz (Keren et al., 2010), which would have been decomposed from low frequency activities by DWT. Finally, global group differences in power could also be an artifact of group differences in conductance properties of the tissue between the scalp and brain. If true, then the groups would differ consistently in the same direction across conditions, which was not the case here (Figure 2).
In conclusion, medicated schizophrenia patients are characterized by pervasive oscillatory abnormalities across most frequencies, with abnormally elevated oscillatory activities being a prominent feature. Among them, two heuristically related theta-alpha biomarkers are present in non-medicated family members of the patients and are characterized by their shared genetic effects. It supports a hypothesis that augmented theta-alpha neural oscillation may be an elementary abnormal rhythm marking aspects of the genetic liability for schizophrenia.
Highlights.
Compared single trial based neural oscillations in resting EEG and pair-click auditory evoked potentials in schizophrenia patients and their families
Augmented theta-alpha range oscillations in resting EEG, and less suppression of the same band in the pair-click paradigm were the only abnormal oscillatory components shared by medicated schizophrenia patients and their unmedicated, non-ill first degree relatives
The theta-alpha measures for resting and pair-click conditions were heritable and also genetically correlated, suggesting that abnormally augmented theta-alpha rhythm may be an elementary neurophysiological problem associated with genetic liability of schizophrenia
Acknowledgement
Supports were received from NIH grants MH049826, MH077852, MH085646, DA027680 and the Neurophysiology Core of the University of Maryland General Clinical Research Center (# M01-RR16500). .
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: a twin study. Schizophr Res. 2007;89:312–319. doi: 10.1016/j.schres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Basar E. Relation between EEG and Brain Evoked Potentials. Elsevier; Amsterdam: 1980. EEG-Brain Dynamics. [Google Scholar]
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina KK, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int.J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Schmiedt-Fehr C, Marbach S, Brand A, Mathes B. Altered oscillatory alpha and theta networks in schizophrenia. Brain Res. 2008;1235:143–152. doi: 10.1016/j.brainres.2008.06.114. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc.Natl.Acad.Sci.U.S.A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry. 1998;155:1691–1694. doi: 10.1176/ajp.155.12.1691. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31:486–494. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Cohen R. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1983. [Google Scholar]
- Daubechie I. Ten lectures on wavelets. Society for Industrial and Applied Mathematics; Philadelphia,PA: 1992. [Google Scholar]
- Fehr T, Kissler J, Wienbruch C, Moratti S, Elbert T, Watzl H, et al. Source distribution of neuromagnetic slow-wave activity in schizophrenic patients--effects of activation. Schizophr Res. 2003;63:63–71. doi: 10.1016/s0920-9964(02)00213-x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Publishing, Inc; Arlington: 1997. [Google Scholar]
- Flynn G, Alexander D, Harris A, Whitford T, Wong W, Galletly C, et al. Increased absolute magnitude of gamma synchrony in first-episode psychosis. Schizophr Res. 2008;105:262–271. doi: 10.1016/j.schres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biological Psychiatry. 2008a;63:736–743. doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Hoffman RS, Mathalon DH. The dependence of P300 amplitude on gamma synchrony breaks down in schizophrenia. Brain Res. 2008b;1235:133–142. doi: 10.1016/j.brainres.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, et al. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry. 1996;53:1114–1121. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Mucci A, Mignone ML, Maj M, Kemali D. CEEG mapping in drug-free schizophrenics. Differences from healthy subjects and changes induced by haloperidol treatment. Schizophr Res. 1991;6:15–23. doi: 10.1016/0920-9964(91)90016-k. [DOI] [PubMed] [Google Scholar]
- Goutagny R, Jackson J, Williams S. Self-generated theta oscillations in the hippocampus. Nat.Neurosci. 2009;12:1491–1493. doi: 10.1038/nn.2440. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29:9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, et al. Heritability and reliability of P300, P50 and duration mismatch negativity. Behav Genet. 2006;36:845–857. doi: 10.1007/s10519-006-9091-6. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Hong LE, Buchanan RW, Thaker GK, Shepard PD, Summerfelt A. Beta (~16 Hz) frequency neural oscillations mediate auditory sensory gating in humans. Psychophysiology. 2008;45:197–204. doi: 10.1111/j.1469-8986.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, et al. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry. 2008;9:1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Archives of General Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- Jansen BH, Hegde A, Boutros NN. Contribution of different EEG frequencies to auditory evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2004;115:523–533. doi: 10.1016/j.clinph.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. An oscillatory short-term memory buffer model can account for data on the Sternberg task. J Neurosci. 1998;18:10688–10699. doi: 10.1523/JNEUROSCI.18-24-10688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren AS, Yuval-Greenberg S, Deouell LY. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. Neuroimage. 2010;49:2248–2263. doi: 10.1016/j.neuroimage.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Karson CN, Coppola R, Morihisa JM, Weinberger DR. Computed electroencephalographic activity mapping in schizophrenia. The resting state reconsidered. Arch Gen Psychiatry. 1987;44:514–517. doi: 10.1001/archpsyc.1987.01800180024003. [DOI] [PubMed] [Google Scholar]
- Kay SM. Modern Spectral Estimation: Theory and Application. Prentic Hall, Inc.; Englewood Cliffs, NJ: 1988. [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biological Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Manseau F, Goutagny R, Danik M, Williams S. The hippocamposeptal pathway generates rhythmic firing of GABAergic neurons in the medial septum and diagonal bands: an investigation using a complete septohippocampal preparation in vitro. J Neurosci. 2008;28:4096–4107. doi: 10.1523/JNEUROSCI.0247-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Organization of hippocampal cell assemblies based on theta phase precession. Hippocampus. 2006;16:785–794. doi: 10.1002/hipo.20202. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Freedman R. Sensory gating in schizophrenics and normal controls: effects of changing stimulation interval. Biological Psychiatry. 1989;25:549–561. doi: 10.1016/0006-3223(89)90215-1. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality. 1997.
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc.Natl.Acad.Sci.U.S.A. 2010;107:7054–7059. doi: 10.1073/pnas.0911184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc.Natl.Acad.Sci.U.S.A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Strauss JS, Carpenter WT., Jr. Prediction of outcome in schizophrenia. III. Five-year outcome and its predictors. Arch Gen Psychiatry. 1977;34:159–163. doi: 10.1001/archpsyc.1977.01770140049005. [DOI] [PubMed] [Google Scholar]
- Tekell JL, Hoffmann R, Hendrickse W, Greene RW, Rush AJ, Armitage R. High frequency EEG activity during sleep: characteristics in schizophrenia and depression. Clin EEG.Neurosci. 2005;36:25–35. doi: 10.1177/155005940503600107. [DOI] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc.Natl.Acad.Sci.U.S.A. 2009 doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat.Rev.Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- Welch PD. The use of fast fourier transform for estimation of power spectra: A method based on time averaging over short, mod ed periodograms. IEEE Trans.on Audio Electroacoustics AU-15. 1967:70–73. [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, Both M, Tort AB, Kopell NJ, Wisden W, Monyer H. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc.Natl.Acad.Sci.U.S.A. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]