Abstract
Several populations of memory T cells have been described that differ in their migration and function. Here, we have identified a unique subset of memory T cells, which we have named recirculating memory T cells (TRCM). By exposing Kaede transgenic mouse skin to violet light, we tracked the fate of cutaneous T cells. One population of memory CD4+ T cells remained in the skiñA second population migrated from the skin into draining lymph nodes (LNs) in a CCR7-dependent manner. These migrating CD4+ T cells expressed a novel cell surface phenotype (CCR7int/+ CD62Lint CD69− CD103+/− E-selectin ligands+), distinct from memory T cell subsets described to date. Unlike memory T cell subsets that remain resident within tissues long-term, or that migrate either exclusively between lymphoid tissues or into peripheral nonlymphoid sites, CD4+ TRCM migrate from the skin into draining LNs. From the draining LN, they reenter into the circulation, distal LNs and sites of non-specific cutaneous inflammation. Additionally, CD4+ TRCM up-regulated CD40L and secreted IL-2 following polyclonal stimulation. Together, our results identify a novel subset of recirculating memory CD4+ T cells equipped to deliver help to both distal lymphoid and cutaneous tissues.
INTRODUCTION
Naïve T cells migrate through lymphoid tissues where they scan dendritic cells for cognate antigen. Upon antigen recognition, naïve T cells are activated, expand and differentiate into effector T cells that can migrate into inflamed peripheral tissues. The majority of effector T cells then die by apoptosis. However, a heterogeneous pool of memory T cells survives to provide local and systemic protection in case of pathogen re-exposure.
Early studies examining memory T cells in human blood identified two distinct memory T cell subsets that could be distinguished by their homing and effector capacities (1). By definition, central memory T cells (TCM) express LN tissue homing receptors and circulate exclusively between the blood and secondary lymphoid tissues. In contrast, effector memory T cells (TEM) migrate from the blood into non-lymphoid peripheral tissues. However, with the recent discovery of resident memory T cells (TRM) (2-4), we now know that the division of memory T cell subsets is more complex than initially appreciated. Memory T cells within extra-lymphoid tissues may include both TEM and TRM. Both of these memory T cell subsets lack CCR7 expression, and so are unable to reenter normal draining LNs. While TRM may provide rapid response to pathogen re-challenge at a local site, depending on the stimulus, these cells migrate little and so may be ineffective at providing protection at a distal site of antigen exposure (3, 5).
Although it has been suggested that the great majority of cutaneous T cells is resident within the skin (2), several data suggest that a subset of memory T cells exits from the skin and reenters draining LNs (6-8). For example, large numbers of T cells have been identified in the afferent lymph of sheep. While the majority of these T cells were found to express CD4, their phenotype and migratory fate remain incompletely defined (6). These recirculating memory T cells may provide extensive immune surveillance, delivering help not only at an initial cutaneous site of antigen challenge, but also within distal tissues. In support of this possibility, a recent study determined that memory CD4+ T cells migrated rapidly within the dermis and out of skin explant cultures (9), while memory CD8+ T cells were quite immobile within the skin following infection with HSV. Accordingly, CD4+ T cells but not CD8+ T cells were required to provide protection against re-infection at a site distal to the primary inoculation site (9). Although this study did not conclusively demonstrate that the CD4+ T cells that mediate this protection originated from the primary skin site, it did suggest that non-resident memory CD4+ T cells contribute to cutaneous immune protection. Therefore, elucidating the receptors expressed by recirculating memory CD4+ T cells, and defining their migration potential may be beneficial for the study of memory CD4+ T cell responses.
Previous studies have demonstrated that adoptively transferred splenocytes or in vitro-activated T cells exit from peripheral tissues and enter into draining LNs in a CCR7-dependent manner (10, 11). However, these adoptively transferred cells may not reflect the cell populations present in the skin. Here, we determined that endogenous memory CD4+ T cells exit from the skin in a CCR7-dependent manner. We identified their surface receptor phenotype, migratory fate and function, and suggest that they are a distinct memory T cell subset.
MATERIALS AND METHODS
Mice
Kaede transgenic mice were obtained from Dr. Osami Kanagawa (RIKEN Institute) (12), rederived at Taconic and then bred at Massachusetts General Hospital. CCR7-deficient (13) and C57Bl/6 mice were obtained from the Jackson Laboratory. Mice were housed in a specific pathogen-free microisolator environment. Animal studies were approved by the institutional care and use committee at Massachusetts General Hospital.
Photoconversion of mice
Kaede transgenic mice were anesthetized and a ~2 cm x 2 cm patch of abdominal skin was shaved. All remaining hair within the shaved patch was removed by application of Veet or Nair for 1 minute, followed by washing with PBS. The shaved abdominal skin was exposed to violet light (420 nm) using a Bluewave LED visible light curing unit (Dymax) equipped with a 420 nm bandpass filter (Andover Corp) with the light source at maximum power, 7.5 cm away from the skin. As reported previously, exposure of the shaved skin to violet light did not induce IL-1β mRNA expression, affect lymphocyte proliferation in response to polyclonal stimulation, or diminish CD4+ T cell chemotaxis to the chemokine, CCL21 (8).
Tissues were isolated 24 h after photoconversion of abdominal skin for analysis, except where noted. For experiments analyzing the reentry of cutaneous T cells into the circulation and distal tissues, mice were photoconverted on 3 consecutive days and tissues were isolated 48 h after the final photoconversion. Similarly mice were photoconverted on 3 consecutive days and draining LNs were isolated after 8 d for analysis of the persistence of CD44, CCR7, CD62L, CCR4 and E-selectin ligands expression.
Bone marrow chimeras
Recipient CD45.1 mice were irradiated with 1000 rads prior to bone marrow injection. Single cell suspensions of hind leg bone marrow from WT CD45.1 CD45.2 mice, WT CD45.2 mice and CCR7-deficient CD45.2 mice were prepared. Recipient mice were injected with either a 1:1 mix of 5 × 106 WT CD45.1 CD45.2 and 5 × 106 CCR7-deficient CD45.2 bone marrow cells, or with a 1:1 mix of 5 × 106 WT CD45.1 CD45.2 and 5 × 106 WT CD45.2 bone marrow cells via lateral tail vein injection. After eight weeks, analysis of cells in blood and lymph nodes demonstrated that mice were reconstituted with both WT and CCR7-deficient T cells, with fewer CCR7-deficient than WT CD4+ T cells in LNs, but more in blood.
Antibodies and flow cytometry
Recovered tissue leukocytes were incubated for 20 minutes with TruStain FcX (Biolegend) and then were stained with fluorochrome-conjugated antibodies. The following monoclonal antibodies were used: anti-mouse CD3, CD4, CD44, CD62L, CD103, CD69, CCR4, CCR7 and CCR9 (Biolegend or eBioscience). Staining for E-selectin ligands was performed using a chimeric E-selectin-Fc fusion protein (R&D Systems) as described (14), with APC-conjugated anti-human IgG Fc-specific (Jackson Immunoresearch) as the secondary staining reagent. All samples were stained with 7-AAD before acquiring data on an LSRII (BD Biosciences) to exclude dead cells from analysis. Data were analyzed using FlowJo (TreeStar). MFI (mean fluorescence intensity) was determined by subtracting the MFI of the FMO (fluorescence minus one) control from the MFI of the stained sample.
Isolation of cells from tissues
Blood (0.5 mL) was collected from mice by cardiac puncture. Mononuclear cells were then isolated by centrifugation over lympholyte-mammal density gradients (CedarLane Laboratories). Abdominal skin was excised, scraped to remove fat, minced, and digested in 5 mL HBSS 1% FBS containing 154 U/mL collagenase type IV (Sigma) at 37°C for 45 minutes. Samples were then vortexed for 30 seconds and washed through a 70 μm nylon filter. Draining axillary LNs and non-draining cervical LNs were isolated, processed and recovered live cells were counted as described (11).
Cytokine secretion assay
24 h after Kaede transgenic mouse abdominal skin photoconversion, draining axillary LNs were isolated and recovered cells cultured at 107 cells/mL in RPMI 5% FBS in 24-well plates coated with anti-mouse CD3 (2 μg/mL) (Biolegend) and addition of anti-mouse CD28 (1 μg/mL) (Biolegend) for 4 h at 37°C. 100 μM TAPI-2 (Peptides International) was added to each well to preserve CD62L expression during stimulation. Cells were then analyzed for secretion of IL-2, IL-10 and IFN-γ using the appropriate cytokine secretion assay-detection kits (APC) (Miltenyi Biotec) following the manufacturer’s instructions.
CD40L staining
24 h after Kaede transgenic mouse abdominal skin photoconversion, draining axillary LNs were isolated and CD4+ T cells were purified using CD4 Dynabeads (Dynal) according to the manufacturer’s instructions. Purified CD4+ T cells were then cultured at 106 cells/mL in RPMI 5% FBS in 24-well plates with or without PMA (50 ng/mL) and ionomycin (500 ng/mL) stimulation for 2 h at 37°C. Cells were then removed from plates, washed extensively and co-stained with APC-conjugated antibody directed against mouse CD40L (Biolegend).
Statistics
Comparisons were analyzed for statistical significance by Student’s t-test with Microsoft Excel software, with P < 0.05 being considered significant.
RESULTS
CCR7-dependent lymphocyte egress from normal skin
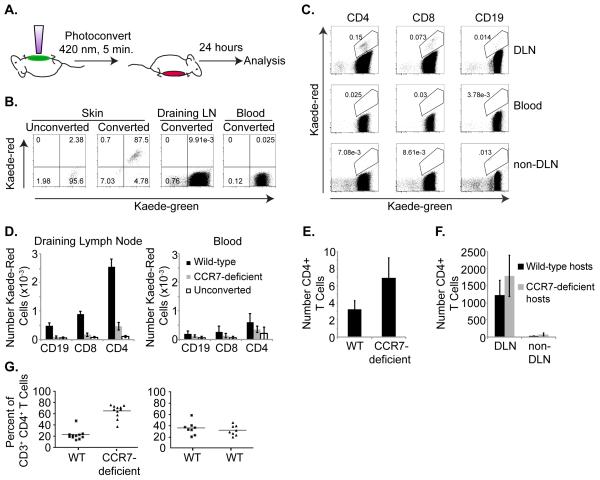
Using Kaede transgenic mice, we have measured the exit of endogenous lymphocyte subsets from the skin in vivo. Briefly, the abdominal skin of Kaede mice was exposed to violet light for five minutes in order to induce the irreversible photoconversion of cutaneous cells (Figure 1a). Initially, lymphocytes within the skin emit green fluorescence. However, immediately following exposure to violet light, cutaneous cells present within the skin during the five-minute period of photoconversion stably emit red fluorescence (8) and Figure 1b). In this manner, we were able to track red fluorescent cells originating in the skin and migrating to the draining LN. 24 h after abdominal skin photoconversion, the draining axillary LNs, blood and non-draining cervical LNs were isolated and recovered lymphocytes were counted, stained with antibodies directed against CD3, CD4, CD8 and CD19, and analyzed by flow cytometry to identify exited Kaede-red+ cells among CD4+, CD8+ and CD19+ lymphocytes. Significantly more CD4+ T cells than CD8+ T cells or CD19+ B cells circulate from the skin to draining LNs (Figure 1c, d). These data demonstrate that endogenous lymphocytes exit from the skin at baseline and correlate with recent studies demonstrating that more CD4+ T cells than CD8+ T cells migrate out of skin in explant cultures (9) and in T cell adoptive transfer studies (10).
Figure 1. Lymphocyte migration from skin to draining LNs in the steady-state is CCR7-dependent.
(a) Schematic of Kaede transgenic mouse photoconversion. Shaved abdominal skin of Kaede transgenic mice is exposed to 420 nm light for 5 minutes. After 24 h, leukocytes are recovered from tissues and analyzed by flow cytometry for CD3, CD4, CD8, CD19 and Kaede-green or Kaede-red expression. (b) Flow cytometric analysis of Kaede-green and Kaede-red expression by CD3+ CD4+ T cells recovered from unconverted skin or photoconverted skin, blood and draining axillary LN immediately after exposure to violet light. (c) Flow cytometric analysis of Kaede-green and Kaede-red expression by draining axillary LN CD3+ CD4+ T cells, CD3+ CD8+ T cells and CD3−CD19+ B cells 24 h after photoconversion of Kaede transgenic mouse abdominal skin. Data are representative of 3 experiments with 6 mice total. (d) Numbers of Kaede-red CD3+ CD4+ T cells, CD3+ CD8+ T cells and CD3− CD19+ B cells within draining axillary LNs (left) or blood (right) 24 h after photoconversion of WT-(black bars) or CCR7-deficient-(gray bars) Kaede transgenic abdominal skin. Open bars are lymphocytes that fall within the Kaede-red gate from draining axillary LNs or blood of control unconverted Kaede transgenic mice. Data are from 3 experiments with 6 mice total for each genotype. A total of 3 control unconverted Kaede transgenic mice were analyzed. (e) The shaved abdominal skin of WT or CCR7-deficient mice was isolated, frozen in OCT, and 10 μm sections were stained with anti-CD4 antibodies. Slides were imaged and numbers of CD4+ T cells in 200× fields were counted. Data are the average of 4-6 mice, with at least 3 fields counted per mouse. (f) Five million WT CD4+ Thy1.1+ T cells were injected s.c. into the right footpads of either WT congenic Thy1.2+ or CCR7-deficient Thy1.2+ hosts. After 16 h, right draining popliteal LNs (DLN) and left contralateral popliteal LNs (non-DLN) were isolated, recovered cells were counted, stained with anti-CD3, anti-CD4 and anti-Thy1.1 antibodies, and analyzed by flow cytometry. Data are from 3 experiments with 9 mice total per genotype. (g) Bone marrow chimeras were generated by reconstituting irradiated WT CD45.1 mice with a 1:1 mix of either WT CD45.1 CD45.2 and CCR7-deficient CD45.2 bone marrow cells or with WT CD45.1 CD45.2 and WT CD45.2 bone marrow cells. After eight weeks, leukocytes were recovered from skin and the percent of WT or CCR7-deficient CD3+ CD4+ T cells of total cutaneous CD3+ CD4+ T cells was calculated. Data are compiled from three experiments with WT: CCR7-deficient chimeras (left graph) and WT:WT chimeras (right graph) and include 11 or 8 chimeric mice total, respectively.
To determine whether the exit of resting endogenous lymphocytes from the skin is CCR7-dependent, we photoconverted the abdominal skin of WT Kaede and CCR7-deficient Kaede mice. After 24 h, we quantitated numbers of Kaede-red WT and Kaede-red CCR7-deficient CD4+ T cells, CD8+ T cells, and CD19+ B cells recovered from draining axillary LNs. Significantly more Kaede-red WT than Kaede-red CCR7-deficient CD4+, CD8+ and CD19+ cells were detected in draining LNs (Figure 1d). The difference in accumulation of Kaede-red WT and Kaede-red CCR7-deficient lymphocytes within the draining LN does not appear to be due to increased exit of Kaede-red CCR7-deficient lymphocytes from the LN (15), since we did not detect increased numbers of Kaede-red CCR7-deficient lymphocytes compared to Kaede-red WT lymphocytes within the blood (Figure 1d). Additionally, we detected as many CD4+ T cells in skin sections of CCR7-deficient mice as of WT mice (Figure 1e), suggesting that CCR7-deficient CD4+ T cells are present within the skin but failed to exit. Furthermore, the lack of CCR7-deficient lymphocyte egress from the skin is not explained by abnormal LN architecture in CCR7-deficient mice (13), as adoptively transferred WT CD4+ T cells migrated equally from the skin to draining LNs of WT and CCR7-deficient mice (Figure 1f). To further demonstrate that the decreased exit of CCR7-deficient T cells from the skin is a result of the lack of CCR7 on T cells and not the result of other differences between WT and CCR7-deficient host mice, we generated WT/CCR7-deficient mixed bone marrow chimeras. In this competitive setting, a greater percent of endogenous CCR7-deficient CD3+ CD4+ T cells than WT CD3+ CD4+ T cells accumulated within the skin of the same WT host (Figure 1g). Collectively, these data suggest that the exit of endogenous CD4+, CD8+ and CD19+ lymphocytes from the skin requires CCR7.
CCR7− CD103+ CD69+ CD4+ T Cells Remain in Skin
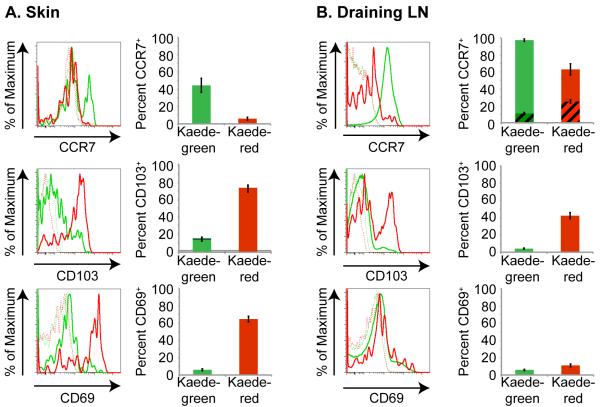
TRM cells have been identified in skin (2, 3, 9, 16) and are characterized by high CD103 and CD69 surface expression, and lack of CCR7 expression. Here, we examined whether CD4+ T cells that remain in the skin following photoconversion also express these cell surface receptors. Because we determined that CCR7 regulates the exit of endogenous CD4+ T cells from the skin (Figure 1d), we first examined whether we could identify differences in CCR7 surface expression on CD4+ T cells that remained in the skin 24 h following photoconversion versus on CD4+ T cells that migrated out of the skin and into draining LNs. We observed that approximately 40% of CD4+ T cells in normal mouse skin express CCR7. This figure is similar to previous investigations demonstrating that approximately 50% of T cells within normal human skin express CCR7 (2). Within 24 h following photoconversion, Kaede-green cells (which likely entered the area of photoconverted skin between the time of photoconversion and tissue harvest either from the blood or from adjacent, unconverted skin) included both CCR7+ and CCR7− CD4+ T cells. In contrast, few Kaede-red CCR7+ T cells are detected within the skin, suggesting that CCR7+ T cells exit the tissue efficiently. Whereas Kaede-red CD4+ T cells remaining in the skin were CCR7− (Figure 2a), Kaede-red CD4+ T cells that migrated from the skin to draining LN expressed intermediate CCR7 (Figure 2b). Together, these results suggest that CCR7− CD4+ T cells are retained in the skin while CCR7+ CD4+ T cells are able to exit into the afferent lymphatic vessels and draining LNs.
Figure 2. Kaede-red CD4+ T cells residing in skin are CCR7− CD103+ CD69+.
Flow cytometric analysis of CCR7, CD103 and CD69 expression by Kaede-green CD3+ CD4+ T cells (green solid line) and Kaede-red CD3+ CD4+ T cells (red solid line) remaining within the abdominal skin (a) or migrated from skin to draining LN (b) 24 h after abdominal skin photoconversion. Dashed lines are control “fluorescence minus one” stains of Kaede-green- and Kaede-red CD3+ CD4+ T cells. Graphs depict the average percent of Kaede-green (green bars) and Kaede-red (red bars) CD3+ CD4+ T cells in the skin or draining LN that express CCR7, CD103 or CD69. For CCR7 expression in the draining LN, striped bars depict the percent of CD3+ CD4+ T cells that express intermediate CCR7 and solid bars show the percent of CD3+ CD4+ T cells that express high CCR7. Data are representative of 3 experiments with 9 mice total.
In parallel, we determined whether Kaede-red CD4+ T cells remaining in the skin following photoconversion expressed higher levels of CD103 and/or CD69 than those Kaede-red CD4+ T cells that exited from the skin. CD103 binds to E-cadherin, which is expressed mainly by epithelial cells (17). Several data suggest that CD103 retains T cells in tissue epithelium (18-20). Here, we determined that Kaede-red CD4+ T cells remaining in the skin after photoconversion express CD103, consistent with a role for this molecule in the persistence of CD4+ T cells within the skin (Figure 2a). However, we observed bimodal CD103 expression on CD4+ T cells that migrated from the skin to draining LN (Figure 2B), suggesting that CD103 expression alone may not be a reliable marker for cutaneous resident memory CD4+ T cells, as it is also expressed on CD4+ T cells that exit from the skin.
The chemoattractant receptor S1P1 regulates the exit of T cells from thymic and LN tissues (21, 22). Additionally, S1P1 may regulate T cell exit from peripheral tissues. Treatment of mice with FTY720 down-regulates S1P1 expression and function, resulting in a partial reduction in CD4+ T cell exit from the skin (23). CD69 suppresses S1P1 function, and thereby promotes the retention of activated (24) and naïve (25) T cells within LNs. CD69 expression has also been detected on CD8+ TRM in the skin (5) and could act similarly to retain T cells within peripheral tissues. Here, we examined CD69 expression on Kaede-red CD4+ T cells remaining in skin 24 h after photoconversion. While Kaede-red CD4+ T cells remaining in skin express CD69 (Figure 2a), CD4+ T cells that migrate to draining LNs lack CD69 expression (Figure 2b). These findings are consistent with a role for CD69 in the persistence of CD4+ T cells within the skin. Together, these results suggest that a CCR7− CD103+ CD69+ phenotype identifies CD4+ T cells that fail to exit from the skin.
TRCM Express Both LN- and Skin-Homing Receptors
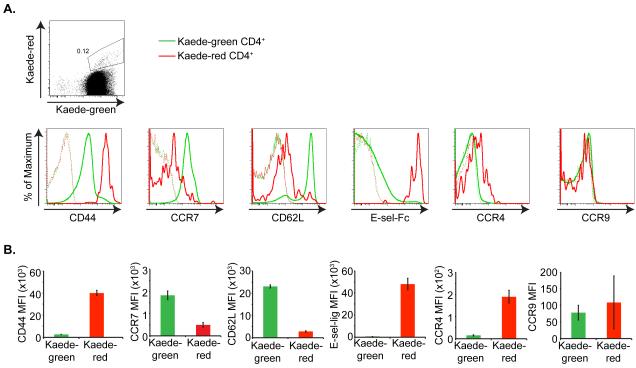
Distinct populations of memory T cells have been defined based on their homing receptor expression and function (1). TCM express the LN homing receptors CCR7 and CD62L, and thus recirculate through secondary lymphoid tissues. In contrast, TEM lack CCR7 and CD62L but express receptors for migration into peripheral tissues. To define the homing receptors expressed by T cells that exit the skin, we isolated lymphocytes from the draining axillary LNs 24 h after photoconversion of the abdominal skin. Recovered cells were stained with antibodies directed against CD3, CD4, the memory marker CD44 as well as tissue-specific homing receptors and analyzed by flow cytometry. Almost all Kaede-red CD4+ T cells that migrated from the skin to draining LNs were CD44hi CCR7intermediate(int) CD62Lint. Additionally, these cells express the skin homing receptors CCR4 and E-selectin ligands, but lack expression of the intestine homing receptor CCR9 (Figure 3a, b).
Figure 3. Kaede-red CD4+ T cells express lymph node- and skin-homing receptors.
(a) Top panel illustrates Kaede-green and Kaede-red expression by CD3+ CD4+ T cells within the draining LN 24 h after photoconversion of Kaede transgenic mouse abdominal skin. Bottom panels depict flow cytometric analysis of CD44, CCR7, CD62L, E-selectin ligands, CCR4 and CCR9 expression by Kaede-green CD3+ CD4+ (green solid line) and Kaede-red CD3+ CD4+ T cells (red solid line) within the draining LNs 24 h after photoconversion of Kaede transgenic mouse abdominal skin. Dashed lines are control “fluorescence minus one” stains of Kaede-green- and Kaede-red CD3+ CD4+ T cells. (b) MFI for receptor expression on Kaede-green CD3+ CD4+ T cells and Kaede-red CD3+ CD4+ T cells are displayed for one experiment with three mice per receptor analyzed and is representative of three experiments with at least eight mice total per receptor.
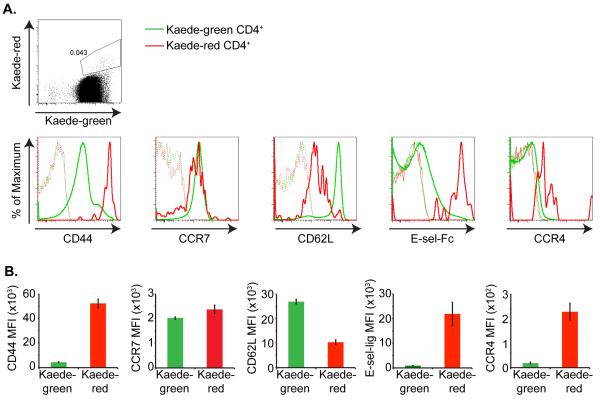
We next examined whether this CCR7int CD62Lint phenotype persists, or whether TRCM convert to a TCM or TEM phenotype with time. Eight days after photoconversion, the recirculating memory CD4+ T cells up-regulate CCR7, but remain CD62Lint (Figure 4a, b). Additionally, these CD4+ memory T cells maintain expression of the skin homing receptors E-selectin ligands and CCR4 (Figure 4a, b). Together, these results suggest that unlike TCM and TEM, recirculating CD4+ memory T cells have the potential to migrate through both cutaneous and lymphoid tissues.
Figure 4. Kaede-red CD4+ T cells up-regulate CCR7 expression over time.
Shaved abdominal skin of Kaede transgenic mice was exposed to 420 nm light for 5 minutes on 3 consecutive days. (a) Top panel illustrates Kaede-green and Kaede-red expression by CD3+ CD4+ T cells within the draining LNs 8 d after the final photoconversion of Kaede transgenic mouse abdominal skin. Bottom panel depicts flow cytometric analysis of CD44, CCR7, CD62L, E-selectin ligands, and CCR4 expression on these Kaede-green CD3+ CD4+ (green solid line) and Kaede-red CD3+ CD4+ T cells (red solid line). Dashed lines are control “fluorescence minus one” stains of Kaede-green- and Kaede-red CD3+ CD4+ T cells. b) MFI for receptor expression on Kaede-green CD3+ CD4+ T cells and Kaede-red CD3+ CD4+ T cells are displayed for one experiment with three mice per receptor analyzed and is representative of three experiments with at least eight mice per receptor.
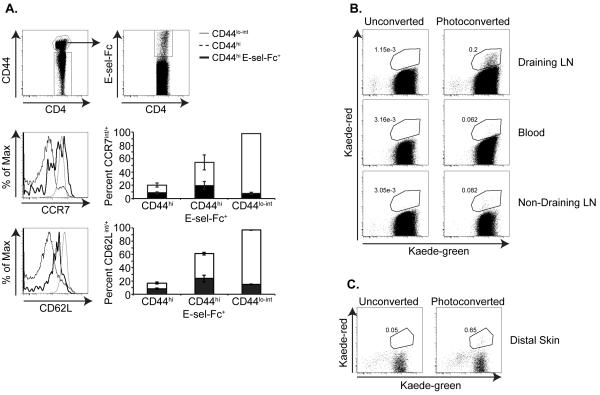
TRCM Reenter the Circulation and Distal Tissue
The homing receptor expression on Kaede-red CD4+ T cells that migrate from skin to draining LN suggests that they circulate between these tissues. Therefore, we examined whether we could identify CD4+ CD44hi E-sel-ligands+ CD62Lint CCR7int/+ T cells within the circulation at steady state. We did detect E-sel-ligands+ memory T cells with intermediate/+ expression of CCR7 and CD62L in the blood of resting C57Bl/6 mice (Figure 5a). In parallel, we examined whether the Kaede-red CD4+ memory T cells that have migrated from skin to draining LNs subsequently enter into the circulation. To address this question, we photoconverted the abdominal skin of Kaede transgenic mice every 24 h for 3 d. We photoconverted the skin multiple times to generate sufficient numbers of Kaede-red CD4+ T cells to track. 48 h after the final photoconversion, we isolated the blood, draining axillary LNs, and non-draining cervical LNs. Then, recovered leukocytes were analyzed by flow cytometry in order to detect Kaede-red CD4+ T cells. Kaede-red CD4+ T cells were detected in the blood, demonstrating that these memory T cells exit from draining LNs and reenter the circulation (Figure 5b). Additionally, these CD4+ TRCM were detected in non-draining cervical LNs (Figure 5b). Therefore, despite our finding that these cells express only intermediate CD62L, they are still able to gain entry into distal LNs, either via HEV or afferent lymphatic vessels draining distal skin sites. However, we were unable to identify Kaede-red CD4+ T cells within normal distal ear skin. The skin is a large tissue through which the migrating T cells can disperse. Nonetheless, because these memory CD4+ T cells enter into the circulation and express the skin-homing receptors E-selectin ligands and CCR4, it remained possible that they were able to migrate into normal skin, but were present in numbers too low to recover and detect. Indeed, studies in sheep demonstrate that T cells isolated from normal skin-draining afferent lymph and injected directly into the bloodstream migrate back into skin (26). Consistent with this, we did detect recirculating memory CD4+ T cells within a distal site of non-specific cutaneous inflammation induced by injecting the ear with CFA (Figure 5c). These results suggest that unlike TCM, TEM or TRM, these TRCM are able to migrate between distal lymphoid and cutaneous sites to provide widespread cutaneous immune surveillance.
Figure 5. Kaede-red CD4+ T cells reenter the circulation from draining LNs.
(a) CCR7 and CD62L expression on CD4+ CD44hi E-sel-Fc+ T cells within the blood of resting C57Bl/6 mice. Leukocytes recovered from the blood were gated on CD3+ CD4+ CD44hi memory T cells and on CD3+ CD4+ CD44lo-int naïve T cells (top left panel). The CD44hi T cell population was then gated on E-sel-Fc+ cells (top right panel). Middle and bottom histograms depict CCR7 and CD62L expression on CD4+ CD44lo-int naïve T cells, CD4+ CD44hi bulk memory T cells, and on CD4+ CD44hi E-sel-Fc+ memory T cells. Each plot is from 6 mice pooled and is representative of 3 independent experiments. Graphs depict the average percent of each CD3+ CD4+ T cell population in the blood that express CCR7 or CD62L. Black bars depict the percent of CD3+ CD4+ T cells that express intermediate CCR7 or CD62L and white bars show the percent of CD3+ CD4+ T cells that express high CCR7 or CD62L. (b) Shaved abdominal skin of Kaede transgenic mice was exposed to 420 nm light for 5 minutes on 3 consecutive days. Flow cytometric analysis of Kaede-green and Kaede-red expression on CD3+ CD4+ T cells recovered from the blood, non-draining cervical LNs and draining axillary LNs 48 h after the final photoconversion. Data are representative of 3 experiments with 8 mice/group total. (c) The right ear of Kaede transgenic mice was injected with 10 μL of a 1:1 PBS:CFA emulsion, and then the shaved abdominal skin was photoconverted as in (a). Flow cytometry plots depict Kaede-green and Kaede-red expression by CD3+ CD4+ T cells recovered from the CFA-injected distal ear skin 48 h after the final photoconversion. Numbers in plots are average percent Kaede-red T cells of CD3+ CD4+ T cells recovered from the ear, and are calculated from 3 experiments with 8-14 mice total/group. Data shown are of 6 mice pooled/group.
TRCM Secrete IL-2 and up-regulate CD40L
Previous studies demonstrated that CD4+ TCM and TEM cells display distinct effector functions. Whereas TCM produce IL-2 and only negligible IFN-γ, TEM cells secrete IFN-γ following stimulation (1). To determine the effector functions mediated by recirculating memory CD4+ T cells, we examined their cytokine secretion following in vitro stimulation with plate-bound anti-CD3 and soluble anti-CD28. Both central memory Kaede-green CD4+ T cells as well as recirculating memory Kaede-red CD4+ T cells secreted IL-2, but negligible IFN-γand IL-10 (Figure 6a). Additionally, both Kaede-green and Kaede-red memory CD4+ T cells up-regulated CD40L expression upon activation (Figure 6b). Based on their cytokine secretion and CD40L expression, CD4+ TRCM resemble TCM. However, unlike TCM that circulate only between lymphoid tissues, TRCM express chemokine receptors and adhesion molecules that enable their delivery of help to both lymphoid as well as non-lymphoid tissues.
Figure 6. Recirculating Kaede-red CD3+ CD4+ CD44hi T cells up-regulate CD40L and secrete IL-2.
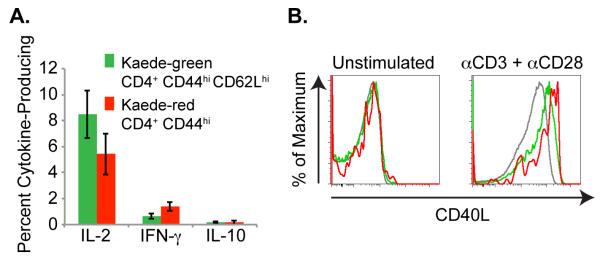
Shaved abdominal skin of Kaede transgenic mice was exposed to 420 nm light for 5 minutes. After 24 h, draining LNs were isolated and (a) recovered cells were stimulated for 4 h with plate-bound anti-CD3 and soluble anti-CD28. Cytokine secretion by Kaede-green CD3+ CD4+ CD44hi CD62Lhi memory T cells and Kaede-red CD3+ CD4+ CD44hi memory T cells was analyzed by flow cytometry. Data are pooled from 3 experiments with 9 mice total for each cytokine. (b) Or, CD4+ T cells were purified from draining LNs and stimulated for 2 h with PMA and ionomycin, or left untreated. Then, surface CD40L expression was analyzed on Kaede-green CD44lo naïve T cells (gray lines), Kaede-green CD44hi memory T cells (green lines) or Kaede-red CD44hi memory T cells (red lines). Data are representative of 2 experiments with 6 mice total.
DISCUSSION
Using Kaede transgenic mice, we have tracked the migration of endogenous cutaneous lymphocytes in vivo. We find that one population of CD4+ T cells remains in the skin after photoconversion and is CD44hi CD69+ CD103+. Additionally, we have identified a unique population of memory CD4+ T cells whose phenotype and migratory pattern differs from central memory, effector memory and resident memory T cells. These CD4+ TRCM cells migrate from normal skin to draining LNs in a CCR7-dependent manner. From the draining LNs, they reenter the circulation, distal LNs and sites of non-specific cutaneous inflammation. Following polyclonal stimulation, these memory T cells up-regulate CD40L and secrete IL-2. Our results identify a recirculating memory CD4+ T cell subset equipped to deliver help to both distal lymphoid and cutaneous tissues.
While previous studies have identified a population of resident memory T cells within the skin (2) (3), our results suggest the presence of a subset of memory T cells that is present only transiently within the skin, displaying dynamic migration through the skin and into afferent lymph. We observe that approximately forty percent of CD4+ T cells in normal mouse skin express CCR7. Within 24 hours following photoconversion, few Kaede-red CCR7+ T cells are detected within the skin. However, CCR7int cells originating from the skin are detected in dLN, suggesting that CCR7+ T cells exit the tissue efficiently. A prior investigation revealed that adult human skin contains about two billion memory T cells (2). Greater than fifty percent of these cutaneous memory T cells express CCR7 (2), suggesting that a substantial number of human memory T cells may also have the potential to exit from normal skin. Of note, CCR7+ memory T cells in human blood have been found to express tissue / inflammatory chemokine receptors, including CCR4 (1). It is possible that like the TRCM cells we have characterized in this study, these human CCR7+ memory T cells also migrate between cutaneous and lymphoid tissues.
We have determined that recirculating memory CD4+ T cells may be identified based on their surface receptor expression: CD44hi, CCR7int-pos, CD62Lint, CD103+/−, CD69−, CCR4+/−, e-sel-ligands+. Previous studies have identified regulatory T cells with a similar cell surface phenotype that migrate from skin to draining LNs during a DNFB-mediated hypersensitivity response (8). Thus, this homing receptor expression profile may define both recirculating cutaneous memory CD4+ T cells as well as regulatory T cells at homeostasis and inflammation. Of note, while these recirculating memory CD4+ T cells are able to migrate into distal skin sites, these T cells lack expression of the intestine-homing receptor CCR9. Early studies performed in sheep have identified memory T cells not only in cutaneous lymph (7), but also intestinal lymph (27), suggesting that separate populations of memory T cells may exist that recirculate through additional tissues, and may be characterized by distinct homing receptor expression profiles.
Previous studies have discovered populations of memory T cells with distinct homing potentials (1). By definition, TCM cells express LN tissue homing receptors and circulate exclusively between the bloodstream and secondary lymphoid tissues. In contrast, TEM cells migrate from blood into extra-lymphoid tissues, but lack CCR7 expression, and so are unable to enter into resting LNs. Whether CD4+ TRCM are a subset completely distinct from TCM or TEM is unknown. While CD4+ T were CCR7int CD62Lint RCM shortly after their exit from the skin, over time, they up-regulated CCR7 expression but remained CD62Lint, E-selectin ligands+, CCR4+/−. Based on this unique homing receptor expression profile and migratory pattern, we propose that they are a distinct memory T cell subset, but future experiments will determine the relationship of this subset to other memory T cell subsets.
In conclusion, our data identifies a distinct population of memory CD4+ T cells that circulate between lymphoid and cutaneous tissues and that can be identified by their surface receptor expression. Re-entry of memory T cells into the circulation might allow for surveillance and delivery of help distal to the site of initial antigen exposure, for example in case of pathogen spread or reinfection. In the current work, we have identified TRCM surface receptors that may be useful in the study of memory CD4+ T cell response and for the development of vaccination strategies requiring widespread surveillance of cutaneous tissues by T cells.
ACKNOWLEDGEMENTS
We thank members of the Luster laboratory for helpful discussions.
Abbreviations used
- TRM
resident memory T cell
- TCM
central memory T cell
- TEM
effector memory T cell
- TRCM
recirculating memory T cell
- int
intermediate
- LN
lymph node
- MFI
mean fluorescence intensity
- FMO
fluorescence minus one
Footnotes
*Designated author to communicate with editors.
REFERENCES
- 1.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 2.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 3.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 4.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 5.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay CR, Kimpton WG, Brandon MR, Cahill RN. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J Exp Med. 1988;167:1755–1765. doi: 10.1084/jem.167.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y, Waldmann H, Hori S, Cyster JG, Watanabe T, Miyachi Y, Kanagawa O, Kabashima K. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest. 2010;120:883–893. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 10.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 12.Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, Kanagawa O. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc Natl Acad Sci U S A. 2008;105:10871–10876. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 15.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, Carter JB, Fisher DC, Kupper TS. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 18.Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 19.Pauls K, Schon M, Kubitza RC, Homey B, Wiesenborn A, Lehmann P, Ruzicka T, Parker CM, Schon MP. Role of integrin alphaE(CD103)beta7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J Invest Dermatol. 2001;117:569–575. doi: 10.1046/j.0022-202x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- 20.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 21.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 22.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 23.Brown MN, Fintushel SR, Lee MH, Jennrich S, Geherin SA, Hay JB, Butcher EC, Debes GF. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J Immunol. 2010;185:4873–4882. doi: 10.4049/jimmunol.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 25.Tomura M, Itoh K, Kanagawa O. Naive CD4+ T lymphocytes circulate through lymphoid organs to interact with endogenous antigens and upregulate their function. Journal of immunology. 2010;184:4646–4653. doi: 10.4049/jimmunol.0903946. [DOI] [PubMed] [Google Scholar]
- 26.Issekutz TB, Chin W, Hay JB. The characterization of lymphocytes migrating through chronically inflamed tissues. Immunology. 1982;46:59–66. [PMC free article] [PubMed] [Google Scholar]
- 27.Cahill RN, Poskitt DC, Frost DC, Trnka Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J Exp Med. 1977;145:420–428. doi: 10.1084/jem.145.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]