Summary
Plasmodium parasites infect the liver and replicate inside hepatocytes before they invade erythrocytes and trigger clinical malaria. Analysis of host signaling pathways affected by liver stage infection could provide critical insights into host-pathogen interactions and reveal targets for intervention. Using protein lysate microarrays we found that Plasmodium yoelii rodent malaria parasites perturb hepatocyte regulatory pathways involved in cell survival, proliferation and autophagy. Notably, the pro-death protein p53 was substantially decreased in infected hepatocytes, suggesting it could be targeted by the parasite to foster survival. Indeed, mice that express increased levels of p53 showed reduced liver stage parasite burden whereas p53 knockout mice suffered increased liver stage burden. Furthermore, boosting p53 levels using the small molecule Nutlin-3 dramatically reduced liver stage burden in vitro and in vivo. We conclude that perturbation of the hepatocyte p53 pathway critically impacts parasite survival. Thus, host pathways might constitute potential targets for host-based antimalarial prophylaxis.
Introduction
Parasites of the genus Plasmodium are the causative agents of the deadly disease malaria, afflicting 350–500 million people annually and causing 800,000 deaths world-wide (Snow et al., 2005). After transmission by an infected Anopheles mosquito, the parasite travels quickly through the blood stream to the liver and infects hepatocytes. The parasite then grows and replicates within hepatocytes, presumably evading detection by the host, and ultimately spawns tens of thousands of daughter merozoites, which are released into the blood stream and infect red blood cells, leading to symptomatic infection (Vaughan et al., 2008).
One feature of host manipulation that has been previously suggested is the ability of Plasmodium berghei rodent malaria parasites to render their host hepatocyte partially resistant to artificial induction of apoptosis in vitro, both early (van de Sand et al., 2005) and late (Leiriao et al., 2005) during liver stage development. It remains unclear however, how broadly the host hepatocyte responds to infection, and if the parasite attempts to counteract responses that might impact its survival. One potential mechanism that could explain how parasitized hepatocytes become resistant to apoptosis involves activation of the hepatocyte growth factor receptor, but this mechanism appears unique to the rodent malaria parasite P. berghei (Carrolo et al., 2003; Kaushansky and Kappe, 2011). Furthermore, some studies have measured transcriptional changes that occur in P. yoelii and P. berghei infected hepatocytes after infection (Albuquerque et al., 2009; Tarun et al., 2008) but perturbations in the translational and post-translational host cell environment that occur upon parasite liver stage infection have not been elucidated.
Results
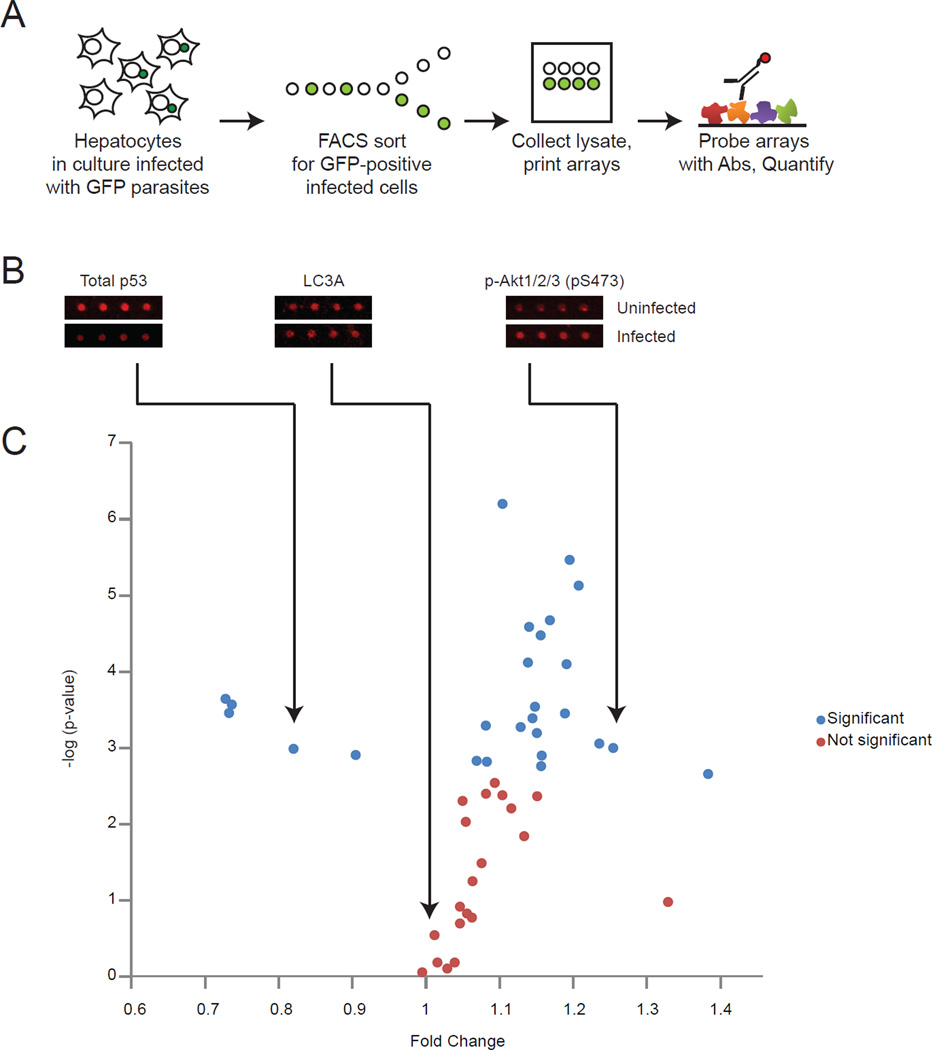
It remains technically challenging to study protein-level cellular responses to liver stage infection, as infection rates are low and thus, infected cells can only be isolated in limited quantities. To circumvent this roadblock, we used reverse-phase protein microarray technology, which enables broad but targeted proteomic investigations on small sample sizes (Sevecka et al., 2011). The platform uses cellular lysates deposited in nanoliter droplets on nitrocellulose-coated glass slides in which levels of specific proteins or their post-translational modifications can be detected by probing the lysates with appropriate antibodies (Figure 1A). We assembled a diverse set of antibodies, many of which have been previously validated for use in reverse phase arrays (Sevecka et al., 2011). These antibodies recognize proteins involved in numerous cellular outcomes, including survival, apoptosis, proliferation, cell cycle control and autophagy. Approximately 10,000 parasite-infected HepG2 CD81 cells as well as uninfected cells were isolated by fluorescence activated cell sorting (FACS), making use of green fluorescent protein (GFP)-tagged P. yoelii parasites (Tarun et al., 2006). Protein extracts from each sample were prepared and printed in quadruplicate on 48 separate nitrocellulose pads followed by probing the arrays with the set of antibodies to obtain quantitative information on changes in host cell protein abundance and/or modifications (Table S1, Figure 1C).
Figure 1. The use of protein microarrays to study liver stage malaria infection.
(A) Schematic representing steps required to obtain lysate microarray data from HepG2-CD81 cells either infected or uninfected with P. yoelii-GFP liver stages. Liver stages were allowed to develop for 24 hours. (B) Representative array images from three antibodies, total p53, LC3A and p-Akt1/2/3 (pS473). (C) Graph representing the ratio of infected (GFP-positive) cells to uninfected (GFP-negative) host cells for signals obtained for 46 separate antibodies, plotted against the log of the p-value obtained. Each point represents a single antibody. Significant differences that pass multiple hypothesis testing (Holm-Bonferroni method) are shown in blue, non-significant differences are shown in red. Also see Figure S1.
Strikingly, the resulting data showed that numerous signaling proteins are perturbed in parasite-infected cells (Table S1, Figure 1B). Multiple pathways were simultaneously impacted at significant but varying levels, indicating that the liver stage parasite drives a multipronged approach to modulate signaling by the infected host cell. When we examined which signals were most significantly changed in infected cells, we found pronounced increases in the anti-apoptotic signaling proteins p-Bcl-2 (P=0.001) and p-Akt/PKB (P = 0.0008, P = 0.000003 for two separate antibodies) and the pro-proliferative phosphorylated states of the mammalian target of rapamycin (mTor) (P = 0.000008) and Retinoblastoma (Rb) (P = 0.003) (Table S1). Furthermore, we identified decreases in phosphorylated forms of the pro-apoptotic proteins p53 (P = 0.0004) and Bad (P = 0.001, 0.0002 for two separate antibodies) as well as a decrease in total abundance of p53 (P = 0.0003, P=0.001 for two separate antibodies) (Table S1, Figure 2A).
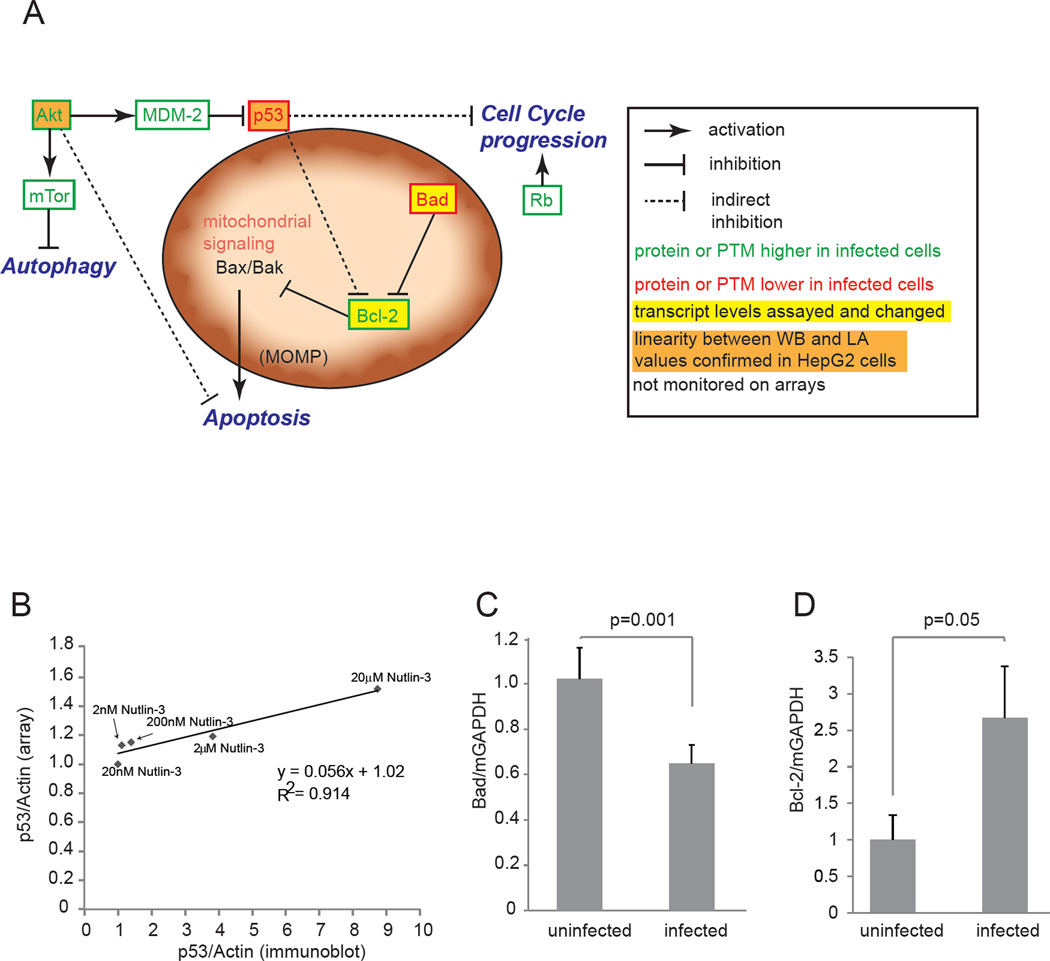
Figure 2. Key host signaling pathways in Plasmodium infection.
(A) Schematic showing the connectivity between host proteins significantly perturbed in liver stage-infected cells as measured by protein lysate microarrays. Proteins for which total level or post-translational modifications are increased in parasitized cells are shown in green, decreases levels in red. Select antibodies tested demonstrate a linear relationship between immunoblotting (WB) and lysate array (LA) are colored orange. Proteins for which transcripts where tested and changed are colored yellow. (B) Demonstration that total p53 antibody (#9282) produces a linear relationship for data obtained using western blotting and lysate array. Data was fit using a linear regression. (C) Quantitative PCR showing that transcript levels of Bad are decreased in infected hepatocytes (P=0.001). (D) Transcript levels of Bcl-2 are elevated in infected hepatocytes (P=0.05). Also see Figure S1.
Remarkably, these data are consistent with the hypothesis that a cohesive network of parasite-mediated signaling changes renders the infected host cell more hospitable for the parasite. The increases of activated Akt and Bcl-2 along with the decrease in Bad indicate a multi-faceted survival response, which assists the parasite in protecting its host cell. The increase in mTor suggests protection against autophagy, which could severely impede intracellular parasite development. The activation of Rb suggests the infected hepatocyte is pushed towards a proliferative state. Finally, the decrease in p53 levels fits into both the proliferative and anti-apoptotic framework (Figure 2A, Table 1). Together, the observed perturbations are consistent with a general anti-apoptotic, pro-proliferative, anti-autophagic environment within the infected host cell.
Table 1.
Lysate arrays demonstrate a variety of host signaling proteins are perturbed in response to Plasmodium liver stage infection.
| Proteins with Most elevated levels in infected cells vs uninfected cells | |||
|---|---|---|---|
| Infected/Uninfected | Std | p-value | |
| Antibody | |||
| p-Rb (S807/S811) | 1.39 | 0.15 | 2.21E-03 |
| p-BCL-2 (S70) | 1.26 | 0.05 | 1.01E-03 |
| p-Akt1/2/3 (S473) | 1.24 | 0.06 | 8.85E-04 |
| p-mTOR (S2448) | 1.21 | 0.03 | 7.45E-06 |
| p-Akt1/2/3 (S473) | 1.20 | 0.03 | 3.43E-06 |
| Proteins with most reduced levels in infected cells vs uninfected cells | |||
| Infected/Uninfected | Std | p-value | |
| Antibody | |||
| p-BAD (S112) | 0.91 | 0.03 | 1.25E-03 |
| p53 | 0.82 | 0.04 | 1.04E-03 |
| p53 | 0.74 | 0.05 | 2.72E-04 |
| p-p53 (S15) | 0.73 | 0.09 | 3.51E-04 |
| p-BAD (S136) | 0.73 | 0.04 | 2.28E-04 |
Because any screen must be validated with alternative approaches, we next sought to confirm the major pathways that were impacted by parasite infection. Both Akt antibodies have been previously validated in a variety of cell lines, including HepG2 cells (Luckert et al., 2012). Encouragingly, signal for all four antibodies in the Akt/mTor pathway recorded increases in response to parasite infection (Figure S1C). In order to further validate additional screen hits, we first explored reproducibility between biological samples, and scalability with data obtained from immunoblotting. We found that one p53 antibody was highly reproducible across biological replicates (Figure S1A). To determine if this antibody also gave a linear relationship between western blotting and lysate array measurements, we generated cellular lysates with variable levels of p53 using a range of concentrations of the MDM-2 inhibitor Nutlin-3. By monitoring p53 levels in these lysates by both western blot and lysate array, we determined that the relationship between these signals was linear (Figure 2B). Interestingly, while total p53 levels were decreased in infected cells, levels of phosphorylated p53 appeared moderately elevated, suggesting residual p53 in infected cells may have altered activity. (Figure S1D). Additionally, these experiments allowed us to calculate that the lysate array difference of 0.82 we observed in infected over uninfected cells corresponded to a decrease of 68% in p53 protein levels in infected cells over uninfected cells (Figure 2B). In addition to determining the magnitude of the p53 response in infected cells, these data highlight a commonly observed feature of lysate array experiments; that the magnitudes of changes observed by lysate arrays are often less pronounced when compared to equivalent changes observed by alternative means (Figure 2B, (Sevecka et al., 2011)).
Overall antibodies, which monitor Bcl-2 phosphorylation were elevated whereas antibodies monitoring the Bcl-2 antagonist Bad, were diminished in infected cells (Figure S1E). Of the three antibodies monitoring Bad phosphorylation, which had statistically significant p-values comparing infected and uninfected samples and also passed the Holm-Bonferroni test for multiple hypothesis testing, two antibodies showed diminished Bad levels. To confirm this trend, and also determine if the effect we observed was regulated on the transcriptional, translational or post-translational level, we chose to assess Bad transcript levels. The results corroborated reduced Bad levels upon parasite infection since we found Bad transcript significantly decreased (P=0.001) in infected cells. This also suggests that total Bad levels are decreased rather than only specific phosphorylated sites (Figure 2C). To additionally validate the effect of liverstage parasites on host cell mitochondrial signaling proteins, as well as the elevated levels of Bcl-2 we observed on the arrays, we also assessed the transcript levels of Bcl- 2, which like its protein levels, were elevated (P=0.05) (Figure 2D). Taken together, we conclude that P. yoelii liver stage infection reduces Bad and elevates Bcl-2 transcript abundance as well as phosphorylated protein abundance in the infected host cell.
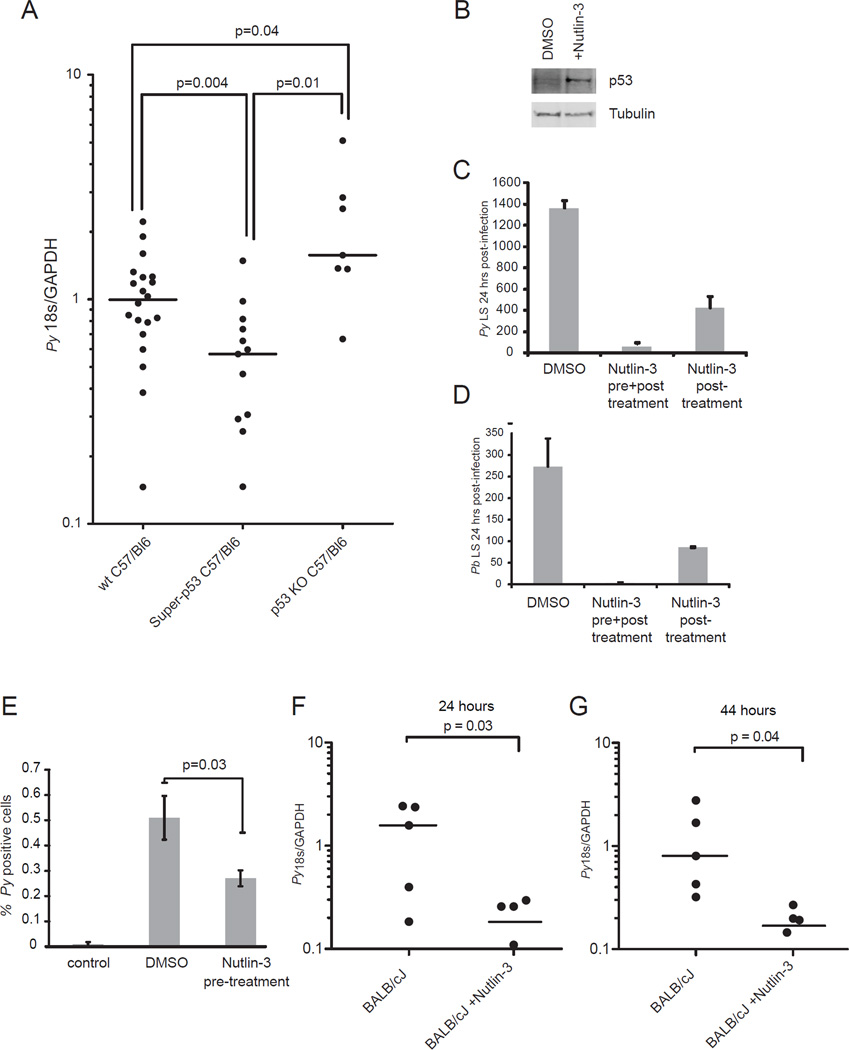
Because of its connectivity with many of the other pathways, we next chose to probe the functional importance of the host cell p53 pathway in liver stage infection. To do this, we first utilized transgenic mice (called ‘super-p53 mice’) that express an additional copy of p53 under its endogenous promoter (Garcia-Cao et al., 2002) as well as mice that lack p53 entirely (‘p53 KO mice’). The levels of p53 increase about two-fold in the ‘super-p53’ mice because only one additional copy of p53 is present. When we infected C57BL/6 control mice and super-p53 mice with P. yoelii sporozoites, then monitored liver stage burden, we found that super-p53 mice showed a significantly reduced liver stage burden when compared to wild-type mice (Figure 3A). Next, we utilized p53 KO mice (Jacks et al., 1994) and predicted that they would be more susceptible to liver stage infection. Indeed, p53 KO mice suffered significantly increased liver stage burden when compared to wild-type mice (Fig 3A). This is consistent with our initial finding that liver-stage parasites are capable of reducing p53 levels in their host hepatocyte, likely to foster their survival and ensure life cycle progression. This interference however might not reach its potential maximum (68% reduction of p53 as measured by lysate arrays of infected cells). By artificially eliminating p53 in the mouse genome, liver-stage parasites have one less host defense to combat, and are thus even more successful in the hepatocyte environment.
Figure 3. Transgenic mice and pharmacological perturbations demonstrate a critical role for host p53 in liver stage infection.
(A) Mice with an additional copy of p53 (‘super-p53 mice’) (n= 13), without p53 (‘p53 KO mice’) (n=7) or wild-type C57BL/6 mice (n=20) were infected with 100,000 P. yoelii sporozoites. Liver stage burden was monitored 42-44 h after infection using quantitative RT-PCR. Parasite burden was significantly reduced in super-p53 mice (P= 0.004) and significant elevated in p53 KO mice (P= 0.04). (B) p53 levels increase in response to 48 h Nutlin-3 treatment, as demonstrated by western blot using an anti-p53 antibody. An anti-αTubulin antibody was used as a loading control. Nutlin-3 treatment (20µM) 24h before and during infection (pre and post), dramatically reduces liver-stage burden of P. yoelii (C, middle) and P.berghei (D, middle). When treatment is applied begining at time of infection and continuing until 24h after infection (post) liver-stage liver stage burden is also reduced, albeit less substantially for both P. yoelii (C, right) P. berghei parasites (D, right). All liver stages were quantified 24 h post-infection in HepG2 CD81 cells. Error bars represent standard deviation between biological replicates. (E) To monitor the effects of Nutlin-3 on P. yoelii (Py) sporozoite early infection of HepG2 CD81 cells, cells were trypsinized 90 min postinfection, fixed and then stained with and antibody to CSP, and subjected to flow cytometric analysis. Wells that were not infected with sporozoites were used as a control. Error bars represent standard deviation of biological replicates. (F, G) Nutlin-3 treatment dramatically reduces liver-stage burden in mice. 50mg/kg Nutlin-3 was administered once daily for two days to BALB/cJ mice. At the time of the last administration of Nutlin-3, mice were infected with 50,000 P. yoelii sporozoites. Livers were removed at 24 h (E) or 44 h (F) post-infection and parasite 18S ribosomal RNA was assessed by qRT-PCR. Signal was normalized to mouse GAPDH. For in vivo experiments, the mean is represented by a horizontal line and the level of Py18s/GAPDH is shown for each individual mouse. Also see Figure S2.
While changes in parasite burden observed in p53-transgenic mice were consistent with our lysate array findings, we next asked if more substantial increases in p53 could cause a more dramatic effect on Plasmodium liver-stage burden. We took advantage of the small molecule Nutlin-3, which is under clinical development for treatment of multiple cancer types (Brown et al., 2009). Nutlin-3 induces growth arrest and apoptosis in a variety of cancer cell lines in vitro, including hepatoma cell lines (Wang et al., 2011), and is also highly effective in inhibiting tumor growth in vivo (Vassilev et al., 2004). Nutlin-3 exerts its effects by binding selectively to the p53- binding region of the E3-ubiquitin ligase MDM-2 and blocks the interaction, which in turn prevents degradation of p53 and in consequence increases its protein levels. Interestingly, in our lysate array analysis we observed that protein levels of MDM-2 were increased in response to parasite infection (P = 0.005; Table S1), indicating that the parasite might promote MDM-2 mediated p53 degradation. We found that the HepG2 CD81 hepatoma cell line, which is susceptible to both P. yoelii and P. berghei infection (Silvie et al., 2003), was responding to Nutlin-3 in the predicted fashion, as p53 levels were dramatically elevated in response to Nutlin-3 treatment (Figure 3B). To determine if increased p53 levels affected liver stage burden, we treated HepG2 CD81 cells with 20 µM Nutlin-3 before and after sporozoite infection or only after sporozoite infection. Each treatment resulted in substantial reduction of liver stage burden, however pre- and post- infection treatment reduced liver stage burden more substantially than postinfection treatment alone. (Figure 3C, D). Furthermore, remaining liver-stages in Nutlin-3-treated cultures were smaller in size than liver stages in untreated cultures (Figure S2G). The dramatic decrease in liver stage burden after Nutlin-3 treatment was not caused by off-target effects that directly impacted parasite fitness because Nutlin-3 treatment had no effect on asexual blood stage growth (Figure S2A) or host cell traversal activity of sporozoites, as measured by a cell wounding assay (Figure S2C). Finally, to test if the cellular changes induced by Nutlin-3 treatment prior to infection are sufficient to curtail parasite infection, we treated HepG2 CD81 cells with Nutlin-3 for 24 h, washed out the drug, and then infected the cells with sporozoites. A significant decrease in liver stage burden (Figure S2B) at 24 hours after infection (Figure S2D) was still observed, further supporting the notion that Nutlin-3 interferes with liver stage infection by targeting the host cell.
We next hypothesized that, in addition to impacting development of liver stages, increased p53 levels due to Nutlin-3 treatment might also contribute to decreased initial infection. To test this, we pre-treated HepG2 CD81 cells with Nutlin-3 and then measured P. yoelii infection rates at an early time point (90 min). Interestingly, we observed significantly fewer infected HepG2 CD81 cells, showing that boosting p53 also alters the host cells’ susceptibility to initial infection by sporozoites (P = 0.03; Fig. 3E). In total, these data indicate that elevated p53 levels render host cells more refractory to initial infection and also reduce subsequent liver stage burden.
Finally, to determine if our in vitro findings extend to in vivo liver stage infection, we treated BALB/cJ mice with 50 mg/kg Nutlin-3 once daily for two days and infected each mouse with 5×104 P. yoelii sporozoites after the first day of treatment. To confirm that Nutlin-3 had the desired effect of increasing p53 activity in vivo, we also monitored transcript abundance of p21, a well characterized positively regulated target of activated p53, which indeed increased in the liver of Nutlin-3-treated mice (Figure S2F). The infected mice were sacrificed and parasite ribosomal 18S RNA was monitored in their livers by quantitative real-time PCR 24 h and 44 h after infection. We noted a dramatically lower liver stage parasite burden in Nutlin-3-treated mice (Figure 3E–F), suggesting that targeting p53 in the host creates an anti-parasitic environment that curtails parasite liver stage infection in vivo.
Discussion
We have shown that lysate array technology provides a unique opportunity to probe the perturbations that pathogens cause in their host cells on the protein level, particularly when it is not possible to obtain a large number of infected cells. Our analysis of malaria parasite liver stage infection suggests that Plasmodium parasites select for, and/or shape, a hepatocytic environment that is pro-proliferative, antiapoptotic and anti-autophagic. Some of our findings are supported by previous work showing that liver stage-infected cells are resistant to at least some apoptotic stimuli (van de Sand et al., 2005). In addition, it was shown that that inhibition of the proliferative PI3K/Akt pathway can reduce liver stage infection (Leiriao et al., 2005) further reinforcing our findings. However, the broad changes in the signaling environment of the infected hepatocyte have remained elusive. Although several of the pathways we identified must be further interrogated for precise mechanism of action, our data lays the framework for a more thorough understanding of host-pathogen interactions that the malaria parasite has evolved for successful replication in hepatocytes.
Specifically, our findings demonstrate that p53 is a key host cell-signaling node perturbed by the parasite, whose regulation is critical to successful liver stage infection. Host p53 is significantly but not completely suppressed by liver stage parasites, and thus promotes survival of the infected cell. However, pharmacological or genetic boosting of p53, which the parasite has not encountered throughout its evolution, counteracts this suppression and impedes parasite proliferation. Conversely, removing p53 genetically provides the parasite with a more hospitable environment for development. Consistent with this, we find no evidence that malaria parasites lower p53 levels to a degree that promotes host cell transformation, as has been described for the related apicomplexan parasite Theileria (Haller et al., 2010). However, the long-term consequences of hepatocyte infection cannot be observed as infected cells die during parasite egress from the liver (Sturm et al., 2006).
The pleotropic functions of p53 in cellular signaling suggest a number of mechanisms for the role of p53 during liver-stage infection. Diminishing levels of p53 in the host hepatocyte could be an obvious advantage for Plasmodium survival as low p53 levels are a major player in developing hepatocyte resistance to apoptosis (Eferl et al., 2003). Although p53 also mediates cell cycle arrest, to date there has been no evidence that parasites prefer or benefit from actively cycling host cells. Finally, our finding that elevated levels of p53 not only have a deleterious effect on liver stage development but also on initial sporozoite infection suggests that p53 affects multiple factors influencing pre-erythrocytic progression. These factors could include the modulation of hepatocyte receptors affecting sporozoite invasion and changes to the hepatocyte cytoskeleton, which could affect liver stage growth.
The roles of p53 in both cell cycle arrest and apoptosis are well understood. However, striking recent evidence suggests that the most dramatic cellular outcomes mediated by p53 function can occur independently of these roles (Li et al., 2012) and that p53 has a substantial role in modulating cellular metabolism and the regulation of reactive oxygen species. These alternatives suggest enticing hypotheses to explain the role of p53 in controlling liver stage parasite development. Plasmodium parasites undergo rapid growth during liver stage infection, suggesting a significant metabolic requirement. Future studies will fully elucidate the interplay between host p53 levels and liver stage parasite survival.
Insights into the host cell-signaling milieu that promotes liver stage survival critically inform host-pathogen interaction studies but might also present potential opportunities for prophylaxis. Efforts to pharmacologically target malaria parasites have focused on intrinsic parasite pathways that are prone to the development of drug resistance (Fidock, 2010; Mackinnon and Marsh, 2010). Here, we demonstrate that the parasite dampens detrimental host signaling, while boosting pro-survival and proliferative signaling. These changes in host signaling represent key features in the intracellular environment required for efficient parasite development. Accordingly, perturbed nodes also represent points of susceptibility. More specifically, Nutlin-3 greatly elevates p53 levels and thus dramatically diminishes liver stage parasite burden. Encouraging, Nutlin-3 has been used in early clinical trials for cancer indications (Secchiero et al., 2011), suggesting that a host-based prophylaxis approach for malaria could be readily translatable to clinical studies. Host-targeted interventions, capable of blocking infection and eliminating the parasite in the liver, are worthy of investigation as a new avenue for anti-malarial prophylaxis.
Materials and Methods
Isolation of PyGFP-infected HepG2 CD81 cells
Cells were cultured as described in supplemental methods. 5×106 cells were infected with 2×106 P.yoelii transgenic parasites that express GFP as described previously (Tarun et al., 2008). Cells were detached using 0.25% Trypsin-EDTA and resuspended in DMEM complete media with 5mM EDTA. Cells were passed through a cell strainer to prepare for FACS. Flow cytometric analyses and cell sorting of PyGFP-infected hepatocytes were carried out with a Cytopeia Influx Cell Sorter using the Spigot Operating Software Version 5.0.3.1 (Cytopeia).
Microarray fabrication
Cells were lysed in SDS lysis buffer (2% SDS, 50 mM Tris-HCl, 5% Glycerol, 5mM EDTA, 1mM NaF, 10mM β-glycerophosphate, 1mM PMSF, 1mM activated Na3VO4, 1mM DTT, 1% phosphatase inhibitor cocktail 2 (Sigma-Aldrich), 1% PhosSTOP Phosphatase Inhibitor Cocktail Tablet (Roche) and stored at −80°C. Custom lysate microarrays were printed in house using an Aushon Biosystems 2470 arrayer (Aushon Biosystems) on 16-pad nitrocellulose-coated glass slides (Grace Bio- Labs). Lysates were arrayed at 333 µM spacing using solid 110 µM pins, which resulted in an average feature diameter of 170 µM. Lysates were arrayed in quadruplicate technical replicates. Slides were then stored dry, in the dark, and at room temperature until probing. Slides were probed and quantified as previously described (Sevecka et al., 2011).
Quantification of p53 by western Blotting
We plated 106 HepG2 CD81 cells in each well of a 6-well plate in DMEM complete and treated with 20µM Nutlin-3 (Cayman Chemical). We made cellular lysates with SDS array buffer (described above). western blots were performed according to standard protocols using an antibody to p53 (Clone 1C12, Cell Signaling Technology) then normalized to signal from an anti-α-tubulin (Invitrogen) western blot. Signals from immunoblots were detected using either an Alexa-680-conjugated anti-rabbit antibody or an Alexa-800-conjugated anti-mouse antibody (LI-COR Biosciences). We visualized membranes using an Odyssey infrared imaging system (LI-COR Biosciences).
Immunofluorescence
105 HepG2 CD81cells were infected with 104 P. berghei or P. yoelii sporozoites as previously described. Cells were fixed and stained as described previously (Kaushansky and Kappe, 2011). We stained cells using anti-sera to Plasmodium heat shock protein 70 (HSP70) Upregulated in infectious sporozoites 4 protein (UIS4). Sporozoites that had not invaded and/or developed in hepatoma cells were distinguished by UIS4 non-circumferential staining and morphology.
Quantification of liver stages after Nutlin-3 treatment by manual counting
105 HepG2 CD81cells were treated with DMSO alone or 20µM Nutlin-3 for the indicated period of time. Cells were infected with 104 P. berghei or P. yoelii sporozoites. Parasites were stained and visualized as describe above. All liver stages in each well were counted, and each assay was performed in biological triplicate.
Quantification of liver stages by FACS
Cells were cultured as described above. 2×105 HepG2-CD81 cells were plated in each well of a 24-well plate, and infected with 5×104 P. yoelii sporozoites. Cells were treated with our without Nutlin-3 as describe above. At the desired time point, cells were harvested and stained as described previously (Luckert et al., 2012). All experimental conditions were tested in biological triplicate. All data is representative of three independent experiments.
Cell Wounding Assay
105 HepG2 CD81 cells were plated into each well of a 48-well plate and allowed to adhere overnight in DMEM complete media. We mixed 5 × 104 P. yoelii sporozoites, or media alone with 1mg/mL AlexaFluor-488 labeled dextran (10,000 MW, lysine fixable, Invitrogen), then performed the traversal assays as described previously (Luckert et al., 2012). Wounded cells (identified by positive AlexaFluor-488 signal) were analyzed by FACS.
In vivo Nutlin-3 experiments
26 BALB/cJ mice (Jackson) were treated with either vehicle control or 50 mg/kg of Nutlin-3 once daily for two days. On the second day of treatment, mice were injected with 5×104 P. yoelii sporozoites. Livers were excised from mice at either 24 or 44 h after infection. Animal handling was conducted according to Institutional Animal Care and Use Committee-approved protocols.
Super-p53 and p53 KO mice experiments
20 male C57BL/6 (Jackson), 13 C57BL/6- super-p53, and 7 C57BL/6-p53-KO (Jackson) mice were injected with 105 P. yoelii-GFP-luciferase sporozoites. At 42–44 hrs post-infection, livers were excised from mice and lysed with TRIzol reagent (Invitrogen). Animal handling was conducted according to Institutional Animal Care and Use Committee-approved protocols.
Supplementary Material
Highlights.
Protein lysate arrays are a useful tool for the study of host-pathogen interactions
Hepatocyte signaling is substantially perturbed in response to Plasmodium infection
Regulation of host-p53 is required for efficient Plasmodium development
Elevated levels of p53 eliminates Plasmodium parasites during liver stage
Acknowledgements
We are grateful to Heather S. Kain, William W. Benz, Mark F. Kennedy and Jen C.C. Hume for mosquito and sporozoite production. We thank Hieu Nguyen for technical assistance with FACS. We thank Patrick McDougall and Seattle Biomedical Research Institute vivarium staff for their work with mice. We are extremely grateful to Manuel Serrano for the kind gift of the ‘super-p53’ mice. A.K. is a recipient of a NRSA Ruth L. Kirschstein National Research Service Award (F32 AI091129), which has partially funded this work. G.M. is an employee of Merrimack Pharmaceuticals Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: AK, ASY, GM and SHIK designed the research, AK, ASY, LSA, SAM, AMV, NC and PGM performed experiments, GM and SHIK supervised the research, AK and SHIK wrote the paper with contributions from ASY and GM.
References
- Albuquerque SS, Carret C, Grosso AR, Tarun AS, Peng X, Kappe SH, Prudencio M, Mota MM. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics. 2009;10:270. doi: 10.1186/1471-2164-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- Carrolo M, Giordano S, Cabrita-Santos L, Corso S, Vigario AM, Silva S, Leiriao P, Carapau D, Armas-Portela R, Comoglio PM, et al. Hepatocyte growth factor and its receptor are required for malaria infection. Nat Med. 2003;9:1363–1369. doi: 10.1038/nm947. [DOI] [PubMed] [Google Scholar]
- Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development, c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Fidock DA. Drug discovery: Priming the antimalarial pipeline. Nature. 2010;465:297–298. doi: 10.1038/465297a. [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. "Super p53" mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller D, Mackiewicz M, Gerber S, Beyer D, Kullmann B, Schneider I, Ahmed JS, Seitzer U. Cytoplasmic sequestration of p53 promotes survival in leukocytes transformed by Theileria. Oncogene. 2010;29:3079–3086. doi: 10.1038/onc.2010.61. [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Kaushansky A, Kappe SH. The crucial role of hepatocyte growth factor receptor during liver-stage infection is not conserved among Plasmodium species. Nat Med. 2011;17:1180–1181. doi: 10.1038/nm.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiriao P, Albuquerque SS, Corso S, van Gemert GJ, Sauerwein RW, Rodriguez A, Giordano S, Mota MM. HGF/MET signalling protects Plasmodium-infected host cells from apoptosis. Cell Microbiol. 2005;7:603–609. doi: 10.1111/j.1462-5822.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckert K, Gujral TS, Chan M, Joos TO, Sorger PK, Macbeath G, Potz O. A dual array-based approach to assess the abundance and posttranslational modification state of signaling proteins. Sci Signal. 2012;5:pl1. doi: 10.1126/scisignal.2002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon MJ, Marsh K. The selection landscape of malaria parasites. Science. 2010;328:866–871. doi: 10.1126/science.1185410. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Bosco R, Celeghini C, Zauli G. Recent advances in the therapeutic perspectives of Nutlin-3. Current pharmaceutical design. 2011;17:569–577. doi: 10.2174/138161211795222586. [DOI] [PubMed] [Google Scholar]
- Sevecka M, Wolf-Yadlin A, MacBeath G. Lysate microarrays enable high-throughput, quantitative investigations of cellular signaling. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.005363. M110 005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, Hannoun L, Eling W, Levy S, Boucheix C, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- Tarun AS, Baer K, Dumpit RF, Gray S, Lejarcegui N, Frevert U, Kappe SH. Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int J Parasitol. 2006;36:1283–1293. doi: 10.1016/j.ijpara.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sand C, Horstmann S, Schmidt A, Sturm A, Bolte S, Krueger A, Lutgehetmann M, Pollok JM, Libert C, Heussler VT. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol Microbiol. 2005;58:731–742. doi: 10.1111/j.1365-2958.2005.04888.x. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zheng T, Chen X, Song X, Meng X, Bhatta N, Pan S, Jiang H, Liu L. MDM2 antagonist can inhibit tumor growth in hepatocellular carcinoma with different types of p53 in vitro. J Gastroenterol Hepatol. 2011;26:371–377. doi: 10.1111/j.1440-1746.2010.06440.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.