Abstract
Purpose
A multi-institutional phase II trial assessed the utility of dose-painted IMRT (DP-IMRT) in reducing grade 2+ combined acute gastrointestinal and genitourinary adverse events (AEs) of 5-fluorouracil (5FU) and mitomycin-C (MMC) chemoradiation for anal cancer by at least 15% as compared to the conventional radiation/5FU/MMC arm from RTOG 9811.
Methods and Materials
T2-4N0-3M0 anal cancer patients received 5FU and MMC days 1 and 29 of DP-IMRT, prescribed per stage - T2N0: 42Gy elective nodal and 50.4Gy anal tumor planning target volumes (PTVs) in 28 fractions; T3-4N0-3: 45Gy elective nodal, 50.4Gy ≤ 3cm or 54Gy > 3cm metastatic nodal and 54Gy anal tumor PTVs in 30 fractions. The primary endpoint is described above. Planned secondary endpoints assessed all AEs and the investigator’s ability to perform DP-IMRT.
Results
Of 63 accrued patients, 52 were evaluable. Tumor stage included: 54% II, 25% IIIA, 21% IIIB. In primary endpoint analysis, 77% experienced grade 2+ gastrointestinal/genitourinary acute AEs (9811 77%). There was, however, a significant reduction in acute grade 2+ hematologic, 73% (9811 85%, P=0.032), grade 3+ gastrointestinal, 21% (9811 36%, P=0.0082), and grade 3+ dermatologic AEs 23% (9811 49%, P<0.0001) with DP-IMRT. On initial pre-treatment review, 81% required DP-IMRT re-planning, while final review revealed only three cases with normal tissue major deviations.
Conclusions
Although the primary endpoint was not met, DP-IMRT was associated with significant sparing of acute grade 2+ hematologic, and grade 3+ dermatologic and gastrointestinal toxicity. While DP-IMRT proved feasible, the high pre-treatment planning revision rate emphasizes the importance of real-time radiation quality assurance for IMRT trials.
INTRODUCTION
Radiation therapy with concurrent 5-fluorouracil (5FU) and mitomycin-C (MMC) serves as the standard of care for patients with non-metastatic squamous cell cancer of the anal canal (1–5). This treatment results in long-term disease-free survival and sphincter preservation, but often with significant acute toxicity, due in part, to the large nonconformal radiation fields used to encompass the elective nodal regions.
As small retrospective series suggested acute toxicity sparing with the use of intensity-modulated radiation therapy (IMRT) (6–8), Radiation Therapy Oncology Group (RTOG) 0529 prospectively assessed the utility of IMRT in reducing the acute morbidity of 5FU/MMC chemoradiation for anal canal cancer in a cooperative group trial setting. A single phase dose-painted IMRT (DP-IMRT) technique in which different fraction sizes of radiation are delivered to high- and low-risk tumor volumes was employed(8). Building upon pre-clinical dosimetric analysis(9), the primary endpoint was to determine if the addition of DP-IMRT would reduce the combined rate of grade 2+ gastrointestinal and genitourinary acute adverse events by at least 15%, as compared to the radiation/ 5FU/MMC arm of RTOG 9811, a randomized phase III trial evaluating the efficacy of nonconformal radiation, 5FU and cisplatin vs. conventional radiation/5FU/MMC. Two important secondary endpoints of RTOG 0529 were to evaluate the potential reduction of all adverse events with the use of DP-IMRT, and to assess the investigator’s ability to perform DP-IMRT within the radiation planning guidelines delineated.
This represents the first report of RTOG 0529. Acute toxicity and radiation planning compliance for patients receiving DP-IMRT with concurrent 5FU and MMC chemotherapy as definitive treatment for anal canal cancer are presented.
METHODS AND MATERIALS
Patient Eligibility
This study was coordinated by the U.S. RTOG and performed with the approval of the institutional review board for human research at each institution. Patients with histologically documented squamous or basaloid carcinoma of the anal canal were eligible if they were at least 18 years of age with a Zubrod performance status of ≤ 1, 2002 American Joint Committee on Cancer clinical stage T2-4 disease with any N category, adequate organ function, history/physical examination within 14 days prior to registration, anal and groin evaluation and staging imaging studies 42 days prior to registration, and informed written consent.
Patients were excluded if they were females who were pregnant or lactating, or had T1 or M1 tumors, severe co-morbid conditions (including AIDS or other immunocompromised states), previous major malignancy (unless disease-free for 3 years), and prior pelvic radiotherapy or chemotherapy or complete macroscopic anal cancer resection.
Evaluation
Pre-treatment evaluation included complete history/physical; blood chemistries to determine the adequacy of hepatic, renal and marrow functions; chest x-ray or chest computed tomography (CT), abdominal/pelvic CT, and/or 2-deoxy-2[F-18]-fluoro-D-glucose positron emission tomography CT to establish the disease stage; and colonoscopy, sigmoidoscopy, or rigid proctoscopy for evaluation of the primary.
DP-IMRT Planning and Delivery
Each institution was required to meet specific credentialing and complete a protocol-directed dry run test (the DP-IMRT plan for the first patient) pre-treatment. Electronic real-time planning review was performed pre-treatment on all cases.
CT-based planning (axial images at ≤ 5 mm intervals from the upper lumbar spine to the mid-femur) was performed. Oral and IV contrast was recommended to allow better visualization of the bowel, iliac and inguinal vessels. A radio-opaque marker at the anal verge and wire to outline the caudad extent of the anal tumor were suggested to assist in target delineation. Patients were scanned in either a supine, “arms-up,” frog-legged position using custom immobilization or in the prone position with bowel displacement.
According to the International Commission on Radiological Units 50 guidelines, all target and normal tissue structures were contoured on the planning CT slices. The gross tumor volume (GTV) was contoured using exam, imaging and endoscopy findings. GTVA included the primary anal tumor; GTVN50 metastatic nodal regions ≤ 3 cm; and GTVN54 metastatic nodal regions > 3 cm. The Clinical Target Volume (CTV) was defined as the GTV plus areas of potential microscopic disease. A 2.5 cm and 1 cm expansion was added to the primary and nodal GTVs, respectively, to create CTVs which were then manually edited to avoid overlap into non-target muscles or bone, considered natural barriers to tumor infiltration. Elective nodal CTVs (mesorectum, presacrum, bilateral internal and external iliac, and bilateral inguinal) have been previously published, and are shown in Figure1a (10). This atlas was developed and placed on the RTOG website after the first five 0529 cases displayed inaccurate contouring of the mesorectum, Figure 1b. A 1 cm expansion was added to all CTVs to create the planning target volumes (PTVs). The PTVs were not edited in any way except to avoid overlap with the skin.
Figure 1.
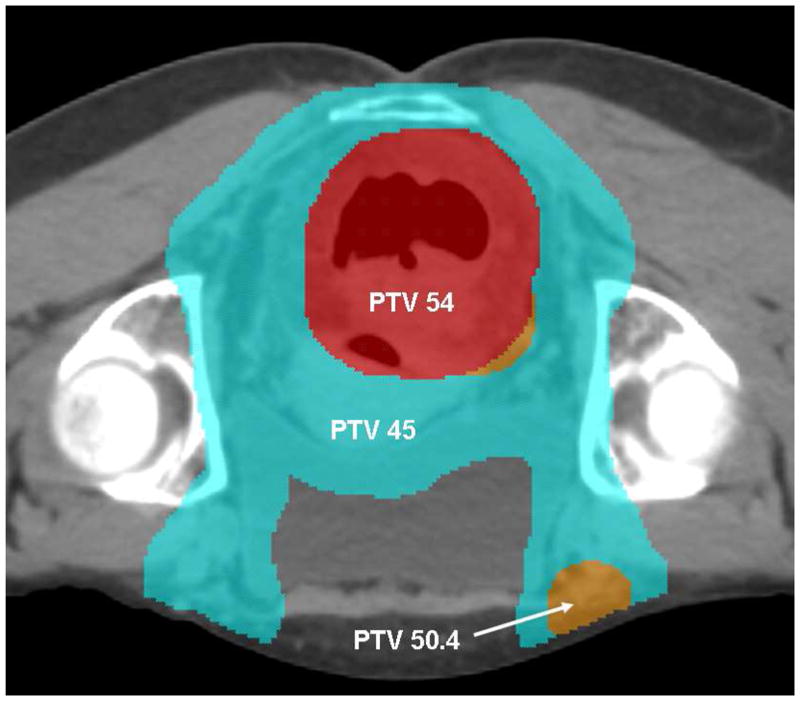
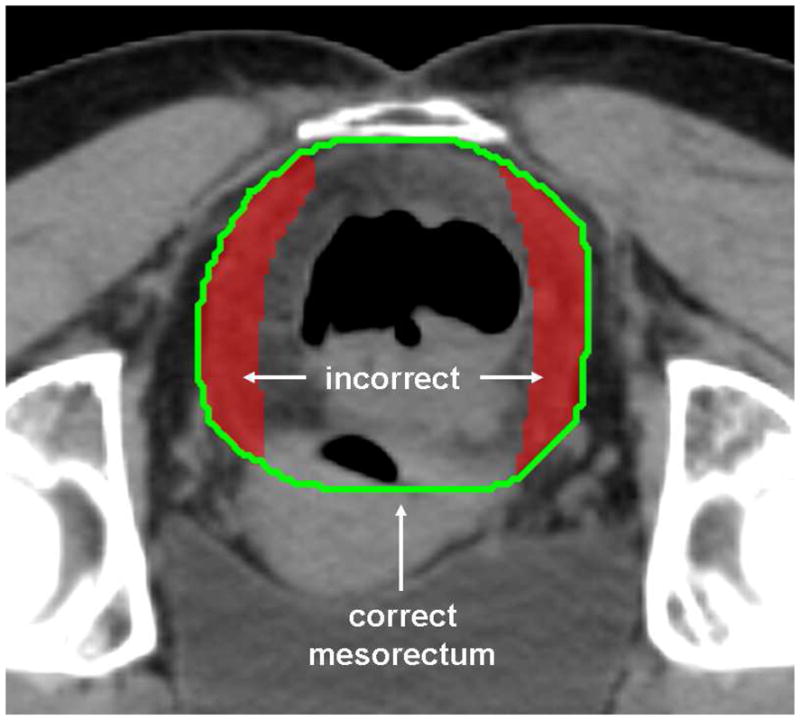
Figure 1a. DP-IMRT Target Volumes and Doses
A representative image of target volumes and doses in a cT3N3M0, stage IIIB case. The primary tumor PTV receives 54 Gy (red colorwash), and the elective nodes 45 Gy (blue colorwash). An involved right-sided inguinal node was dose-painted to 50.4 Gy (orange colorwash).
Figure 1b. Mesorectal Contouring
An illustration of incorrect (red colorwash) and correct (green line) investigator contouring of the mesorectum. The rectum with its surrounding lymphatic tissue is an important elective target.
Normal structures (small bowel, large bowel, bladder, femoral heads, iliac bones, perianal skin, genitalia) were also contoured, the bowel as individual loops to 2 cm above the most superior extent of the target CTVs. The entire rectum was considered a target structure and therefore was excluded from large bowel contouring.
Dose prescriptions for target volumes varied according to stage, Figure 1b; T2N0: 42Gy elective nodal and 50.4Gy anal tumor PTVs in 28 fractions, with T3-4N0-3: 45Gy elective nodal, 50.4Gy ≤ 3cm or 54Gy > 3cm metastatic nodal and 54Gy anal tumor PTVs in 30 fractions. Normal tissue dose constraints are listed in Table 1. The planning priority was maximal PTV coverage, with the prescription isodose surface encompassing at least 90% of the primary and involved nodal PTVs, and at least 85% of the uninvolved nodal PTVs. Dose distributions included tissue heterogeneity corrections.
Table 1.
DP-IMRT Dose Constraints for Normal Tissues
| Organ | Dose (Gy) at < 5% volume | Dose (Gy) at < 35% volume | Dose (Gy) at < 50% volume |
|---|---|---|---|
| Small bowel*† | 45 (< 20cc) | 35 (< 150cc) | 30 (< 200cc) |
| Femoral heads* | 44 | 40 | 30 |
| Iliac crest | 50 | 40 | 30 |
| External genitalia | 40 | 30 | 20 |
| Bladder | 50 | 40 | 35 |
| Large bowel† | 45 (< 20cc) | 35 (< 150cc) | 30 (< 200cc) |
Organs are listed in order of decreasing priority.
assigned criteria for major and minor violations; major violations were considered as part of the feasibility secondary endpoint
dose constraints based on absolute volume instead of % volume
DP-IMRT treatments were delivered once daily, 5 fractions per week. Daily image guidance (IGRT) was recommended, but not mandated, for prone delivery. Radiation was held for grade 3–4 non-hematologic acute toxicity (with the exception of dermatologic) as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 [NCI-CTCAE v3], (MedDRA version 6.0), until the toxicity was reduced to ≤ grade 2. Radiation was held for grade 4 dermatologic morbidity and resumed when improved to grade ≤ 3. Radiation was held for grade 3 white blood cell (wbc) and platelet morbidity and re-started when the absolute neutrophil count was ≥ 500/μL and platelets ≥ 50,000/μL.
Chemotherapy
Two cycles of 5-fluorouracil (1000 mg/m2/d as a 96 hour infusion, days 1–5 and 29–33 of DP-IMRT) and mitomycin-C (10 mg/m2 bolus, days 1 and 29) were administered. Chemotherapy was held for grade 3+ acute toxicities until the morbidity was resolved to ≤ grade 2. If the toxicity occurred before the second cycle, then the second cycle dose was reduced to 50%. For dermatologic toxicity, the chemotherapy second cycle was omitted if grade 4 dermatitis was reported before the fifth treatment week.
Toxicity Assessment
Acute adverse events (AEs) were assessed per the NCI-CTCAE v3 weekly during chemoradiation and in follow-up (4 and 8 weeks post treatment). The incidence of the worst grade toxicity sustained up to 90 days from DP-IMRT initiation was recorded as an acute toxicity event.
Statistics
The primary endpoint was to determine if the combined rate of treatment-related acute grade 2+ gastrointestinal/genitourinary AEs was decreased by at least 15% as compared to the RTOG 9811 conventional radiation/5FU/MMC arm (77%)(5). Fifty-four patients provided 80% power to detect this 15% reduction, using a one-sided chi-squared test with a 0.05 type I error rate. Fifty-nine patients were targeted to allow for an 8% ineligibility/lost rate. As the sites of worst dermatologic toxicity are not discriminated using the NCI-CTCAE v3, and as it was hypothesized that a similar peri-anal skin reaction would be reported, dermatologic toxicity was not chosen for primary endpoint analysis. An important secondary endpoint was to determine the feasibility of performing DP-IMRT in a cooperative group setting. Using Simon’s two-stage design(11), p is the true probability of an acceptable review. A p close to 1 implies that DP-IMRT is reproducible. With 42 patients, if p is ≤ 80%, there is < 5% probability of concluding that the technique is reproducible, and < 10% probability of concluding that the technique is not reproducible if p is ≥ 95%. If < 5 cases had major radiation planning deviations, than DP-IMRT would be considered reproducible. Supplemental Table 1 shows the power to detect 15% and 20% absolute reductions in the specified AEs, defined aprior in the protocol.
All analyses were performed using SAS/STAT® software(12). Z-tests were used to compare AE proportions. Reported p-values for these comparisons are 1-sided. The Kruskal-Wallis test was used to test for differences in median treatment duration and median days of treatment interruptions between RTOG 0529 and 9811. Logistic regression models were used to assess correlation between patient/tumor characteristics and grade 2+ AEs, with grade < 2 AEs as the reference group. Characteristics evaluated included: gender (male vs. female), T stage (T1/T2 vs. T3/T4), N stage (N+ vs. N0), and small bowel score (per protocol vs. minor/major deviation).
RESULTS
Patient and Tumor Characteristics
This trial opened on December 21, 2006 and closed on March 21, 2008; accruing 63 patients from 38 institutions. Fifty-two patients were evaluable. Reasons for exclusion included history/exam performed > 14 days prior to registration (n=6), biopsy performed > 42 days prior to registration (n=1), wbc count < 3,000/μL (n=1), no protocol treatment (n=1), tumor stage T1 (n=1), and incorrect protocol registration (RTOG 0529 instead of 0522), (n=1).
Table 2 describes baseline patient and treatment characteristics for the 52 evaluable patients. The median age was 58 years (min-max, 34–82 years); 81% female. The median anal tumor size was 4 cm (min-max, 2.1–20.0 cm). The stage of disease was II in 54%, IIIA in 25% and IIIB in 21%.
Table 2.
Baseline Patient and Treatment Characteristics (n=52)
| Characteristics | Number of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 58 | |
| Min-Max | 34–82 | |
| Primary Tumor Size, cm | ||
| Median | 4.0 | |
| Min-Max | 2.1–20.0 | |
| Gender | ||
| Male | 10 | 19.2 |
| Female | 42 | 80.8 |
| Race | ||
| White | 44 | 84.6 |
| Black or African American | 5 | 9.6 |
| Other | 3 | 5.8 |
| Zubrod | ||
| 0 | 40 | 76.9 |
| 1 | 12 | 23.1 |
| Tumor Histology | ||
| Squamous | 48 | 92.3 |
| Basaloid | 4 | 7.7 |
| Tumor Differentiation | ||
| Low grade | 5 | 9.6 |
| Intermediate | 22 | 42.3 |
| High grade | 14 | 26.9 |
| Unknown | 11 | 21.2 |
| Tumor Stage | ||
| T2 | 32 | 61.5 |
| T3 | 16 | 30.8 |
| T4 | 4 | 7.7 |
| Nodal Stage | ||
| N0 | 29 | 55.8 |
| N1 | 13 | 25.0 |
| N2 | 5 | 9.6 |
| N3 | 5 | 9.6 |
| AJCC Staging, 6th Edition | ||
| II | 28 | 53.8 |
| IIIA | 13 | 25.0 |
| IIIB | 11 | 21.2 |
Radiation Planning Quality Assurance
Of the 52 DP-IMRT cases, 81% required planning revisions pre-treatment on initial submission, 46% required multiple re-submissions, of which 4 cases never passed. Incorrect investigator contouring included inaccurate delineation of gross tumor (21%), miscontouring of elective nodal volumes (mesorectum 55%, presacrum 43%, inguinal fossa 33%, iliac nodal groups 31%), and/or misidentification of normal structures (small bowel 60%, large bowel 45%). Each DP-IMRT treatment was reviewed for target dose prescription and normal tissue constraint compliance, Table 3. Minor deviations were infrequent for tumor dosing, and 39% experienced minor deviations for small bowel and 22% for femoral head coverage. On final pre-treatment review, there were no major deviations concerning target dosing, and only three cases with normal tissue major deviations (two from investigators who did not attain final pre-treatment plan approval). As such, DP-IMRT was considered reproducible in a cooperative group setting.
Table 3.
| PTV Anal | PTV Nodal | PTV Small Bowel | PTV Femoral Heads | |
|---|---|---|---|---|
| Per Protocol | 44 (86%) | 48 (94%) | 28 (55%) | 39 (76%) |
| Minor Deviation | 7 (14%) | 3 (6%) | 20 (39%) | 11 (22%) |
| Major Deviation | 0 (0%) | 0 (0%) | 3 (6%)‡ | 1 (2%)‡ |
Abbreviations: PTV=planning tumor volume
For target coverage (anal and nodal), a minor deviation included 6–10% of the PTV receiving < 90% of the radiation prescription dose, or 2–5% < 80%, or > 2% receiving > 115%. A major deviation included ≥ 10% of the PTV receiving < 90% of the prescription dose, or ≥ 5% < 80%. For normal structures, a minor deviation for the small bowel included treating 150–300 cc of small bowel above 35 Gy, or 20–30 cc > 45 Gy. A major deviation was ≥ 300 cc receiving above 35 Gy, or ≥ 30 cc exceeding 45 Gy. For the femoral heads, a minor deviation was treating 5–10% of either hip above 44 Gy, with a major deviation designated to ≥ 10% receiving > 44 Gy.
One patient did not receive DP-IMRT.
Of note, 4 major deviations occurred in 3 patients.
Acute Toxicity
Fifty-two patients are included in the analysis, of which 51 patients (95%) completed DP-IMRT as prescribed and one patient did not receive DP-IMRT unrelated to toxicity. All patients started protocol chemotherapy, but 8 (16%) patients were scored as not completing per protocol on both cycles. The median duration of DP-IMRT was 43 days (min-max, 32–59), as compared to 49 days (min-max, 4–100) for the RTOG 9811 radiation/5FU/MMC arm (P<0.0001). Treatment breaks due to toxicity were needed in 49%, as compared to 62% on 9811 (P=0.09). The median duration of treatment interruption was 0 days (min-max, 0–12), as compared to 3 days (min-max, 0–33) on 9811 (P=0.0047).
Acute AEs are summarized in Table 4. All patients had complete AE data. Seventy-seven percent of patients experienced a combined grade 2+ gastrointestinal/genitourinary rate of acute AEs, equivalent to the rate reported on RTOG 9811’s radiation/5FU/MMC arm, Table 5. While, the primary endpoint hypothesis was not met and overall AE rates were similar, there was a significant reduction in acute grade 2+ hematologic toxicity from 85% on 9811 to 73% with DP-IMRT, P=0.032, as well as in grade 3+ gastrointestinal/genitourinary morbidity, 21% with DP-IMRT vs. 37% on 9811, P=0.0052. When analyzing gastrointestinal and genitourinary acute AEs independently, the rate of grade 3+ genitourinary toxicity was similar to 9811, while grade 3+ gastrointestinal AEs showed a significant reduction with DP-IMRT. In assessment of dermatologic toxicity, there was also a significant reduction in grade 3+ dermatitis to 23% from the 49% reported on 9811, P<0.0001.
Table 4.
Number of DP-IMRT Patients with an Acute Adverse Event by Category and Grade* (n=52)
| Category | Grade | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Allergy/immunology | 1 | 1 | 0 | 0 | 0 |
| Blood/bone marrow | 4 | 8 | 16 | 14 | 0 |
| Cardiac arrhythmia | 0 | 0 | 2 | 0 | 0 |
| Cardiac general | 0 | 1 | 0 | 0 | 0 |
| Coagulation | 0 | 0 | 0 | 1 | 0 |
| Constitutional symptoms | 10 | 16 | 6 | 0 | 0 |
| Dermatology/skin | 5 | 27 | 11 | 1 | 0 |
| Endocrine | 1 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 10 | 27 | 11 | 0 | 0 |
| Hemorrhage/bleeding | 10 | 1 | 0 | 0 | 0 |
| Infection | 0 | 5 | 11 | 1 | 0 |
| Lymphatics | 4 | 0 | 0 | 0 | 0 |
| Metabolic/laboratory | 9 | 6 | 11 | 0 | 0 |
| Neurology | 5 | 3 | 1 | 0 | 0 |
| Pain | 8 | 15 | 10 | 2 | 0 |
| Pulmonary/upper respiratory | 4 | 0 | 3 | 0 | 0 |
| Renal/genitourinary | 10 | 7 | 1 | 0 | 0 |
| Sexual/reproductive function | 2 | 2 | 2 | 0 | 0 |
| Vascular | 0 | 1 | 0 | 0 | 0 |
|
| |||||
| Worst overall | 3 | 6 | 27 | 16 | 0 |
Adverse events were scored as definitely, probably, or possibly related to protocol treatment and include adverse events where relationship to protocol treatment is missing.
Adverse events were graded with CTCAE version 3.0.
Table 5.
Comparisons of Acute Treatment-Related Adverse Events*
| 0529 (n=52) | 98-11 (Arm 1†) (n=325) | p-value (one-sided proportions test§) | |
|---|---|---|---|
| Grade 2+ Adverse Events | |||
| GI/GU‡ | 40 (77%) | 249 (77%) | 0.50 |
| Derm | 39 (75%) | 271 (83%) | 0.10 |
| GI | 38 (73%) | 237 (73%) | 0.50 |
| GU | 8 (15%) | 66 (20%) | 0.18 |
| Heme | 38 (73%) | 275 (85%) | 0.032 |
| Overall | 49 (94%) | 318 (98%) | 0.12 |
| Grade 3+ Adverse Events | |||
| GI/GU | 11 (21%) | 120 (37%) | 0.0052 |
| Derm | 12 (23%) | 159 (49%) | <0.0001 |
| GI | 11 (21%) | 117 (36%) | 0.0082 |
| GU | 1 (2%) | 11 (3%) | 0.32 |
| Heme | 30 (58%) | 201 (62%) | 0.29 |
| Overall | 43 (83%) | 283 (87%) | 0.23 |
Abbreviations: GI, gastrointestinal; GU, genitourinary; Derm, dermatologic; Heme, hematologic
Graded using CTCAE version 3.0 in 0529 and CTC version 2.0 in 9811.
9811 Arm 1: conventional radiation, 5-Fluorouracil and Mitomycin-C
Represents the primary endpoint analysis, while others are secondary analyses.
This is a Z-test for decrease in adverse events from 98-11.
In univariate analysis of acute toxicity predictors, patients who had a minor or major deviation in small bowel radiation dose had an increased rate of grade 2+ gastrointestinal morbidity, compared to patients meeting the constraints (90% vs. 65%, P=0.04). Univariate and multivariate analyses of other characteristics did not reveal any further significant associations, (data not shown).
DISCUSSION
This represents the first report of a phase II multi-institutional trial of IMRT for the treatment of anal cancer. Although the primary endpoint was not met, DP-IMRT was associated with a significant reduction in acute toxicity for grade 3+ dermatologic and gastrointestinal, and grade 2+ hematologic events. Grade 3+ skin toxicity was demonstrated in 23% of patients, in contrast to 49% of patients on the 5FU/MMC arm of RTOG 9811, which used conventional radiation(5). With DP-IMRT, skin bolus was not necessary as the oblique incidence of the radiation beams increases superficial dose, and the PTVs were trimmed away from the surface to avoid superficial skin dose in areas not involved with gross disease. Given the proximity of the primary tumor to the perianal tissues, grade 2 perianal skin reactions still occurred; however, the inguinal fossa and genitalia were spared significant toxicity. This skin toxicity savings may be important for trials combining epidermal growth factor inhibitors with standard chemotherapy in attempts to improve disease-free survival.
With conventional chemoradiation, acute gastrointestinal morbidity in anal cancer is also substantial. In RTOG 9811, 36% of patients experienced grade 3+ gastrointestinal toxicity, primarily diarrhea (5). With DP-IMRT, intentional sparing of dose to the small bowel resulted in a decrease of grade 3+ gastrointestinal toxicity to 21%. Of note, cases with a minor deviation in small bowel dose had increased grade 2+ gastrointestinal morbidity, compared to cases meeting this constraint. Further analysis of dose-volume predictors of gastrointestinal toxicity with DP-IMRT, as well as the effect of patient positioning (supine vs. prone), will be the subject of a separate publication.
The rate of grade 2+ acute hematologic toxicity with DP-IMRT was lower than those reported by RTOG 9811, despite the large volume of pelvic bone marrow receiving low radiation doses(5). Investigators have shown that it is the volume of bone marrow receiving 10 and 20 Gy that is associated with hematologic morbidity (13). Further reductions in hematologic toxicity may be gained if increased priority was placed on reducing the DP-IMRT dose to the pelvic bone marrow receiving 10–20 Gy.
Treatment interruptions due to toxicity were less frequent with DP-IMRT. The median duration of DP-IMRT was 43 days, as compared to 49 days for the RTOG 9811 radiation/5FU/MMC arm (P<0.0001). Reducing breaks in treatment is of paramount importance for maintaining local control and sphincter-preservation in anal cancer, given that patients enrolled onto RTOG 9208, a phase II trial exploring higher radiation doses with a mandatory two week break, had a 30% two-year colostomy rate, as compared to a 12% 5-year colostomy rate on RTOG 9811(14).
Critical to the use of IMRT is an understanding of the elective CTV. In approximately 80% of RTOG 0529’s plans, incorrect investigator contouring was identified, with undercontouring of the mesorectum in over 50%. While the rectum and its associated mesentery are avoided in IMRT planning of gynecologic or genitourinary malignancies, they represent the first echelon of nodal drainage for anal cancer and, therefore, must be carefully contoured for treatment. This finding emphasizes the importance of continuing education for IMRT volume contouring for anal cancer, as well as real-time radiation quality assurance for future IMRT trials. It was, however, reassuring that major deviations to the radiation planning directives were identified in only three cases, demonstrating that IMRT to this disease site is feasible in a cooperative group setting.
While our study represents the first cooperative group analysis of IMRT for the treatment of anal cancer and the only study incorporating real-time QA, this report has several limitations. Firstly, our results are being compared to historical data (in contrast to randomized data) from RTOG 9811. Secondly, in order to perform a single IMRT plan, our elective nodal doses slightly varied from those of RTOG 9811, which may bias the toxicity data and impact local-regional control. Thirdly, with highly conformal therapy, comes the risk of underdosing both subclinical and gross disease. Marginal misses have been demonstrated in head and neck cancer treated with IMRT (15). While investigators have reported excellent rates of local control in their retrospective IMRT series (6–8), it will be important to demonstrate comparable two-year efficacy control and local-regional failure patterns with DP-IMRT and its elective nodal doses to that of the larger conventional radiation series. Lastly, as daily IGRT was not mandated in this trial, doses to both normal tissues and PTVs may have been affected.
In conclusion, dose-painted IMRT with 5-fluorouracil and mitomycin-C for the treatment of anal canal cancer is feasible in a multi-institutional setting. Although the primary endpoint was not met, DP-IMRT was associated with a significant sparing of acute grade 2+ hematologic, and grade 3+ dermatologic and gastrointestinal toxicity. Analysis of efficacy, local-control patterns and late effects will be forthcoming in attempts to validate this approach. If local-regional control appears equivalent or improved to RTOG 9811 historical rates, IMRT (with 5FU and MMC) will become the standard arm for future RTOG studies, and may become standard practice. It is also important to note that the high pre-treatment revision rate of volume contouring indicates an educational need and emphasizes the importance of real-time radiation quality assurance for future IMRT trials.
Supplementary Material
SUMMARY.
RTOG 0529 assessed the utility of dose-painted IMRT (DP-IMRT) in reducing the acute morbidity of 5-fluorouracil/mitomycin-C chemoradiation for T2-4N0-3M0 anal cancer. With 52 evaluable patients, the primary endpoint of reducing ≥ grade 2 combined gastrointestinal and genitourinary acute adverse events by 15% as compared to the RTOG 9811 5-fluorouracil/mitomycin-C arm using standard radiation techniques was not met. However, DP-IMRT yielded significant sparing of acute grade 2+ hematologic, and grade 3+ dermatologic and gastrointestinal toxicity.
Acknowledgments
This work was supported by RTOG U10 CA21661, CCOP U10 CA3742 and ATC U24 CA 81647 grants from the National Cancer Institute.
The authors would like to acknowledge analysis support from Jennifer Moughan, M.S.
Footnotes
Conflict of Interest to Disclose: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nigro ND, Vaitkevicius VK, Considine B., Jr Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–356. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 2.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin, UKCCCR Anal Cancer Trial Working Party, UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 3.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 4.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 5.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 6.Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2005;63:354–361. doi: 10.1016/j.ijrobp.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007;25:4581–4586. doi: 10.1200/JCO.2007.12.0170. [DOI] [PubMed] [Google Scholar]
- 8.Kachnic LA, Tsai HK, Coen JJ, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys. 82:153–158. doi: 10.1016/j.ijrobp.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Tsai HK, Hong TS, Willins J, et al. Dosimetric comparison of dose-painted intensity modulated radiation therapy vs. conventional radiation therapy for anal cancer. J Clin Oncol. 2006;27:18s. (suppl; abstr 338) [Google Scholar]
- 10.Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 12.The analysis for this paper was generated using SAS/STAT software, Version 9.2 of the SAS System for Windows. Copyright © 2009 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
- 13.Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1431–1437. doi: 10.1016/j.ijrobp.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 14.John M, Pajak T, Flam M, et al. Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92-08. Cancer J Sci Am. 1996;2:205–211. [PubMed] [Google Scholar]
- 15.Cannon DM, Lee NY. Recurrence in region of spared parotid gland after definitive intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:660–665. doi: 10.1016/j.ijrobp.2007.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


