Abstract
Overexpression of vascular endothelial growth factor (VEGF) and VEGF receptors (VEGFRs) indicates poor prognosis for cancer patients in a variety of clinical studies. Our goal is to develop a tracer for positron emission tomography (PET) imaging of VEGFR expression using recombinant human VEGF121 with three lysine residues fused to the N-terminus (denoted as K3-VEGF121), which can facilitate radiolabeling without affecting its VEGFR binding affinity. K3-VEGF121 was conjugated with 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) and labeled with 61Cu (t1/2: 3.3 h; 62% β+). The IC50 value of NOTA-K3-VEGF121 for VEGFR-2 was comparable to K3-VEGF121 (1.50 and 0.65 nM, respectively) based on cell binding assay. 61Cu labeling was achieved with good yield (55 ± 10 %) and specific activity (4.2 GBq/mg). Serial PET imaging showed that the 4T1 tumor uptake of 61Cu-NOTA-K3-VEGF121 was 3.4 ± 0.5, 4.9 ± 1.0, 5.2 ± 1.0, and 4.8 ± 0.8 %ID/g (n = 4) at 0.5, 2, 4, and 8 h post-injection respectively, which was consistent with biodistribution data measured by gamma counting. Blocking experiments and ex vivo histology confirmed VEGFR specificity of 61Cu-NOTA-K3-VEGF121. Extrapolated human dosimetry calculation showed that liver was the organ with the highest radiation dose. The use of 61Cu as the radiolabel is desirable for small proteins like K3-VEGF121, which has much higher β+ branching ratio than the commonly used 64Cu (62% vs. 17%) thereby offering stronger signal intensity and lower tracer dose for PET imaging.
Keywords: Vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR), 61Cu, Positron emission tomography (PET), Tumor angiogenesis, Molecular imaging
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a potent mitogen in embryonic and somatic angiogenesis, the formation of new blood vessels.1, 2 The VEGF/VEGF receptor (VEGFR) signaling pathway plays a crucial role in both normal vasculature development and disease processes such as tumor development and metastasis.3, 4 VEGF-A is a homodimeric, disulfide-bound glycoprotein which exists in several isoforms with different numbers of amino acid residues, such as VEGF121 and VEGF165. Different VEGF-A isoforms exhibit varying biological properties such as the ability to bind to cell surface heparin sulfate proteoglycans. VEGF121, commonly existing as a homodimer, is freely diffusible without heparin binding. The two VEGFRs, namely Flt-1 (VEGFR-1) and Flk-1/KDR (VEGFR-2), are endothelium-specific tyrosine kinase receptors that mediate most of the angiogenic actions of the VEGF family.5 It has been reported that VEGFs and/or VEGFRs are overexpressed in various tumor biopsy specimens, which is indicative of poor prognosis for cancer patients.2, 6, 7 Therefore, non-invasive imaging and quantification of VEGFR expression is of paramount importance in cancer patient management.
Many strategies have been adopted to block the VEGF/VEGFR signaling pathway for cancer treatment, such as agents that can bind to VEGF-A to prevent its interaction with VEGFRs (bevacizumab, VEGF-trap, etc.),8, 9 antibodies/antibody fragments that target VEGFR-2 (ramucirumab, CDP791, etc.),10, 11 and small molecule inhibitors that interrupt the downstream signaling of VEGFR-2 (axitinib, sunitinib, sorafenib, etc.).12–14 Many of these agents have been approved by the Food and Drug Administration (FDA) for various medical indications in cancer therapy.2, 15 Non-invasive imaging and measurement of VEGFR expression can provide important information in future anti-angiogenic drug development and clinical trials, such as patient stratification and evaluating the therapeutic response/efficacy. Due to the soluble and dynamic nature of VEGF proteins, imaging of VEGF expression was not studied as extensively as imaging of VEGFR expression.3, 16 To date, the strategies used for VEGF imaging are almost exclusively based on anti-VEGF antibodies or reporter gene approaches.
Tremendous effort has been devoted to non-invasive imaging of VEGFR expression in cancer over the last two decades and various agents have been developed for different imaging modalities, such as single photon emission computed tomography (SPECT),17–20 positron emission tomography (PET),18, 21–25 optical imaging,18, 26 magnetic resonance imaging (MRI),27 and ultrasound.28, 29 Because of the high affinity to VEGFRs, VEGF121 has emerged as a particularly desirable candidate for tracer development in the literature.3, 30 To avoid significant interference with VEGFR binding, site-specific labeling of VEGF-based proteins has been adopted in many literature reports which typically utilizes a cysteine residue for radiolabeling.4,18 However, in many of the reported studies, kidney uptake of the tracer was very high (in some cases > 100 percentage of injected dose per gram of tissue [%ID/g]) which significantly hampered the clinical translation/applications of these tracers.
The goal of this study is to develop a PET tracer for the imaging of VEGFR expression using lysine-tagged recombinant human VEGF121 (denoted as K3-VEGF121). The three lysine residues at the N-terminus, far from the VEGFR binding sites, can facilitate radiolabeling without affecting the biological activity and receptor binding. Since the commonly used PET isotope for protein labeling, 64Cu, has low β+ branching ratio (17%), in this study we used 61Cu as a PET label which is ideal for small proteins like VEGF121. The significantly shorter half-life than that of 64Cu (3.3 h vs. 12.7 h) is more suitable for the pharmacokinetics of VEGF-based tracers and can give lower radiation dosimetry to normal organs. In addition, the much higher β+ branching ratio of 61Cu (62% vs. 17% for 64Cu) leads to stronger signal intensity and requires lower injection dose of the PET tracer. These features (shorter decay half-life, stronger signal, lower dose needed for PET imaging, etc.) are desirable for future clinical translation. In this work, K3-VEGF121 was conjugated to 2-S-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) for 61Cu labeling and in vivo investigation in a 4T1 murine breast cancer model.
EXPERIMENTAL SECTION
Reagents
K3-VEGF121 was synthesized through recombinant DNA technology and purified by GenScript Corp. (Piscataway, NJ). p-SCN-Bn-NOTA (Macrocyclics, Inc., Dallas, TX), PD-10 desalting columns (GE Healthcare, Piscataway, NJ), Chelex 100 resin (50–100 mesh; Sigma-Aldrich, St. Louis, MO), and FITC/Cy3-labeled secondary antibodies (Jackson Immunoresearch Laboratories, Inc., West Grove, CA) were all purchased from commercial sources. Water and all buffers were of Millipore grade and pre-treated with Chelex 100 resin to ensure that the aqueous solution was heavy metal-free. All other reaction buffers and chemicals were acquired from Thermo Fisher Scientific (Fair Lawn, NJ).
Cell Lines and Animal Model
4T1 murine breast cancer cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum at 37 °C with 5% CO2. Porcine aortic endothelial cells expressing human KDR (PAE/KDR) were cultured in Ham’s F-12 medium containing 10% fetal calf serum. Cells were used for in vitro and in vivo experiments when they reached ~75% confluence. All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. The 4T1 tumor model was generated by subcutaneous injection of 2 × 106 cells in 100 μl of phosphate-buffered saline (PBS) into the front flank of twelve-week-old female BALB/c mice (Harlan, Indianapolis, IN).31 Tumor sizes were monitored every other day and mice were used for in vivo experiments when the diameter of tumors reached 5–8 mm (typically 1–2 weeks after inoculation).
NOTA-Conjugation, Cell Binding Assay, and 61Cu-Labeling
NOTA-conjugation was carried out at pH 9.0 (in PBS adjusted with 0.1 N sodium carbonate), with the reaction ratio of p-SCN-Bn-NOTA:K3-VEGF121 being 10:1. NOTA-K3-VEGF121 was purified using PD-10 columns with PBS as the mobile phase. Detailed procedure for the cell-binding assay has been reported previously.21, 22 VEGFR-2 binding affinity of K3-VEGF121 and NOTA-K3-VEGF121 was analyzed in PAE/KDR cells using 125I-VEGF165 as the radioligand.
61Cu was produced in a GE PETtrace cyclotron using the 60Ni(d,n)61Cu reaction, with specific activity of ~2 Ci/μmol at the end of bombardment. 61CuCl2 (74 MBq) was diluted in 300 μL of 0.1 M sodium acetate buffer (pH 5.5) and added to 10 μg of NOTA-K3-VEGF121 for radiolabeling. The reaction mixture was incubated for 30 min at 37 °C with constant shaking. 61Cu-NOTA-K3-VEGF121 was purified using PD-10 columns with PBS as the mobile phase. The radioactive fractions containing 61Cu-NOTA-K3-VEGF121 was collected and passed through a 0.2 μm syringe filter before in vivo experiments.
Imaging and Biodistribution Studies
PET/CT scans were performed using an Inveon microPET/microCT rodent model scanner (Siemens Medical Solutions USA, Inc.). Each 4T1 tumor-bearing mouse was intravenously injected with 3–5 MBq of 61Cu-NOTA-K3-VEGF121 and five-minute static PET scans were performed at various time points post-injection (p.i.). Details for data acquisition, image reconstruction, and region-of-interest (ROI) analysis have been reported previously.32–34 Quantitative data were presented as %ID/g. Blocking studies were carried out to evaluate VEGFR specificity of 61Cu-NOTA-K3-VEGF121 in vivo, where a group of four mice was each injected with 61Cu-NOTA-K3-VEGF121 after intravenous injection of 100 μg of K3-VEGF121.
After the last PET scans at 8 h p.i., biodistribution studies were carried out to confirm that the quantitative tracer uptake values derived from PET imaging truly represented radioactivity distribution in tumor-bearing mice. Blood, 4T1 tumor, and major organs/tissues were collected and weighed. The radioactivity in each tissue was measured using a gamma-counter (Perkin Elmer) and presented as %ID/g. The 4T1 tumor, liver, and kidneys (i.e. tissues with significant uptake of 61Cu-NOTA-K3-VEGF121) were also frozen for histological analysis.
Radiation Dosimetry Extrapolation to Humans
Estimated human dosimetry was calculated from serial PET imaging results on BALB/c female mice injected with 61Cu-NOTA-K3-VEGF121. It was assumed that the biodistribution of the tracer in BALB/c mice was the same as in adult humans. ROI analysis was performed on major organs and time-activity curves were generated from the mean values obtained for each organ of interest. The source organ residence times were then calculated for the human model by integrating a mono-exponential fit to the experimental distribution data for major organs. Organ Level Internal Dose Assessment (OLINDA; Vanderbilt University) was used for estimating the radiation dosimetry.35
Histology
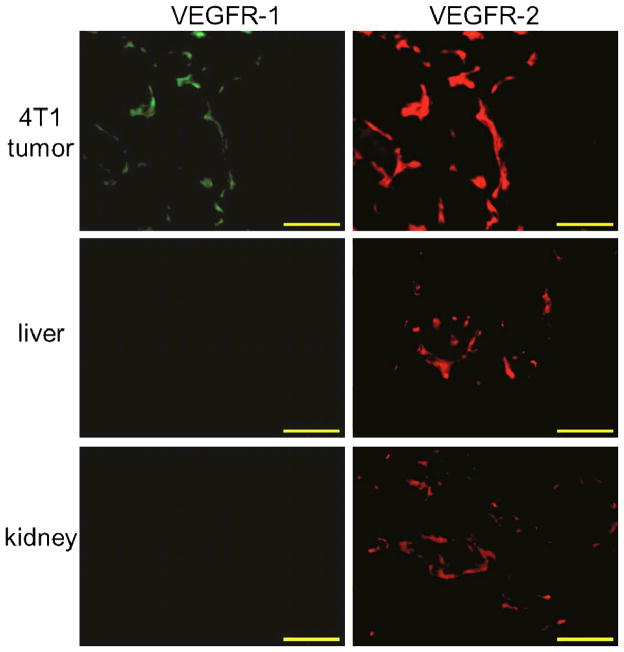
Frozen tissue slices of 5 μm thickness were fixed with cold acetone for 10 min and dried in the air for 30 min. After rinsing with PBS for 2 min and blocking with 10% donkey serum for 30 min at room temperature, the tissue slices were incubated with rabbit anti-mouse VEGFR-1 antibody (2 μg/mL, Thermo Fisher Lab Vision, Kalamazoo, MI) for 1 h at 4 °C and visualized using FITC-labeled donkey anti-rabbit secondary antibody. After washing with PBS, the slices were incubated with rat anti-mouse VEGFR-2 antibody (2 μg/mL) for 1 h at 4 °C and visualized with Cy3-conjugated donkey anti-rat secondary antibody. All images were acquired with a Nikon Eclipse Ti microscope.
Statistical Analysis
Quantitative data were expressed as mean ± SD. Means were compared using Student’s t-test. P values < 0.05 were considered statistically significant.
RESULTS
In Vitro Studies
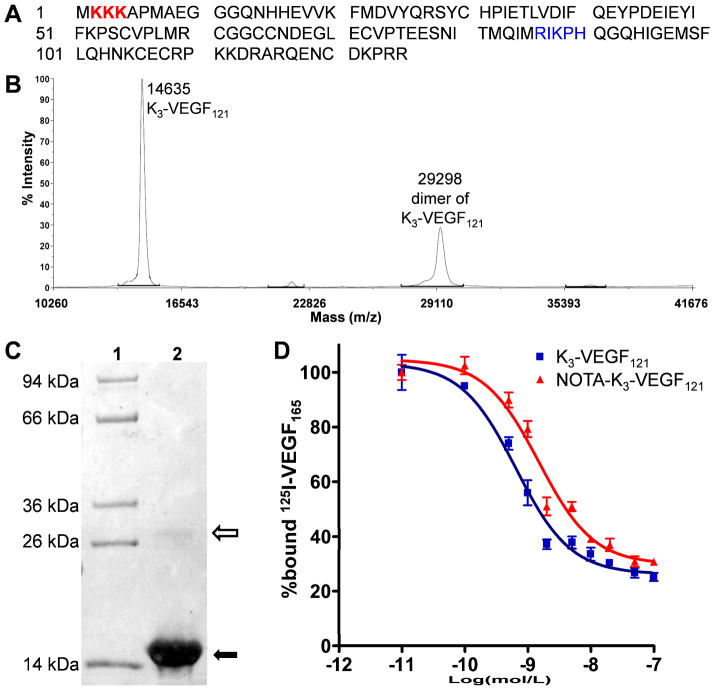
The amino acid sequence of K3-VEGF121 is shown in Figure 1A. Mass spectrometry indicated the expected molecular weight of ~14.6 kDa for K3-VEGF121 and another peak at ~29.3 kDa for the homodimer (Figure 1B). K3-VEGF121 exhibited > 95% purity as indicated by a Coomassie Blue-stained SDS-PAGE gel (Figure 1C), with a very light band of K3-VEGF121 homodimer above the 26 kDa marker band. The binding of K3-VEGF121 and NOTA-K3-VEGF121 to cells expressing VEGFR-2 was evaluated using 125I-VEGF165 as the competitive radioligand (Figure 1D). The measured 50% inhibitory concentration (IC50) values of K3-VEGF121 and NOTA-K3-VEGF121 were 0.65 and 1.50 nM, respectively, indicating minimal interference with VEGFR-2 binding after NOTA conjugation. As a reference, the IC50 values of VEGF121 were measured to be 1–3 nM in our previous studies using the same cell binding assay.21, 22 This observation indicated that the lysine residues used for NOTA conjugation were not within the VEGFR-2 binding domain (shown in blue in Figure 1A).36 Through the addition of three lysine residues at the N-terminus of VEGF121, which did not interfere with VEGFR-2 binding as indicated by the IC50 values, the possibility of modifying the lysine residue in the VEGFR-2 binding domain is significantly reduced.
Figure 1.
Characterization of K3-VEGF121 and NOTA-K3-VEGF121. (A) The amino acid sequence of K3-VEGF121. The 3 added lysine residues are coded in red and the amino acid residues involved in VEGFR-2 binding are shown in blue, which contain a lysine residue. (B) Mass spectrometry of K3-VEGF121 which shows the expected peak (14635 Da) as well as the homodimer (29298 Da). (C) SDS-PAGE gel indicated > 95% purity of K3-VEGF121 (solid arrow) with a very light homodimer band (empty arrow). Lane 1: molecular weight marker; Lane 2: K3-VEGF121. (D) Cell binding assay in PAE/KDR cells with 125I-VEGF165 as the competitive ligand. Data represent triplicate samples. The IC50 values are 0.65 and 1.50 nM for K3-VEGF121 and NOTA-K3-VEGF121, respectively.
61Cu-Labeling
61Cu-labeling including final purification using PD-10 columns took 60 ± 10 min (n = 8). The decay-corrected radiochemical yield was 55 ± 10 %, based on 5 μg of NOTA-K3-VEGF121 per 37 MBq of 61Cu, with radiochemical purity of > 95%. The specific activity of 61Cu-NOTA-K3-VEGF121 was 4.2 GBq/mg protein, assuming complete recovery of the NOTA-K3-VEGF121 conjugate after size exclusion chromatography.
Small Animal PET Imaging
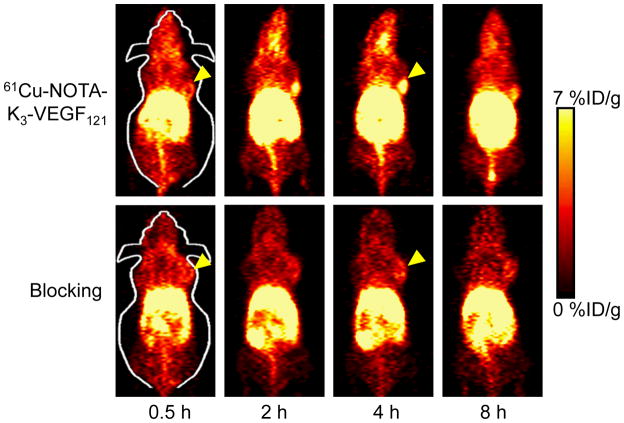
Based on our previous studies on PET imaging of VEGFR expression with radiolabeled VEGF12121, 22 and the 3.3 h decay half-life of 61Cu, the time points of 0.5, 2, 4, and 8 h p.i. were chosen for serial PET scans of 4T1 tumor-bearing mice after intravenous injection of 61Cu-NOTA-K3-VEGF121. Coronal PET images that contain the 4T1 tumor are shown in Figure 2, with the quantitative data obtained from ROI analysis and representative PET/CT fused image of a mouse at 4 h p.i. of 61Cu-NOTA-K3-VEGF121 shown in Figure 3.
Figure 2.
Serial coronal PET images of 4T1 tumor-bearing mice at 0.5, 2, 4, and 8 h post-injection of 61Cu-NOTA-K3-VEGF121, or a 100 μg dose of K3-VEGF121 before 61Cu-NOTA-K3-VEGF121 administration (i.e. blocking). Images are representative of 4 mice per group and the 4T1 tumors are indicated by arrowheads.
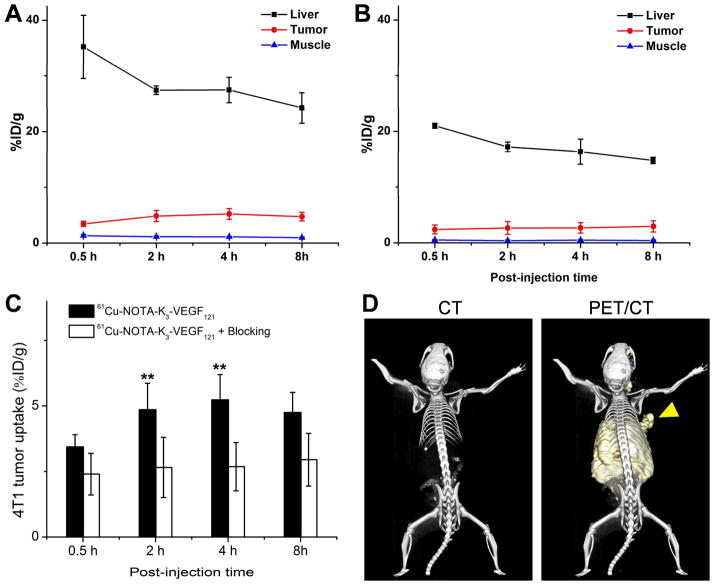
Figure 3.
Quantitative analysis of the PET data. (A) Time-activity curves of the liver, 4T1 tumor, and muscle upon intravenous injection of 61Cu-NOTA-K3-VEGF121 into 4T1 tumor-bearing mice (n = 4). (B) Time-activity curves of the liver, 4T1 tumor, and muscle upon intravenous injection of 61Cu-NOTA-K3-VEGF121, after a 100 μg dose of K3-VEGF121, into 4T1 tumor-bearing mice (n = 4). (C) Comparison of 4T1 tumor uptake between the two groups (n = 4). D. Representative PET/CT images of a 4T1 tumor-bearing mouse at 4 h post-injection of 61Cu-NOTA-K3-VEGF121. Arrowhead indicates the tumor. **: P < 0.01.
61Cu-NOTA-K3-VEGF121 cleared from the mouse body through both the hepatobiliary and renal pathways (with the former being more dominant). The uptake of 61Cu-NOTA-K3-VEGF121 in the liver was prominent at early time points and decreased gradually over time. The %ID/g values of the liver was 35.2 ± 5.6, 27.4 ± 1.0, 27.4 ± 2.2, and 24.2 ±2.7 %ID/g at 0.5, 2, 4, and 8 h p.i. respectively (n = 4; Figure 3A). Importantly, the 4T1 tumor uptake of 61Cu-NOTA-K3-VEGF121 was clearly visible starting from 2 h p.i. and remained stable over time (3.4 ± 0.5, 4.9 ± 1.0, 5.2 ± 1.0 and 4.8 ± 0.8 %ID/g at 0.5, 2, 4, and 8 h p.i. respectively; n = 4; Figure 2, 3A&C). Although the tumor uptake remained stable after 2 h p.i., the tumor-to-muscle ratio increased significantly from 2.6 ± 0.5 at 0.5 h p.i. to 4.9 ± 1.0 at 8 h p.i. (n = 4) due to tracer clearance from normal tissues.
Administering 100 μg of K3-VEGF121 at 30 min before 61Cu-NOTA-K3-VEGF121 injection significantly reduced the tumor uptake (P < 0.01 at 2 and 4 h p.i. when compared with mice injected with 61Cu-NOTA-K3-VEGF121 alone; Figure 2, 3B&C), indicating VEGFR specificity of the tracer in vivo. Radioactivity in the blood pool was comparably low for both groups. However, liver uptake of 61Cu-NOTA-K3-VEGF121 in the “blocking” group (21.0 ± 0.4, 17.2 ± 0.8, 16.3 ± 2.3, and 14.8 ± 0.6 %ID/g at 0.5, 2, 4, and 8 h p.i. respectively; n = 4) was significantly lower at all time points examined (P < 0.01) than that of mice injected with 61Cu-NOTA-K3-VEGF121 alone. Taken together, these data suggested faster renal/hepatic clearance of 61Cu-NOTA-K3-VEGF121 when most VEGFR in the mice was already bound by pre-injected K3-VEGF121, thereby leaving fewer receptors available for the tracer to interact with. Successful blocking of the 4T1 tumor uptake with 100 μg/mouse of K3-VEGF121 confirmed the VEGFR specificity of 61Cu-NOTA-K3-VEGF121 in vivo.
Biodistribution Studies and Radiation Dosimetry
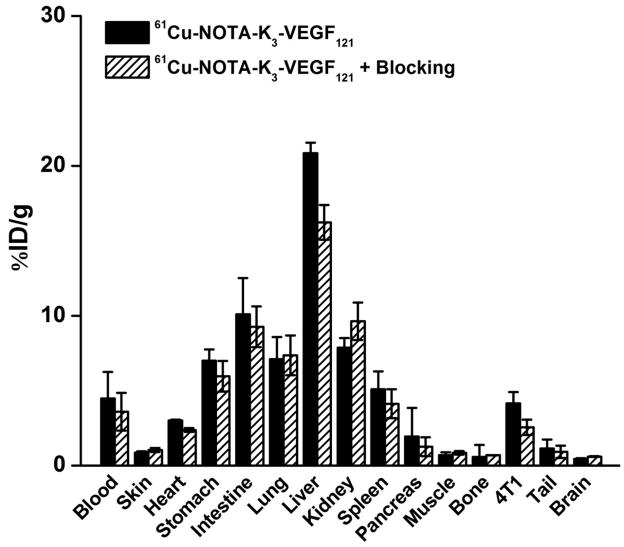
All mice were euthanized after the last PET scans at 8 h p.i. The tissues were collected for biodistribution and immunofluorescence staining studies to validate the in vivo PET data. Figure 4 shows the biodistribution data of 61Cu-NOTA-K3-VEGF121 at 8 h p.i. Besides the liver, the kidneys and intestine also had significant uptake of 61Cu-NOTA-K3-VEGF121. Based on the biodistribution studies, the tumor-to-muscle ratio at 8 h p.i. was 5.9 ± 1.8 (n = 4) which is similar to that obtained from PET imaging (4.9 ± 1.0; n = 4). Pre-injection of a blocking dose of K3-VEGF121 led to a tumor-to-muscle ratio of 2.5 ± 0.4 (n = 4) at 8 h p.i., which was significantly lower than mice injected with 61Cu-NOTA-K3-VEGF121 alone (P < 0.05) and corroborated the in vivo PET findings. Overall, the quantitative results obtained from biodistribution studies and PET scans matched very well, confirming that quantitative ROI analysis of non-invasive PET scans truly reflected tracer distribution in vivo.
Figure 4.
Biodistribution data in 4T1 tumor-bearing mice at 8 h post-injection of 61Cu-NOTA-K3-VEGF121, or 61Cu-NOTA-K3-VEGF121 after a 100 μg pre-injected dose of K3-VEGF121. n = 4 per group.
Estimated human absorbed doses to normal organs from 61Cu-NOTA-K3-VEGF121 are presented in Table 1. The highest radiation-absorbed dose is to the liver (0.23 ± 0.022 mGy/MBq; n = 4) and most other organs have very low level of radiation-absorbed doses. When compared to our previous studies on 64Cu-DOTA-VEGF121,21 the radiation doses to most normal organs (e.g. brain, stomach, lungs, ovaries, spleen, uterus, etc.) were much lower. For the kidney which were the dose-limiting organ for 64Cu-DOTA-VEGF121 (1.05 ± 0.27 mGy/MBq, n = 3), this is not the case for 61Cu-NOTA-K3-VEGF121 due to much lower kidney uptake of the tracer. The whole-body absorbed dose was found to be 0.024 ± 0.001 mGy/MBq administered 61Cu-NOTA-K3-VEGF121, which is less than half of that for 64Cu-DOTA-VEGF121 (0.05 ± 0.006 mGy/MBq; n = 3). Together, the much improved estimated human dosimetry of 61Cu-NOTA-K3-VEGF121 over 64Cu-DOTA-VEGF121 suggested that the use of 61Cu as the PET isotope was more advantageous than 64Cu for labeling small proteins such as VEGF121 derivatives. The three additional lysine residues at the N-terminus may also have contributed to the more desirable biodistribution of the tracer, in addition to facilitating the radiolabeling procedure.
TABLE 1.
Estimated radiation absorbed doses to an adult human after intravenous injection of 61Cu-NOTA-K3-VEGF121 based on PET imaging data obtained in female mice (n = 4).
| Organ | mGy/MBq (SO) | rad/mCi (SO) |
|---|---|---|
| Adrenals | 2.25E-02 (1.15E-03) | 8.33E-02 (4.24E-03) |
| Brain | 1.54E-04 (1.51E-05) | 5.71E-04 (5.53E-05) |
| Breasts | 4.49E-03 (7.14E-04) | 1.66E-02 (2.63E-03) |
| Gallbladder | 3.28E-02 (1.68E-03) | 1.22E-01 (6.35E-03) |
| LLI Wall | 5.08E-03 (2.34E-03) | 1.88E-02 (8.64E-03) |
| Small Intestine | 6.93E-02 (4.28E-02) | 2.57E-01 (1.59E-01) |
| Stomach | 9.88E-03 (2.60E-04) | 3.66E-02 (9.95E-04) |
| ULI Wall | 1.62E-02 (4.96E-03) | 5.98E-02 (1.83E-02) |
| Heart | 6.33E-02 (2.52E-02) | 2.34E-01 (9.33E-02) |
| Kidneys | 2.04E-01 (8.68E-03) | 7.54E-01 (3.29E-02) |
| Liver | 2.29E-01 (2.17E-02) | 8.47E-01 (8.07E-02) |
| Lungs | 1.03E-02 (1.41E-03) | 3.82E-02 (5.21E-03) |
| Muscle | 5.43E-03 (1.55E-04) | 2.01E-02 (5.89E-04) |
| Ovaries | 7.67E-03 (3.42E-03) | 2.84E-02 (1.27E-02) |
| Pancreas | 1.85E-02 (9.11E-04) | 6.83E-02 (3.44E-03) |
| Skin | 6.11E-03 (3.72E-04) | 2.26E-02 (1.37E-03) |
| Spleen | 4.30E-03 (1.03E-04) | 1.59E-02 (3.74E-04) |
| Thymus | 2.46E-03 (7.75E-05) | 9.10E-03 (2.74E-04) |
| Thyroid | 1.02E-02 (1.70E-04) | 3.77E-02 (6.50E-04) |
| Urinary | 6.27E-03 (1.72E-03) | 2.32E-02 (6.35E-03) |
| Uterus | 1.01E-03 (1.45E-04) | 3.72E-03 (5.35E-04) |
| Effective dose | 2.42E-02 (1.04E-03) | 8.96E-02 (3.14E-03) |
LLI = lower large intestine; ULI = upper large intestine.
Histology
The frozen tissue slices of 4T1 tumor, liver, and kidneys were stained for VEGFR-1 and VEGFR-2 after decay of the radioactivity. High VEGFR-2 expression and detectable VEGFR-1 expression was observed in the 4T1 tumor (Figure 5), which corresponded to prominent tracer uptake within the tumor. The observable level of VEGFR-2, but not VEGFR-1, expression in the liver and kidneys may also have contributed partly to tracer accumulation in these two organs. However, the majority of 61Cu-NOTA-K3-VEGF121 uptake in the liver and kidneys is likely related to hepatic/renal clearance, nonspecific capture, and possibly metabolites of the tracer after VEGFR-mediated internalization into cells.
Figure 5.
Immunofluorescence VEGFR-1/VEGFR-2 staining of the 4T1 tumor, liver, and kidney tissue sections. All images were acquired under the same conditions and displayed at the same scale. Magnification: 200×. Scale bar: 50 μm.
DISCUSSION
PET imaging has been widely for patient management in clinical oncology.37–39 Non-invasive imaging of VEGFR expression holds enormous potential to accelerate anti-angiogenic drug development and improve the management of cancer patients.3, 4, 30 In this study, we developed a PET tracer by labeling lysine-tagged VEGF121 with 61Cu for imaging of VEGFR expression in a murine breast cancer model and demonstrated the specificity of 61Cu-NOTA-K3-VEGF121 in vitro, in vivo and ex vivo. Many radiotracers have been reported for in vivo imaging of VEGFR expression previously,3, 4, 30 among which VEGF121 was one of the most popular ligands used for tracer development. However, direct labeling of VEGF121 for VEGFR imaging faces several challenges such as potential interference with the VEGFR-2 binding affinity (VEGFR-1 binding is of less concern for cancer imaging since VEGFR-2 has been shown to be more important than VEGFR-1 for tumor angiogenesis2, 22), very high renal uptake in mouse models which hampers clinical translation,3 the choice of the optimal PET isotope, in vivo stability of the PET label, among others. Our study addresses several of these issues and 61Cu-NOTA-K3-VEGF121 exhibits many desirable characteristics for future clinical translation.
There are 10 lysine residues in K3-VEGF121, with 3 at the N-terminus and 7 in the naturally occurring VEGF121. Among these lysine residues that are amenable for radiolabeling, Lys84 is critical for VEGFR-2 binding and should not be modified in order to preserve VEGFR-2 binding affinity.36 By adding 3 lysine residues at the N-terminus, the possibility of compromising the biological activity of VEGF121 after NOTA conjugation is significantly reduced. With the reaction ratio of p-SCN-Bn-NOTA:K3-VEGF121 being 10:1, it was estimated that there are 1~2 NOTA residues in each NOTA-K3-VEGF121. Such minimum modification of the protein did not alter the binding affinity of VEGF121 to VEGFR-2, as cell binding assay confirmed that the IC50 values of both K3-VEGF121 and NOTA-K3-VEGF121 were comparable to the naturally occurring VEGF121 at the nM range. The in vivo and ex vivo experiments further confirmed the binding affinity and target specificity of the tracer.
Radiation dose to both patients and healthcare personnel is a major concern for radiopharmaceuticals.40–42 In the design of novel radiotracers, it is important to minimize the radiation dose to normal organs without compromising the imaging characteristics. VEGF121 and its derivatives have been labeled with many PET/SPECT isotopes.3, 4, 30 However, some tracers resulted in relatively low tumor uptake, while several others showed high radiation dose to normal organs such as kidneys and the liver. 61Cu, with a 3.3 h decay half-life, 62% β+ branch ratio, and 1.22 MeV maximum β+ energy, is an excellent PET isotope for labeling of small molecules or proteins. However, development of 61Cu-based PET tracers has been severely understudied due to limited commercial availability of the isotope, despite the fact that tumor images obtained using 61Cu were found to be superior to those using 64Cu.43 The relatively short half-life and high β+ branch ratio requires lower radiotracer dose for imaging applications and can lead to shorter organ residence time than other PET isotopes such as 64Cu. In our study, the liver was the organ with the highest radiation dose (which is still at a relatively low level) because 61Cu-NOTA-K3-VEGF121 was cleared from mice primarily through the hepatic route. The estimated radiation doses to the other normal organs were even lower, which makes this tracer suitable for future clinical translation.
In comparison with the clinical “gold standard” PET isotope 18F, which is typically time consuming for protein labeling and often gives low radiochemical yield,40, 44 labeling K3-VEGF121 with 61Cu was achieved rapidly (~ 30 minutes) with good yield (> 50%). In addition, radiometal can often give higher tumor uptake than similar 18F-labeled tracers, due to much higher intracellular trapping efficiency of the radiometal than 18F after receptor-mediated internalization of the tracer into tumor (vascular endothelial) cells and subsequent metabolism.45 The 4T1 tumor uptake of 61Cu-NOTA-K3-VEGF121 reached > 5 %ID/g at 4 h p.i. in our study, which gave good tumor contrast. Even though 61Cu is not commercially available yet, it is feasible to distribute it to sites without a cyclotron similar as how 18F and 18F-FDG are currently supplied through commercial carriers, once the broad clinical potential of 61Cu is demonstrated. Of note, cyclotron production of 61Cu has a relatively low cost with the use of a deuteron beam for 61Cu production (60Ni is significantly less expensive than 64Ni, which are commonly used for 64Cu production with a proton beam). For imaging/diagnostic purposes using radiolabeled small proteins, 61Cu is a preferred choice over the more widely available 64Cu. Several other PET isotopes also have desirable characteristics for protein-based imaging (e.g. 45Ti and 44Sc, both with similar half-lives as 61Cu and higher β+ branching ratio), which deserve more research effort in the near future. Since the tumor uptake of 61Cu-NOTA-K3-VEGF121 peaks at around 4 h p.i., 68Ga is not suitable for labeling of K3-VEGF121 because of its short decay half-life (68.3 minutes). Furthermore, the much higher energy of β+ emitted by 68Ga will also lead to lower quality PET images than 61Cu-based tracers.
The in vivo stability of radiometal-based tracers is always a concern, such as those based on copper radioisotopes. An elegant study compared the effect of several bifunctional chelators on the biodistribution of a 64Cu-labeled antibody,46 which concluded that thermodynamic stability of 64Cu-chelator complexes did not significantly influence tumor uptake of the tracer. However, there were significant differences in tracer concentration in other tissues, including those involved in tracer clearance (e.g. liver and spleen). Similar findings were also observed in our previous studies of 64Cu-based tracers34, 47 and it is now generally agreed that NOTA is one of the best chelators for 61/64Cu-labeling of proteins,46, 48 which was used in this work.
The endogenous isoforms of VEGF may compete with 61Cu-NOTA-K3-VEGF121, which can potentially influence tracer uptake in the tumor and makes quantitative correlation of VEGFR expression with tracer uptake more difficult. Nonetheless, the intact VEGFR binding affinity of NOTA-K3-VEGF121 and the fact that the endogenous VEGF concentration is far from saturating the VEGFR resulted in prominent 61Cu-NOTA-K3-VEGF121 uptake in the 4T1 tumors. Lastly, rodent kidneys typically express significant level of VEGFR-1 during development which can cause very high renal uptake of VEGF-based tracers in mouse models,21, 22 since VEGF121 binds to VEGFR-1 with higher affinity than VEGFR-2. Such VEGFR-1 expression in rodent kidneys is age dependent. With the use of adult mice (12–14 weeks) in this study, the kidney uptake is acceptable (~10 %ID/g) and much lower than previous studies of VEGF-based tracers (usually > 30%ID/g).3, 21 It is likely that the use of these mice is more clinically relevant since most cancer patients are adults. Furthermore, VEGFR-1 expression in human kidneys is not as prominent as that in rodents.
CONCLUSION
We have successfully synthesized 61Cu-labeled K3-VEGF121, a recombinant VEGF121 with three lysine residues fused to the N-terminus to facilitate radiolabeling without compromising its biological activity. The binding affinity, tumor targeting efficiency, and VEGFR specificity of the tracer was investigated both in vitro and in vivo. Small animal PET imaging revealed fast, prominent, and VEGFR-specific uptake of 61Cu-NOTA-K3-VEGF121 in the 4T1 tumor model, which were validated by biodistribution, blocking, and histology studies. By using 61Cu as the PET isotope, the estimated radiation dose to humans could be significantly reduced when compared with similar 64Cu-labeled tracers. In addition, high β+ branching ratio and desirable half-life of 61Cu also makes the tracer suitable for future investigations in multiple scenarios such as clinical translation and evaluating the biological responses to various anti-cancer drugs.
Acknowledgments
The authors acknowledge financial support from the University of Wisconsin Carbone Cancer Center, the Department of Defense (W81XWH-11-1-0644), and the Elsa U. Pardee Foundation.
Footnotes
The authors declare no competing financial interest.
References
- 1.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Chen X. Multimodality imaging of vascular endothelial growth factor and vascular endothelial growth factor receptor expression. Front Biosci. 2007;12:4267–79. doi: 10.2741/2386. [DOI] [PubMed] [Google Scholar]
- 4.Backer MV, Backer JM. Imaging key biomarkers of tumor angiogenesis. Theranostics. 2012;2:502–15. doi: 10.7150/thno.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, Shibuya M. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci. 2000;902:201–5. doi: 10.1111/j.1749-6632.2000.tb06314.x. discussion 205–7. [DOI] [PubMed] [Google Scholar]
- 6.Underiner TL, Ruggeri B, Gingrich DE. Development of vascular endothelial growth factor receptor (VEGFR) kinase inhibitors as anti-angiogenic agents in cancer therapy. Curr Med Chem. 2004;11:731–45. doi: 10.2174/0929867043455756. [DOI] [PubMed] [Google Scholar]
- 7.Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–77. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 9.Moroney JW, Sood AK, Coleman RL. Aflibercept in epithelial ovarian carcinoma. Future Oncol. 2009;5:591–600. doi: 10.2217/fon.09.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O’Bryant C, Chow LQ, Serkova NJ, Meropol NJ, Lewis NL, Chiorean EG, Fox F, Youssoufian H, Rowinsky EK, Eckhardt SG. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–7. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ton NC, Parker GJ, Jackson A, Mullamitha S, Buonaccorsi GA, Roberts C, Watson Y, Davies K, Cheung S, Hope L, Power F, Lawrance J, Valle J, Saunders M, Felix R, Soranson JA, Rolfe L, Zinkewich-Peotti K, Jayson GC. Phase I evaluation of CDP791, a PEGylated di-Fab’ conjugate that binds vascular endothelial growth factor receptor 2. Clin Cancer Res. 2007;13:7113–8. doi: 10.1158/1078-0432.CCR-07-1550. [DOI] [PubMed] [Google Scholar]
- 12.Fruehauf J, Lutzky J, McDermott D, Brown CK, Meric JB, Rosbrook B, Shalinsky DR, Liau KF, Niethammer AG, Kim S, Rixe O. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res. 2011;17:7462–9. doi: 10.1158/1078-0432.CCR-11-0534. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 14.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 16.Cai W, Hong H. Peptoid and positron emission tomography: an appealing combination. Am J Nucl Med Mol Imaging. 2011;1:76–79. [PMC free article] [PubMed] [Google Scholar]
- 17.Chan C, Sandhu J, Guha A, Scollard DA, Wang J, Chen P, Bai K, Lee L, Reilly RM. A human transferrin-vascular endothelial growth factor (hnTf-VEGF) fusion protein containing an integrated binding site for 111In for imaging tumor angiogenesis. J Nucl Med. 2005;46:1745–52. [PubMed] [Google Scholar]
- 18.Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007;13:504–9. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 19.Blankenberg FG, Backer MV, Levashova Z, Patel V, Backer JM. In vivo tumor angiogenesis imaging with site-specific labeled 99mTc-HYNIC-VEGF. Eur J Nucl Med Mol Imaging. 2006;33:841–8. doi: 10.1007/s00259-006-0099-1. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Peck-Radosavljevic M, Kienast O, Preitfellner J, Havlik E, Schima W, Traub-Weidinger T, Graf S, Beheshti M, Schmid M, Angelberger P, Dudczak R. Iodine-123-vascular endothelial growth factor-165 (123I-VEGF165). Biodistribution, safety and radiation dosimetry in patients with pancreatic carcinoma. Q J Nucl Med Mol Imaging. 2004;48:198–206. [PubMed] [Google Scholar]
- 21.Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, Chen X. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006;47:2048–56. [PubMed] [Google Scholar]
- 22.Wang H, Cai W, Chen K, Li ZB, Kashefi A, He L, Chen X. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging. 2007;34:2001–10. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- 23.Hsu AR, Cai W, Veeravagu A, Mohamedali KA, Chen K, Kim S, Vogel H, Hou LC, Tse V, Rosenblum MG, Chen X. Multimodality molecular imaging of glioblastoma growth inhibition with vasculature-targeting fusion toxin VEGF121/rGel. J Nucl Med. 2007;48:445–54. [PubMed] [Google Scholar]
- 24.Eder M, Krivoshein AV, Backer M, Backer JM, Haberkorn U, Eisenhut M. ScVEGF-PEG-HBED-CC and scVEGF-PEG-NOTA conjugates: comparison of easy-to-label recombinant proteins for [68Ga]PET imaging of VEGF receptors in angiogenic vasculature. Nucl Med Biol. 2010;37:405–12. doi: 10.1016/j.nucmedbio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Gao H, Guo N, Niu G, Ma Y, Kiesewetter DO, Chen X. Site-Specific Labeling of scVEGF with Fluorine-18 for Positron Emission Tomography Imaging. Theranostics. 2012;2:607–617. doi: 10.7150/thno.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Chen K, Niu G, Chen X. Site-specifically biotinylated VEGF121 for near-infrared fluorescence imaging of tumor angiogenesis. Mol Pharm. 2009;6:285–94. doi: 10.1021/mp800185h. [DOI] [PubMed] [Google Scholar]
- 27.De Leon-Rodriguez LM, Lubag A, Udugamasooriya DG, Proneth B, Brekken RA, Sun X, Kodadek T, Dean Sherry A. MRI detection of VEGFR2 in vivo using a low molecular weight peptoid-(Gd)8-dendron for targeting. J Am Chem Soc. 2010;132:12829–31. doi: 10.1021/ja105563a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willmann JK, Paulmurugan R, Chen K, Gheysens O, Rodriguez-Porcel M, Lutz AM, Chen IY, Chen X, Gambhir SS. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246:508–18. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323–30. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 30.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S–28S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 31.Hong H, Yang Y, Zhang Y, Engle JW, Barnhart TE, Nickles RJ, Leigh BR, Cai W. Positron emission tomography imaging of CD105 expression during tumor angiogenesis. Eur J Nucl Med Mol Imaging. 2011;38:1335–43. doi: 10.1007/s00259-011-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Hong H, Engle JW, Yang Y, Barnhart TE, Cai W. Positron Emission Tomography and Near-Infrared Fluorescence Imaging of Vascular Endothelial Growth Factor with Dual-Labeled Bevacizumab. Am J Nucl Med Mol Imaging. 2012;2:1–13. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Hong H, Engle JW, Yang Y, Theuer CP, Barnhart TE, Cai W. Positron Emission Tomography and Optical Imaging of Tumor CD105 Expression with a Dual-Labeled Monoclonal Antibody. Mol Pharm. 2012;9:645–53. doi: 10.1021/mp200592m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong H, Benink HA, Zhang Y, Yang Y, Uyeda HT, Engle JW, Severin GW, McDougall MG, Barnhart TE, Klaubert DH, Nickles RJ, Fan F, Cai W. HaloTag: a novel reporter gene for positron emission tomography. Am J Transl Res. 2011;3:392–403. [PMC free article] [PubMed] [Google Scholar]
- 35.Sgouros G. Dosimetry of internal emitters. J Nucl Med. 2005;46(Suppl 1):18S–27S. [PubMed] [Google Scholar]
- 36.Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996;271:5638–46. doi: 10.1074/jbc.271.10.5638. [DOI] [PubMed] [Google Scholar]
- 37.Eary JF, Hawkins DS, Rodler ET, Conrad EUI. 18F-FDG PET in sarcoma treatment response imaging. Am J Nucl Med Mol Imaging. 2011;1:47–53. [PMC free article] [PubMed] [Google Scholar]
- 38.Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. The clinical use of PET with 11C-acetate. Am J Nucl Med Mol Imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- 39.Iagaru A. 18F-FDG PET/CT: timing for evaluation of response to therapy remains a clinical challenge. Am J Nucl Med Mol Imaging. 2011;1:63–64. [PMC free article] [PubMed] [Google Scholar]
- 40.Nolting DD, Nickels ML, Guo N, Pham W. Molecular imaging probe development: a chemistry perspective. Am J Nucl Med Mol Imaging. 2012;2:273–306. [PMC free article] [PubMed] [Google Scholar]
- 41.Thorek DLJ, Robertson R, Bacchus WA, Hahn J, Rothberg J, Beattie BJ, Grimm J. Cerenkov imaging - a new modality for molecular imaging. Am J Nucl Med Mol Imaging. 2012;2:163–173. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Chang RC, Chu L, Mak HK. Current neuroimaging techniques in Alzheimer’s disease and applications in animal models. Am J Nucl Med Mol Imaging. 2012;2:386–404. [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy DW, Bass LA, Cutler PD, Shefer RE, Klinkowstein RE, Herrero P, Lewis JS, Cutler CS, Anderson CJ, Welch MJ. High purity production and potential applications of copper-60 and copper-61. Nucl Med Biol. 1999;26:351–8. doi: 10.1016/s0969-8051(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 44.Alauddin MM. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am J Nucl Med Mol Imaging. 2012;2:55–76. [PMC free article] [PubMed] [Google Scholar]
- 45.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–73. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 46.Dearling JLJ, Voss SD, Dunning P, Snay E, Fahey F, Smith SV, Huston JS, Meares CF, Treves ST, Packard AB. Imaging cancer using PET -- the effect of the bifunctional chelator on the biodistribution of a 64Cu-labeled antibody. Nucl Med Biol. 2011;38:29–38. doi: 10.1016/j.nucmedbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Hong H, Engle JW, Bean J, Yang Y, Leigh BR, Barnhart TE, Cai W. Positron emission tomography imaging of CD105 expression with a 64Cu-labeled monoclonal antibody: NOTA is superior to DOTA. PLoS One. 2011;6:e28005. doi: 10.1371/journal.pone.0028005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper MS, Ma MT, Sunassee K, Shaw KP, Williams JD, Paul RL, Donnelly PS, Blower PJ. Comparison of 64Cu-Complexing Bifunctional Chelators for Radioimmunoconjugation: Labeling Efficiency, Specific Activity, and in Vitro/in Vivo Stability. Bioconjug Chem. 2012;23:1029–1039. doi: 10.1021/bc300037w. [DOI] [PMC free article] [PubMed] [Google Scholar]