Abstract
Postural sway is defined as the movement of a body’s center of mass within the base of support to maintain postural equilibrium. Deficits in postural sway are present after ACL injury; however, current evidence linking it to future injury risk is unclear. The purpose of this study was to determine if postural sway deficits persist after ACL reconstruction (ACLR). The hypothesis tested was that after ACLR, patients who return to sport (RTS) would demonstrate differences in postural sway compared to control (CTRL) subjects. Fifty-six subjects with unilateral ACLR released to RTS, and 42 uninjured CTRL subjects participated. Dynamic postural sway was assessed and 3-way (2×2×2) ANOVA was used to analyze the variables. A side X group X sex (p=0.044) interaction in postural sway was observed. A side X group analysis also revealed an interaction (p=0.04) however, no effect of sex was observed (p=0.23). Analysis within the ACLR cohort showed less (p=0.001) postural sway on the involved side (1.82 ± 0.84°) versus the uninvolved side (2.07 ± 0.96°). No side-to-side differences (p=0.73) were observed in the CTRL group. The involved limb of subjects after ACLR demonstrated the least postural sway. In conclusion, these findings indicate that dynamic postural sway may be significantly altered in a population of athletes after ACLR and RTS compared to CTRL subjects. Further investigation is needed to determine if deficits in postural sway can be used as an effective criterion to assist in the decision to safely RTS after ACLR.
Keywords: Anterior Cruciate ligament reconstruction, Postural Sway, postural stability, return to sport
INTRODUCTION
The incidence of ACL injury is high among young, active, individuals 1 with the most frequent medical management being surgical reconstruction. Injury to the ACL results in biomechanical changes at the tibiofemoral joint as well as deficits in proprioceptive feedback and sensorimotor function.2–4 While ACLR may successfully restore the mechanical stability of the knee; the resolution of certain proprioceptive measures and its importance in return to sport decision-making, remains controversial.5,6
Proprioception of the knee joint as defined by Lephart et al7 is afferent information from the joint that contributes to sensation, posture and joint stability. Various assessment tools were traditionally used to quantify deficits in proprioceptive function after ACL injury and focused on static measures of joint position sense or the patient’s ability to detect the onset of passive motion.2–4 Collectively, these authors suggested the presence of altered position sense and deficits in movement perception after ACL injury2,8 as well residual impairments after ACLR.3,4 However, some authors have argued that these assessment methods lacked applicability to assess functional status, as they frequently use passive movements, assessed in non-weight bearing positions.7
Recent attempts to quantify proprioception with corollary measures have included assessment of dynamic postural stability and postural sway. Deficits in postural stability (total motion of the center of pressure of the foot) are reported post ACL injury,9 with subsequent improvement after surgical reconstruction.9,10 In these cases, postural stability was defined as a dynamic postural response to an applied or volitional perturbation and was assessed by measurement of the deviation from a level position on a moveable force platform. Although this methodology represents a functional dynamic tool, what was not gleaned from these studies was the patient’s variability of movement within each test trial. Postural sway is a distinct measure and is defined as the movement of a body’s center of mass within the base of support to maintain postural equilibrium.7 The magnitude and pattern of postural sway is the result of a dynamic incorporation of sensory inputs from the trunk and lower extremity, in addition to a coordinated neuromuscular response. Objective measures of postural sway in a variety of patient populations are prevalent in the literature. These measures included linear and non-linear measures to determine the optimal variability of movement in both normal and pathologic conditions.11–13 These data indicate that normal, healthy individuals have an optimal range of postural sway between abnormal states of excessive or insufficient sway.12
Early investigations of postural sway after ACL injury were controversial. The literature regarding the effect of variables that increase the complexity of the task, such as single limb stance or visual occlusion, on measures of postural sway after ACLR remain contradictory, but may be critical for assessing the complexity of postural deficits after ACL injury and ACLR.14,15 Initial investigations of postural sway on a fixed force plate did not report altered sway in patients after ACL injury14,15 or ACLR. 16 Conversely, a significant increase in postural sway while standing on a flat, unmovable force plate existed when the difficulty of the task was increased by removing visual input. 14,15 Furthermore, as studies increased the challenge of the balance task with the use of movable force plates, additional deficits were revealed. Finally, more robust measures that capture the complexity and difficulty of postural sway maintenance in a “less controlled environment” such as the playing field situation may reveal deficits that may be otherwise missed in simple controlled task.
The purpose of this study was to determine if postural sway deficits during single limb stance on a dynamic, movable platform persist in subjects following ACLR and completion of rehabilitation prior to their return to sport (RTS). The hypothesis tested was that after ACLR, young athletes who returned to sport would demonstrate significant differences in single limb postural sway compared to a cohort of healthy control subjects.
METHODS
Subjects
We recruited 98 subjects between the ages of 10 and 25 years old to participate in this prospective, cohort study. The ACLR group included 56 subjects (35 females) who had recently undergone ACL reconstruction, completed their rehabilitation and had been cleared to return to sports.17 Patients were eligible for inclusion if they intended to return to greater than 50 hours per year of jumping, pivoting or cutting activity (Level I/II sports per Daniel et al.18), no prior history of a contralateral ACL injury and no recent history of an ankle, hip, spine or contralateral knee injury in the past 12 months. The control (CTRL) group included 42 subjects (29 females) recruited from the community, who also participated in comparable activities. The control group had no prior history of ACL injury and otherwise identical inclusion criteria. Demographic data for the study sample are displayed in table 1. All testing was approved by the Institutional Review Boards.
Table I. Mean (±SD) characteristics of study sample.
| ACL Reconstructed (N=56) | Controls (N= 42) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Female n=35 (62.5%) | Male n=21 (37.5%) | p-value* | Total | Female n=29 (69.0%) | Male n=13 (31.0%) | pvalue* | p-value† | |
| Age (yrs) | 16.4±3.0 | 16.2±2.2 | 16.8±4.0 | 0.51 | 16.8±2.3 | 16.9±1.9 | 16.4±3.1 | 0.57 | 0.528 |
| Height(cm) | 167.3±11.7 | 164.2±6.4 | 172.3±16.2 | 0.04 | 166.8±8.9 | 164.3±5.7 | 172.4±12.1 | 0.04 | 0.826 |
| Weight(kg) | 66.8±18.1 | 62.4±10.0 | 74.2±25.4 | 0.05 | 62.2±13.1 | 59.1±7.6 | 69.3±19.2 | 0.09 | 0.173 |
| BMI | 23.5±4.5 | 23.2±3.6 | 24.2±5.8 | 0.44 | 22.3±3.4 | 22.0±2.7 | 23.0±4.5 | 0.48 | 0.129 |
Difference between genders, Independent t-test
Difference between ACLR and control groups, Independent t-test
Testing Protocol
Postural Sway Assessment
After demographic data were collected, dynamic postural sway was assessed using the Biodex Balance System SD (BSS) (Biodex, Shirley, NY). The subject was positioned and balanced centrally on a single limb in the center of the dynamic, unstable platform. The subject stood with the test limb in slight flexion (less than 10 degrees) with the contralateral limb flexed and both arms crossed (Figure 1). The subject was instructed to maintain a stable posture on the platform for 20 seconds while the stability system was set at a level 4 stability setting. The stability setting of the Biodex SD system ranges from 1 to 8 with 1 being the least stable setting and 8 being the most stable setting. The subject executed this 20 second trial 3 times on each limb. Limb testing order was randomized and all testing was completed with eyes open with no visual feedback on performance. During each trial, the Balance System recorded the displacement of the platform away from a level position in degrees. This displacement represented the patient’s postural stability. In addition, the standard deviation of the movement was recorded to represent the variability of movement in degrees. This standard deviation represented postural sway and was the variable of interest in this study. Figure 2 provides examples of postural stability and postural sway outcome tracings provided by the BSS. Subjects may demonstrate altered postural stability and postural sway as seen in figure 2A, or an independent deficit in postural stability with minimal variability or postural sway as seen in figure 2C. Figure 2B represents optimal postural stability and minimal postural sway. Altered postural stability as measured on the BSS can be the result of erratic movement of the platform (figure 2a) or a consistently deviated position of the platform (figure 2c). The generated data represented the overall stability as well as deviations in the anterior-posterior and medial and lateral direction. These methods have been previously reported with high reliability.19
Figure 1.
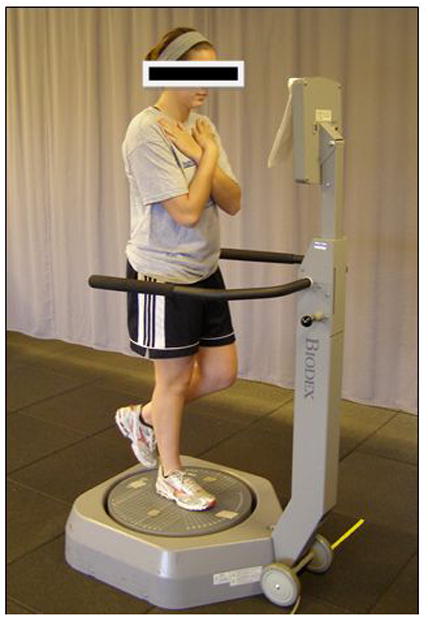
Demonstration of postural sway assessment
Figure 2.
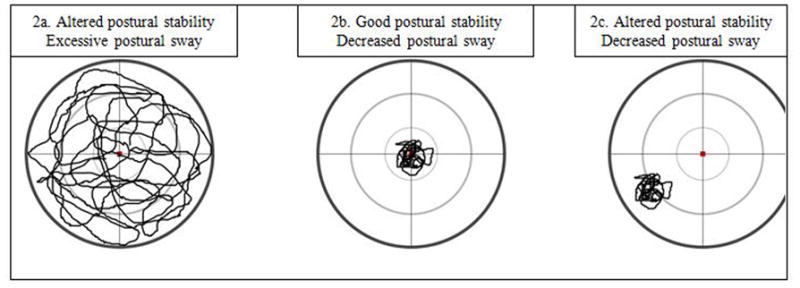
Examples of postural control tracing representative of ranges of postural stability and postural sway.
Statistical Analysis
Independent t-tests were used to assess mean differences in demographic characteristics between ACLR and CTRL subjects. A 3-way (2×2×2) analysis of variance (ANOVA) was used to analyze the relationships between side (involved vs. uninvolved), group (ACLR vs. CTRL) and sex (female vs. male) on postural sway. The involved limb of the ACLR subjects was matched to the non-preferred limb of the CTRL subjects, and the uninvolved limb of the ACLR group was matched to the preferred limb of the CTRL group. Additional analyses inclusive of ANOVA’s and paired t-tests were used to examine group differences. The preferred limb of the CTRL group was determined by which foot initially hit the ground during a bipedal, drop vertical jump task, as was previously reported in the literature.17 Data were analyzed using PASW (SPSS version 17.0, Chicago, IL).
RESULTS
Selected baseline characteristics by ACLR or CTRL status are reported in Table 1. Independent t-tests indicated no differences in mean age, height, weight and body mass index between the ACLR and CTRL groups (p>0.05). When sex differences were assessed independently within the ACLR and CTRL groups, a significant sex difference in height was seen within both ACLR and CTRL groups (p=0.04) and weight within the ACLR group (p=0.05). The participants in the ACLR group returned to competitive sport participation after surgery (mean ± SD RTS time, 6.9 ± 1.7 months) and were tested within 4 weeks of RTS.
The analysis of postural sway showed a significant side X group X sex (F (1, 94) = 4.180, p=0.04) interaction. A 2 × 2 analysis of the effect of side X group also revealed a significant interaction (F (1, 94) = 4.362, p=0.04). However, a sex X side interaction was not observed (F (1, 94) = 1.469, p=0.23) in this cohort, which indicated that sex did not have an effect on the dependent variable. Additional analyses within the ACLR cohort demonstrated a significant main effect of side (p=0.001). Specifically, the involved limb in the ACLR cohort displayed decreased postural sway (1.82 ± 0.84°) relative to the uninvolved limb (2.07 ± 0.96°) (Figure 2). Conversely, no side-to-side differences (p=0.73) were observed in the control group. Postural sway while standing on the non-preferred limb within the control group (1.96 ± 0.94°) was not significantly different from the postural sway on the preferred limb (2.01 ± 1.02°). (Figure 3) The involved limb of subjects after ACLR demonstrated less postural sway compared to the contralateral limb and both limbs of the control subjects.
Figure 3.
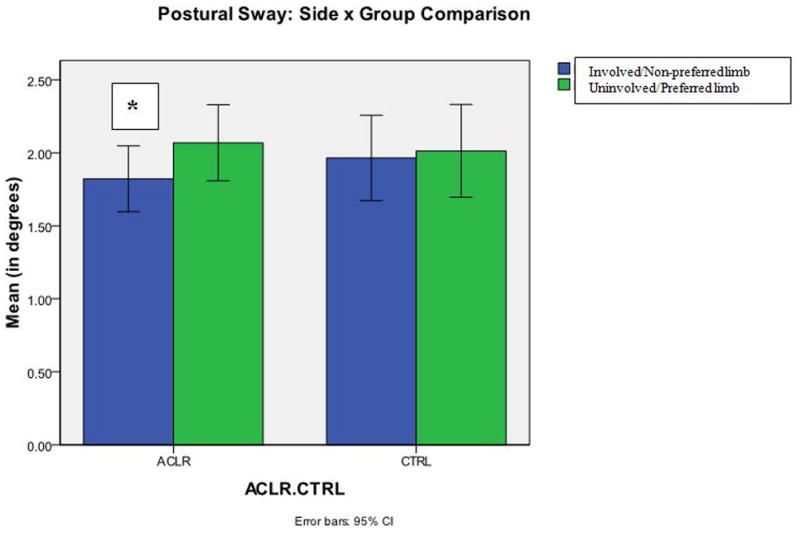
Postural sway (ACLR vs. Control) during a single limb stance on an unstable platform. (Blue=involved/non-preferred limb; Green=uninvolved/preferred limb) (* = p<0.05)
Discussion
The results support the hypotheses that postural sway was altered after ACLR despite completion of a rehabilitation program and clearance to RTS. Specifically, the involved limb in patients after ACLR presented with less amplitude of postural sway when compared to their contralateral limb as well as both limbs of the healthy, control group. These results failed to elucidate any sex differences in postural sway after ACLR at the time of RTS.
Given the novel methodology of our study, these results provide unique evidence to the postural sway debate in patients after ACL injured. Methodological inconsistencies among previous studies in the literature have led to contradictory results related to postural control.20 Specifically, the methods used to assess postural control are widely varied. Our study represents a novel attempt to report the variability of postural sway amplitude using the BSS in subjects after ACLR. Prior studies, which used the BSS reported postural stability, as a measure of deviation from a level platform but did not account for the variability of the postural sway amplitude. A prior report that used the BSS set at Level 4 to assess single limb stability on a dynamic surface in patients less than 12 months after ACLR noted altered postural stability, that is, an increase amplitude of deviations of the platform relative to an activity matched control group.9 Similar deficits in postural stability and sway were consistently identified in populations of ACL deficient athletes while standing on a single limb.15,21–23 In studies using a similar or more stable platform setting, these deficits were no longer present when patients were 12–18 month post-ACLR.9,10
Alonso et al.11 assessed postural stability and sway in patients after ACLR, activity-matched subjects and sedentary controls using the BSS on a more unstable setting (level 2). They reported subjects in the ACLR group presented with less postural stability and postural sway in their ACLR limb compared to the contralateral limb and both limbs of the activity matched control group. Unlike our findings, these authors noted that reduction in magnitude was consistent with both limbs of the sedentary control group. Lamoth et al12 also reported similar differences in postural control between control subjects with varied levels of athletic abilities. Alonso et al.11 reported an increased stability with a more complex task, which may be indicative of the presence of less compensatory mechanisms or strategies to maintain stability on the dynamic platform. As a result, in the absence of the ability to create a more dynamic, coordinated response to the external stress, the subjects may have simply co-contracted their lower extremity musculature in an attempt to create a more static, stable posture.
Attempts to quantify additional measures of postural control in patients after ACLR have yielded a variety of results.24 Several authors measured sway amplitude in patients 18–36 months after ACLR and noted increased sway after ACLR compared to activity-matched controls.14,23 All subjects in these studies were compared to control subjects who participated in similar levels of activity. Unlike findings reported by Alonso et al.,11 these authors did not observe deficits during sway in a double limb stance position.14,15,23 Finally, visual input was identified by several authors as a primary compensatory mechanism in the presence of ACL-deficiency.15,21 Collectively, these studies may demonstrate an optimal condition may exist, with an appropriate level of complexity, to accurately identify deficits in postural sway after ACLR. However, due to the varying methodology and the lack of standardization in regards to the optimal variable to assess postural sway, the optimal amount of postural sway after ACLR remains undetermined.
Additional methods to quantify sway, inclusive of the magnitude and pattern of sway, in a variety of populations have occurred. Historically, an assessment of center of pressure (COP) trajectory was used to assess postural sway. Recent techniques used to analyze the quantity and qualitative pattern of sway have identified unique results.25 In the healthy subjects, Riley et al.25 noted that as the complexity of the task increased the movement became much less random (and more deterministic). Lamoth et al.12 also reported a more deterministic pattern of sway in sedentary subjects compared to more athletic subjects. Similar results of less random movement were seen in populations where the complexity of the movement task was increased inherently due to some pathology or injury such as patients with cerebral palsy,26 post cerebral vascular accident,27 and in patients with Parkinson’s disease.13 This pattern has also been reported in patients with ACL deficiency. More deterministic movements result in less flexibility of the neuromuscular system to adapt to perturbations.28 Together, these studies indicated that a level of variability in movement was healthy and may be related to a greater ability to adapt to stress or perturbation from the environment.12
Corroborative of the present findings, these authors suggested subjects with an inherent injury or those with less skill possessed a lack of dynamic motor strategies to adapt to a change in environment resulting in a more deterministic movement pattern. Conversely, in the absence of injury, an individual may possess greater compensatory strategies when faced with a challenge to their postural balance, and may demonstrate a more random pattern of sway. If a reduction in postural sway as measured by a dynamic stability platform, as observed in our study, in subjects after ACLR at the time of RTS, correlates with a more determinist or less random sway pattern, increased risk of injury may exist in these patients. Future studies should attempt to determine the relationship between postural sway as measured on a dynamic platform and deterministic movement patterns through nonlinear analyses. In addition, the relationship between less random sway patterns and future injury should be examined as this tool could potentially serve as a useful screening tool to identify individuals at increased risk for future injury and ultimately use to determine readiness to RTS after ACLR. Finally, optimal rehabilitation interventions that address these deficits should be identified.
LIMITATIONS
This study represents a novel attempt to report changes in linear measures of postural sway with a strong prospective study design. However, limitations of this study are noted. While this study design allowed for an identification of differences between athletes, it was unable to determine if the deficits in the involved limb of the ACLR group were similar to a sedentary population, as described by Alonso et al,11 due to the athletic nature of the CTRL group. Secondly, while the study was able to quantify the variability of the magnitude of postural sway on a dynamic platform, it was not able to report on the pattern of sway to determine the randomness of the sway. Future investigations need to assess sway patterns to determine the relationships between these variables. Finally, this study did not allow for pre-injury or pre-ACLR assessment to determine if these deficits may have existed prior to ACL injury or ACLR. Future longitudinal assessments of postural sway in young, healthy populations prior to ACL injury will determine if this deficit exists prior to injury.
CONCLUSION
This study demonstrates that dynamic, single limb postural sway is altered in a population of athletes after ACLR released to RTS compared to activity matched control subjects. Future research is required to better define the effects of ACL injury and reconstruction on the magnitude and quality of postural sway and to identify the relationship of postural sway to future injury and ability to safely return to activities. This information will help better define the role of postural sway measures as a screening tool in the return to sport algorithm after ACLR.
Research Highlights.
Dynamic, single limb postural sway is altered in a population of athletes after ACLR released to RTS compared to activity matched control subjects
Patients had a reduced amplitude of sway on their involved limb after ACLR when compared to their contralateral limb and control subjects
These results failed to elucidate any sex differences in postural sway after ACLR at the time of RTS
Acknowledgments
The authors would like to acknowledge the supporting staff at the Sports Medicine Biodynamics Center and the Division of Occupational Therapy and Physical Therapy at Cincinnati Children’s Hospital Medical Center as well as our funding sources. Specifically the National Institutes of Health grants (R01-AR049735, R01-AR056259, R01-AR055563, R03-AR057551, F32-AR055844) and the National Football League Charities.
Footnotes
Conflict of Interest Statement
The authors have no financial or personal relationship with other people or organizations that could inappropriately influence (bias) their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997 Oct;79(10):1556–1576. doi: 10.2106/00004623-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989 Jan-Feb;17(1):1–6. doi: 10.1177/036354658901700101. [DOI] [PubMed] [Google Scholar]
- 3.Co FH, Skinner HB, Cannon WD. Effect of reconstruction of the anterior cruciate ligament on proprioception of the knee and the heel strike transient. J Orthop Res. 1993 Sep;11(5):696–704. doi: 10.1002/jor.1100110512. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald PB, Hedden D, Pacin O, Sutherland K. Proprioception in anterior cruciate ligament-deficient and reconstructed knees. Am J Sports Med. 1996 Nov-Dec;24(6):774–778. doi: 10.1177/036354659602400612. [DOI] [PubMed] [Google Scholar]
- 5.Johansson H, Sjolander P, Sojka P. A sensory role for the cruciate ligaments. Clin Orthop Relat Res. 1991 Jul;(268):161–178. [PubMed] [Google Scholar]
- 6.Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34(4):269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- 7.Lephart S, Riemann BL, Fu FH. Introduction to the Sensorimotor System. In: Lephart S, Fu FH, editors. Proprioception and Neuromuscular Control in Joint Stability. Champaign: Human Kinetics; 2000. [Google Scholar]
- 8.Friden T, Zatterstrom R, Lindstrand A, Moritz U. Disability in anterior cruciate ligament insufficiency. An analysis of 19 untreated patients. Acta Orthop Scand. 1990 Apr;61(2):131–135. doi: 10.3109/17453679009006504. [DOI] [PubMed] [Google Scholar]
- 9.Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clin Orthop Relat Res. 2002 Sep;(402):76–94. doi: 10.1097/00003086-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC., 3rd Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002 Sep;37(3):262–268. [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso AC, Greve JM, Camanho GL. Evaluating the center of gravity of dislocations in soccer players with and without reconstruction of the anterior cruciate ligament using a balance platform. Clinics (Sao Paulo) 2009;64(3):163–170. doi: 10.1590/S1807-59322009000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamoth CJ, van Lummel RC, Beek PJ. Athletic skill level is reflected in body sway: a test case for accelometry in combination with stochastic dynamics. Gait Posture. 2009 Jun;29(4):546–551. doi: 10.1016/j.gaitpost.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Schmit JM, Riley MA, Dalvi A, et al. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp Brain Res. 2006 Jan;168(3):357–367. doi: 10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- 14.Bonfim TR, Jansen Paccola CA, Barela JA. Proprioceptive and behavior impairments in individuals with anterior cruciate ligament reconstructed knees. Arch Phys Med Rehabil. 2003 Aug;84(8):1217–1223. doi: 10.1016/s0003-9993(03)00147-3. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Abe N, Katayama Y, Senda M, Kuroda T, Inoue H. Effect of vision on postural sway in anterior cruciate ligament injured knees. J Orthop Sci. 2005 May;10(3):277–283. doi: 10.1007/s00776-005-0893-9. [DOI] [PubMed] [Google Scholar]
- 16.Harrison EL, Duenkel N, Dunlop R, Russell G. Evaluation of single-leg standing following anterior cruciate ligament surgery and rehabilitation. Phys Ther. 1994 Mar;74(3):245–252. doi: 10.1093/ptj/74.3.245. [DOI] [PubMed] [Google Scholar]
- 17.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010 Oct;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994 Sep-Oct;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 19.Paterno MV, Myer GD, Ford KR, Hewett TE. Neuromuscular training improves single-limb stability in young female athletes. J Orthop Sports Phys Ther. 2004;34(6):305–316. doi: 10.2519/jospt.2004.34.6.305. [DOI] [PubMed] [Google Scholar]
- 20.Palmieri RM, Ingersoll CD, Stone MB, Krause BA. Center-of-Pressure Parameters Used in teh Assessment of Postural Control. Journal of Sports Rehabilitation. 2002;11(1):51–66. [Google Scholar]
- 21.Davids K, Kingsbury D, George K, O’Connell M, Stock D. Interacting Constraints and the Emergence of Postural Behavior in ACL-Deficient Subjects. J Mot Behav. 1999 Dec;31(4):358–366. doi: 10.1080/00222899909601000. [DOI] [PubMed] [Google Scholar]
- 22.Lysholm M, Ledin T, Odkvist LM, Good L. Postural control--a comparison between patients with chronic anterior cruciate ligament insufficiency and healthy individuals. Scand J Med Sci Sports. 1998 Dec;8(6):432–438. doi: 10.1111/j.1600-0838.1998.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 23.Shiraishi M, Mizuta H, Kubota K, Otsuka Y, Nagamoto N, Takagi K. Stabilometric assessment in the anterior cruciate ligament-reconstructed knee. Clin J Sport Med. 1996 Jan;6(1):32–39. doi: 10.1097/00042752-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Moussa AZB, Zouita S, Dziri C, Salah B. Single-leg assessment of postural stability and knee functional outcome two years after anterior cruciate ligament reconstruction. Annals of Physical and Rehabilitation Medicine. 2009;52:475–484. doi: 10.1016/j.rehab.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Riley MA, Balasubramaniam R, Turvey MT. Recurrence quantification analysis of postural fluctuations. Gait Posture. 1999 Mar;9(1):65–78. doi: 10.1016/s0966-6362(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 26.Donker SF, Ledebt A, Roerdink M, Savelsbergh GJ, Beek PJ. Children with cerebral palsy exhibit greater and more regular postural sway than typically developing children. Exp Brain Res. 2008 Jan;184(3):363–370. doi: 10.1007/s00221-007-1105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roerdink M, De Haart M, Daffertshofer A, Donker SF, Geurts AC, Beek PJ. Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Exp Brain Res. 2006 Sep;174(2):256–269. doi: 10.1007/s00221-006-0441-7. [DOI] [PubMed] [Google Scholar]
- 28.Negahban H, Salavati M, Mazaheri M, Sanjari MA, Hadian MR, Parnianpour M. Non-linear dynamical features of center of pressure extracted by recurrence quantification analysis in people with unilateral anterior cruciate ligament injury. Gait Posture. Apr;31(4):450–455. doi: 10.1016/j.gaitpost.2010.01.020. [DOI] [PubMed] [Google Scholar]


