1. Introduction
Cancer remains to be one of the leading causes of death worldwide. Over the past several decades significant advancements have been made in our fundamental understanding of cancer biology; which has in turn lead to better diagnostic and treatment methods. Despite these advancements, the over morality of cancer still remains high with an estimated total of 1,596,670 diagnoses and 571,950 deaths from cancer in United States in 2011 alone [1]. A major reason for this is our inability to administer therapeutic agents selectively to the targeted sites without adverse effects on healthy tissue. Current therapeutic strategies for most cancers involve a combination of surgical resection, radiation therapy, and chemotherapy. These therapies themselves are associated with significant morbidity and mortality primarily due to their non-specific effects on “normal” cells. The increase in efficacy of a therapeutic formulation is directly correlated to its ability to selectively target diseased tissue, overcome biological barriers, and “intelligently respond” to the disease environment to release therapeutic agents.
Nanotechnology coupled with advanced sophisticated therapeutic agents offers the most potential for addressing these challenges (Caldorera-Moore and Peppas, 2009a). In recent years, outstanding progress has been made in using nanovectors, liposomal and polymer-mediated delivery strategies to (a) target drugs to tumor cells through surface ligands and (b) increase localized delivery by increasing serum residence time (Caldorera-Moore and Peppas, 2009a). Although these strategies have reduced systemic toxicity, significant improvement on delivery strategies is still necessary to increase patient compliance and reduce chemotherapy-related side effects in cancer patients. In this review, we will highlight some of the limitations of current clinical treatment methods for cancer while also exploring novel research in nanotechnology for the creation of better targeted treatment moieties that have the potential to serve as drug carriers that can selectively target cancer cells and provide controlled release of chemotherapeutics.
1.1 Chemotherapy and its limitations
Chemotherapeutic agents are, in the broadest sense, small drug-like molecules that disrupt the normal functioning of a cell by inhibiting replication or inducing apoptosis, (Feng and Chien, 2003). Due to their proficiency at provoking cytotoxic effects, chemotherapeutic agents have been almost exclusively utilized in the treatment of cancer, where they exhibit the most deleterious effects to rapidly proliferating cells (Feng and Chien, 2003). Prominent chemotherapeutic agents include paclitaxel, doxorubicin, daunorubicin, cisplatin, and docetaxel. Paclitaxel and docetaxel are both taxanes, components that function by stabilizing the microtubules and preventing mitosis from progressing from metaphase to anaphase (Rowinsky, 1997). Doxorubicin and daunorubicin belong to a class of chemotherapeutics known as the anthracyclines. These molecules are among the most effective drugs available, inducing the greatest degree of cytotoxicity and used to treat the widest variety of tumor types including aggressive lymphoma, breast cancer, and myeloblastic leukemia (Minotti et al., 2004; Weiss, 1992). Doxorubicin has been shown to target the topoisomerase-II-DNA complex, disrupting the DNA and preventing cellular replication (Hurley, 2002). Similarly, cisplatin, a platinum-compound, modifies cellular DNA which activates signaling pathways that triggers apoptosis (Boulikas and Vougiouka, 2003).
The primary concern with utilizing the aforementioned chemotherapeutic agents is their inability to differentiate between healthy and tumor tissue (Maeda, 2001). The drugs will attack all cells without discrimination, being particularly harmful to any rapidly proliferating cells in the body such as hair, intestinal epithelial cells, and bone marrow (Feng and Chien, 2003). The most cytotoxic agents are the most effective but often result in severe side effects. Doxorubicin is widely considered to be best anti-cancer drug available today but results in side effects such as, nausea, fatigue, and extensive and often fatal cardiotoxicity (Minotti et al., 2004). Oncologists must, therefore, optimize the balance between the effectiveness of the drug and a patient’s ability to tolerate the accompanying side effects (Feng and Chien, 2003). Nanoscale carrier systems designed to target specific disease conditions could be utilized to alleviate some if not all of these cytotoxic effects to health cells.
1.2 Nanoparticles as targeted, controlled-release carriers
Nanoparticles have the potential of addressing and remedying some of the most significant limitations of traditional chemotherapy, namely, its lack of specificity and narrow window of therapeutic efficacy. Nanoparticles are colloidal carriers with dimensions on the nano scale (10−9 m). They are particularly attractive for cancer treatment due to their small size, varied composition, surface functionalization, and stability which provide unique opportunities to interact and target the tumor microenvironment (Park et al., 2009; Wang et al., 2008). These interactions of nanoparticles with the tumor include aiding in small molecule transport to the intracellular organelles to induce the greatest cytotoxic effect. (Jones and Harris, 1998). This review will discuss various nanoparticle structures and targeting moieties that have the potential to serve as drug carriers that can selectively target cancer cells and provide controlled release of chemotherapeutics.
2. Tumor Physiology
Tumor biology plays an important role in drug delivery. The growth, structure, and physiology of a tumor all impact the ability of nanoparticle drug carriers to be delivered successfully. Understanding which aspects of tumor biology are beneficial and which are detrimental to delivery leads to the development of more effective and efficient drug carriers.
2.1 Tumor Growth
A tumor grows from a single cell that undergoes some mutation that blocks its apoptotic signaling pathway causing it to uncontrollably proliferate. The rapidly replicating cells displace their healthy counterparts due to an increased demand for nutrients and subsequent waste product elimination (Brannon-Peppas and Blanchette, 2004). During the initial stages of tumor growth the cells rely solely on diffusion to obtain nutrients limiting their size to approximately 2 mm3 (Jones and Harris, 1998). To bypass their diffusion-limited size the tumor cells must begin to recruit new blood vessels in a process called angiogenesis (Brannon-Peppas and Blanchette, 2004; Brown and Giaccia, 1998).
2.2 Tumor Vasculature and Lymphatic System
Once a tumor mass is able to initiate angiogenesis, the blood vessels continue to rapidly grow producing an unorganized and aberrant vasculature (Haley and Frenkel, 2008). Consequently, the tumor contains regions with extensive vasculature and rich blood supply and regions with poor vasculature and little blood supply. The variance in level of vasculature and the tendency of the vessels to have dead-ends and little-to-no smooth muscle or nerve innervation results in significantly heterogeneous blood flow through the tumor tissue (Brown and Giaccia, 1998). Tumor vessels are also inherently leaky due to abnormal basement membranes and incomplete endothelial linings caused by the inability of pericytes to fully line the quickly proliferating cells forming the vessel walls (Baban and Seymour, 1998; Haley and Frenkel, 2008).
Tumors also have a reduced ability to drain fluid and waste from the interstitial space (Brannon-Peppas and Blanchette, 2004). The reduction in drainage is due to a poorly-defined lymphatic system caused by the demand of the quickly proliferating tumor cells (Haley and Frenkel, 2008). Unlike healthy tissue which can rapidly remove macromolecules and lipids from its interstitium, a tumor will accumulate these molecules and retain them for extended periods of time (Maeda, 2001).
Additional factors present at high levels in tumor cells contribute notably to angiogenesis and vessel permeability. These factors include vascular endothelial growth factor (Roberts and Palade, 1995), basic fibroblast growth factor (Dellian et al., 1996), bradykinin (Matsumura et al., 1988), and nitric oxide (Wu et al., 1998). Vascular endothelial growth factor (VEGF) increases the permeability of blood vessels by increasing both the size and quantity of fenestrations between cells (Roberts and Palade, 1995). Elevated concentrations of bradykinin and depletion of nitric oxide both result in increased extravasation of macromolecules through the tumor vasculature (Matsumura et al., 1988; Wu et al., 1998). Basic fibroblast growth factor (bFGF) is active in angiogenesis as it recruits endothelial cells and increases cellular proliferation (Roberts and Palade, 1995).
Combined, the highly permeable vasculature, poorly-defined lymphatic system, and elevated levels of the aforementioned factors, result in a phenomenon called the enhanced permeability and retention effect (EPR). This effect was first defined by Maeda and colleagues and explains the observed accumulation of drugs, lipids and other macromolecules (MW > 50 kDa) at the tumor site (Maeda, 2001). The EPR has been the focus of much research due to its ability to passively target macromolecules including nanoparticles.
2.3 Barriers to Drug Delivery in Tumors
Most chemotherapeutic drugs are given via a systemic injection and circulate in the bloodstream prior to reaching the tumor site. A disadvantage of this type of delivery scheme is that the agent is allowed to come into contact with both healthy tissue and the tumor. This interaction between healthy tissue and the chemotherapeutic agent is what often leads to the debilitating side effects that accompany treatment. Another detriment to systemic delivery is that the agent will encounter numerous extra-and intracellular barriers prior to reaching the tumor site. Furthermore, the drug must retain its biological activity and reach the target site at high enough concentrations to have therapeutic efficacy. In this section we will examine the significant systemic, extra- and intracellular barriers therapeutic agents encounter.
2.3.1 Reticuloendothelial System and Mononuclear Phagocytic System
The reticuloendothelial system (RES) also known as the mononuclear phagocytic system (MPS) are a group of organs and circulating macrophages whose primary function is to rid the body of foreign objects, such as bacteria (Owens and Peppas, 2006). Nanoparticles that enter the bloodstream are also subject to rapid clearance by the RES/ MPS. These foreign bodies are not directly recognized by the macrophages, typically liver macrophages or Kupffer cells, and must first be coated by a layer of proteins in a process called opsonization. The proteins involved in this process are termed opsonins, a class of proteins available in the circulation. Opsonins include immunoglobulins, components of the complement system (C3, C4, and C5), fibronectin, type I collagen, and many others. These proteins, when encountering a foreign particle, adhere by a variety of interactions such as ionic, electrostatic, hydrophobic, hydrophilic and van der Waals forces (Owens and Peppas, 2006). The macrophages then identify the surface layer of bound opsonin proteins coating the foreign body and proceed to engulf the particle by phagocytosis, “cell-eating”, then degrading it within an intracellular vesicle such as the lysosome (Jones and Harris, 1998).
2.3.2 First Pass Renal Filtering
The human body is a carefully designed system that is particularly adept at recognizing and removing foreign particles from circulation. The renal system is an essential component in the purification of the blood and is an important consideration when designing carriers for drug delivery. The kidneys filter blood through a structure known as the glomerular capillary wall. Particles with a diameter of less than 10 nm are subject to first pass renal filtration through this structure (Davis et al., 2008; Venturoli and Rippe, 2005).
2.3.3 Heterogeneous Blood Flow
As mentioned previously, due to the rapid proliferation of tumor tissue, tumor vasculature is highly aberrant and unorganized. In conjunction with irregular vasculature structure is a lack of nerve enervation and smooth muscle which leads to a heterogeneous and variable blood flow. This becomes a barrier to systemic drug delivery as the macromolecular therapeutic agent will not be evenly dispersed throughout the tumor tissue (Jang et al., 2003). It has been shown that areas of tumor tissue with poor blood flow are often resistant to treatment (Hori et al., 1991).
2.3.4 High Tumor Interstitial Pressure
The tumor interstitium comprises the bulk of tumor mass and consists of a collagen network and highly viscous fluid (Haley and Frenkel, 2008). The fluid within the interstitium has some quantifiable pressure that increases with tumor size and proximity to the tumor center. This pressure increase is due to a combination of factors such as rapid cellular proliferation in a confined area, high vascular permeability into the interstitium, and lack of lymphatic drainage from the interstitium (Jain, 1987, 1998). Drug diffusion into the interstitium is depleted as the pressure increases. For this reason, there tends to be a lack of drug accumulation in the center of the tumor mass where the interstitial pressure is the highest (Haley and Frenkel, 2008; Jain, 1998).
2.3.5 Extracellular Matrix (ECM)
The extracellular matrix is composed of fibrous proteins such as collagen and elastin, as well as a highly viscous polysaccharide-containing fluid. Its primary functions are to maintain cellular structure and integrity, modulate cellular interaction with the external milieu – including neighboring cells, regulate macromolecular transport and serve as a barrier to bacterial infiltration. In the context of drug transport, and, more specifically chemotherapeutic agent delivery, the ECM poses a formidable physical barrier. The tightly woven fibrous proteins and highly viscous ECM fluid, containing both hyaluronan and proteoglycans, each serve to reduce the diffusivity and spatial distribution of drug molecules within the tumor interstitium. (Jain, 1987; Jang et al., 2003).
2.3.6 Intracellular Transport
Once the drug component reaches the cell it must be internalized. This internalization process is termed phagocytosis, or cell eating, and consists of actin protrusions of the cellular membrane surrounding and engulfing a particle (Jones and Harris, 1998). The particle is now contained within an intracellular vesicle for transport through the cytoplasm. The particle is shuttled from the early endosome to the late endosome and finally the lysosome for degradation. Throughout this pathway the pH decreases from 7.4 to approximately 5.0. Additionally, contained within the intracellular components are enzymes that aid in foreign body degradation. The drug must maintain its activity through both decreased pH and rampant enzymatic activity (Jones and Harris, 1998).
3. Nanoparticles
Nanoparticles are particularly attractive for drug delivery due to their varied composition, structure, and surface characteristics (Liechty and Peppas, 2012). The vast array of nanoparticle compositions and structures allow the carriers to be fine-tuned for specific applications and targets. The most common architectures for targeted drug delivery applications include: liposomes, micelles, dendrimers, nanospheres, and nanocapsules. This section will highlight the benefits and detriments of these different nanoparticle systems for their use as drug delivery vehicles.
3.1 Liposomes
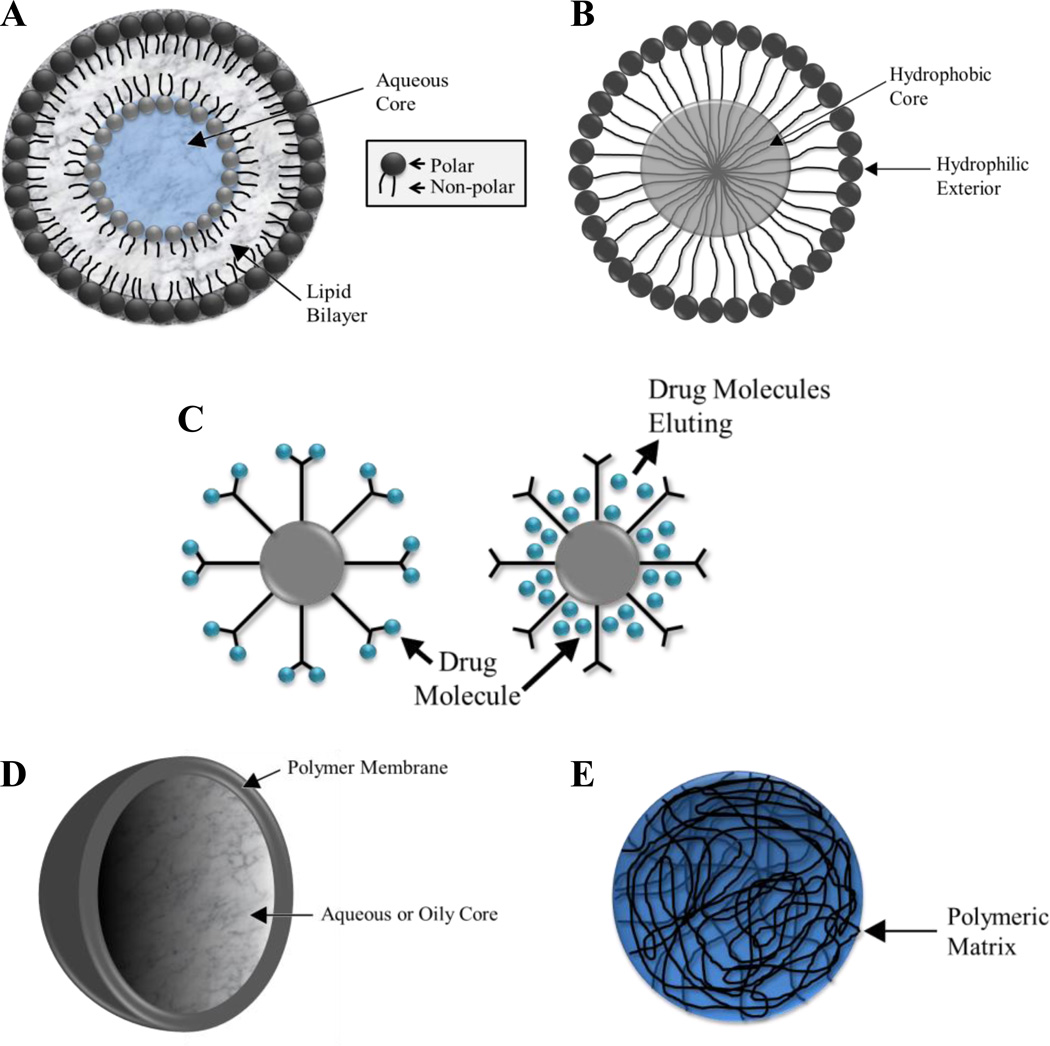
Liposomes are composed of amphiphilic molecules that are comprised of both polar and nonpolar components that self-assemble into colloidal particles (Figure 1a). This self-assembly produces a spherical structure with the polar components of the molecule contacting the polar environment and the nonpolar components contacting the nonpolar environment (Lasic, 1998). The most common classification of liposomes is by the number of lipid bilayers present in the colloidal structure, with unilamellar liposomes containing one lipid bilayer and multilamellar liposomes containing multiple lipid bilayers. Due to their amphiphilic nature liposomes are capable of encapsulating both polar and nonpolar compounds for delivery (Lasic, 1998).
Figure 1.
Particle Schematics. (A) liposome, (B) micelle, (C) dendrimers functionalized with complexed (left) and encapsulated (right) drug molecules, (D) nanosphere, and (E) nanocapsule.
Liposomes are attractive for drug delivery applications for numerous reasons, including their resemblance to cell membranes in both structure and composition. Additionally, liposomes can be readily formed with nontoxic, nonimmunogenic, natural and biodegradable amphiphilic molecules (Haley and Frenkel, 2008; Lasic, 1998). Liposomes by themselves tend to be slightly sterically unstable and are cleared rapidly from the bloodstream. For drug delivery applications, this behavior is remedied by functionalizing the liposomal surface with poly(ethylene glycol) tethers to impart increased steric stabilization (PEG discussed in greater detail later in this review) (Lasic, 1998). The surface of the liposome can also be modified with ligands for active targeting. A pegylated biodegradable liposome was used to encapsulate doxorubicin and became the first liposome-based treatment for cancer (Doxil) (Haley and Frenkel, 2008). While liposomes have been
3.2 Micelles
The micelle is composed of amphiphilic molecules that self-assemble into a structure with a hydrophobic core and a hydrophilic exterior (Figure 1b) (Liechty and Peppas, 2012). Micellar structure lends itself well to drug delivery applications for multiple reasons. Micelles typically have diameters of less than 100 nm, allowing them to participate in extravasation through the fenestrations in tumor vessels and limiting their uptake by the MPS/RES system. Their hydrophilic surface characteristics also shield them from immediate recognition and subsequently increase circulation time (Lavasanifar et al., 2002). Hydrophobic drugs can be loaded into the core of the micellar structure and protected by the hydrophilic corona during transport to the tumor site (Kwon and Kataoka, 1995).
3.3 Dendrimers
Dendrimers are highly branched molecules that display a high degree of monodispersity and a well-defined structure (Hughes, 2005). They are stable and have surfaces that can be readily functionalized with targeting ligands and molecules such as folic acid (Majoros et al., 2006). Drug molecules can be encapsulated in the dendrimer’s multifunctional core and protected by the extensive branching. Drug molecules, such as paclitaxel, can also be attached to the exterior of the dendrimer (Figure 1c) (Majoros et al., 2006).
3.4 Nanospheres and Nanocapsules
Nanospheres consist of a spherical polymeric matrix within which a drug is encapsulated (Figure 1d). The drug is typically distributed evenly throughout this matrix and released into the environment via diffusion. The composition of the polymer matrix and its ability to imbibe fluids will determine how rapidly the drug will be released (Brigger et al., 2002; Ratner B.D. et al., 2004).
Nanocapsules are often referred to as reservoir systems as they contain the active ingredient in a core separated from the environment by a polymeric membrane (Figure 1e) (Haley and Frenkel, 2008). By saturating the core the active ingredient can diffuse through the membrane with an approximately constant release rate (Ratner et al., 2004). This release behavior is attractive for drug delivery applications.
The above nanoparticle systems have been widely explored for diffusion driven drug release due to their large surface-to-volume ratios which allow for drug release at feasible and clinically relevant time scales. There is a surge in the development of nanoparticle systems that do not rely solely on diffusion mechanisms for drug release. Instead, this new class of nanoparticle is able to respond to environmental, chemical, thermal, or biological triggers (Caldorera-Moore and Peppas, 2009b; Liechty et al., 2011; Peppas et al., 2012; Schoener et al., 2012). These ‘smart materials’ will release their therapeutic payload only when triggered. A more complete review on environmentally responsive carriers was recently published by Liechty et al. Although the diffusion-driven nanoparticles are unable to respond directly to their environment there are means by which these systems can target and accumulate in the tumor interstitium.
4. Targeting
4.1 Passive Targeting
Passive targeting of nanoparticles takes advantage of the abnormal tumor physiology and structure that results in the EPR effect. The permeability of the vasculature and retention by an insufficient lymphatic system can passively accumulate macromolecules and increase their tumor concentration by 70-fold (Duncan, 2003). This accumulation will only be observed if the macromolecules avoid clearance by mechanisms such as renal clearance and uptake by the MPS/RES. Two of the most important properties of effective nanocarriers are the carriers’ ability to (a) remain circulating in the blood stream for a significant amount of time and (b) target specific tissues and cells (Duncan, 2003). Particle circulation time, targeting, and the ability to overcome biological barriers is also dependent on a particle’s shape, size, and surface characteristics. The lifespan of a nanoparticle within circulation is modulated by its interactions with the environment and can be modified by changing its size, particle shape, and surface characteristics (Davis et al., 2008).
4.1.1 Size
The size of nanoparticles has an extremely important impact on its interaction with its environment. As stated previously, a particle must be at least 10 nm in diameter to avoid clearance by first pass renal filtration (Davis et al., 2008; Venturoli and Rippe, 2005). The largest size of a nanoparticle to be used for drug delivery to a tumor is determined by a multitude of factors. As passive targeting is entirely dependent on diffusion-mediated transport into the tumor, size is important. Dreher and colleagues have shown that particles on the order of hundreds of nanometers in diameter can accumulate in the tumor tissue. Using dextran as a model macromolecule they showed that increasing the molecular weight from 3.3 kDa to 2 MDa reduced permeability by two orders of magnitude. Larger molecules were able to accumulate but were primarily contained close to the vascular surface within the tumor. Conversely, smaller molecules could penetrate more deeply into the tumor interstitium and achieve a more homogenous distribution. These observed behaviors are attributed to the effective interstitial diffusion coefficient, which decreases as the molecular weight of the diffusing molecule increases (Dreher et al., 2006). Extrapolating from macromolecules to nanoparticles, it has been determined that the upper bound size for nanoparticles participating in the EPR effect is approximately 400 nm (Alexis et al., 2008). Particles larger than 400 nm are simply unable to diffuse through the tumor interstitium in sufficient quantities to have any clinical or therapeutic effect.
While 400 nm is the upper bound for harnessing the effect of EPR there are other important factors that narrow the effective size range of nanoparticles. The leaky vasculature in tumors is highly permeable due to the increased size and quantity of fenestrations as well as incomplete or abnormal basement membranes (Haley and Frenkel, 2008; Roberts and Palade, 1995). These fenestrations are typically 50– 100 nm in size and, although not the only mechanism of permeating into the tumor interstitium, an important pathway for nanoparticle accumulation [40]. Looking solely at clearing mechanisms, it has been shown that particles with diameters less than 200 nm will be cleared much less rapidly than particles with diameters over 200 nm (Alexis et al., 2008; Matsumura et al., 1988; Moghimi et al., 1993). With all of the above factors taken into consideration, an approximate upper bound of 150 nm has been determined (Liechty and Peppas, 2012). Therefore, in order to be an effective drug carrier the nanoparticle should have a diameter between 10– 150 nm. This size range will ensure longer circulation time and increased accumulation in the tumor interstitium.
4.1.2 Particle Shape
Development of novel particle fabrication methods that allow for precise control over particle shape and size (Caldorera-Moore et al., 2011a; Champion et al., 2007; Glangchai et al., 2008; Rolland et al., 2005) has allowed for researchers to explore the effects of particle shape on particle bio-distribution and cellular internalization. The effects of micro- and nanoscale particle shape on particle localization and uptake was recently review (Caldorera-Moore et al., 2010) and therefore in the interest of this review only the effects of shape of nanoscale particles will be presented. The effects of particle shape and potentially the particles’ curvature on cellular internalization was shown by Chan et al. (Chithrani and Chan, 2007). It was reported that 14 and 75 nm spherical nanoparticles were up-taken by cells 3.75–5 times more than 74-by-14 nm rod-shaped particles. The group hypothesized that the significant difference in uptake could be due to the difference in particle curvature which will affect the contact area with the cell membrane receptors as well as the distribution of targeting ligands on the particles. Using PRINT-fabricated nanoparticles of various shapes and sizes, Gratton et al. have also demonstrated the effects of in vitro cellular internalization in HeLa cells. The group reported that cylindrical nanoparticles had the highest percentage of cellular internalization (Gratton et al., 2008). Specifically, nanoparticles with 150 nm diameter and 450 nm height showed the highest internalization percentage and were taken up 4 times faster than symmetrical particles (aspect ratio of 1, 200 by 200 nm cubes). These findings suggest that nanoparticles’ aspect ratio also plays an important role in cellular uptake. However, in the same studies, 100 nm diameter particles with an aspect ratio of 3 had a lower degree of internalization compared with 150 nm particles with the same aspect ratio. The group also observed that cylindrical-shaped particles with 500 nm or 1 µm diameters and 1 µm height had reduced internalization in comparison with smaller particles but showed higher uptake than micrometer-sized square cross-section particles. This result suggests that the uptake kinetics is probably a function of both size and shape.
4.1.3 Surface Characteristics
The surface of a particle is the primary medium by which it interacts with its environment. This is of even greater important with nanoparticles because of their large surface-to-volume ratio and relatively large surface area (Storm et al., 1995). The surface can be modified by polymer content or functionalization which will impact how the environment “sees” the particle. When contemplating the question of drug delivery it is essential to consider how to modify the particle so it remains in circulation for the longest possible time to ensure tumor accumulation. It has been determined that modifying the surface of nanoparticles by adding hydrophilic polymers results in decreased clearance by the MPS/RES system (Storm et al., 1995). One such hydrophilic polymer is poly(ethylene glycol) (PEG). When attached to the surface of nanoparticles PEG imparts stealth characteristics by shielding the nanoparticles from opsonin adsorption and subsequent clearance by the MPS/RES (Alexis et al., 2008). The shape, density and length of the PEG chains can be modified and have various effects on the rate of clearance. It has been shown that increasing the molecular weight of PEG chains above 2 kDa increases the half-life of the PEGylated particle (Owens and Peppas, 2006). A dense covering of PEG chains over the surface, particularly of negative particles easily recognized by the MPS/RES, is also necessary to prevent rapid clearance (Fang et al., 2006).
4.1.4 Limitations of Passive Targeting
Passive targeting can be achieved by modulating the size, shape, and surface characteristics of the nanoparticle drug carriers. However, there remain significant barriers to transport that often result in insufficient drug concentrations at the tumor site and, consequently, little therapeutic efficacy (Brigger et al., 2002; Gu et al., 2007). Furthermore, passive targeting suffers from some of the same limitations of traditional chemotherapy such as an inability to actively distinguish healthy tissue from tumor tissue.
4.2 Active Targeting
Active targeting takes advantage of ligand-receptor, antigen-antibody and other forms of molecular recognition to deliver a particle or drug to a specific location (Haley and Frenkel, 2008). For cancer therapy active targeting moieties are particularly beneficial because they reduce or eliminate the delivery of potentially toxic drugs to healthy tissue. Targeted nanoparticles delivering chemotherapeutics are of interest because they can increase therapeutic effectiveness and reduce potential side effects (Gu et al., 2007). Active targeting takes advantage of the over-expression of receptors, such as folate and transferrin, on the tumor cell surface (Liechty and Peppas, 2012). These targeted nanodelivery devices have performed significantly better than their non-targeted counterparts resulting in an increased cytotoxicity to tumor cells and reduction of side effects (Phillips et al., 2010). This section will focus on the most widely utilized active targeting ligands for tumor therapy including folate, transferrin, aptamers, antibodies, and peptides.
4.2.1 Folate
Folate has been one of the most extensively utilized ligands for targeted drug delivery devices. The folate receptor (FR), or the high affinity membrane folate binding protein, binds the folate molecule with extremely high affinity (KD~10−9) (Gu et al., 2007; Hilgenbrink and Low, 2005). This receptor is also over-expressed in a variety of tumors such as ovarian carcinomas, choricarcinomas, meningiomas, uterine sarcomas, osteosarcomas, and non-Hodgkin’s lymphomas (Sudimack and Lee, 2000). Particles conjugated with folate or folic acid and bound to a folate receptor are internalized by the cell and introduced to the cytoplasm (Figure 2a). The drug is then released by the nanoparticle in the cytoplasm of the tumor cell and proceed to interact with intracellular components (Haley and Frenkel, 2008; Stella et al., 2000).
Figure 2.
Targeted Particles: (A) Example of a folate receptor targeted particle. Liposome functionalized with PEG tethers to impart STEALTH characteristics and folate for tumor targeting (Hilgenbrink et al., 2005), (B) Folate-conjugated PLGA-PGA polymeric micelle loaded with encapsulated doxorubicin (Sudimak et al., 2000) and (C) cRGD-functionalized PCL-PEG polymeric micelle containing encapsulated doxorubicin (Nasongkla et al., 2004).
One such folate conjugated nanoparticle is a folate receptor targeted biodegradable polymeric micelle loaded with doxorubicin developed by Yoo and colleagues. Micelles were created from a copolymer of poly(D,L-lactic-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG). The PLGA allows the particle to biodegrade after delivery of its drug payload and the PEG increases the circulation time of the particles. Doxorubicin was conjugated via a chemical linkage to the PLGA while the folate was added to the PEG. The micelle (Figure 2b) was tested for cytotoxicity and cardiotoxicity (a common side effect of DOX) compared to free DOX on folate-receptor-positive cell lines. It was determined that these particles exhibited increased cellular uptake, circulation time, and decreased cardiotoxicity (Yoo and Park, 2004). The decrease of cardiotoxicity indicates that the targeting moiety was able to differentiate between healthy and tumor tissue with greater specificity than untargeted DOX. Furthermore, the increased cytotoxicity and cellular uptake shows that the folate-receptor actively internalized the conjugated particle into the cytoplasm (Yoo and Park, 2004).
4.2.2 Transferrin
Transferrin is another receptor-ligand pair that has been utilized for tumor targeting applications. Transferrin is a membrane glycoprotein that functions with its receptor, TfR, to aid in uptake of iron by the cell (Ponka and Lok, 1999; Yoo and Park, 2004). Much like folate, when transferrin binds to its receptor it initiates endocytosis and is internalized into the cellular cytoplasm (Ponka and Lok, 1999). The transferrin receptor is overexpressed by as much as 10-fold on tumor cells making it an attractive option for targeted delivery of chemotherapeutics via nanoparticle carriers (Sahoo et al., 2004).
Sahoo and colleagues have focused a great deal of attention on developing transferrin-conjugated paclitaxel-loaded nanoparticles. The nanoparticles were made using copolymerized PLGA and poly(vinyl alcohol) (PVA), both well-studied and defined materials for drug delivery. Transferrin was conjugated to the nanoparticle surface and loaded with paclitaxel. The conjugated and loaded nanoparticles were introduced to a human prostate cancer cell line. These particles were compared to a simple solution of paclitaxel and loaded particles without transferrin. The transferrin-conjugated particles exhibited a sustained release profile and a cellular uptake three times greater than the unconjugated nanoparticles. Furthermore, the conjugated NPs reduced cellular proliferation by 70%, while the unconjugated NPs only reduced it by 35%. The free paclitaxel, by comparison, only reduced proliferation by 20% (Sahoo et al., 2004). Transferrin-conjugated nanoparticles have been shown to inhibit cellular proliferation and tumor growth while participating in sustained release profiles and increased cellular uptake. The effectiveness of the conjugated nanoparticles is most likely due to their ability to be taken up by receptor-mediated endocytosis, which enhances the amount of drug delivered to tumor cells and limiting the amount delivered to healthy cells (Sahoo and Labhasetwar, 2005; Sahoo et al., 2004).
4.2.3 Aptamers
Aptamers are short oligonucleotides of RNA or DNA that can fold into various conformations and engage in ligand binding (Gu et al., 2007). However, finding such sequences is akin to finding a needle in a haystack, with only one in 1010 random RNA sequences folding into a configuration able to participate in ligand binding (Wilson and Szostak, 1999). SELEX, or systematic evolution of ligands by exponential amplification, is a process by which researchers can comb through vast populations of RNA and DNA sequences to find new aptamers to act as targeting ligands (Wilson and Szostak, 1999). Benefits of aptamers include their small size (~15 kD), lack of immunogenicity, and the potential to readily penetrate and target tumor cells (Gu et al., 2007). It has been shown that, much like folate and transferrin, aptamers result in increased targeting specificity and more efficient drug delivery to tumor cells (Gu et al., 2007).
An aptamer-conjugated nanoparticle has been created for the delivery of cisplatin to prostate cancer cells (Dhar et al., 2008). The selected target is a prostate-specific membrane antigen (PSMA) that is highly overexpressed in prostate cancer cells and can be readily targeted by a PSMA aptamer. A traditional nanoparticle carrier composed of poly(D,L-lactic-co-glycolic acid) and poly(ethylene glycol) tethers was used to encapsulate cisplatin. Cisplatin is a platinum-based chemotherapeutic that functions by interfering with DNA transcription but is normally ineffective against prostate cancer cells when administered systemically. It is thought that targeted delivery of cisplatin could increase its therapeutic effectiveness. In fact, when compared to free cisplatin the PSMA aptamer-targeted Pt(IV)-encapsulated PLGA-b-PEG nanoparticles are 80 times more toxic to prostate cancer cells expressing PSMA [54]. Aptamer-conjugated nanoparticles have significant potential as cancer-drug-delivery vehicles.
4.2.4 Antibodies (Monoclonal Antibodies)
Like aptamers, antibodies attached to the surfaces of nanoparticles target specific antigens present on the cell membrane. The use of antibodies as targeting moieties has been extensively investigated over the past decade and has resulted in numerous available treatments (Table 1) (Adams and Weiner, 2005; Brannon-Peppas and Blanchette, 2004; Gu et al., 2007; Weber, 2007; Weiner et al., 2010). Unconjugated antibodies have been shown to have antitumor effects on lymphomas, breast cancers, non-Hodgkin’s lymphomas, colorectal cancers and chronic lymphocytic leukemias (Mehren et al., 2003; Weiner and Adams, 2000). Antibody-based treatments function by recognizing specific antigens located on the surface of cancer cells. Once an antibody-antigen interaction occurs it can induce antitumor affects by multiple mechanisms including interfering with ligand-receptor binding or suppression of protein expression (Mehren et al., 2003).
Table 1.
Available antibody-based cancer treatments (Adams and Weiner, 2005; Brannon-Peppas and Blanchette, 2004; Weber, 2007; Weiner et al., 2010).
| Drug | Antigen Target |
Cancer | Release Date |
|---|---|---|---|
| Alemtuzumab | CD52 | Chronic lymphocytic leukemia | 2001 |
| Bevacizumab | VEGF | Colorectal, Lung Cancer | 2004 |
| Cetuximab | EGF receptor | Colorectal Cancer | 2004 |
| Gefinitib | EGFR | Advanced non-small cell lung cancer | 2003 |
| Gemtuzumab | CD33 | Acute meylogenous leukemia | 2000 |
| Ibritumomab tiuxetan | CD20 | Non-Hodgkins Lymphoma | 2002 |
| Ipilimumab | CTLA-4 | Advanced Melanoma | 2011 |
| Ofatumumab | CD20 | Chronic lymphocytic leukemia | 2010 |
| Panitumumab | EGFR | Colorectal Cancer | 2008 |
| Rituximab | CD20 | Lymphoma | 1997 |
| Tostiumomab | CD20 | Lymphoma | 2003 |
| Trastuzumab | HER2 | Breast Cancer | 1998 |
Although utilized for multiple successful treatments, antibody-based targeting had several early limitations. The antibodies for human use were often derived from mice and, in some individuals, resulted in an immune response that limited the duration and effectiveness of treatment. Another limitation was the lack of specificity and adequate targeting of the antibodies to their antigen-binding sites (Brissette et al., 2006). Current technology has overcome some of these early limitations. Antibodies derived from murine proteins can now be manipulated into humanized versions that will provoke little to no immune response. Furthermore, the specific binding regions can be molecularly modified to specifically target a wide variety of receptors (Brissette et al., 2006). The IgG molecule is extensively used for this purpose, as it contains a binding region that recognizes antigens and can be readily modified to specifically distinguish a variety of targets (Brissette et al., 2006).
One such target is the epidermal growth factor receptor (EGFR), which is over-expressed in many cancers, and will bind to two separate ligands: epidermal growth factor and transforming growth factor-alpha (Mendelsohn, 1997). When either ligand binds to the EGFR it stimulates growth of cells and is responsible for the rapid proliferation of cells in a variety of cancers. By blocking this ligand-receptor interaction via antibody-interference, the proliferative behavior of the cell is either reduced or stopped (Mendelsohn, 1997). Hoffman and colleagues have determined that combining anti-EGFR antibodies with cisplatin and doxorubicin increases the cytotoxic effects of the drugs and, in some cancers, entirely eradicates the tumor (Hoffmann et al., 1997). Monoclonal antibodies have also been examined as targets for conjugated-nanoparticle drug-delivery vehicles. The Alléman group tested two different biodegradable PLA nanoparticle formulations. The first formulation was conjugated with the trastuzumab mAb (HER2 antigen) and the second with rituximab mAb (CD20 antigen). The conjugated-nanoparticles bound to cells expressing the respective antigens at a frequency 10 times higher than non-targeted nanoparticles (Nobs et al., 2005).
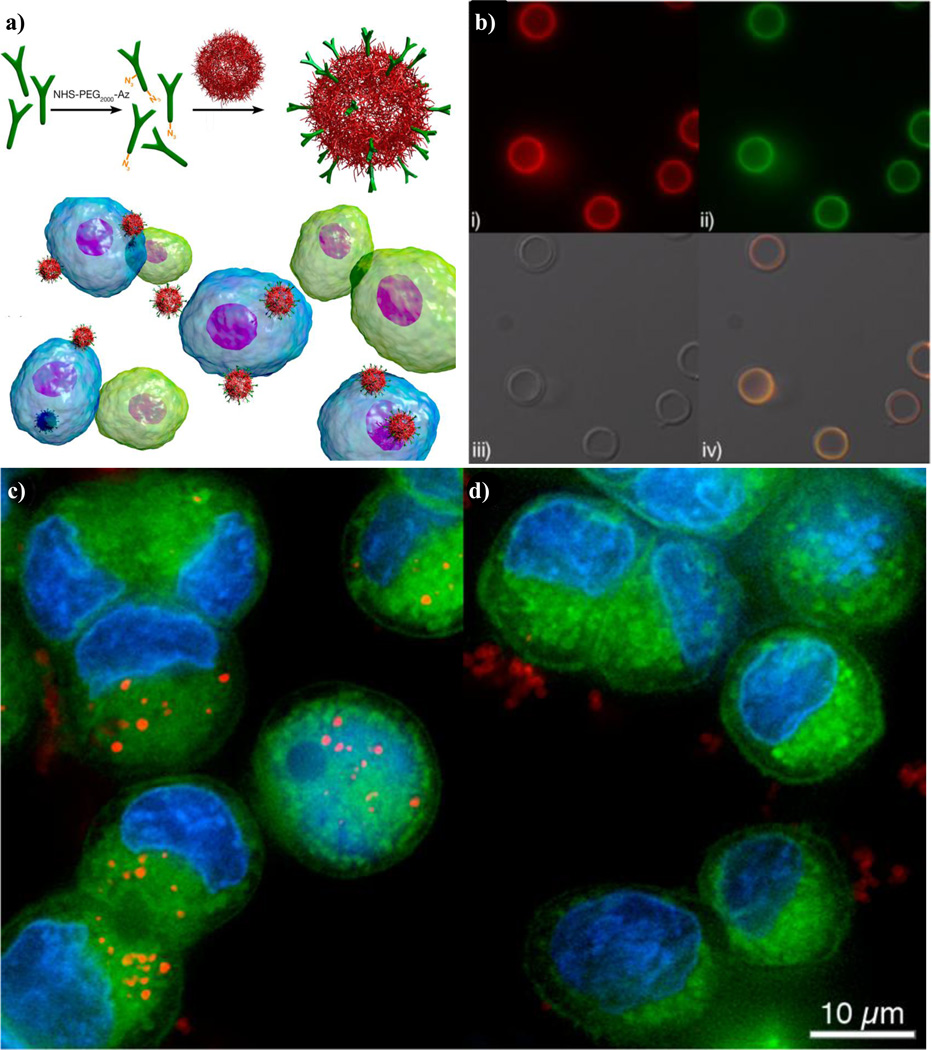
The specificity of antibodies lends particularly well to the active targeting of a variety of tumor types due to their ability to distinguish between healthy and cancerous cells and even amongst cancer cell types. In colorectal cancers, for example, over 95% of cases express the A33 antigen which can be targeted via a humanized A33 monoclonal antibody (huA33 mAb). A number of clinical studies have shown that huA33 mAb is capable of localizing specifically to colorectal cancer cells expressing the A33 antigen (Johnston et al., 2012). Recently, Johnston and colleagues, have reported on the development of a polymeric nanoparticle system composed of a silica core followed by a layer-by-layer deposition of alkyne-modified poly(N-vinylpyrrolidone) (PVPONAlk) and poly(methacrylic acid) (PMA) (Figure 3b). To this particle, the A33 monoclonal antibody was conjugated to the surface via click chemistry and imparted targeting characteristics to the system (Figure 3a). Upon incubating the huA33 mAb-conjugated particles with L1M1899 colorectal cancer cells expressing the A33 antigen, it was observed that extensive internalization of the particles occurred as compared to the particles conjugated with a negative control, IgG (Figures 3c and d). The antibody-conjugated particles not only preferentially interacted with the cancerous cells but were also phagocytosed, which is ideal for the delivery of chemotherapeutic agents (Johnston et al., 2012).
Figure 3.
(a) Top. Antibody functionalization of the surface of nanoparticles composed of a 585nm silica core and a layer-by-layer deposition of alkyne-modified poly-(N-vinylpyrrolidone) and poly(methacrylic acid). Bottom. Antibody-functionalized particles added to a mixed population of cells that either expresses the complementary antigen (blue) or not (green). (b) Fluorescence microscopy images of huA33 mAbAz-functionalized nanocapsules with (i) the antibody labeled with AF647 (red), or (ii) antibody labeled with AF488 (green), (iii) bright-field, and (iv) overlay images. (c) Deconvolution microscopy images of (left) antibody-functionalized or (right) IgG-functionalized nanocapsules. The particles were incubated with L1M1899 CRC cells at 37C for 24 hours. Capsules are labeled with AF647 (red), cells are labeled with LavaCell (green), and the nucleus is labeled with Hoechst 33342 (blue) (Johnston et al., 2012).
While antibody-based cancer therapeutics have shown promise there are several remaining limitations that must be considered in the future. The development and modification of antibodies is a complex and expensive process that is difficult to scale-up to large-scale manufacture (Brissette et al., 2006). Even with fully humanized antibodies an immune response is a potential road block to treatment. Tumor penetration has also been an issue, with observed non-uniform uptake into the tumor mass (Weiner and Adams, 2000). This lack of tumor penetration has been attributed to the increased size of nanoparticles due to the hydrodynamic radius of antibodies (~20nm) and an uneven distribution of antigens (Gu et al., 2007; Weiner and Adams, 2000). Antibody fragments have been posed as a solution, as they are smaller, induce a lesser immune response, and can still selectively target antigen receptors on the surface of tumor cells (Gu et al., 2007).
4.2.5 Peptides
Peptides have also been proposed as a potential targeting moiety for delivering chemotherapeutics. Peptides, much like antibodies, can be used to disrupt ligand-receptor interactions on tumor cells and lead to cessation of cellular proliferation. They have the added benefit of being much less expensive and complex to manufacture than antibodies (Brissette et al., 2006). Screening of potential protein ligands is typically completed using a combinatorial phage library. This technique results in ligands that range from 10– 15 amino acids in length and are able to selectively bind to tumor targets with high affinity (Brissette et al., 2006; Gu et al., 2007; Krag et al., 2006). One such tumor target is the αvβ3 integrin, which is present at elevated levels on tumor cells and is an essential component of angiogenesis (Brooks et al., 1994). This integrin is recognized by the arginine-glycine-aspartic acid (RGD) peptidic sequence (Byrne et al., 2008). The affinity of the RGD sequence to the αvβ3 integrin has potential to be exploited for drug delivery devices. Nasongkla and colleagues have functionalized the surface of polymeric micelles with a cyclic peptide containing the RGD sequence to deliver doxorubicin to Kaposi’s sarcoma cells (Figure 2c) (Nasongkla et al., 2004). The polymer micelle was composed of poly(ε-caprolactone)-poly(ethylene glycol) (PCL-PEG) imparting both biodegradable and long-circulating characteristics to the structure. The doxorubicin (DOX) was loaded into the polymeric micelle and preferentially located into the center of the structure. The polymer ends were then functionalized with a cyclic pentapeptide c(Arg-Gly-Asp-D-Phe-Lys) c(RGD) containing the RGD sequence to allow for selective targeting to the αvβ3 integrin. When introduced to Kaposi’s sarcoma derived cells (displaying an overexpression of the αvβ3 integrin) a 30-fold increase in cellular uptake was observed between the cRGD-containing and non-functionalized micelles (Nasongkla et al., 2004).
Another peptidic sequence capable of targeting behavior is Angiopep-2, the complementary ligand to the low-density lipoprotein receptor-related protein (LRP). The LRP is highly overexpressed both on the blood-brain barrier and on glioblastoma multiforme (GBM), or glioma, a tumor of the pituitary gland which is typically inoperable. The combined targeting effects of Angiopep-2 has the potential to allow a therapeutic to pass through the blood-brain barrier at sufficient concentration to then target the glioma within the brain. Xin et al, have conjugated Angiopep-2 to the surface of poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles to engage in dual-targeting of gliomas. After observing increased cellular uptake of Ang-targeted U87 MG glioma cells as compared to blank controls, the in vivo targeting effects were measured. Fluorescently labeled nanoparticles, blank and conjugated with Angiopep-2, were injected via the tail vein into a mouse bearing an intracranial U87 MG glioma tumor. Figure 4 shows in vivo fluorescence images of the particles after 24 hours of circulation. While the blank nanoparticles are capable of accumulating in the glioma due to the EPR effect, the targeted nanoparticles were present in the tumor at much higher concentrations. This concentration differential indicates that Angiopep-conjugated PEG-PCL nanoparticles can selectively bypass the blood-brain barrier and actively target and accumulate in a glioma (Xin et al., 2011).
Figure 4.
In vivo fluorescence imaging of nude mice bearing intracranial U87 MG glioma tumors after an intravenous injection of nanoparticles either (a) conjugated with Angiopep or (b) left bare. (c) Images of dissected organs from U87 MG glioma tumor-bearing mice, removed 24 hours after an injection of nanoparticles. We observe a collection of nanoparticles both at the tumor site in the brain (indicated by the arrows) and in the MPS/RES organs such as the liver and spleen. (d) TEM image of the PEG-PCL nanoparticles. (e) TEM image of the PEG-PCL nanoparticles after modification with Angiopep (Xin et al., 2011).
4.2.6. Limitations of Active Targeting
Active targeting moieties are capable of reducing off-target effects and improving the bioavailability of the chemotherapeutic agent. In addition, the inclusion of imaging modalities within these nanostructures yield particles that can, theoretically, be used to target and image the tumor, while simultaneously releasing a therapeutic payload (Caldorera-Moore et al., 2011b). However, there are a number of limitations with active targeting that bear some discussion.
The incorporation of active targeting ligands is designed to improve and enhance nanoparticle accumulation at the tumor site. What remains to be seen is whether the increased concentration of carriers and their respective payloads have any bearing upon the delivery of the therapeutic into the interior of the cell. Even if the nanoparticle carriers are capable of preferentially collecting in the tumor site, their efficacy is wholly dependent on their ability to deliver the payload (Phillips et al., 2010). The harnessing of receptor-mediated endocytosis is coupled with the added challenge of encouraging endosomal-escape once the carrier or therapeutic is entrapped (Janson et al., 2006). Additionally, the replacement of stealth polymers, such as PEG, with the active targeting moieties can drastically affect opsonization and clearance of the carrier. In order for the active targeting ligands to perform their function they must encounter tumor cells expressing the motifs of interest. If the carriers are rapidly cleared from the bloodstream, accumulation in the liver, spleen and other RES organs will be observed, while the tumor will amass a lesser amount of the targeted carriers (Phillips et al., 2010). While active targeting ligands overcame a number of limitations seen with their passive targeted counterparts, additional work must be completed to enhance overall biodistribution and therapeutic efficacy of these actively targeted nanoparticlulate carriers.
5. Conclusions
Nanoparticles used as drug carriers for chemotherapeutic agents have the potential to drastically improve the way cancer is treated. Targeted therapy can reduce the extremely severe side effects those undergoing chemotherapy must endure. In addition, targeted therapy can push the boundaries of the therapeutic indices by ensuring that the cytotoxic levels of drug are only observed at the desired tumor site. A wide variety of nanoparticle structures and targeting ligands speaks to the promise of wide-scale use of targeted nanoparticle drug delivery carriers. Increasing the specificity of the carrier and optimizing drug loading and release are essential tasks to improve the quality of these devices. Targeted nanoparticle drug carriers have the potential to revolutionize cancer therapy and improve both the quality and duration of a patient’s life.
6. Acknowledgments
This work was supported in part by grants from the National Institutes of Health NCI Center for Oncophysics Grant CTO PSOC U54-CA-143837, and National Institutes of Health Grant No. EB-00246-18).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol Pharmaceutics. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban DF, Seymour LW. Control of tumour vascular permeability. Adv Drug Delivery Rev. 1998;34:109–119. doi: 10.1016/s0169-409x(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review) Oncol Rep. 2003;10:1663–1682. [PubMed] [Google Scholar]
- Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Delivery Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Brigger In, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Delivery Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Brissette R, Prendergast J, Goldstein N. Identification of cancer targets and therapeutics using phage display. Curr Opin Drug Discov Devel. 2006;9:363–369. [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvÎ23 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brown JM, Giaccia AJ. The Unique Physiology of Solid Tumors: Opportunities (and Problems) for Cancer Therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Delivery Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv. 2010;7:479–495. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldorera-Moore M, Kang MK, Moore Z, Singh V, Sreenivasan SV, Shi L, Huang R, Roy K. Swelling behavior of nanoscale, shape- and size-specific, hydrogel particles fabricated using imprint lithography. Soft Matter. 2011a;7:2879–2887. [Google Scholar]
- Caldorera-Moore M, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Accounts Chem Res. 2011b;44:1061–1070. doi: 10.1021/ar2001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldorera-Moore M, Peppas NA. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv Drug Delivery Rev. 2009a;61:1391–1401. doi: 10.1016/j.addr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldorera-Moore M, Peppas NA. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv Drug Delivery Rev. 2009b;61:1391–1401. doi: 10.1016/j.addr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci U.S.A. 2007;104:11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chithrani BD, Chan WCW. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- Dellian M, Witwer BP, Salehi HA, Yuan F, Jain RK. Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am J Pathol. 1996;149:59–71. [PMC free article] [PubMed] [Google Scholar]
- Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc Natl Acad Sci U.S.A. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J Natl Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- Fang C, Shi B, Pei Y-Y, Hong M-H, Wu J, Chen H-Z. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27:27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Feng S-S, Chien S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem Eng Sci. 2003;58:4087–4114. [Google Scholar]
- Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J Controlled Release. 2008;125:263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U.S.A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, Langer RS, Farokhzad OC. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2:14–21. [Google Scholar]
- Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J Pharm Sci. 2005;94:2135–2146. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Hafner D, Ballo H, Haas I, Bier H. Antitumor activity of antiepidermal growth factor receptor monoclonal antibodies and cisplatin in ten human head and neck squamous cell carcinoma lines. Anticancer Res. 1997;17:4419–4425. [PubMed] [Google Scholar]
- Hori K, Suzuki M, Tanda S, Saito S. Characterization of Heterogeneous Distribution of Tumor Blood Flow in the Rat. Cancer Sci. 1991;82:109–117. doi: 10.1111/j.1349-7006.1991.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1:22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- Jain RK. Transport of Molecules in the Tumor Interstitium: A Review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. J Controlled Release. 1998;53:49–67. doi: 10.1016/s0168-3659(97)00237-x. [DOI] [PubMed] [Google Scholar]
- Jang SH, Wientjes MG, Lu D, Au JLS. Drug Delivery and Transport to Solid Tumors. Pharm Res. 2003;20:1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- Janson CG, During MJ, Liang KW, Liu F, Huang L. Targeted Gene Delivery Peptide Nucleic Acids, Morpholinos and Related Antisense Biomolecules. Springer US; 2006. pp. 30–37. [Google Scholar]
- Johnston APR, Kamphuis MMJ, Such GK, Scott AM, Nice EC, Heath JK, Caruso F. Targeting Cancer Cells: Controlling the Binding and Internalization of Antibody-Functionalized Capsules. ACS Nano. 2012 doi: 10.1021/nn3010476. [DOI] [PubMed] [Google Scholar]
- Jones A, Harris AL. New developments in angiogenesis: a major mechanism for tumor growth and target for therapy. Cancer J Sci Am. 1998;4:209–217. [PubMed] [Google Scholar]
- Krag DN, Shukla GS, Shen G-P, Pero S, Ashikaga T, Fuller S, Weaver DL, Burdette-Radoux S, Thomas C. Selection of Tumor-binding Ligands in Cancer Patients with Phage Display Libraries. Cancer Res. 2006;66:7724–7733. doi: 10.1158/0008-5472.CAN-05-4441. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Kataoka K. Block copolymer micelles as long-circulating drug vehicles. Adv Drug Delivery Rev. 1995;16:295–309. [Google Scholar]
- Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16:307–321. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(l-amino acid) micelles for drug delivery. Adv Drug Delivery Rev. 2002;54:169–190. doi: 10.1016/s0169-409x(02)00015-7. [DOI] [PubMed] [Google Scholar]
- Liechty WB, Caldorera-Moore M, Phillips MA, Schoener CA, Peppas NA. Advanced molecular design of biopolymers for transmucosal and intracellular delivery of chemotherapeutic agents and biological therapeutics. J controlled Release. 2011;155:119–127. doi: 10.1016/j.jconrel.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechty WB, Peppas NA. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm. 2012;80:241–246. doi: 10.1016/j.ejpb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Biol Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Majoros InJ, Myc A, Thomas T, Mehta CB, Baker JR. PAMAM Dendrimer-Based Multifunctional Conjugate for Cancer Therapy: Synthesis, Characterization, and Functionality. Biomacromolecules. 2006;7:572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Kimura M, Yamamoto T, Maeda H. Involvement of the Kinin-generating Cascade in Enhanced Vascular Permeability in Tumor Tissue. Cancer Sci. 1988;79:1327–1334. doi: 10.1111/j.1349-7006.1988.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehren Mv, Adams GP, Weiner LM. Monoclonal Antibody Therapy for Cancer. Annu Rev Med. 2003;54:343–369. doi: 10.1146/annurev.med.54.101601.152442. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J. Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clin Cancer Res. 1997;3:2703–2707. [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hedeman H, Christy NM, Illum L, Davis SS. Enhanced hepatic clearance of intravenously administered sterically stabilized microspheres in zymosan-stimulated rats. J Leukocyte Biol. 1993;54:513–517. doi: 10.1002/jlb.54.6.513. [DOI] [PubMed] [Google Scholar]
- Nasongkla N, Shuai X, Ai H, Weinberg BD, Pink J, Boothman DA, Gao J. cRGD-Functionalized Polymer Micelles for Targeted Doxorubicin Delivery. Angew Chem, Int Ed. 2004;116:6483–6487. doi: 10.1002/anie.200460800. [DOI] [PubMed] [Google Scholar]
- Nobs L, Buchegger F, Gurny R, Allemann E. Biodegradable Nanoparticles for Direct or Two-Step Tumor Immunotargeting. Bioconjugate Chem. 2005;17:139–145. doi: 10.1021/bc050137k. [DOI] [PubMed] [Google Scholar]
- Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Park K, Lee S, Kang E, Kim K, Choi K, Kwon IC. New Generation of Multifunctional Nanoparticles for Cancer Imaging and Therapy. Adv Funct Mater. 2009;19:1553–1566. [Google Scholar]
- Peppas NA, Slaughter BV, Kanzelberger MA. Hydrogels in Polymer Science: A Comprehensive Reference. Amsterdam: Elsevier; 2012. [Google Scholar]
- Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Ratner BD, Hoffman AS, Schoen FJ. Biomaterials Science: An Introduction to Materials in Medicine. 2nd ed. ed. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials Science, Second Edition: An Introduction to Materials in Medicine. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct Fabrication and Harvesting of Monodisperse, Shape-Specific Nanobiomaterials. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- Rowinsky M, Eric K. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Labhasetwar V. Enhanced Antiproliferative Activity of Transferrin-Conjugated Paclitaxel-Loaded Nanoparticles Is Mediated via Sustained Intracellular Drug Retention. Mol Pharmaceutics. 2005;2:373–383. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer. 2004;112:335–340. doi: 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- Schoener CA, Hutson HN, Peppas NA. pH-Responsive Hydrogels with Dispersed Hydrophobic Nanoparticles for the Delivery of Hydrophobic Therapeutic Agents. Polym Intern. 2012;61:874–879. doi: 10.1002/pi.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella B, Arpicco S, Peracchia MT, Desmaële D, Hoebeke J, Renoir M, D'Angelo J, Cattel L, Couvreur P. Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci. 2000;89:1452–1464. doi: 10.1002/1520-6017(200011)89:11<1452::aid-jps8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Delivery Rev. 1995;17:31–48. [Google Scholar]
- Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Adv Drug Delivery Rev. 2000;41:147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol Renal Physiol. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang L, Chen Z, Shin DM. Application of Nanotechnology in Cancer Therapy and Imaging. CA Cancer J Clin. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]
- Weber J. Review: AntiCTLA-4 Antibody Ipilimumab: Case Studies of Clinical Response and Immune-Related Adverse Events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Adams GP. New approaches to antibody therapy. Oncogene. 2000;19:6144–6151. doi: 10.1038/sj.onc.1204000. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–686. [PubMed] [Google Scholar]
- Wilson DS, Szostak JW. In vitro selection of functional nucleic acids. Annu Rev Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- Wu J, Akaike T, Maeda H. Modulation of Enhanced Vascular Permeability in Tumors by a Bradykinin Antagonist, a Cyclooxygenase Inhibitor, and a Nitric Oxide Scavenger. Cancer Res. 1998;58:159–165. [PubMed] [Google Scholar]
- Xin H, Jiang X, Gu J, Sha X, Chen L, Law K, Chen Y, Wang X, Jiang Y, Fang X. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Controlled Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]