Abstract
Amphiphilic polymer carriers were formed by polymerizing a hydrophilic, pH-responsive hydrogel composed of poly(methacrylic – grafted – ethylene glycol) (P(MAA-g-EG)) in the presence of hydrophobic PMMA nanoparticles. These polymer carriers were varied in PMMA nanoparticle content to elicit a variety of physiochemical properties which would preferentially load doxorubicin, a hydrophobic chemotherapeutic, and release doxorubicin locally in the colon for the treatment of colon cancers. Loading levels ranged from 49% to 64% and increased with increasing nanoparticle content. Doxorubicin loaded polymers were released in a physiological model where low pH was used to simulate the stomach and then stepped to more neutral conditions to simulate the upper small intestine. P(MAA-g-EG) containing nanoparticles were less mucoadhesive as determined using a tensile tester, polymer samples, and fresh porcine small intestine. The cytocompatibility of the polymer materials were assessed using cell lines representing the GI tract and colon cancer and were non-cytotoxic at varying concentrations and exposure times.
Keywords: hydrogels, pH-responsive, nanoparticles, doxorubicin, controlled release, oral delivery
INTRODUCTION
Chemotherapeutic agents are primarily administered intravenously for systemic delivery in the treatment against cancer and targets healthy and diseased tissue in an unbiased fashion. Because many chemotherapeutics are hydrophobic, low molecular weight agents, intravenous treatment has remained the chief delivery mechanism and more focus has been placed on targeting the chemotherapeutics as opposed to developing new delivery methods. New delivery methods, particular the oral delivery of chemotherapeutics, could make a dramatic impact to the medical community who administers the cancer treatments and the patients who endure the effects of the cancer treatments.1–3 Oral chemotherapy has been discussed and presented in the past as systems where chemotherapeutics are delivered to the upper small intestine for subsequent uptake into the bloodstream, but only a small number show improved or equal efficacy to intravenous treatment.4–11 This research is focused on developing oral delivery strategies of chemotherapeutics for the direct, local delivery of chemotherapeutics to the colon for the treatment of colon cancer.
The local, oral delivery of chemotherapeutics to the colon maybe made possible by using pre-existing technology and biomaterials, by assembling them into novel structures and architectures that can elicit properties appropriate for the application at hand.12–15 Hydrogels, a type of biomaterial, are three-dimensional networks, insoluble in aqueous environments due to physical and/or chemical crosslinks, and able to imbibe large amounts of water or biological fluids which allow them to interact positively with human physiological systems.16–18 More specifically, pH-Responsive hydrogels are a class of biomaterials that exhibit desirable physicochemical properties at specific pH ranges for the oral delivery of therapeutic agents.19–21 However, the hydrophilicity of pH-responsive hydrogels would not be favorable to hydrophobic chemotherapeutics and would need to be modified with hydrophobic properties to be successful.
In addition to possessing amphiphilic properties, a pH-responsive hydrogel used for the oral delivery of chemotherapeutics needs to be able to load and release an encapsulated chemotherapeutic at amounts which provide therapeutic effects to the tumor. To achieve the proper concentrations, the pH-responsive hydrogel must remain collapsed in low pH conditions to prevent negative interactions the chemotherapeutic would have on the stomach and vice versa. A collapsed network will also prevent leakage or premature release which would ultimately result in insufficient quantities reaching the tumor site. Next, the pH-responsive hydrogel must expand or swell in neutral pH conditions to develop the necessary porous environment to allow diffusion of the encapsulated chemotherapeutic into the surrounding environment. Finally, the pH-responsive hydrogel must exhibit properties which can direct delivery to colon to achieve therapeutic levels of a chemotherapeutic for the treatment of colon cancer.
Doxorubicin, an anthracycline antibiotic, is a chemotherapeutic which causes toxicity to cancer cells by intercalating between DNA and disrupting the replication process.22 Unfortunately, the maximum lifetime dose is not to exceed 500 – 600 mg/m2 as cumulative cardiotoxicity associated with anthracycline treatment has been reported.23 Therefore, great efforts have been made to modify doxorubicin for improved targeting, but still utilize intravenous delivery. Modifications with moderate success include Doxil® (ALZA Corporation), a PEGylated version of doxorubicin loaded into liposomes, and LivaTag® which uses poly(isohexyl cyanoacrylate) nanoparticles loaded with doxorubicin.24–26 Research efforts, in the arena of the oral delivery of doxorubicin, have included polymer nanoparticles, dendrimers, lipid nanocarriers, and smart pectin hydrogels.27–30 Reducing cardiotoxicity of doxorubicin has also been explored through cardioprotective additives such as steroids, antioxidants, and antidiabetics.31–34
In this work, we combined the desirable characteristics of pH-sensitive, hydrophilic networks composed of poly(methyacrylic acid – grafted – ethylene glycol) (P(MAA-g-EG)) with hydrophobic poly(methyl methacrylate) (PMMA) nanoparticles to develop amphiphilic polymer structures for the oral delivery of doxorubicin. These polymer carriers were designed to provide local, direct delivery of doxorubicin to the colon for the treatment of colon cancer. Our understanding of P(MAA-g-EG) and its role in the oral delivery of therapeutics has been extensively characterized.35–44 P(MAA-g-EG) contains methacrylic acid with a pKa value of 4.8 – 4.9 which allows it to swell when it transitions from the stomach (pH 1 – 3) to the small intestine (pH 5) and increase in the degree of swelling as it travels to the colon (pH 7 – 8). P(MAA-g-EG) also contains ethylene glycol tethers to maintain a tight, collapsed network through hydrogen bonding. As for nanoparticles, their small nature exhibits characteristics different from their larger parent counterparts and can be used as polymer carriers themselves45–47 or be incorporated in to the P(MAA-g-EG) network to develop new chemical and physical properties.
By incorporating varying amounts of PMMA nanoparticles in the P(MAA-g-EG) during polymerization, amphiphilic polymer carriers with a distribution of physical properties can be formed. Previously, these polymer carriers were investigated for their physical and chemical properties and their ability to load and release fluorescein, a model solute with properties similar to chemotherapeutics.48 In this paper, PMMA’s effect on the loading and release of doxorubicin, a common chemotherapeutic used in cancer treatment, was determined. The pH change from the stomach to the small intestine could be used as a trigger for releasing encapsulated therapeutic agents and is tested. Cytocompatibility of the polymer materials was assessed on cells which model the GI tract and colon cancer cells.
MATERIALS AND METHODS
Materials
Methacrylic acid (MAA), methyl methacrylate (MMA), tetraethylene glycol dimethacrylate (TEGDMA), 1-hydroxycyclohexyl phenyl ketone (Irgacure® 184), ammonium persulfate (APS), dimethyl sulfoxide (DMSO), Dulbecco’s Modified Eagle’s Medium (DMEM), Roswell Park Memorial Institute Medium (RPMI), fibronectin, and ethanol were purchased from Sigma–Aldrich (St. Louis, MO). 10× phosphate buffer solution (PBS), sodium chloride, and hydrochloric acid were purchased from Fisher Scientific (Fair Lawn, NJ). Poly(ethylene glycol) monomethyl ether monomethacrylate (PEGMMA; 1000 g/mol) was from Polysciences Inc. (Warrington, PA). Doxorubicin was purchased from Selleck Chemicals (Houston, TX). Fetal bovine serum (FBS) and trypsin with EDTA were obtained from Hyclone (South Plainfield, NJ). 1× Phosphate Buffered Saline (PBS) without calcium or magnesium along with penicillin and streptomycin were from MediaTech (Manassas, VA). The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-terazolium (MTS) compound was used for cell proliferation assays purchased from Promega (Madison, WI). Caco-2 and SW620 cells were obtained from American Type Culture Collection (ATCC, Rockwell, MD) and HT29-MTX cells were a gift from Dr. Thecla Lesuffleur, INSERM, Paris, France. All chemicals were used as received except for MAA which was vacuum distilled at 54 °C and 25 mm Hg prior to use to remove the inhibitor hydroquinone. Double distilled water was used in all studies.
Synthesis of P(MAA-g-EG) Hydrogels Dispersed with PMMA
P(MAA-g-EG) hydrogels were dispersed with PMMA nanoparticles by first forming the PMMA nanoparticles and then polymerizing the P(MAA-g-EG) hydrogel in the presence of PMMA nanoparticles and previously discussed in detail.48 Briefly, PMMA nanoparticles were formed by combining MMA, APS, TEGDMA, and water and reacted for 3 hr at 75 °C. The nanoparticles were then dialyzed and freeze dried. Then P(MAA-g-EG) dispersed with PMMA nanoparticles was formed by combining MAA, PEGMMA, TEGDMA, Irgacure® 184, and dry PMMA nanoparticles in ethanol and water. The solution was sonicated, purged with N2, placed between glass slides, and exposed to UV light (Dymax 2000-EC Light Curing System, Torrington, CT). The resulting P(MAA-g-EG) film containing nanoparticles was washed, punched into discs, or dried, crushed, and sieved into particles sized between 75 – 150 µm for future use. Both particles and discs were dried in vacuum at 30 °C for 1 week. P(MAA-g-EG) hydrogels dispersed with 1, 2.5, or 5 wt% PMMA nanoparticles will be identified as P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, P(MAA-g-EG)-5.0NP hence forth.
Loading Doxorubicin in P(MAA-g-EG) and P(MAA-g-EG) dispersed with PMMA Nanoparticles
Doxorubicin was loaded by equilibrium partitioning in the following manner: a stock solution of doxorubicin was prepared in 2 wt.% DMSO in 1× PBS (pH 7.4) at a concentration of 0.25 mg/mL. A 5 mg/mL concentration of crushed particles (75 – 150 µm) of P(MAA-g-EG) or P(MAA-g-EG) containing PMMA nanoparticles to doxorubicin stock solution was allowed to stir slowly for 2 hr. The doxorubicin-loaded particles were filtered and rinsed twice with 0.1 N HCl and once with water to remove any loosely surface-adsorbed doxorubicin. A fluorescent plate reader (Biotek Synergy-HT, Winooski, VT), operating at a 485 nm excitation and 590 nm emission wavelengths, determined the concentration levels and multiplying these concentrations by the volume at each loading step allowed us to calculated loading efficiency:
| (1) |
where Mo is the initial doxorubicin mass in the solution and Mf is the final doxorubicin mass after particles were soaked, but before rinsing because no considerable drug loss was detected after rinsing.
Doxorubicin Release from P(MAA-g-EG) and P(MAA-g-EG) dispersed with PMMA Nanoparticles
Release experiments were performed on a rotary mixer (Glas-Col, Terre Haute, IN) operating at 15 rpm which was placed in a dry oven (Fisher Scientific Isotemp Incubator Model 525D, Pittsburgh, PA) thermostated to 37 °C. For all doxorubicin release experiments, 1.5 mg of doxorubicin loaded P(MAA-g-EG) or P(MAA-g-EG) containing PMMA nanoparticles was added to 3 mL of 1× PBS (pH 2.0 or 7.4). For doxorubicin release in neutral pH, 1× PBS (pH 7.4) was used and over the duration of 6 hr, samples (100 µL) were taken and replaced with fresh 1× PBS to maintain doxorubicin concentration levels below 10 µg/mL so as to continually maintain a concentration gradient appropriate for doxorubicin release from loaded microparticles. Doxorubicin release in low pH was conducted in the same manner as neutral pH except 1× PBS was adjusted to a pH of 2.0 using 1N HCl.
A two-step pH change from low pH (2.0) to high pH (7.0) was used to model the physiological conditions and residence time of the stomach and small intestine.49, 50 Doxorubicin loaded microparticles were first placed in 1× PBS at pH 2.0. After 90 min, 5N NaOH was added to increase the pH to 7.0 where release continued for 6 hr. Samples were obtained as above. The mass of doxorubicin released was determined by the fluorescent plate reader and reported as follows:
| (2) |
where Mt is mass released at a given time and M∞ is total mass released.
Cytocompatibility
Caco-2 and HT29-MTX cells were maintained in DMEM and are used as GI tract cell models. SW620 cells were maintained in RPMI media and were used as a colon cancer cell model. Both medias were supplemented with heat-inactivated FBS, penicillin, and streptomycin. Cytocompatibility experiments were performed in fibronectin coated 96 – well plates (Nunc, Rochester, NY). Caco-2, HT29-MTX, and SW620 cells were seeded at a density of 2.0 × 103 cells/cm2, 3.0 × 104 cells/cm2, and 1.5 × 104 cells/cm2, respectively and incubated for 48 hr before testing.
P(MAA-g-EG) and P(MAA-g-EG) containing PMMA nanoparticles were incubated with all cell lines for 2 hr at concentrations of 1 mg/mL, 2.5 mg/mL, and 5 mg/mL. P(MAA-g-EG) and P(MAA-g-EG)-5.0NP were also exposed to the same cell models at a concentration of 5 mg/mL for 6, 12, and 24 hr to determine cytocompatibility since these materials are designed to reside within the GI tract and not transport into the bloodstream. Cell viability was determined after the microparticles were removed, the cell lines rinsed three times with 1× PBS, and MTS assayed (CellTiter 96® Aqueous One Solution Cell Proliferation Assay).
Mucoadhesion
Mucoadhesion of P(MAA-g-EG) and P(MAA-g-EG)-5.0NP discs were determined by measuring the force required to de-adhere samples from fresh porcine upper small intestine. A texture analyzer (TA.XT plus, Stable Micro Systems, UK) and a mucoadhesive-testing rig (Stable Micro Systems, UK) were used to complete the tests. Before testing, samples equilibrated in 1× PBS adjusted to a pH of 7.0 for 24 hr and attached to a cylindrical probe.
Fresh porcine upper small intestine tissue was obtained immediately after slaughter at the local slaughterhouse and used within 2 – 4 hr. Rectangle tissue samples were placed on the mucoadhesive rig, submerged in 1× PBS (pH 7.0) and thermostated to 37 °C. The probe and disc were attached to the texture analyzer, the polymer sample was lowered at a rate of 5 mm/min until a force of 5 g was sensed between polymer sample and tissue, then proceeded to lower at 0.1 mm/min until a force of 50 g was applied. After 5 min of static loaded force, the probe was withdrawn at a rate of 0.1 mm/min until it was fully detached from the tissue sample. Using texture analyzer software (Texture Exponent 32), the work of adhesion (Wad) could be computed and used to compare mucoadhesive properties between samples.
RESULTS AND DISCUSSION
Synthesis of P(MAA-g-EG) dispersed with PMMA Nanoparticles
PMMA nanoparticles were first formed by utilizing a surfactant free polymerization technique. Then P(MAA-g-EG) hydrogels were formed by free radical UV-initiated polymerization in the presence of the PMMA nanoparticles to form pH-responsive hydrogels dispersed with PMMA nanoparticles. It was previously determined that the amount of nanoparticles present in the P(MAA-g-EG) hydrogel affected material properties and ultimately the ability to load and release hydrophobic therapeutic agents.48
Loading Doxorubicin in P(MAA-g-EG) and P(MAA-g-EG) dispersed with PMMA Nanoparticles
The amount of nanoparticles present in the P(MAA-g-EG) hydrogel corresponded to the loading efficiencies of doxorubicin. Loading efficiencies ranged from 49 – 64% and increased with increasing PMMA nanoparticle content (Table 1). The P(MAA-g-EG)-5.0NP contained the highest weight percentage of nanoparticles which resulted in the highest loading efficiency of 64 ± 1%. P(MAA-g-EG)-2.5NP also demonstrated a high loading level of 63 ± 3%. P(MAA-g-EG)-2.5NP’s higher degree of swelling48 allowed doxorubicin to imbibe more into the polymer network as compared to P(MAA-g-EG)-5.0NP. P(MAA-g-EG) possessed the lowest loading level due to the absence of hydrophobic nanoparticles. Weight percent loading ranged from 2.4 – 3.1% and is defined as the percentage of doxorubicin (by weight) present in the polymer.
Table 1.
Doxorubicin loading efficiency and weight percent loading for P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles (75 – 150 µm).
| Formulation | Loading Efficiency (%) |
Wt. loading (%) |
|---|---|---|
| P(MAA-g-EG) | 49 ± 2 | 2.4 |
| P(MAA-g-EG)-1.0NP | 57 ± 1 | 2.8 |
| P(MAA-g-EG)-2.5NP | 63 ± 3 | 3.1 |
| P(MAA-g-EG)-5.0NP | 64 ± 1 | 3.1 |
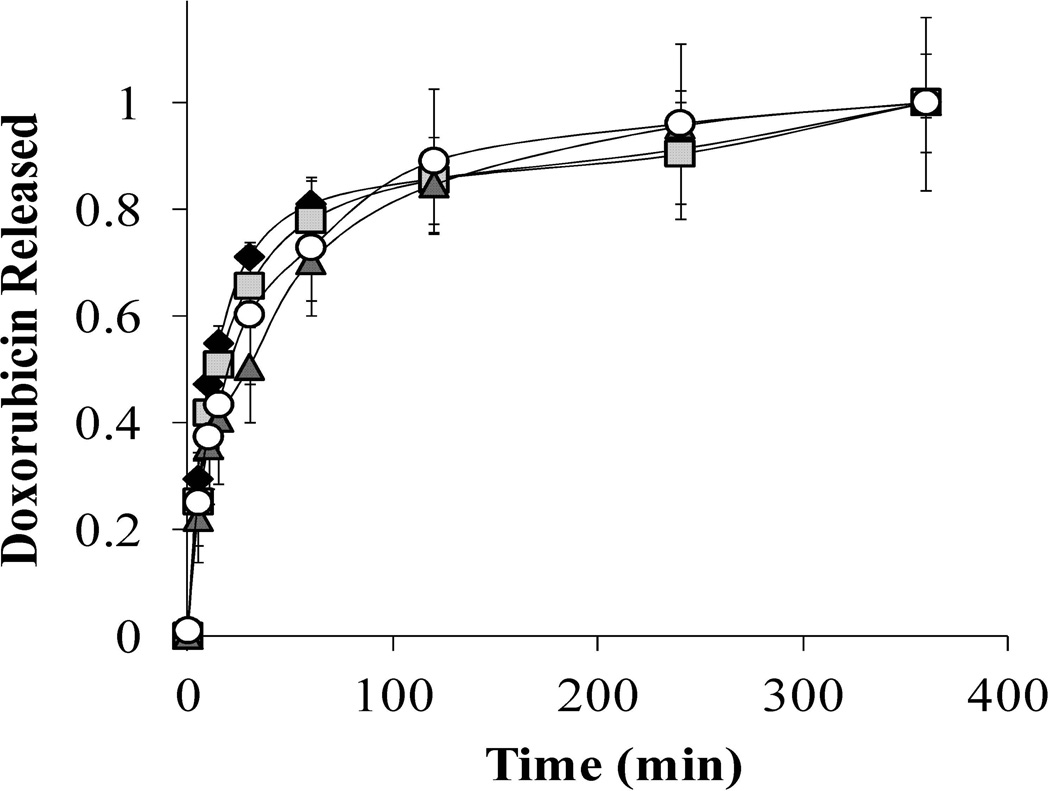
Doxorubicin Release from P(MAA-g-EG) and P(MAA-g-EG) dispersed with PMMA Nanoparticles. In neutral pH, 90 – 95% of doxorubicin released occurred over the course of 4 hr for P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles (Figure 1). These release profiles are expected since the pH of the release media is greater than the pKa value of MAA. M∞ values were 39.0, 28.6, 29.4, and 33.6 µg for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
Figure 1. Doxorubicin release of P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles in neutral pH conditions.
Doxorubicin loaded P(MAA-g-EG) (♦), P(MAA-g-EG)-1.0NP ( ), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 7.4) for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 7.4) for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
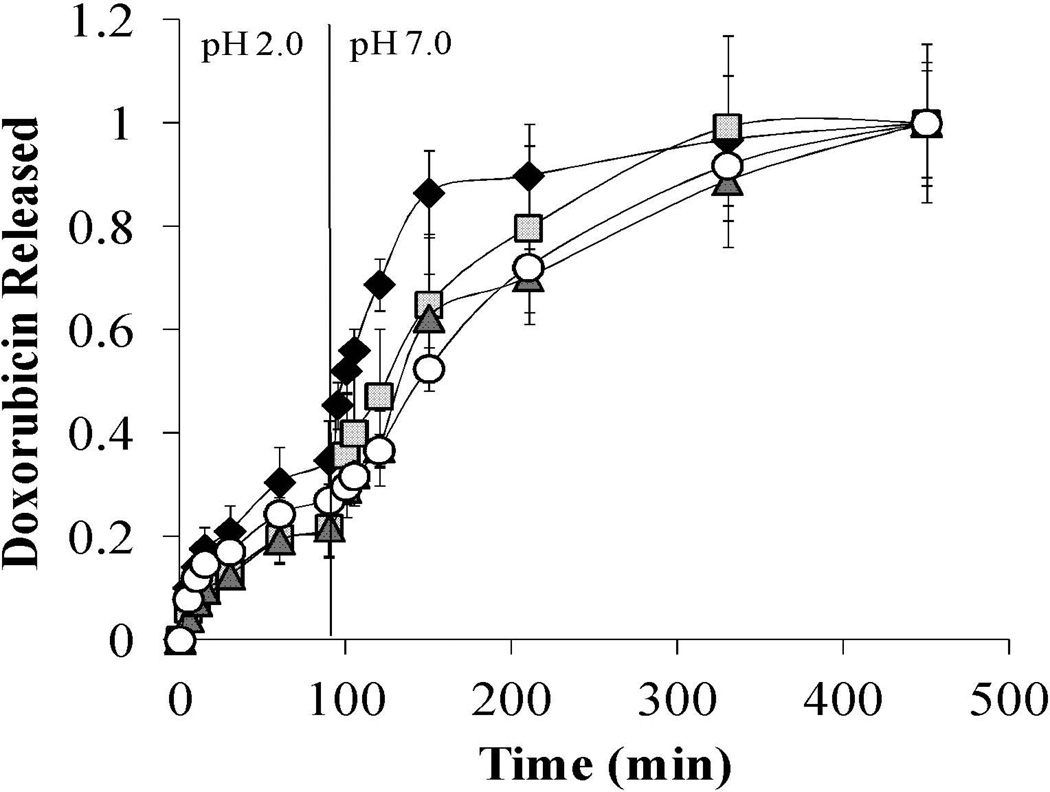
The two – step pH model was used to relate in vitro behavior to in vivo behavior. First, doxorubicin loaded particles were released in pH 2.0 (1× PBS) for 90 min and then increased to pH 7.0 (1× PBS) for an additional 6 hr. P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles all released less than 27% of doxorubicin in the low pH conditions (Figure 2). After the pH was increased from 2.0 to 7.0, the remaining amount of doxorubicin released was completed within 3 hr for P(MAA-g-EG) and P(MAA-g-EG)-1.0NP and within 4 hr for P(MAA-g-EG)-2.5NP and P(MAA-g-EG)-5.0NP (Figure 4). P(MAA-g-EG)-2.5NP and P(MAA-g-EG)-5.0NP contain more hydrophobic nanoparticles which preferentially associate with hydrophobic doxorubicin, resulting in the delayed release in the pH 7.0 as compared to P(MAA-g-EG) and P(MAA-g-EG)-1.0NP. M∞ values were 24.9, 17.6, 14.11, and 26.2 µg for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
Figure 2. Doxorubicin release of P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles in two – step pH conditions.
Doxorubicin loaded P(MAA-g-EG) (♦), P(MAA-g-EG)-1.0NP ( ), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 2.0) for 90 min. Then the pH was increased to 7.0 by adding 5 N NaOH and release continued for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
), P(MAA-g-EG)-2.5NP (▲), and P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 2.0) for 90 min. Then the pH was increased to 7.0 by adding 5 N NaOH and release continued for 6 hr. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞. M∞ ranged was 42.5, 18.7, 29.4, and 33.7 µg/mL for P(MAA-g-EG), P(MAA-g-EG)-1.0NP, P(MAA-g-EG)-2.5NP, and P(MAA-g-EG)-5.0NP, respectively.
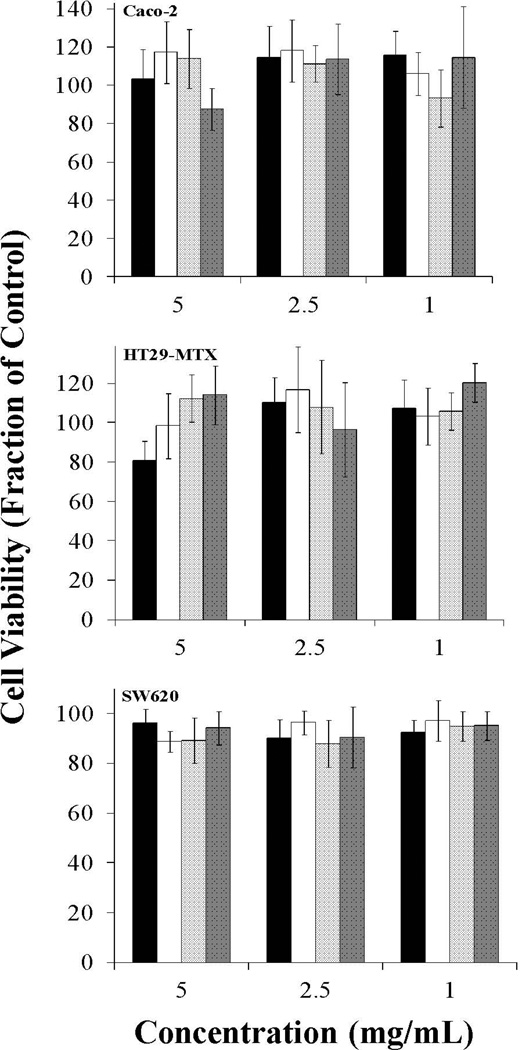
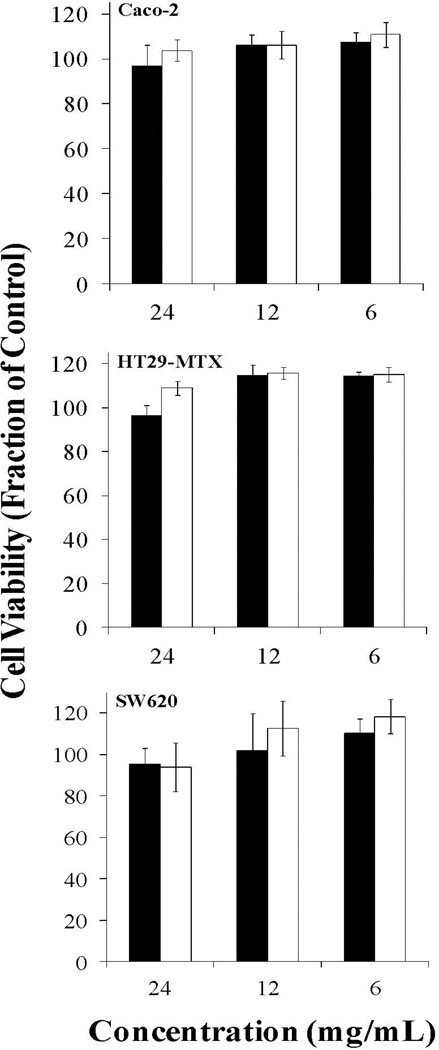
Figure 4. Effect of 2 hr P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles on Caco-2, HT29-MTX, and SW620 cell proliferation.
P(MAA-g-EG) (■), P(MAA-g-EG)-1.0NP (□), P(MAA-g-EG)-2.5NP ( ), and P(MAA-g-EG)-5.0NP (■) microparticles (75 – 150 µm) were added to all cell lines and incubated for 2 hr. Error bars represent error propagated over control cells. n = 6 – 8.
), and P(MAA-g-EG)-5.0NP (■) microparticles (75 – 150 µm) were added to all cell lines and incubated for 2 hr. Error bars represent error propagated over control cells. n = 6 – 8.
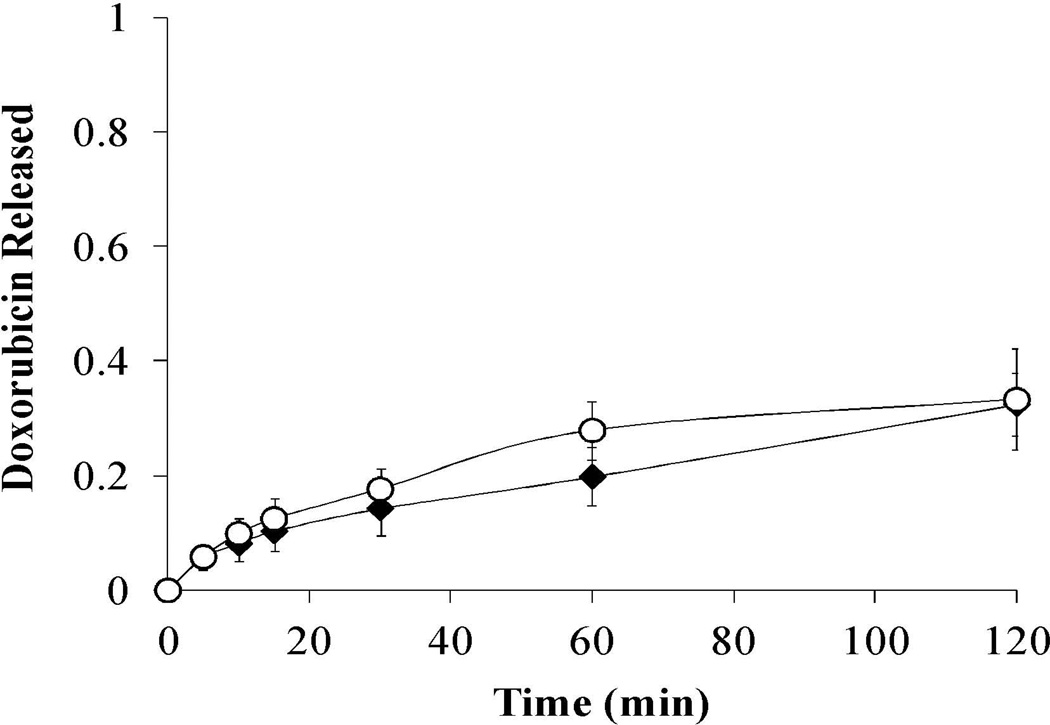
Release studies in low pH conditions were extended to 2 hr for P(MAA-g-EG) and P(MAA-g-EG)-5.0NP samples to provide a reflection of doxorubicin release for longer gastric transit times in the stomach (Figure 3). P(MAA-g-EG) and P(MAA-g-EG)-5.0NP both released 32% after 2 hr in low pH conditions indicating minimal increase in doxorubicin release for longer gastric transit times. Overall, P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles had reduced doxorubicin release in low pH conditions, which is advantageous for oral delivery of chemotherapeutics. M∞ values were 11.02 and 9.98 µg for P(MAA-g-EG) and P(MAA-g-EG)-5.0NP, respectively.
Figure 3. Doxorubicin release of P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles in low pH conditions.
Doxorubicin loaded P(MAA-g-EG) (♦) or P(MAA-g-EG)-5.0NP (○) crushed particles (75 – 150 µm) were released in 1× PBS (pH 2.0) for 120 min. Doxorubicin release is expressed as Mt/M∞. Curves generated are n = 3 and error bars represent error propagation due to ratio of Mt/M∞.
Cytocompatibility
Caco-2, HT29-MTX, and SW620 cells were plated in fibronectin coated 96 – well plates and allowed to grow 48 hr before testing. The cell lines were exposed to increasing concentrations (1 – 5 mg/mL) of P(MAA-g-EG) or P(MAA-g-EG) containing nanoparticles for 2 hr. No significant decrease in cell viability was observed for all P(MAA-g-EG) or P(MAA-g-EG) containing nanoparticles (Figure 4). Cell viability appeared low (80%) for HT29-MTX exposed to 5 mg/mL of P(MAA-g-EG), but visual inspection showed a small sheet of cells dislodged during the washing step.
The same cell lines were also exposed to P(MAA-g-EG) or P(MAA-g-EG) containing nanoparticles for 6, 12, or 24 hr at a concentration of 5 mg/mL. Longer residency times were tested since the design of these polymer carriers are not intended to transport into the bloodstream, but rather stay in the GI tract. Therefore, these polymer carriers should not exhibit toxic effects for the duration of their use. No cytotoxic effects were observed for these longer residency times (Figure 5) indicating these microparticles should be non-cytotoxic with human physiological systems and appropriate for oral delivery of chemotherapeutics. No toxic effects for the HT29-MTX with P(MAA-g-EG) at 5 mg/mL address any further concerns for the toxicity seen in the 2 hr study.
Figure 5. Effect of 6, 12, or 24 hr P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles exposure on Caco-2, HT29-MTX, and SW620 cell proliferation.
P(MAA-g-EG) (■) and P(MAA-g-EG)-5.0NP (□) microparticles (75 – 150 µm) were added to all cell lines and incubated for 6, 12, or 24 hr. Error bars represent error propagated over control cells. n = 6 – 8.
The results published here are in agreement with previous results testing the non-cytotoxicity of the P(MAA-g-EG) hydrogel utilizing the same polymerization technique.51–53 However, it should be noted that polymer materials with an increase in MAA content increase the number of carboxyl groups that could bind to Ca2+ and result in levels too low to allow normal cell function to continue. This hypothesis was confirmed by increasing the molar feed of MAA:EG or AA:EG from 1:1 to 4:1 as well as increasing the P(MAA-g-EG) concentration up to 10 mg/mL using a 1:1 molar ratio.17, 54 MAA in the ionized form and in high concentrations could also result in local acid microenvironments too harmful for cells.
Despite P(MAA-g-EG) history as a cytocompatible polymer, the introduction of PMMA nanoparticles could have influenced the overall cytotoxicity of the polymer carrier. From these studies, it can be determined that the addition of PMMA at these concentration levels did not harm the cells. PMMA use in the medical community is extensive and these results were expected.
Mucoadhesion
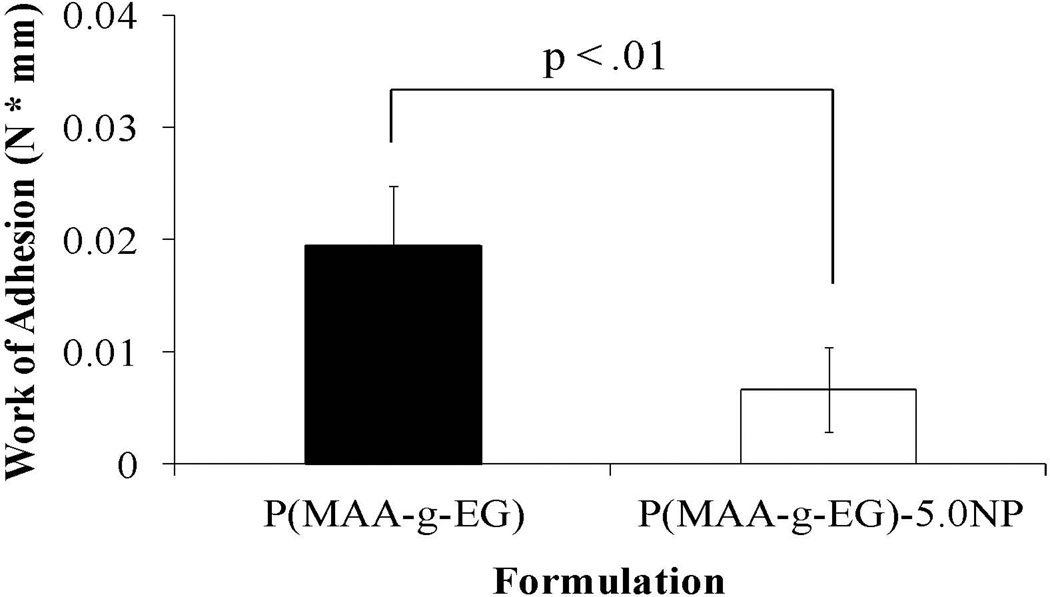
Using a tensile tester and mucoadhesive rig, the mucoadhesion of P(MAA-g-EG) and P(MAA-g-EG)-5.0NP was determined. The P(MAA-g-EG)-5.0NP was chosen because it demonstrated the highest loading efficiencies and most appropriate release profiles for oral drug delivery. In the results obtained from these experiments (Figure 6), the P(MAA-g-EG)-5.0NP demonstrated the lowest degree of adhesion and the P(MAA-g-EG) the highest (t-test, p < .01).
Figure 6. Mucoadhesion for P(MAA-g-EG) and P(MAA-g-EG)-5.0NP.
P(MAA-g-EG) (■) and P(MAA-g-EG)-5.0NP (□) discs were brought into contact with fresh porcine small intestine for 5 min, retracted slowly, the force measured with a tensile tester, and the work of adhesion computed Results demonstrate statistical significance (p < .01). n = 3 – 4 ± SD.
P(MAA-g-EG) was optimized to increase residency time in the upper small intestine by increasing its mucoadhesion through different lengths of the grafted PEG tether.55–61 Increased mucoadhesion at the upper small intestine allows more time for an encapsulated therapeutic to transport from the GI lumen into the bloodstream ultimately affecting the bioavailability. However, by introducing PMMA nanoparticles and the hydrophobicity it imparts into the polymer system, the interaction of the mucosal surface with the polymer system is not favorable resulting in decreased mucoadhesion. Furthermore, the presence of the nanoparticles could have prevented or reduced PEG tether mobility which is critical to penetrating the mucin surface. Since the final target is colon cancer, decreased mucoadhesion is a positive attribute as it allows the polymer carrier to travel further and deliver its loaded contents closer to the final location.
CONCLUSIONS
P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles were designed to possess amphiphilic properties for the oral delivery of chemotherapeutics. The loading and release of doxorubicin verified these polymer systems could be used for oral delivery and to use the transition from the stomach to the upper small intestine as a physiological trigger to release its encapsulated chemotherapeutic agent. Release studies in neutral, low, and two-step pH environments provided an excellent representation of possible in vivo performance.
For the P(MAA-g-EG) containing PMMA nanoparticles, loading levels were increased from 49% for the P(MAA-g-EG) to 64% for P(MAA-g-EG)-5.0NP and increased with increasing nanoparticle content. For release studies, P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles kept doxorubicin release below 27% in low pH conditions. After shifting the pH from low to neutral, the remainder of doxorubicin was released over 3 hr for P(MAA-g-EG) and P(MAA-g-EG)-1.0NP and 4 hr for P(MAA-g-EG)-2.5NP and P(MAA-g-EG)-5.0NP. The larger amount of nanoparticles present in the latter couple helped extend doxorubicin release over 4 hr which could help improve more local release to the colon for colon cancer. Final doxorubicin concentrations were 17.59 – 23.36 µg/mL with P(MAA-g-EG)-5.0NP being the highest.
In vitro assessments of P(MAA-g-EG) and P(MAA-g-EG) containing nanoparticles was determined through cytotoxicity and mucoadhesive experiments. Cytotoxicity was completed on Caco-2 or HT29-MTX cell lines which represented a GI tract model while SW620 operated as a tumor model. Concentrations of P(MAA-g-EG) or P(MAA-g-EG) containing nanoparticles, ranging from 1 – 5 mg/mL, were exposed to all cell lines for 2 hr and determined to be cytocompatible. Long term exposures ranging from 6 – 24 hr were also tested at a concentration of 5 mg/mL since the polymer systems are designed to reside within the GI tract and were also determined to be cytocompatible. Future studies exposing cells to both doxorubicin and the polymer carrier at the same time will help demonstrate that toxicity arises from the chemotherapeutic and not the polymer carrier.
Mucoadhesion experiments indicated that the P(MAA-g-EG) possessed the greatest adhesion and the P(MAA-g-EG)-5.0NP the least. Since P(MAA-g-EG) contains no nanoparticles to prevent or reduce tether mobility, the PEG chain was capable of penetrating the porcine small intestine mucosal surface and increased mucoadhesion. The presence of the nanoparticles also imparts a degree of hydrophobicity that does not interact favorably with a dominantly hydrophilic mucosal surface composed of 70 – 90% of carbohydrates.62 Based on these studies, P(MAA-g-EG) containing nanoparticles can serve as a good system for the oral delivery of chemotherapeutics for the treatment of colon cancer.
ACKNOWLEDGEMENT
This research was supported by a grant from the NIH/NCI Center for Oncophysics (CTOPSOCU54-CA-143837).C.A.S. acknowledges the National Science Foundation for a Graduate Research Fellowship.
References
- 1.Blanchette J, Kavimandan N, Peppas NA. Principles of transmucosal delivery of therapeutic agents. Biomed Pharmacother. 2004;58:142–151. doi: 10.1016/j.biopha.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 3.Irshad S, Maisey N. Considerations when choosing oral chemotherapy: identifying and responding to patient need. Eur J Cancer Care. 2010;19:5–11. [Google Scholar]
- 4.Mao JH, Balmain A. Genomic approaches to identification of tumour-susceptibility genes using mouse models. Curr Opin Genet Dev. 2003;13:14–19. doi: 10.1016/s0959-437x(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohmori K, Sasaki K, Asada S, Tanaka N, Umeda M. An assay method for the prediction of tumor promoting potential of chemicals by the use of Bhas 42 cells. Mutat Res-Gen Tox En. 2004;557:191–202. doi: 10.1016/j.mrgentox.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Schrenk D, Schmitz HJ, Bohnenberger S, Wagner B, Worner W. Tumor promoters as inhibitors of apoptosis in rat hepatocytes. Toxicol Lett. 2004;149:43–50. doi: 10.1016/j.toxlet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Wolfle D. Enhancement of carcinogen-induced malignant cell transformation by prostaglandin F-2 alpha. Toxicology. 2003;188:139–147. doi: 10.1016/s0300-483x(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Twelves C, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: Results of a large phase III study. J Clin Oncol. 2001;19:4097–4106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 9.Boeck S, Wilkowski R, et al. Oral capecitabine in gemcitabine-pretreated patients with advanced pancreatic cancer. Oncology-Basel. 2007;73:221–227. doi: 10.1159/000127413. [DOI] [PubMed] [Google Scholar]
- 10.Beach DF, Somer R. Novel Approach to Gorlin Syndrome: A Patient Treated With Oral Capecitabine. J Clin Oncol. 2011;29:E397–E401. doi: 10.1200/JCO.2010.33.3393. [DOI] [PubMed] [Google Scholar]
- 11.Bowater R, Abdelmalik S, Lilford R. Efficacy of adjuvant chemotherapy after surgery when considered over all cancer types: a synthesis of meta-analyses. Ann Surgl Oncol. 2012 doi: 10.1245/s10434-012-2388-1. [DOI] [PubMed] [Google Scholar]
- 12.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Delivery Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release in nanoscale drug carriers. Expert Opin Drug Deliv. 2010;7:479–495. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoener CA, Hutson HN, Fletcher GK, Peppas NA. Amphiphilic Interpenetrating Networks for the Delivery of Hydrophobic, Low Molecular Weight Therapeutic Agents. Ind Eng Chem Res. 2011;50:12556–12561. doi: 10.1021/ie201593h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Peppas NA. Synthesis and characterization of pH- and temperature-sensitive poly(methacrylic acid)/poly(N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules. 2000;33:102–107. [Google Scholar]
- 16.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 17.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical fooundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 18.Peppas NA, Wood KM, Blanchette JO. Hydrogels for oral delivery of therapeutic proteins. Expert Opin Biol Ther. 2004;4:881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 19.Gil ES, Hudson SM. Stimuli-reponsive polymers and their bioconjugates. Prog Polym Sci. 2004;29:1173–1222. [Google Scholar]
- 20.Marek SR, Conn CA, Peppas NA. Cationic nanogels based on diethylaminoethyl methacrylate. Polymer. 2010;51:1237–1243. doi: 10.1016/j.polymer.2010.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pool-Zobel BL, Van Loo J, Rowland I, Roberfroid M. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br J Nutr. 2002;87:S273–S281. doi: 10.1079/BJNBJN/2002548. [DOI] [PubMed] [Google Scholar]
- 23.Peppas NA, Kavimandan NJ. Nanoscale analysis of protein and peptide absorption: insulin absorption using complexation and pH-senstive hydrogels as delivery vehicles. Eur J Pharm Sci. 2006;29:183–197. doi: 10.1016/j.ejps.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Peppas NA, Kavimandan NJ. Confocal microscopic analysis of transport mechanisms of insulin across the cell monolayer. Int J Pharm. 2008;354:143–148. doi: 10.1016/j.ijpharm.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabizon AA, Catane R, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 26.Torres-Lugo M, Garcia M, Record R, Peppas NA. pH-Sensitive hydrogels as gastrointestinal tract absorption enhancers: transport mechanisms of salmon calcitonin and other model molecules using the Caco-2 model. Biotech Prog. 2002;18:612–616. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benival DM, Devarajan PV. Lipomer of doxorubicin hydrochloride for enhanced oral bioavailability. Int J Pharm. 2012;423:554–561. doi: 10.1016/j.ijpharm.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Ke W, Zhao Y, Huang R, Jiang C, Pei Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J Pharm Sci. 2007;97:2208–2216. doi: 10.1002/jps.21155. [DOI] [PubMed] [Google Scholar]
- 29.Yousefpour P, Atyabi F, Vasheghani-farahani E, Movahedi AM, Dinarvand R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anit-Her2 trastuzumab. Int J Nanomed. 2011;6:1977–1990. doi: 10.2147/IJN.S21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosio VE, Machain V, et al. Binding and encapsulation of doxorubicin on smart pectin hydrogels for oral delivery. Appl Biochem Biotechnol. 2012;167:1365–1376. doi: 10.1007/s12010-012-9641-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang WC, Uen YH, et al. Protective effect of guggulsterone against cardiomyocyte injury induced by doxorubicin in vitro. BMC Complem Alter Med. 2012;12:138. doi: 10.1186/1472-6882-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haemat. 2005;131:561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 33.Viswanatha SH, Wangikar U, Koti BC, AH T, Ronad PM, Manjula DV. Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Ind J Pharm. 2011;43:507–511. doi: 10.4103/0253-7613.84952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asensio-Lopez MC, Lax A, Pascual-Figal DA, Valdes M, Sanchez-Mas J. Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic Biol Med. 2011;51:1861–1871. doi: 10.1016/j.freeradbiomed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. Oral insulin delivery using P(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J Control Release. 2004;95:589–599. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Kavimandan NJ, Losi E, Peppas NA. Novel delivery system based on complexation hydrogels as delivery vehicles for insulin–transferrin conjugates. Biomaterials. 2006;27:3846–3854. doi: 10.1016/j.biomaterials.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Carr DA, Gomez-Burgaz M, Boudes MC, Peppas NA. Complexation Hydrogels for the Oral Delivery of Growth Hormone and Salmon Calcitonin. Ind Eng Chem Res. 2010;49:11991–11995. doi: 10.1021/ie1008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres-Lugo M, Peppas NA. Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules. 1999;32:6646–6651. [Google Scholar]
- 40.Morishita M, Kamei N, Chiba H, Kavimandan NJ, Peppas NA, Takayama K. Complexation hydrogels for intestinal delivery of interferon beta and calcitonin. J Control Release. 2009;134:98–102. doi: 10.1016/j.jconrel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanchette J, Park K, Peppas NA. Use of complexation hydrogels for oral administration of chemotherapeutic agents. Trans Soc Biomater. 2003;89:1606–1613. [Google Scholar]
- 42.Foss AC, Goto T, Morishita M, Peppas NA. Development of acrylic-based copolymers for oral insulin delivery. Eur J Pharm Biopharm. 2004;57:163–169. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 43.Lopez JE, Peppas NA. Effect of poly(ethylene glycol) molecular weight and microparticle size on oral insuline delivery from P(MAA-g-EG) microparticles. Drug Dev Ind Pharm. 2004;30:497–504. doi: 10.1081/ddc-120037480. [DOI] [PubMed] [Google Scholar]
- 44.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62:81–87. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 45.Owens DE, III, Jian Y, Fang JE, Slaughter BV, Chen YH, Peppas NA. Thermally-responsive swelling properties of polyacrylamide/poly(acrylic acid) interpenetrating polymer network nanoparticles. Macromolecules. 2007;40:7306–7310. [Google Scholar]
- 46.Fisher OZ, Kim T, Dietz SR, Peppas NA. Enhanced core hydrophobicity, functionalization and cell uptake of polybasic nanomatrices. Pharm Res. 2009;26:51–60. doi: 10.1007/s11095-008-9704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Schoener CA, Hutson HN, Peppas NA. pH-responsive hydrogels with dispersed hydrophobic nanoparticles for the delivery of hydrophobic therapeutic agents. Polymer Int. 2012;61:874–879. doi: 10.1002/pi.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varaprasad K, Mohan YM, et al. Hydrogel-silver nanoparticle composites: a new generation of microbials. J Appl Polym Sci. 2010;115:1199–1207. [Google Scholar]
- 50.Yamagata T, Morishita M, et al. Characterization of insulin protection properties of complexation hydrogels in gastric and intestinal enzyme fluids. J Control Release. 2006;112:343–349. doi: 10.1016/j.jconrel.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Blanchette J, Peppas NA. Oral chemotherapeutic delivery: Design and cellular response. Ann Biomed Eng. 2005;33:142–149. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- 52.Torres-Lugo M, García M, Record R, Peppas NA. Physicochemical behavior and cytotoxic effects of p(methacrylic acid–g-ethylene glycol) nanospheres for oral delivery of proteins. J Control Release. 2002;80:197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 53.Foss AC, Peppas NA. Investigation of the cytotoxicity and insuline transport of acrylic-based copolymer protein delivery systems in contact with Caco-2 cultures. Eur J Pharm Biopharm. 2004;57:447–455. doi: 10.1016/j.ejpb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Foss AC, Peppas NA. Investigation of the cytotoxicity and insulin transport of acrylic-based copolymer protein delivery systems in contact with caco-2 cultures. Eur J Pharm Biopharm. 2004;57:447–455. doi: 10.1016/j.ejpb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Sahlin JJ, Peppas NA. Enhanced hydrogel adhesion by polymer interdiffusion: Use of linear poly(ethylene glycol) as an adhesion promoter. J Biomater Sci-Polym Ed. 1997;8:421–436. doi: 10.1163/156856297x00362. [DOI] [PubMed] [Google Scholar]
- 56.Jabbari E, Wisniewski N, Peppas NA. Evidence of mucoadhesion by chain interpenetration at a poly(acrylic acid) mucin interface using ATR-FTIR spectroscopy. J Control Release. 1993;26:99–108. [Google Scholar]
- 57.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical, foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 58.Morishita M, Goto T, Takayama K, Peppas NA. Oral insulin delivery systems based on complexation polymer hydrogels. J Drug Del Sci Technol. 2006;16:19–24. doi: 10.1016/j.jconrel.2005.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morishita M, Goto T, Nakamura K, Lowman AM, Takayama K, Peppas NA. Novel oral insulin delivery systems based on complexation polymer hydrogels: Single and multiple administration studies in type 1 and 2 diabetic rats. J Control Release. 2006;110:587–594. doi: 10.1016/j.jconrel.2005.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goto T, Morishita M, Kavimandan N, Takayama K, Peppas NA. Gastrointestinal transit and mucoadhesive characteristics of complexation hydrogels in rats. J Pharm Sci. 2006;95:462–469. doi: 10.1002/jps.20566. [DOI] [PubMed] [Google Scholar]
- 61.Serra L, Domenech J, Peppas NA. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur J Pharm Biopharm. 2009;71:519–528. doi: 10.1016/j.ejpb.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlstedt I, Sheehan JK, AP C, Gallagher JT. Mucus glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]