Abstract
Adult-born neurons in crayfish (Procambarus clarkii) are the progeny of 1st-generation precursor cells (functionally analogous to neuronal stem cells in vertebrates) that are located in a neurogenic niche on the ventral surface of the brain. The daughters of these precursor cells migrate along the processes of bipolar niche cells to proliferation zones in the cell clusters where the somata of the olfactory interneurons reside. Here they divide again, producing offspring that differentiate into olfactory local and projection neurons. The features of this neuronal assembly line, and the fact that it continues to function when the brain is isolated and perfused or maintained in organotypic culture, provide opportunities unavailable in other organisms to explore the sequence of cellular and molecular events leading to the production of new neurons in adult brains. Further, we have determined that the 1st-generation precursor cells are not a self-renewing population, and that the niche is, nevertheless, not depleted as the animals grow and age. We conclude, therefore, that the niche is not a closed system and that there must be an extrinsic source of neuronal stem cells. Based on in vitro studies demonstrating that cells extracted from the hemolymph are attracted to the niche, as well as the intimate relationship between the niche and vasculature, we hypothesize that the hematopoietic system is a likely source of these cells.
Keywords: hematopoietic system, hemocytes, neurogenic niche, olfactory pathway, serotonin, stem cell
Introduction
According to current understanding, stem cells by definition are “capable of dividing and renewing themselves for long periods” in vivo, although adult stem cells are not capable of long-term self-renewal in vitro, as are embryonic stem cells (National Institutes of Health, http://stemcells.nih.gov/info/basics/). A second fundamental tenet is that adult stem cells in vivo “generate the cell types of the tissue in which they reside. For example, a blood-forming adult stem cell in the bone marrow normally gives rise to …blood cells. …..a hematopoietic stem cell…cannot give rise to the cells of a very different tissue, such as nerve cells in the brain.” Our studies concerning the lineage of precursor cells that generates neurons in the adult crayfish brain challenge both of these principles. In this paper, we review what is currently known about the precursor cells underlying adult neurogenesis in the crayfish brain. These findings are discussed in relation to studies of bone marrow stromal (i.e., mesenchymal) stem cells and the generation of new neurons in adult mammalian brains.
1.1 Adult neurogenesis in the crayfish brain
Our studies focus on life-long neurogenesis among interneuronal populations in the olfactory pathway of the crustacean brain (Fig. 1A; Schmidt, 1997; Harzsch et al., 1999; Schmidt and Harzsch, 1999). The sensory, local and projection neurons of the crustacean midbrain are functionally analogous to groups of neurons in the vertebrate olfactory system that have has a similar capacity for life-long neurogenesis (Lois and Alvarez-Buylla, 1994; Hildebrand and Shepherd, 1997).
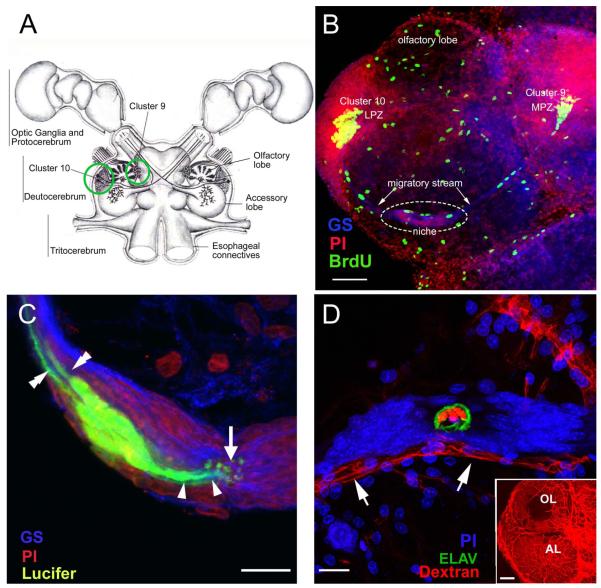
Figure 1.
(A) Diagram of the eureptantian (crayfish, lobster) brain including the optic ganglia, and showing the locations of the proto-, trito- and deutocerebral neuropils. The soma clusters 9 and 10 (circles), locations of neurogenesis in the adult brain, flank two prominent neuropil regions of the deutocerebrum, the olfactory and accessory lobes. (Names of brain areas are according to Sandeman et al., 1992.) (B) Left side of the brain of Procambarus clarkii labeled immunocytochemically for the S-phase marker BrdU (green). Labeled cells are found in the lateral proliferation zone (LPZ) contiguous with Cluster 10 and in the medial proliferation zone (MPZ) near Cluster 9. The two zones are linked by a chain of cells in the migratory stream, labeled immunocytochemically for glutamine synthetase (GS; blue). These streams originate in the oval region ‘niche’ (dotted circle) containing cells labeled with the nuclear marker propidium iodide (PI, red). (C) Several niche cells are labeled by intracellular injection of Lucifer yellow. Each of these has a short process (arrowheads) projecting to the vascular cavity (arrow) and longer fibers (double arrowheads) that fasciculate to form the tracts projecting to the LPZ and MPZ, along which the daughters of the niche cells (2nd-generation neuronal precursors) migrate (the ‘streams’). Blue, GS; red, propidium iodide (PI). (D) The vascular connection of the cavity in the center of the glial soma cluster was demonstrated by injecting a dextran dye into the dorsal artery. The cavity, outlined in green by its reactivity to an antibody to ELAV, contains the dextran dye (red), which is also contained within a larger blood vessel that runs along beneath the niche. PI (blue) labeling of nuclei in the niche cells is also shown. Inset: dextran-filled vasculature in the olfactory (OL) and accessory (AL) lobes on the left side of the brain. Scale bars: B, 100 μm; C and D, 20 μm; inset in D, 100 μm. [C and D from Sullivan et al., 2007a].
The crustacean olfactory system consists of sensory neurons that synapse on local and projection interneurons within the glomeruli of the olfactory lobes (OL), which are involved in the primary processing of olfactory information. The cell bodies of olfactory interneurons are clustered in functional groups: the local interneurons located medial to the OL in cell clusters 9 and 11, and the projection neurons lateral to the OL in Cluster 10 (Fig. 1A; terminology of Sandeman et al., 1992). Cluster 9 interneurons innervate both the OL and accessory lobe (AL); Cluster 10 projection neurons innervate either the OL or AL (Sullivan et al., 2000), and their axons project via the olfactory globular tract (OGT) to neuropil regions in the lateral protocerebrum (Sullivan and Beltz, 2001). The AL is involved in higher-order integration of olfactory, visual and mechanosensory information (Sandeman et al., 1995; Sullivan and Beltz, 2005).
Neuronal proliferation in most regions of the decapod brain ceases in the period around hatching when the embryonic precursor cells (neuroblasts) disappear (Beltz and Sandeman, 2003). The exception to this is in the central olfactory pathway where mitotic activity continues throughout life (Harzsch and Dawirs, 1996; Schmidt, 1997; Schmidt and Harzsch, 1999; Harzsch et al., 1999). Adult neurogenesis also occurs in the visual pathway (Sullivan and Beltz, 2005), but has been studied in much less detail. In the olfactory pathway, life-long neurogenesis is found among the sensory (Steullet et al., 2000), local (Cluster 9) and projection (Cluster 10) neurons (Fig. 1A, B). Until our discovery of the 1st-generation neuronal precursor cells (functionally analogous to mammalian neuronal stem cells) in a neurogenic niche located on the ventral surface of the brain in crayfish (Fig. 1B-D) (Sullivan et al., 2005; 2007a), the source of these adult-born neurons had not been identified.
1.2 Mechanisms of proliferation of adult-born neurons in the crayfish brain
Adult neurogenesis occurs in the brains of a phylogenetically diverse array of animals. In the higher (amniotic) vertebrates, the precursor cells are glial cells that reside within specialized regions, known as neurogenic niches, the elements of which both support and regulate neurogenesis (Garcia-Verdugo et al., 2002; Doetsch, 2003). The in vivo identity of the precursor cells responsible for adult neurogenesis in crayfish was revealed using cell cycle and glial markers. We have demonstrated that the 1st-generation precursor cells in crayfish reside within a specialized niche containing a vascular cavity (Fig.1C, D), located on the ventral surface of the brain (Sullivan et al., 2005; 2007a). The progeny of these 1st-generation cells migrate from the niche along fibers of the bipolar niche cells, to the lateral (LPZ) and medial (MPZ) proliferation zones in cell clusters 9 and 10. Here they divide at least once more, and their descendants differentiate into neurons (Sullivan and Beltz, 2005). Anatomical differentiation has been confirmed using fluorescently-labeled dextran to backfill cells in clusters 9 and 10 from their terminals in the AL, in animals that were previously labeled with BrdU (Fig. 2A); double labeling with both BrdU and dextran identified neurons born during the BrdU labeling period that had developed processes in the AL (Fig. 2B). Chemical differentiation was confirmed by exposing crayfish to BrdU followed by several months in pond water, after which brains were labeled immunocytochemically for the transmitters expressed by mature Cluster 9 and Cluster 10 neurons (e.g., crustacean SIFamide; Fig. 2C) (Sullivan et al., 2007a).
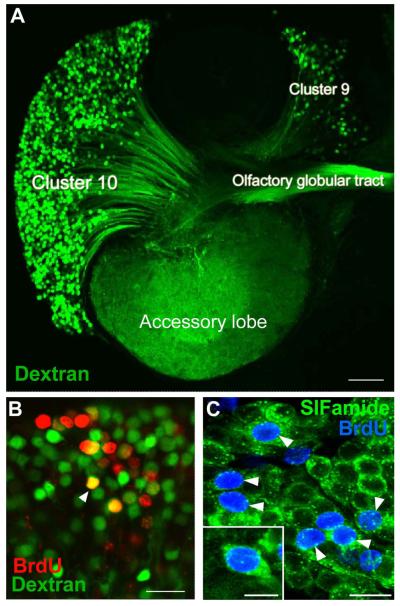
Figure 2.
A. The left side of a brain of P. clarkii in which dextran was applied to the accessory lobe using the technique of Utting et al. (2000). The dextran (green) enters neurons that have their terminals in the accessory lobe and labels the corresponding cell bodies and axons. From this it is clear that both projection neurons in Cluster 10, and local interneurons in Cluster 9, have their terminals in the accessory lobes and the axons from the projection neurons lie in the olfactory globular tract. B. Cluster 10 cell bodies from an animal that was exposed to BrdU for 12 days and sacrificed 4 months later, at which time dextran fluorescein 3000 MW was applied to the accessory lobe. Cells labeled red indicate that they passed through the cell cycle in the presence of BrdU. Cells labeled green indicate that they have terminals in the accessory lobe but did not pass through a cell cycle in the presence of BrdU. Double-labeled cells (orange) are cells that passed through a cell cycle in the presence of BrdU and have differentiated into neurons with their terminals in the accessory lobe. C. Cluster 10 cell bodies with BrdU (blue) and crustacean-SIFamide (green) labeled six months after being exposed to BrdU. Arrowheads point to double-labeled cells, green (cytoplasm) and blue (nuclei). Crustacean-SIFamide immunoreactivity is known to be expressed in olfactory interneurons in P. clarkii (Yasuda-Kamatani and Yasuda, 2006) and the presence of double labeling indicates that these cells were born in the adult animal and have differentiated into olfactory interneurons. Scale bars: A, 10 μm; B, C, 20 μm; C insert, 10 μm. [From Sullivan et al., 2007b; based on the experiments published in Sullivan and Beltz, 2005]
A study of the dynamics of the neurogenic niche, migratory streams and proliferation zones showed that the neuronal precursor cell generations are spatially separated, allowing studies on specific parts of the lineage (see model, Fig. 3) (Sullivan et al., 2007b). A repeating theme is the involvement of serotonin in adult neurogenesis in crayfish: from cell cycle regulation among specific precursor cell generations (Zhang et al., 2011), to the presence of serotonin in the rim of the vascular cavity and its role in the attraction of cells to the niche (Benton et al., 2011). These features of the crustacean system producing adult-born neurons and the fact that this apparatus continues to function in vitro (Benton et al., 2008; 2011) provide opportunities unavailable in other organisms to explore the sequence of cellular and molecular events leading to the production of new neurons in adult brains.
Figure 3.
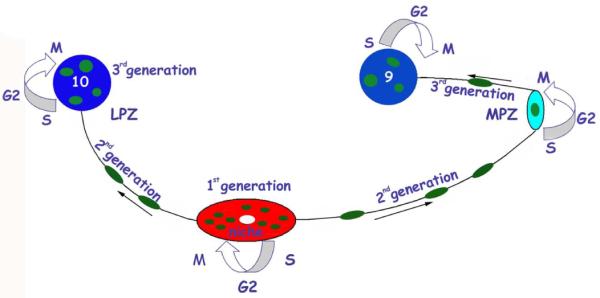
A model summarizing the events leading to the production of new olfactory interneurons in adult crayfish. Neuronal precursor (1st generation) cells reside within a neurogenic niche where they divide symmetrically. Their daughters (2nd generation precursors) migrate along tracts created by the fibers of the niche cells, towards either the LPZ or the MPZ. At least one more division will occur in the LPZ and MPZ before the progeny (3rd and subsequent generations) differentiate into neurons. [From Beltz et al., 2011]
This system has many features in common with the process of adult neurogenesis in vertebrate organisms, including the association of the 1st-generation neuronal precursors with a niche that has a close association with the brain vasculature, directed migration of neuronal precursors and specialized basal laminae. Aspects of the cellular machinery maintaining adult neurogenesis appears, therefore, to be shared by widely disparate taxa, suggesting a common strategy for the generation of new neurons in adult brains (Sullivan et al., 2007a). Our work also suggests, however, a novel hypotheses related to the function of the neurogenic niche and the identity of the 1st-generation neuronal precursor cells.
1.3 The 1st-generation neuronal precursors in the niche are not self-renewing
In the mammalian brain the 1st-generation neuronal precursors residing in neurogenic niches are reported to undergo self-renewing divisions, thereby providing a source of new neurons throughout life (Zhao et al., 2008). In contrast, the 1st-generation neuronal precursors in the crayfish niche undergo geometrically symmetrical divisions and it appeared that both daughters migrate away to the two proliferation zones where further divisions occur (Fig. 4); this was first suggested by the observation that BrdU-labeled (S-phase) cells are frequently encountered in the margins of the niche near the emergence of the streams, and these are often in pairs (Zhang et al., 2009). The dividing cells label immunocytochemically for glutamine synthetase (GS), a marker of the niche cells, thus indicating that they are descendants of GS-labeled cells residing in the niche. However, in spite of this continuous efflux of BrdU-labeled cells from the niche, the neuronal precursor cells in crayfish are not depleted. In fact, the total number of niche cells continues to expand as the animals age (Zhang et al., 2009), although it is not known what proportion of these cells is competent to become neuronal precursors. We proposed, therefore, that (1) primary neuronal precursor cells in the crayfish niche are not self-renewing, and (2) a source extrinsic to the niche provides cells that replenish the niche precursor pool (Beltz et al., 2011; Benton et al., 2011). This proposal is in contrast with the view that large mitotic cells in the crayfish niche (putative neuroblasts) persist beyond embryonic life, providing a self-renewing source of neurons (Song et al., 2009; Schmidt and Derby, 2011). To resolve this issue, we directly tested the self-renewal capacity of niche precursor cells. We also examined the development of the niche, to ask whether the emergence of the niche is associated with embryonic neuroblasts.
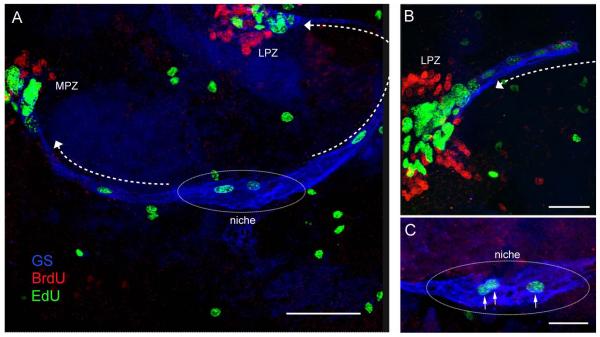
Figure 4.
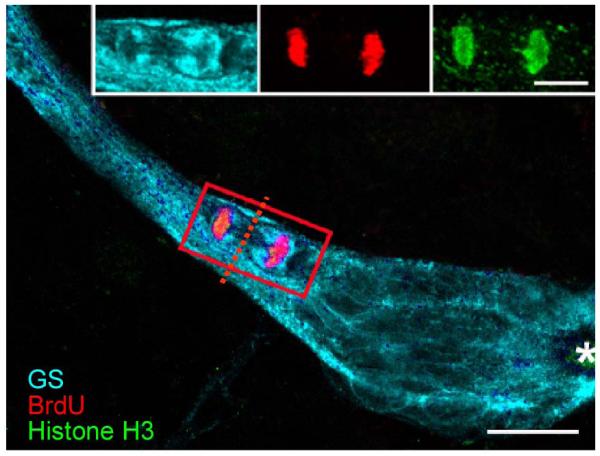
Triple-labeled M-phase cell near the emergence of the streams immunolabeled for glutamine synthetase (cyan), phosphohistone-H3 (green) and BrdU (red). The cleavage planes of niche cells are always oriented perpendicular to the track of the stream. Cytoplasmic labeling of the dividing cell for GS confirms that GS-labeled niche cells are the precursors of the neuronal lineage. Outlines of other niche cells also are GS-labeled. Asterisk marks vascular cavity. Scale bar 20 μm. [From Zhang et al., 2009]
First, the fate of 1st- and 2nd-generation neuronal precursors in the crayfish brain was tracked using sequential double-nucleoside labeling of the niche cells. Brains were labeled with 5-bromo-2′-deoxyuridine (BrdU), followed by 3.5-7 days in pond water without BrdU prior to incubation in 5-ethynyl-2′-deoxyuridine (EdU). Immunocytochemical labeling was used to distinguish BrdU and EdU. The results were decisive: (1) BrdU labeling is not retained in the niche but instead is found only in the 2nd-generation cells in the streams and 3rd generation cells in Clusters 9 and 10. (2) Proliferating cells in the niche are labeled with only the second nucleoside, EdU (Fig. 5). The interpretation of these data is that all of the initially-labeled BrdU cells in the niche migrated away, leaving no BrdU-labeled daughter behind (i.e., no self-renewal). Rapid cycling of niche precursors, and hence the dilution and extinction of the BrdU label in cells possibly remaining in the niche, cannot explain this result, because we know that 5-7 days (dependent on animal size) are required for cells to traverse the streams (Sullivan et al., 2007b; Benton et al., 2011) and there are no more than 12 cells in the streams at any given time. These numbers indicate, because the streams are the only egress from the niche, that the 1st-generation precursors cycle relatively slowly (roughly once every 48 hrs). We also tested the size of the labeled niche cell pool following increasing incubation times in BrdU (6 hrs-10 days); if divisions are self-renewing (as in embryos), one might expect increasing numbers of BrdU-labeled cells in the niche with increasing incubation times, provided that the speed of migration is relatively slow (as indicated by our double-nucleoside labeling studies). However, no more than 2-4 labeled cells were found in the niche regardless of BrdU incubation time (Benton et al., 2011).
Figure 5.
Double-nucleoside labeling of the niche, streams and proliferation zones in the crayfish brain. Crayfish were incubated in BrdU (red) for 6 hrs and then maintained in fresh pond water for 7 days. Just before sacrifice, they were treated with EdU (green) for 6 hrs. Fixed brains were labeled for glutamine synthetase (blue) to reveal the niche and streams. (A) Only EdU labels S phase cells in the niche and streams, demonstrating that migration is uni-directional (away from the niche [in the direction of the arrows]) and that the earlier-labeled BrdU cells do not remain in the niche, suggesting that niche cell divisions are not self-renewing. (B) Higher magnification image of the LPZ. BrdU-labeled cells (labeled first in the sequence) are found only in the proliferation zones (MPZ, LPZ) and not in the niche or streams. Arrow indicates direction of migration. (C) Higher magnification image of a neurogenic niche reveals that cells in S-phase labeled with EdU also labeled for glutamine synthatase (arrows), as do the other cells residing in the niche. Scale bars: A, 100 μm; B, 50 μm. C, 25 μm.
Our results therefore provide no evidence of self-renewal of the niche precursor cells, and indicate instead that the niche is not a closed system because the niche precursors are not depleted throughout the relatively long life of the crayfish (Benton et al., 2011). We conclude that the pool of neuronal precursor cells in the niche must be replenished from an extrinsic source as those in the niche divide and migrate away.
1.4 Cells circulating in the hemolymph are attracted to the niche in vitro
Given the extensive vascularization of the brain and the close relationship of the niche and vascular system via the vascular cavity, we assumed that cells recruited to the niche would at first be present in the hemolymph. We therefore chose blood cells (hemocytes) and cells from three different tissue types as controls, namely the green gland, hepatopancreas and hematopoietic tissue (Benton et al., 2011). All four cell types were isolated from their respective tissues and labeled with the fluorescent marker CellTracker™ Green CMFDA (CTG; Invitrogen). The labeled cell types were then introduced into separate culture dishes containing freshly dissected, desheathed crayfish brains, followed by a 6-hr incubation period at 18°C. The distribution of labeled cells in each culture dish was subsequently visualized to determine whether cells showed any affinity for the brains and/or associated niches. Of the cell types tested, all except the hemocytes remained evenly distributed in the culture dishes and showed little (less than 10%) or no attraction to the brains or niches. However, the hemocytes showed a remarkable affinity for the niche. In 77% of niche-hemocyte co-cultures, CTG-labeled hemocytes were found in the vascular cavity or among the precursor cells in the niches (Fig. 6); some of these cells also co-labeled for glutamine synthetase, a marker of the 1st generation niche precursor cells. Serotonergic mechanisms appear to be involved in this attraction, as serotonin or agents that interfere with serotonergic mechanisms (e.g., the P. clarkii 5-HT2β-specific antagonist methiothepin mesylate salt [MMS]) alter the behavior of the hemocytes, significantly reducing their affinity for the niche (Benton et al., 2011).
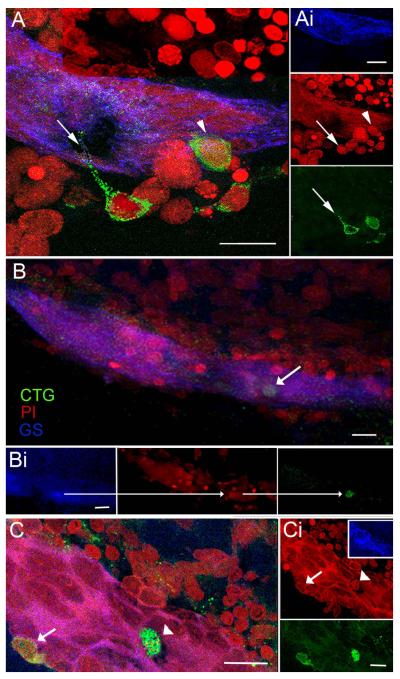
Figure 6.
Cells circulating in the hemolymph are attracted to the niche in vitro. CellTracker™ Green (CTG)-labeled hemocytes are found in the vascular cavity and in, and on, the neurogenic niche. (A) Niche on a desheathed brain co-cultured with CTG-labeled cells extracted from the hemolymph: merged confocal fluorescent channels of stacked images. Several CTG-labeled cells reside just outside the niche, one with a long process extending into the niche (arrow), and another that is inserting on the outer margin of the niche (arrowhead). (Ai) Separate channels: GS outlining the niche (top); PI revealing cell nuclei, arrow/arrowhead pointing to the respective CTG-labeled cells in A (middle); and CTG-labeled cells with arrow pointing to the fine process from the CTG-labeled cell (bottom). (B) Projection of stacked images from a more dorsal region of the neurogenic niche than in (A) reveals a CTG-labeled cell just below the surface of the niche. (Bi) Separate confocal channels of the region in (B) with arrows pointing to the same CTG-filled cell also labeled for GS (left); PI, revealing cell nucleus (middle); and CTG-labeling (right). (C) In another example, a CTG-labeled cell (arrowhead) resides in the cavity and a second CTG-labeled cell (arrow) is embedded in the outer edge of the niche. (Ci) PI labeling of cell nuclei with arrowhead and arrow pointing to the corresponding nuclei with CTG-labeling in C. Insert, GS labeling of the niche. Bottom, separate channel, CTG-labeled cells. Scale bars: A, 20 μm; Ai, 10 μm; B, Bi, C and Ci, 20 μm.
1.5 Neurovascular relationships: developmental and morphological studies
The niche and streams in P. clarkii lie on a blood vessel and the vascular cavity is confluent with the circulation, a feature that has been demonstrated by injection of dye-conjugated dextran into the pericardial sinus (Benton et al., 2011) or into the dorsal artery that vascularizes the brain (Sullivan et al., 2007a). In addition, the development of the neurogenic niche demonstrates that from the beginning, the niche maintains a close association with the vasculature. Fluorescently-labeled dextran was micro-injected into the dorsal sinus in P. clarkii embryos just prior to and at hatching, the period when the anlagen of the niche first appears (Sintoni et al., 2012). The protoniche, which is revealed by immunocytochemical labeling for tyrosinated tubulin, is visualized as a tuft of fine fibers surrounding a central cavity (Fig. 7A) that is intertwined with dextran-labeled vascular elements (Fig. 7B). A series of timed studies suggests that the protoniche is associated with the deutocerebral vasculature in a region that seems to be undergoing angiogenesis (Sintoni et al., 2012). These events occur in parallel with the appearance of the deutocerebral proliferative system, a transverse band of mitotically active cells that during the molt to the second post-embryonic stage (POII) differentiates into the MPZ and LPZ in cell clusters 9 and 10, respectively. While these proliferation zones are highly mitotically active from the beginning of post-embryonic life, the developing niche contains only very few dividing cells, a characteristic that persists in the adult organism. Our data suggest that the MPZ and LPZ are primarily responsible for the production of new neurons in the early post-embryonic stages, and that the neurogenic niche plays a subordinate role. However, as the neuroblasts in the proliferation zones disappear during early post-embryonic life, the neuronal precursors in the niche gradually become the dominant and only mechanism for generation of new neurons in the adult brain (Sintoni et al., 2012).
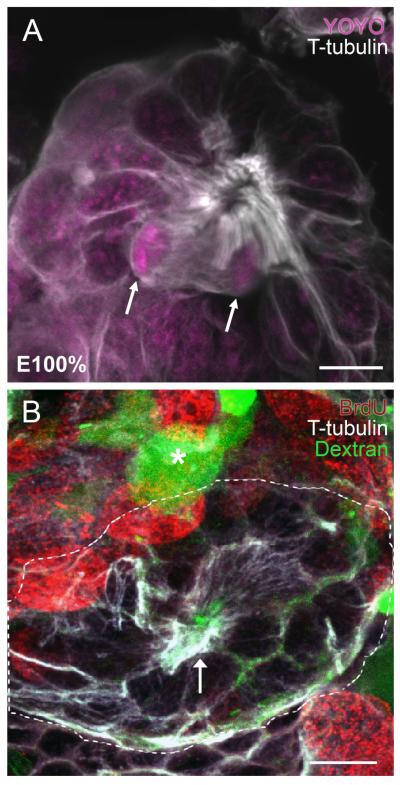
Figure 7.
(A) The protoniche at hatching (embryonic stage 100%; E100%) in the marbled crayfish, Procambarus fallax, labeled immunocytochemically for tyrosinated tubulin (white) and the nuclear stain YOYO (magenta). Dividing cells in the protoniche (arrows) are close to a central fibrous area that demarcates the emerging vascular cavity. (B) P. clarkii embryos at E95-100% and postembryonic stage 1 (POII) were micro-injected into the dorsal sinus with fluorescently-labeled dextran (green), which fills the brain vasculature (for technical details see Sintoni et al., 2012). Embryos were subsequently labeled immunocytochemically for tyrosinated tubulin (white), which delineates the protoniche (outlined with a broken white line, with an arrow pointing to the central pore). Dextran-filled fine vascular elements (green) run throughout the protoniche. A fine capillary with an expanded blind ending (lacuna; Sandeman, 1967) near the protoniche is noted with an asterisk. BrdU (red) labels cells associated with the deutocerebral proliferative system. Scale bars: A, 10 μm; B, 20 μm. [A from Sintoni et al., 2012]
Morphological studies confirm that the close relationship between the niche and vasculature persists in adult crayfish. Semi-thin sections demonstrate that the niche in the mature brain is nearly encompassed by blood vessels that are embedded in connective tissues (Fig. 8A), suggesting a retia-like complex of fine channels between the niche and the vasculature emerging from the underlying accessory lobe (Chaves da Silva et al., 2012). Sagittal sections through the brain show what appears to be a direct connection between a blood vessel and the most dorsal layers of the niche that adhere to the accessory lobe (Fig. 8B, C). Further, fluorescently-labeled dextran injected into the dorsal sinus reveals a network of fine blood vessels that approach from the ventral surface to infiltrate the niche (Fig. 8D); it is intriguing that the blood vessels adjacent to and contacting the niche are immunoreactive for glutamine synthetase, as are the niche cells. These vessels are only seen when the sheath covering the ventral surface of the brain is left intact, suggesting that these vessels adhere to this connective tissue layer. In our standard immunocytochemical studies, the brain sheath is completely removed in order to facilitate antibody penetration. These sheath-intact niche preparations therefore have revealed additional connections with the vasculature that were not previously recognized.
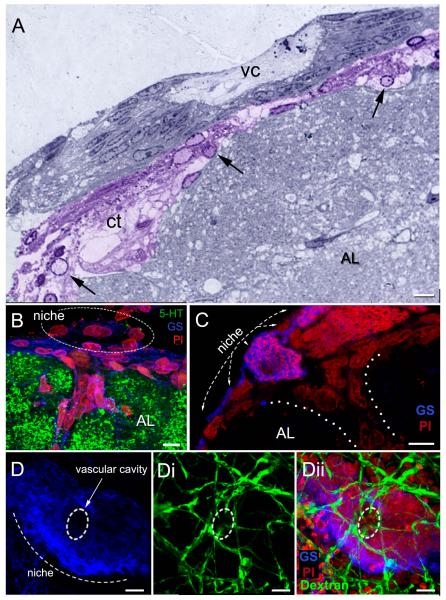
Figure 8.
The relationship between the neurogenic niche and vascular tissues. (A) Semi-thin section stained with toluidine blue, showing the niche and central vascular cavity (vc). The connective tissue (ct) below the niche (colorized purple) has many cells (arrows) with features resembling hemocytes. (B, C) Sagittal sections through the brain. These images show the niche lying on the ventral surface of the accessory lobe (AL), which is labeled immunocytochemically for serotonin (5-HT; green) in B. A blood vessel (dotted lines in C) emerges from the accessory lobe, Some cells within the blood vessel and others forming a layer between the niche and accessory lobe (B) are immunoreactive for glutamine synthetase, as are the niche cells. (D) The dorsal sinus in adult crayfish (15-20 carapace length) was injected with fluorescently-labeled dextran, which rapidly filled the brain vasculature. Fine blood vessels (dextran, green) associated with the niche are revealed on the ventral surface of the niche (Di, Dii), with some of these infiltrating the edge of the vascular cavity (broken line circle). Scale bars: A and C,10 μm; B, 6 μm; D, 20 μm. [A from Chaves da Silva et al., 2012]
The developmental emergence of the niche in concert with vascularization in the deutocerebrum (Fig. 7, from Sintoni et al., 2012), presence of the vascular cavity that is confluent with the circulation (Fig. 1D, from Sullivan et al., 2007a; see also Benton et al., 2011), and direct connections between the vasculature and the niche dorsally (Fig. 8A-C; Chaves da Silva et al., 2012) and ventrally (Fig. 8D) are consistent with the possibility that a stem cell circulating in the hemolymph could readily gain access to the niche.
1.6 Atypical neuronal stem cells: hypotheses and future directions
Our experiments on the lineage of cells producing adult-born neurons in the crayfish clearly show that the 1st-generation neuronal precursors, which are functionally analogous to neuronal stem cells in vertebrates, are not self-renewing. Nevertheless, the niche is not depleted and adult neurogenesis continues throughout the animal’s lifetime. The corollary of these findings is that the niche is not a closed system and that pre-neuronal stem cells originating from a site extrinsic to the niche must replenish the neuronal stem cells as they divide and migrate away. Therefore, in the crayfish, the contribution of stem cells from a source external to the niche is clear, although the identification of that source is still pending. Our in vitro experiments testing the attraction between the niche and different cell types provide evidence that cells recruited to the niche may be of hematopoietic origin. The close anatomical relationship between the vascular system and the niche provides an avenue by which cells circulating in the hemolymph could approach and find their way into the niche. The next step is to bring these ideas together with experiments testing the behaviour of specific cell types and their competence to become neuronal precursors in an in vivo, whole organism situation.
Of particular interest for our future studies is the work of Noonin et al. (2012) in the crayfish Pacifasticus leniusculus. These investigators have identified a specialized region of the hematopoietic system that is located near the brain and which they propose constitutes a stem cell center. This anterior proliferation center (APC) is different in several ways from the rest of the hematopoietic tissue (HPT), which is located in a layer just beneath the dorsal carapace on both sides of the dorsal artery (the posterior HPT) and extends laterally and anteriorly towards the brain (anterior HPT). The APC is a distinct tissue located between the anterior HPT and the brain. The highest rate of BrdU incorporation was found in the most anterior part of the HPT and the APC. In addition, the majority of cells in the APC contain nuclei with loose euchromatin, whereas most cells in the dorsal HPT contain cells with condensed heterochromatin. It is suggested that this difference in chromatin structure may be related to the degree of differentiation of the cells, with DNA becoming more condensed as the cells differentiate (Meshorer and Misteli, 2006; Noonin et al., 2012). Further, reactive oxygen species (ROS), which induce the differentiation of hematopoietic stem cells in Drosophila (Owusu-Ansah and Banerjee, 2009) and in the mammalian myeloid lineage (Ito et al., 2006), are produced in high levels only in the most anterior part of the HPT, between the APC and the brain. ROS labeling increased throughout the APC at 30 minutes following laminarin injection into crayfish, a treatment that mimics fungal infection. This timing corresponds with the decrease in circulating hemocytes and subsequent recruitment of new hemocytes from the HPT associated with laminarin treatment, suggesting that an increase in metabolic activity may be related to the differentiation of cells in the APC. Based on these and additional data, Noonin et al. propose that cells in the APC are the multipotent stem cells of the crustacean HPT. Because of their properties and their location, these cells are of special interest in our efforts to identify the source of neuronal precursors in the niche, and tests utilizing these cells are currently underway. Implicit in the model proposed here (Fig. 9) is that the neurogenic niche would provide instructive cues directing the transformation of multipotent stem cells into 1st-generation neuronal precursors, rather than nurturing long-lived stem cells. Indeed, the neuronal stem cells residing in the crayfish niche appear to be a highly transient cell type, rather than the long-lived neuronal stem cells described in mammalian neurogenic regions. Second, the provision of multipotent stem cells, potentially from a hematopoietic source, implies that cells of a non-ectodermal origin may contribute to building neural structures throughout life, although the developmental origin of hematopoietic tissues in crayfish and related decapod crustaceans is not known.
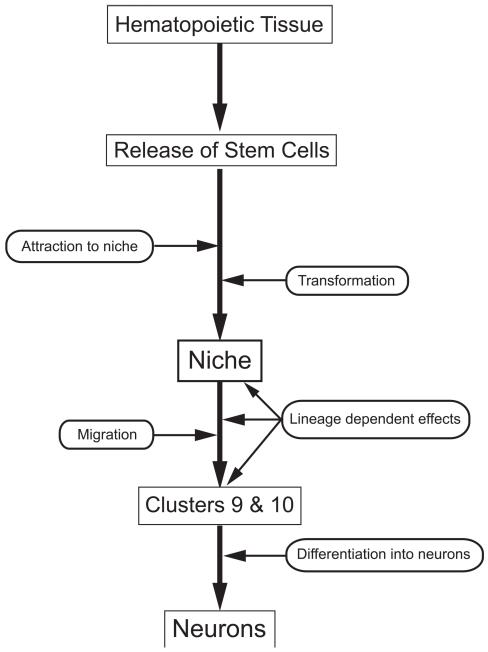
Figure 9.
Our current model of the sequence of events involved in the production of adult born neurons, beginning with hematopoietic tissue, the release of stem cells, their attraction to the niche and transformation into 1st-generation neuronal precursor cells. These aspects of the model are hypothetical. These precursor cells in the niche label for glutamine synthetase and produce daughters that migrate along processes of the niche cells towards Cluster 9 or 10. As these cells migrate, lineage-dependent changes in their physiological status are apparent, for example that they begin to express specific serotonin receptor subtypes as they approach the proliferation zones (Zhang et al., 2011). These 2nd-generation precursors divide at least once more in the proliferation zones in Clusters 9 and 10, and then differentiate into neurons (Sullivan and Beltz, 2005). These aspects of the model are supported by published data (Sullivan et al., 2007a, b; Beltz et al., 2011).
There are, therefore, distinctions between the 1st-generation neuronal precursors in the crayfish brain and neuronal stem cells in mammals, although these cell types appear to be functionally equivalent in terms of their position in the precursor cell lineage. We have been careful in recent publications to refer to cells in the crayfish lineage according to their generation, to avoid the confusion that these cells do not self-renew, as do true “stem cells”. But, is this a semantic issue or is there also a mechanistic problem? If the 1st-generation neuronal precursors in the crayfish niche are provided from an extrinsic source, for example the APC that is part of the hematopoietic system, then these are presumably multipotent stem cells that would become biased towards a neural fate while interacting with the niche cells. Are there, then, no committed neuronal stem cells in the adult crayfish brain-----only multipotent stem cells that become 1st-generation neuronal precursors? The resolution to this conundrum—whether semantic or mechanistic---awaits the results of current experiments tracking the fate of APC cells.
1.7 Mesenchymal stem cells in mammals
The proposal that stem cells derived from a non-neural source are transformed, rather than sustained, by a neurogenic niche are features consistent with current findings in the crayfish adult neurogenic system, but that contradict the basic tenets proposed for stem cells cited in the introduction to this paper. In this regard, there are tantalizing studies demonstrating that mesenchymal stem cells (MSCs) in rodents and humans are capable of differentiating not only into a variety of mesodermal cell types, but also into neurons both in vitro (Sanches-Ramos et al., 2000; Woodbury et al., 2000; see also review in Chen et al., 2006) and in vivo (Mezey and Chandross, 2000; Li et al., 2002; Mezey et al., 2003; Cogle et al., 2004; Munoz-Elias et al., 2004). A great deal of work has been done with MSCs derived from bone marrow precisely because these cells have been shown to transdifferentiate into a number of different cell types and therefore hold great promise for regenerative medicine. Indeed, MSC transplantation in animal models of neurological damage and degeneration often results in dramatic functional improvement. However, the rate of MSC transdifferentiation is very low, raising doubts about whether transdifferentiation can account for the observed recovery. Further, the differentiation of MSCs in vitro is rapid, on a scale of hours, causing concern about whether the neural derivatives are authentic or an artificial response to the culture conditions. In addition, MSCs spontaneously express neural proteins (Tondreau et al., 2004; Deng et al., 2006; Lamoury et al., 2006; Blondheim et al., 2006), provoking questions about whether transdifferentiation in vitro is genuine or rather a default mode.
Because of these doubts regarding the contribution of MSC transdifferentiation to functional neurological recovery, two other attributes of MSCs have received significant attention (see review in Maltman et al., 2011): cell fusion and trophic effects. The ability of MSCs to spontaneously fuse with neural cells, and with hybrid cells adopting the traits of the recipient cell (Terada et al., 2002), has been used to explain the apparent transdifferentiation phenomena. However, when bone marrow is transplanted into human brains, human hematopoietic cells can transdifferentiate into neurons in vivo and, further, cell fusion events have been ruled out (Mezey et al., 2003; Cogle et al., 2004).
Perhaps least controversial among these theories regarding the role of MSCs in functional recovery from neurological damage, is the idea that MSCs exert trophic influences on the nervous system. It is well known that MSCs secrete a variety of neurotrophins, growth factors, cytokines and other soluble factors that are capable of altering neuronal differentiation and function (Chen and Chopp, 2006; Crigler et al., 2006), and it is likely that trophic actions mediated by such molecules at least contribute to the documented effects of MSCs on neurological recovery following damage.
Regardless of the debate surrounding the specific mechanism(s) by which MSCs exert their influence over the nervous system, it is clear that the strict boundaries once proposed to separate the immune and nervous systems in vertebrate organisms are more flexible than previously thought (e.g. a “brain-bone-blood axis”, Spiegel et al., 2008). Indeed, reciprocal influences between these tissues maintain homeostasis and promote rapid responses to stress, underscoring the highly dynamic nature of this relationship (Spiegel et al., 2008). Studies of invertebrate species may offer an evolutionary perspective on these interactions. For example, Drosophila melanogaster (Charroux and Royet, 2010) and the crayfish P. leniusculus (Cerenius et al., 2008; Söderhäll et al., 2005) have contributed to our understanding of hematopoiesis and innate immunity in non-vertebrates, providing a window through which common properties and conserved molecular mechanisms have been revealed (Lin et al., 2010). The recent studies in P. leniusculus identifying hematopoietic tissue near the crayfish brain that appears to contain multipotent stem cells (Noonin et al., 2012) expands the potential for examining transdifferentiation phenomena and the relationship between the immune and nervous systems in crustacean species. Our studies in the crayfish P. clarkii suggest that one point of intersection between hematopoietic and nervous tissues may lie in the stem cell niche that supports adult neurogenesis, and our studies will continue to explore the potential interplay between these systems.
Highlights.
Lifelong neurogenesis is a characteristic of the decapod crustacean brain.
Neurogenesis persists among interneurons in the olfactory pathway.
Neuronal stem cells are located in a niche on the surface of the adult brain.
Neuronal stem cells in crayfish divide symmetrically and are not self-renewing.
Hemolymph cells are attracted to and incorporated into the crayfish niche.
Acknowledgements
The authors thank P. Carey and V. LePage for care of the animals used in these studies. The studies reviewed in this paper were supported by NIH R01 MH67157, NSF IBN 0344448 and 0091092, NSF IOS 0818259 and 1121345, Brazilian Financial Agency CAPES, and a Brachman Hoffman Fellowship from Wellesley College.
Abbreviations
- 5-HT
serotonin
- AL
accessory lobe
- APC
anterior proliferation center
- BrdU
5-bromo-2′-deoxyuridine
- CTG
CellTracker™ Green CMFDA
- EdU
5-ethynyl-2′deoxyuridine
- GS
glutamine synthetase
- HPT
hematopoietic tissue
- LPS
lipopolysaccharide
- LPZ
lateral proliferation zone
- MPZ
medial proliferation zone
- MMS
methiothepin mesylate salt
- MSC
mesenchymal stem cell
- OGT
olfactory globular tract
- OL
olfactory lobe
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod. Struct. Dev. 2003;32:39–60. doi: 10.1016/S1467-8039(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Zhang Y, Benton JL, Sandeman DC. Adult neurogenesis in the decapod crustacean brain: a hematopoietic connection? Eur. J. Neurosci. 2011;34:870–883. doi: 10.1111/j.1460-9568.2011.07802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Goergen EM, Rogan SC, Beltz BS. Hormonal and synaptic influences of serotonin on adult neurogenesis. Gen. Comp. Endocrin. 2008;158:183–190. doi: 10.1016/j.ygcen.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Zhang Y, Kirkhart CR, Sandeman DC, Beltz BS. Primary neuronal precursors in adult crayfish brain: replenishment from a non-neuronal source. BMC Neurosci. 2011;12:53. doi: 10.1186/1471-2202-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondheim NR, Levy YS, Ben-zur T, Burshtein A, Cherlow T, Kan I, Barzilai R, Bahat-Stromza M, Barhum Y, Bulvik S, Melamed E, Offen D. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15:141. doi: 10.1089/scd.2006.15.141. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Lee BL, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Charroux B, Royet J. Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract. Fly. 2010;4:40–47. doi: 10.4161/fly.4.1.10810. [DOI] [PubMed] [Google Scholar]
- Chaves da Silva PG, Benton JL, Beltz BS, Allodi S. Adult neurogenesis: Ultrastructure of a neurogenic niche and neurovascular relationships. PLoS One. 2012;7:e39267. doi: 10.1371/journal.pone.0039267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Teng FYH, Tang BL. Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation: progress and uncertainties. Cell Mol. Life Sci. 2006;63:1649. doi: 10.1007/s00018-006-6019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, Scot EW. Bone marrow transdifferentiation in brain afier transplantation: a retrospective study. Lancet. 2004;363:1432. doi: 10.1016/S0140-6736(04)16102-3. [DOI] [PubMed] [Google Scholar]
- Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Deng YB, Liu XG, Liu ZG, Liu XL, Liu Y, Zhou GQ. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: evidence from a study in rhesus monkeys. Cytotherapy. 2006;8:210–214. doi: 10.1080/14653240600760808. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds and mammals. Brain Res. Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Dawirs RR. Neurogenesis in the developing crab brain: Postembryonic generation of neurons persists beyond metamorphosis. J. Neurobiol. 1996;29:384–398. doi: 10.1002/(SICI)1097-4695(199603)29:3<384::AID-NEU9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. J. Neurosci. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Ann. Rev. Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Lamoury FM, Croitoru-Lamoury J, Brew BJ. Undifferentiated mouse mesenchymal stem cells spontaneously express neural and stem cell markers Oct-4 and Rex-1. Cytotherapy. 2006;8:228–242. doi: 10.1080/14653240600735875. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Lin X, Novotny M, Söderhäll K, Söderhäll I. Ancient cytokines, the role of astakines as hematopoietic growth factors. J. Biol. Chem. 2010;285:28577–28586. doi: 10.1074/jbc.M110.138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem. Int. 2011;59:347–356. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Mistelli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat. Rev. Mol. Cell Biol. 2006;7:540–546. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ. Bone marrow: a possible alternative source of cells in the adult nervous system. Eur. J. Pharmacol. 2000;405:297–302. doi: 10.1016/s0014-2999(00)00561-6. [DOI] [PubMed] [Google Scholar]
- Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. PNAS. 2003;100:1364–1369. doi: 10.1073/pnas.0336479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J. Neurosci. 2004;24:4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonin C, Lin X, Jiravanichpaisal P, Söderhäll K, Söderhäll I. Invertebrate hematopoiesis: An anterior proliferation center as a link between the hematopoietic tissue and the brain. Stem Cells Dev. 2012 Jun 13; doi: 10.1089/scd.2012.0077. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila hematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches-Ramos J, Song S, Cardozo-Perez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Sandeman DC. The vascular circulation in the brain, optic lobes and thoracic ganglia of the crab Carcinus. Proc. Roy. Soc. B. 1967;168:82–90. doi: 10.1098/rspb.1967.0052. [DOI] [PubMed] [Google Scholar]
- Sandeman D, Beltz B, Sandeman R. Crayfish brain interneurons that converge with serotonin giant cells in accessory lobe glomeruli. J. Comp. Neurol. 1995;352:263–279. doi: 10.1002/cne.903520209. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biol. Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus maenas. Brain Res. 1997;762:131–143. doi: 10.1016/s0006-8993(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Harzsch S. Ontogeny of the ventral nerve cord in malacostracan crustaceans: a common plan for neuronal development in Crustacea, Hexapoda and other Arthropoda? Arthropod Struct. Dev. 1999;32:17–37. doi: 10.1016/S1467-8039(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Derby CD. Cytoarchitecture and ultrastructure of neural stem cell niches and neurogenic complexes maintaining adult neurogenesis in the olfactory midbrain of spiny lobsters, Panulirus argus. Journal of Comparative Neurology. 2011;519:2283–2319. doi: 10.1002/cne.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintoni S, Benton JL, Beltz BS, Hansson BS, Harzsch S. Neurogenesis in the central olfactory pathway of adult decapod crustaceans: development of the neurogenic niche in the brains of Procambarid crayfish. Neur. Dev. 2012;7:1. doi: 10.1186/1749-8104-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderhäll I, Kim YA, Jiravanichpaisal P, Lee SY, Söderhäll K. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J. Immunol. 2005;174:6153–6160. doi: 10.4049/jimmunol.174.10.6153. [DOI] [PubMed] [Google Scholar]
- Song CK, Johnstone LM, Edwards DH, Derby CD, Schmidt M. Cellular basis of neurogenesis in the brain of crayfish, Procambarus clarkii: neurogenic complex in the olfactory midbrain from hatchlings to adults. Arthropod Struct. Dev. 2009;38:339–60. doi: 10.1016/j.asd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–492. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cate HS, Derby CD. A spatiotemporal wave of turnover and functional maturation of olfactory receptor neurons in the spiny lobster Panulirus argus. J. Neurosci. 2000;20:3282–3294. doi: 10.1523/JNEUROSCI.20-09-03282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Neural pathways connecting the deutocerebrum and lateral protocerebrum in the brains of decapod crustaceans. J. Comp. Neurol. 2001;441:9–22. doi: 10.1002/cne.1394. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J. Neurobiol. 2005;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Beltz BS. Serotonin depletion in vivo inhibits the branching of olfactory projection neurons in the lobster deutocerebrum. J. Neurosci. 2000;20:7716–7721. doi: 10.1523/JNEUROSCI.20-20-07716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Beltz BS. Characterization of a putative stem/progenitor cell niche in the brain of an adult invertebrate, the crayfish Procambarus clarkii. Soc. Neurosci. Abstr. 2005;31:366.4. [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult Neurogenesis: A Common Strategy Across Diverse Species. J. Comp. Neurol. 2007a;500:574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Benton JL, Beltz BS. Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons. J. Mol. Histol. 2007b;38:527–542. doi: 10.1007/s10735-007-9112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, Bron D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- Utting M, Agricola H-J, Sandeman R, Sandeman D. Central complex in the brain of crayfish and its possible homology with that of insects. J. Comp. Neurol. 2000;416:245–261. [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yasuda-Kamatani Y, Yasuda A. Characteristic expression patterns of allatostatin-like peptide, FMRFamide-related peptide, orcokinin, tachykinin-related peptide, and SIFamide in the olfactory system of crayfish Procambarus clarkii. J. Comp. Neurol. 2006;496:135–147. doi: 10.1002/cne.20903. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Allodi S, Sandeman DC, Beltz BS. Adult neurogenesis in the crayfish brain: proliferation, migration and possible origin of precursor cells. Dev. Neurobiol. 2009;69:415–436. doi: 10.1002/dneu.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Benton JL, Beltz BS. 5-HT receptors mediate lineage-dependent effects of serotonin on adult neurogenesis in Procambarus clarkii. Neur. Dev. 2011;6:2. doi: 10.1186/1749-8104-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:s645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]