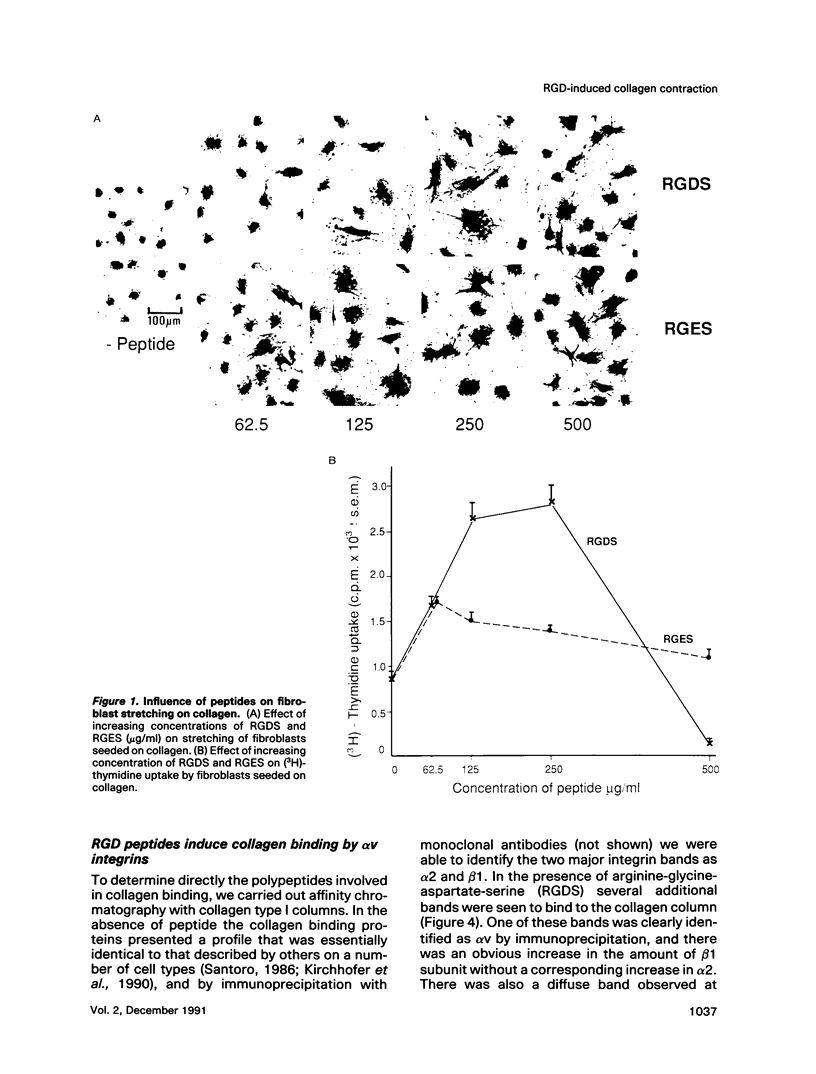
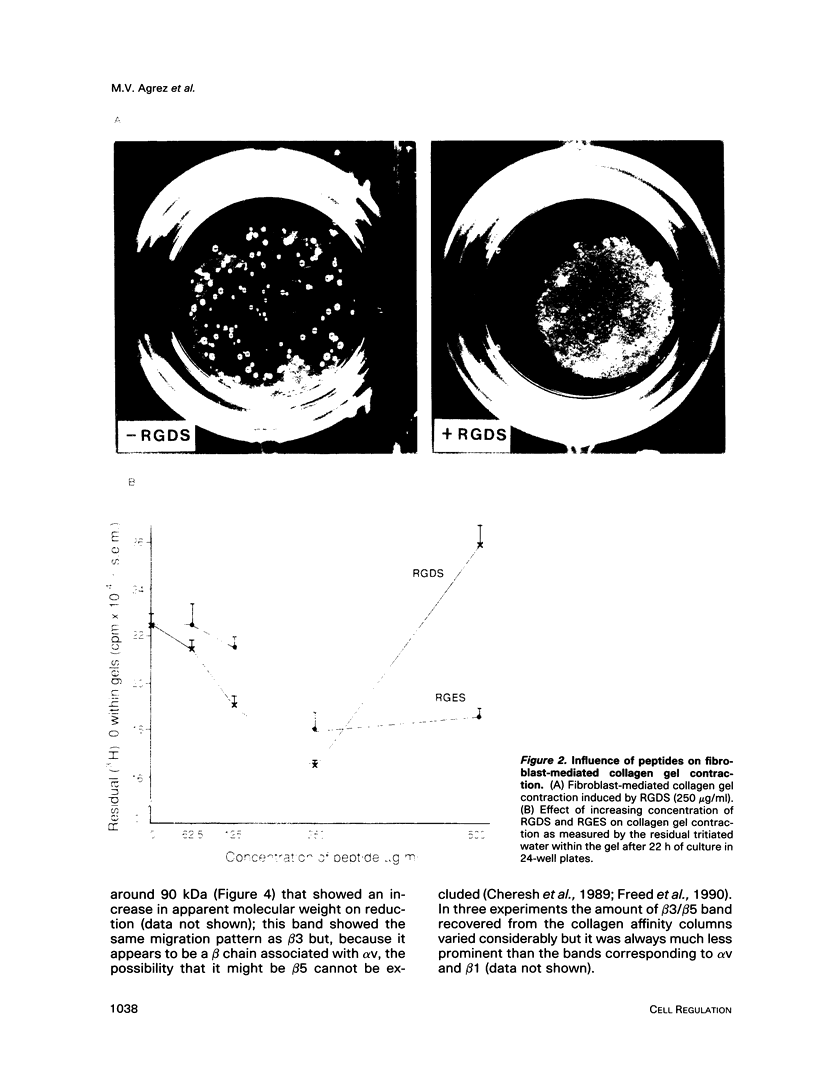
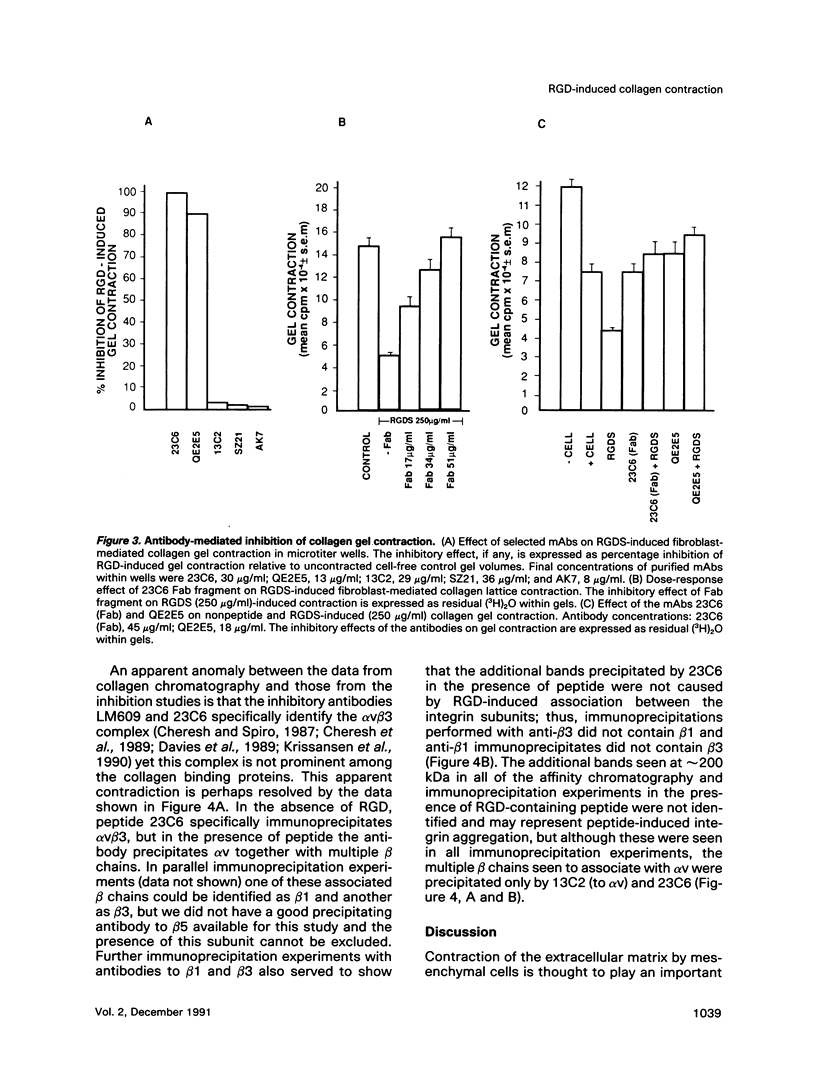
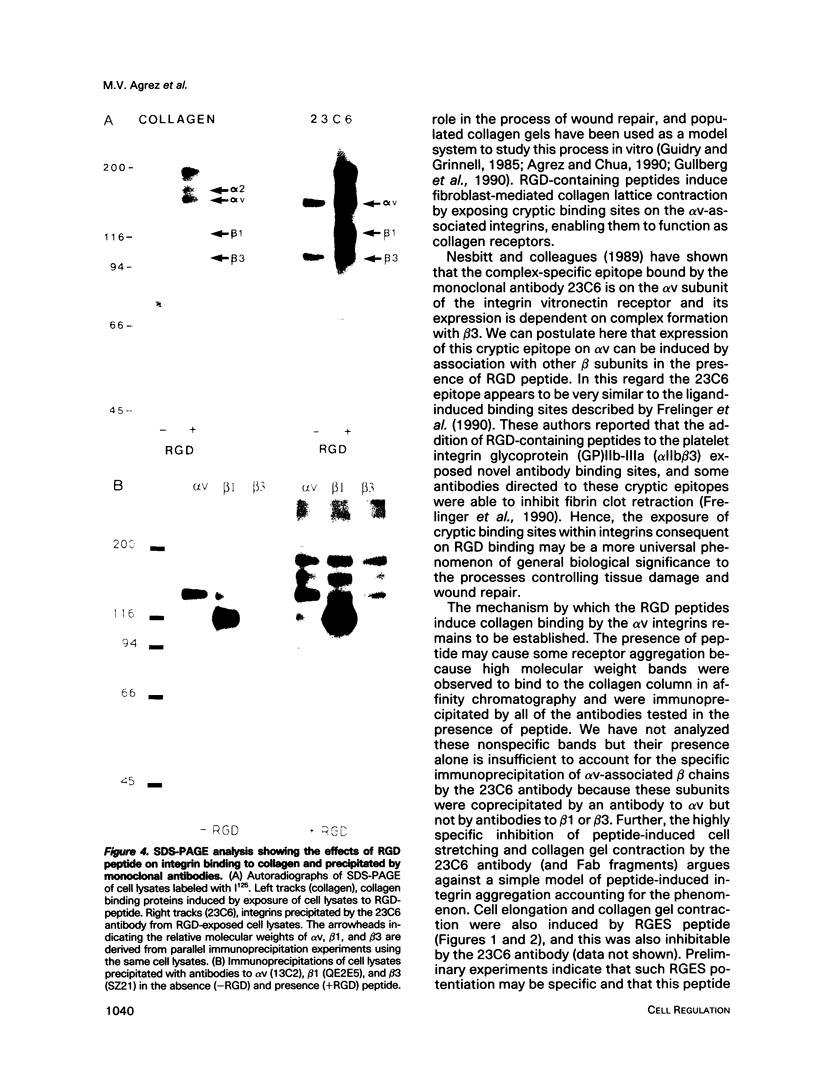
Abstract
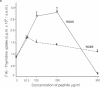
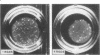
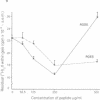
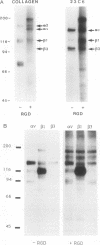
Integrins are a family of cell-surface receptors intimately involved in the interactions of cells with their extracellular matrix. These receptors comprise an alpha and beta subunit in noncovalent association and many have been shown to recognize and bind an arginine-glycine-aspartate (RGD) sequence contained within their specific extracellular matrix ligand. Fibroblasts express integrin receptors belonging to two major subfamilies. Some of the members within the subfamily defined by beta 1 (VLA) are receptors for collagen but, perhaps surprisingly, the other major subfamily of integrins on fibroblasts--that defined by the alpha chain of the vitronectin receptor, alpha v--all appear to bind primarily vitronectin and/or fibronectin. In the present study we show that RGD-containing peptides expose cryptic binding sites on the alpha v-associated integrins enabling them to function as collagen receptors. The addition of RGD-containing peptides to fibroblasts cultured on type I collagen induced dramatic cell elongation and, when the cells were contained within collagen matrices, the peptides induced marked contraction of the gels. These processes were inhibited by Fab fragments of a monoclonal antibody against an alpha v integrin. Also, alpha v-associated integrins from cell lysates bound to collagen I affinity columns in the presence, but not in the absence, of RGD-containing peptides. These data suggest a novel regulatory control for integrin function. In addition, because the cryptic collagen receptors were shown to be implicated in the contraction of collagen gels, the generation of such binding forces suggests that this may be the major biological role for these integrins in processes such as wound healing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrez M. V. A collagen matrix microassay for use in tumour-stromal cell co-cultures. Immunol Cell Biol. 1989 Apr;67(Pt 2):101–105. doi: 10.1038/icb.1989.14. [DOI] [PubMed] [Google Scholar]
- Agrez M. V., Chua F. K. The role of colon fibroblasts in malignant large bowel obstruction--an experimental in vitro model. Br J Cancer. 1990 Oct;62(4):567–572. doi: 10.1038/bjc.1990.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Biggs B. A., Goozé L., Wycherley K., Wilkinson D., Boyd A. W., Forsyth K. P., Edelman L., Brown G. V., Leech J. H. Knob-independent cytoadherence of Plasmodium falciparum to the leukocyte differentiation antigen CD36. J Exp Med. 1990 Jun 1;171(6):1883–1892. doi: 10.1084/jem.171.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990 Apr 15;265(11):5938–5941. [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerman E., Hemler M. E. Regulation of proteins in the VLA cell substrate adhesion family: influence of cell growth conditions on VLA-1, VLA-2, and VLA-3 expression. Exp Cell Res. 1988 Jul;177(1):132–142. doi: 10.1016/0014-4827(88)90031-6. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Freed E., Gailit J., van der Geer P., Ruoslahti E., Hunter T. A novel integrin beta subunit is associated with the vitronectin receptor alpha subunit (alpha v) in a human osteosarcoma cell line and is a substrate for protein kinase C. EMBO J. 1989 Oct;8(10):2955–2965. doi: 10.1002/j.1460-2075.1989.tb08445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger A. L., 3rd, Cohen I., Plow E. F., Smith M. A., Roberts J., Lam S. C., Ginsberg M. H. Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J Biol Chem. 1990 Apr 15;265(11):6346–6352. [PubMed] [Google Scholar]
- Grinnell F., Toda K., Takashima A. Activation of keratinocyte fibronectin receptor function during cutaneous wound healing. J Cell Sci Suppl. 1987;8:199–209. doi: 10.1242/jcs.1987.supplement_8.11. [DOI] [PubMed] [Google Scholar]
- Guidry C., Grinnell F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci. 1985 Nov;79:67–81. doi: 10.1242/jcs.79.1.67. [DOI] [PubMed] [Google Scholar]
- Gullberg D., Tingström A., Thuresson A. C., Olsson L., Terracio L., Borg T. K., Rubin K. Beta 1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990 Feb;186(2):264–272. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhofer D., Languino L. R., Ruoslahti E., Pierschbacher M. D. Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem. 1990 Jan 15;265(2):615–618. [PubMed] [Google Scholar]
- Krissansen G. W., Elliott M. J., Lucas C. M., Stomski F. C., Berndt M. C., Cheresh D. A., Lopez A. F., Burns G. F. Identification of a novel integrin beta subunit expressed on cultured monocytes (macrophages). Evidence that one alpha subunit can associate with multiple beta subunits. J Biol Chem. 1990 Jan 15;265(2):823–830. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Loftus J. C., Du X. P., Glass A. A., Ruggeri Z. M., Shattil S. J., Plow E. F., Ginsberg M. H. Affinity modulation of the alpha IIb beta 3 integrin (platelet GPIIb-IIIa) is an intrinsic property of the receptor. Cell Regul. 1990 Nov;1(12):883–893. doi: 10.1091/mbc.1.12.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986 Sep 12;46(6):913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Walsh J. J., Pexton T., Santoro S. A. The alpha 2 beta 1 integrin cell surface collagen receptor binds to the alpha 1 (I)-CB3 peptide of collagen. J Biol Chem. 1990 Mar 25;265(9):4778–4781. [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]