Abstract
Our recent findings of a weaning-related pattern of oxytocin (OT) and OT receptor (OTR) expression in the rat enteric nervous system and in villus-crypt enterocytes, together with the known high level and stability of OT in breast milk support that OT may play a role in gut function and development. We previously described a biphasic dose- response of the PI3K/Akt pathway in gut cells treated with OT. Activation peaked at 62.5 nM OT (30 min) and coincided with OTR internalization. Here we use automated Western blotting to further explore OT-elicited changes in Akt and pAktT308, as well as in downstream substrates p70 S6 kinase-1 (S6K1) and eIF-4E binding protein 1 (4E-BP1). Relative to fresh growth medium (FGM) alone, our results showed OT in FGM reduced the abundance and phosphorylation of S6K1 and the phosphorylation of 4E-BP1, both substrates of mammalian target of rapamycin complex 1 (mTORC1). Phosphorylation of mTORC1 regulator, RaptorS792, was increased by high and low OT concentrations, with predicted inhibitory effects on mTORC1. OT thus downregulates anabolic effects induced by FGM activity catalyzed by mTORC1. OT is a regulator of the PI3K/Akt/mTORC1 pathway in Caco2BB cells and may modulate translation in gut cells.
Keywords: 4E-BP1, Caco2BB, Enterocyte, PI3K/Akt, Autism
INTRODUCTION
The known high level and stability of oxytocin (OT) in breast milk [1] in conjunction with the weaning-related pattern of OT and OT receptor (OTR) expression that we have observed in the rat enteric nervous system and in villus-crypt enterocytes [2] raises intriguing questions about the role that OT plays in gut function and development. Our group found that when combined with secretin OT reduced rat colonic inflammation [3]. This finding is consistent with studies showing that OT can reduce inflammatory and cellular oxidative stress [4,5]. We have also found that mice lacking the OTR have altered gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance (Welch et al., in submission). Our discoveries suggest that, in addition to its role in the brain, OT may also have an important neuromodulatory role in the gut. However, the relevant cell signaling mechanism associated with OT in the gut remains to be elucidated.
We recently demonstrated that OT activates the phosphoinositide 3-kinase (PI3K)/Akt pathway in a dose and time-dependent manner in Caco2BB cells (in vitro model of enterocytes). We further established that the activation peaked at 62.5 nM (high) OT [6]. In the present study, we extend our investigation of the PI3K/Akt pathway by looking at mammalian target of rapamycin complex-1 (mTORC1) and its substrates. mTORC1 is important in protein synthesis through its modulation of ribosomal biogenesis [7], cell proliferation and cell size [8] by way of sensing nutrient sufficiency signals [9] and cellular responses to stressors [10].
The relationship between Akt and mTORC1 is very important and most certainly involves crosstalk, although a full understanding of this complex relationship is just beginning to emerge. Increased pAkt activity increases phosphorylation of hamartin/tuberin complex (TSC1/TSC2), which attenuates its inhibitory effect on mTORC1 (i.e., increases mTORC1 activity) [11,12]. Modulation of mTOR can also have upstream effects. A recent study demonstrated that chronic rapamycin treatment, which inhibits mTORC1, differentially phosphorylates Akt on residues T308 vs S473 and impairs insulin action and glucose tolerance [13]. Interestingly, disruption of the negative feedback loop upon mTORC1 mediated by S6 kinase, a substrate of mTORC1, results in increased insulin sensitivity [14].
The present study investigates a possible role for OT in regulating mTORC1 and its substrates. Because of their known roles in downstream signaling pathways, we examined Raptor, part of the mTORC1 complex, as well as mTORC1 substrates S6K1 and eIF4E binding protein 1 (4E-BP1). pS6K1 enhances downstream translation activity [15] while 4E-BP1 functions as a natural inhibitor of translation initiation factor 4E (eIF4E) in protein synthesis [16,17]. The phosphorylation of 4E-BP1S65 is a signaling marker for disrupted inhibition of eIF4E; the less 4E-BP1S65 is phosphorylated, the more it inhibits eIF4E translation activity [18].
Here, we show that OT has an overall dampening effect on the PI3K/Akt/mTORC1 pathway. We also show that OT increases the phosphorylation of Raptor S792 while downregulating both the abundance and phosphorylation of S6K1 and 4E-BP1S65.
MATERIALS AND METHODS
Cells and Culture Reagents
Caco2BB cells (C2BBe1 clone; American Type Culture Collection, Manassas, VA) were grown (5% CO2 and 37°C in a humid atmosphere) in Dulbecco modified essential medium (DMEM, glucose 4.5 g/L) fortified with bovine transferrin 10 ng/ml that was supplemented with standard penicillin and streptomycin, 2 mM glutamine, and 10% fetal calf serum (GIBCO, Grand Island, NY).
Reagents
Human OT (Phoenix Pharmaceuticals Inc., Burlingame, CA). OTR antagonist (OTA; desGly-NH2-d(CH2)5[D-Tyr2, Thr4]OVT (ST-11-61); donated by Dr. Maurice Manning, University of Toledo, OH [19]).
Antibodies
Studies used: mouse anti-αtubulin (mAb) (T6074, Sigma-Aldrich, St Louis, MO), goat anti-rabbit IgG horseradish peroxidase (HRP) conjugate, goat anti-mouse IgG HRP conjugate (ProteinSimple Santa Clara CA), rabbit mAb anti-pAktS473 (XP, 4060; Cell Signaling Technology (CST), Inc., Danvers, MA), rabbit anti- pAktT308 (9265; CST), rabbit mAb anti-(pan)Akt (4691;CST), rabbit anti-p70S6 kinase (mAb) (2708; CST), mouse anti-pS6K1 (mAb-Thr389; 9206; CST), rabbit anti phospho-Raptor (Ser792, 2083; CST), rabbit mAb anti GAPDH (2118; CST), rabbit anti-phospho-4E-BP1 Ser65 (9451; CST), rabbit anti-4E-BP1 (9452; CST).
OTR Stimulation and Protein Extraction
OT stimulation experiments were performed in cell cultures 24 h after seeding of 25 × 104 cells/cm2. Continuous stimulation times (10 to 60 min, as indicated) were terminated by placing the cultures on ice. The cultures were washed twice with ice cold phosphate-buffered saline (PBS) and cold wash buffer provided by the kit described below. Subsequently 0.1 ml of ice cold protein extraction cocktail prepared from the Cell Lysis Kit Bicine/Chaps (p/n CBS403, ProteinSimple, Santa Clara CA) was added for 15 min. The extraction cocktail, containing protease inhibitors and phosphatase inhibitors, was used according to the supplier instructions. The protein extracts were scraped, cooled on ice for 5 min and spun at 10,000 × g for 30 min at 4°C. A sample of each extract was processed for protein determination and the remainder was stored at −70°C. Protein concentrations were measured by a paper spot protein assay against a bovine serum albumin (BSA) standard curve. Protein samples (4 μl) were applied to 3 MM filter paper, stained with Coomassie blue in 40% methanol and 10% acetic acid, washed with the same solution without dye and dried. Proteins were eluted with 3 ml of 2% SDS and concentrations were quantified on an ELISA reader at 650 nm.
Simon™ Automated Western Blotting and Analysis
All reagents were prepared and used according to manufacturer’s recommendations for use on Simon™ (ProteinSimple, San Jose, CA, www.proteinsimple.com/simon.html). Reagents included: biotinylated molecular weight ladder, streptavidin-HRP, fluorescent standards, luminol-S, hydrogen peroxide, sample buffer, DTT, stacking matrix, separation matrix, running buffer, wash buffer, matrix removal buffer, capillaries, containing a proprietary UV-activated chemical linked reagent, and antibody diluent and antibodies (goat-anti rabbit secondary antibody, and goat-anti mouse secondary antibody).
Samples were diluted to adjust protein concentration to 3–4 μg in 2.5 μl with sample buffer and further diluted 1:2 by adding 2.5μl of the 2X master mix (containing 80 mM DTT, 2X Sample buffer and 2X fluorescent standards). The final samples of 5 μl each were boiled 5 min, placed on ice for 5 min, briefly centrifuged and applied to proper wells. Both a stock of 1M DTT and 1:1 mixture of luminol-S and peroxide (150uL) were prepared fresh daily and kept on ice until use. Aliquots were stored at −20 C and removed for each run on Simon™.
Simon ™ Instrumentation
The instrument was prepared by adding 2 ml of matrix removal buffer to trough 1, 2 ml of wash buffer to trough 2, and 0.8 ml of running buffer to trough 3. Capillaries and the 384-well plate containing samples, antibodies, and matrices were then placed inside the instrument. The Simple Western was run with capillaries (12) filled with separation matrix for 100 sec, stacking matrix for 16 sec and protein extracts for 12 sec. The samples were then separated with 250V for 40 min and then immobilized to the capillary wall using default immobilization conditions and washed with matrix removal buffer for 140 sec to remove the separation matrix.
Capillaries were then washed with wash buffer for 150 sec and blocked with antibody diluent for 15 min. Next, capillaries were incubated with primary antibody (3 hours), washed, and incubated with HRP conjugated secondary antibodies for 1 hour. After removal of unbound secondary antibody, the capillaries were incubated with the luminol-S/peroxide substrate and chemiluminescent signal was collected using the Charge-Coupled Device (CCD) camera of Simon™ with 6 different exposure times (30, 60, 120, 240, 480, and 960 seconds). Data analysis was performed using the Compass Software (ProteinSimple) on Simon™
Statistical Analysis
Band density differences at each time point or each reagent concentration were computed against controls using a paired Student’s t-test (2-tailed or 1-tailed in the absence or presence of expectation, respectively; α=0.05). Data were collected from 3–5 replicates per condition and analyzed using SPSS Base 9.0 (SPSS, Chicago, IL). All plots present mean ± standard error.
RESULTS
Downregulation of pAkt in presence of medium by OT is time dependent
We have previously shown that high (62.5nM) OT has a differential effect on PI3K compared with Akt, especially after 30 min stimulation of Caco2BB cells (a sub-line of the Caco2 colon adenocarcinoma human cell line selected for its brush border structures) [6]. Our preliminary experiments suggested that fresh growth medium (FGM) (rich in growth factors) may enhance phosphorylation of Akt. In this experiment we stimulated Caco2BB cells with OT in the presence of FGM and, with FGM alone, at different concentrations and timepoints.
Low OT (7.8 nM) attenuated phosphoisoform induction compared with FGM alone, with the maximum effect at 30 min (Figure 1). We observed that levels of pAktT308 and pAktS473 were enhanced in the presence of FGM in a time-dependent manner. This result suggests that OT decreases the anabolic effects of the medium. FGM by itself also elicited phosphoisoform changes over time.
Figure 1. Timecourse of Akt and S6K1 phosphorylation response to low OT.
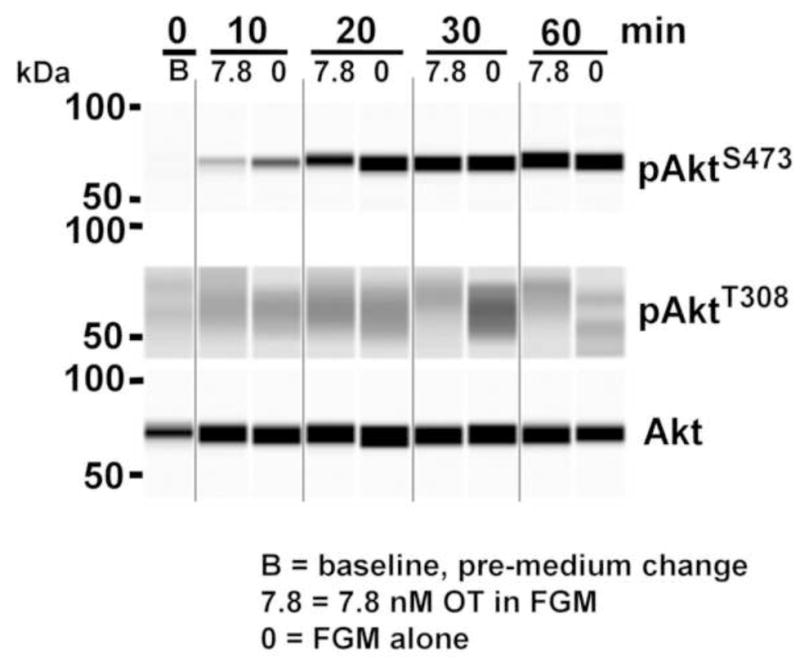
Caco2BB cultures were stimulated with low OT (7.8 nM) in fresh growth medium (FGM) and FGM alone (0). Electrophoretic bands were developed with anti-pAktT308, with anti pAkt S473 and anti-total Akt antibodies. Note that low OT downregulates pAktT308, the maximal effect occurring at 30 min. FGM also has a time-dependent effect on pAktT308. Different time dependence is seen for pAktS473. The phosphorylation of S6K1 observed after FGM was attenuated by OT.
Like low OT, high OT (62.5 nM) attenuated phosphoisoform induction, albeit with a different time course. (Figure 2A). Furthermore, the data suggested that high OT has a differential effect on kinases that target residues S473 (mTOR complex 2; mTORC2) and T308 (3-phosphoinositide-dependent kinase 1; PDK1). While high OT had a minimal effect on mTORC2, significant effects were observed for PDK1. Total Akt levels were also reduced at 10 min by high OT. We next showed Akt phosphoisoform induction was dependent upon the OTR. Partial recovery of pAktT308 after 30 min of high OT stimulation was inhibited by the potent OTR antagonist OTA. This implies significant dependence upon OTR activation for phosphoisoform induction beyond that stimulated by the addition of FGM (Figure 2B, C).
Figure 2. Timecourse of Akt phosphorylation response to high OT and dependence upon the OTR.
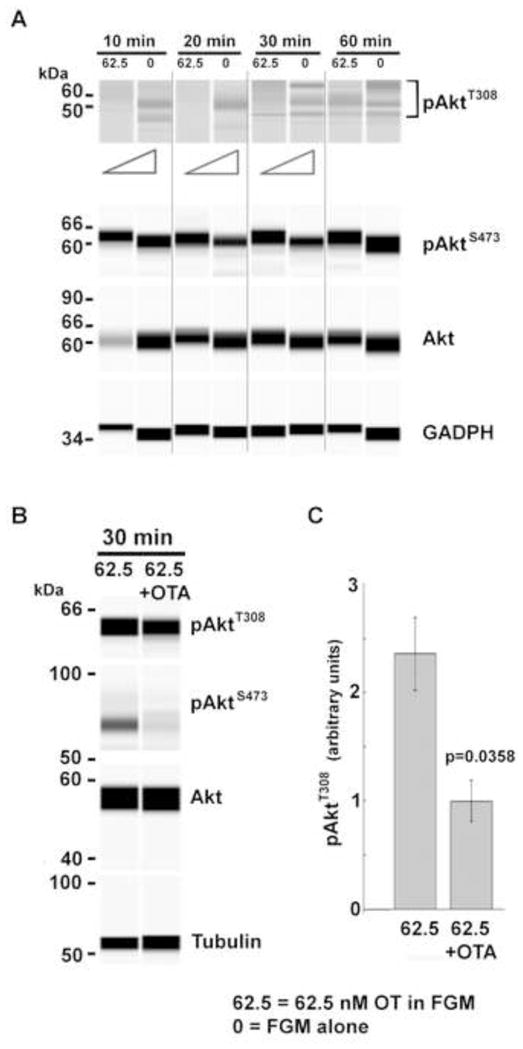
(A) Caco2BB cultures were stimulated with high OT (62.5 nM) in FGM vs FGM alone (0) at indicated timepoints. Representative electrophoretic bands developed with anti-pAktT308, anti pAkt S473 and anti-total Akt antibodies are shown. Triangles denote differential pT308 band densities detected by chemiluminescence. OT attenuated the effect of growth factors in the medium on pAkt and total Akt. The time course of FGM’s effect is complex and varies from that of OT. (B, C) Cell cultures were stimulated for 30 min with 62.5 nM OT in the absence or presence of 5 nM of the OTR antagonist, OTA. In western blot (B) and with quantification of data (C) a differential decrease in AktT308 phosphorylation was observed in the presence of OTA (p=0.0358, n=3).
Effects of OT on substrates of mTORC1
We explored downstream effects of OT upon the mTORC1 pathway. With FGM alone, phosphorylation of S6K1 was observed at 20 and 30 min, but not at 60 min. Low OT attenuated pS6K1 levels at 20 and 30 min and resulted in some S6K1 phosphorylation at 60 min (Figure 1). We further assessed the induction and phosphorylation of S6K1 and translation initiation factor 4E-binding protein (4E-BP1) because reduced phosphorylation of 4E-BP1 is associated with greater inhibition of eIF4E translation activity [16].
Treatment with FGM alone resulted in increased abundance of S6K1 at all times (peaking at 20 min), and increased pS6K1 levels at 10 and 60 min (Figure 3A). High OT reduced the expression of S6K1 at all times and attenuated pS6K1 levels at 10 and 60 min, but increased pS6K1 relative to control at 20 and 30 min of stimulation. This inconsistency during the 20–30 min time period may be the consequence of OTR internalization as observed in our previous study [6]. 4E-BP1 and phosphorylated 4E-BPS65 (p4E-BPS65) showed slight similarity to the biphasic progression of S6K1 and pS6K1. Low OT significantly decreased pS6K1 while high OT brought it closer to the levels observed with FGM alone, and the OT receptor antagonist (OTA) prevented this high OT-induced increase (Figure 3B, C), which suggests that OTA interferes with the interaction of high OT with the OTR such that it resembles the effect of low OT. This implies that high OT-elicited reductions in pS6K1 are mainly OTR-dependent.
Figure 3. Timecourse of S6K1 and 4E-BP1 expression and phosphorylation in response to high OT and dose-dependent effect of OT on pS6K1 at 30 min.
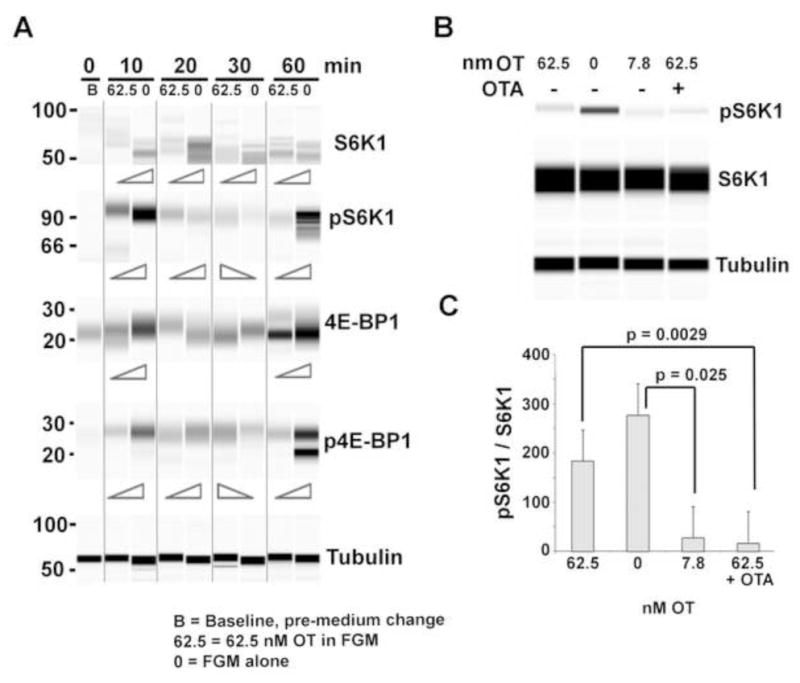
(A) Automated Western blots showing Caco2BB cultures stimulated with high OT (62.5nM) in FGM vs FGM alone (0). Electrophoretic bands were developed with anti S6K1, pS6K1T389, 4E-BP1, and p4E-BP1S65. Notice that S6K1 is downregulated by OT at all timepoints. However, the other 3 were all downregulated at 10 and 60 min. The apparent inconsistency during the 20–30 min time period may result from OTR internalization that takes place during this time interval. (B, C) Caco2BB cells were stimulated in FGM; FGM alone (0), low OT (7.8nM), high OT (62.5nM) and high OT in the presence of the OTR receptor antagonist, OTA (5nM). Note the decrease of pS6K1 under low OT and its increase under high OT in western blot (B) and when normalized to total S6K1 levels (C). High OT in presence of OTA has an effect similar to low OT.
OT induces pRaptorS792, a marker of mTORC1 downregulation
pRaptorS792 disrupts mTORC1 functioning in complex with Raptor protein [20]. In this study, both high and low OT significantly increased pRaptorS792 abundance after 30 min relative to FGM (p=0.02 and 0.038, respectively; n=5) (Figure 4). This implies that mTORC1 activity may be inhibited by both an upstream decrease in pAkt and by Raptor phosphorylation.
Figure 4. Both Low and high OT increase pRaptorS792.
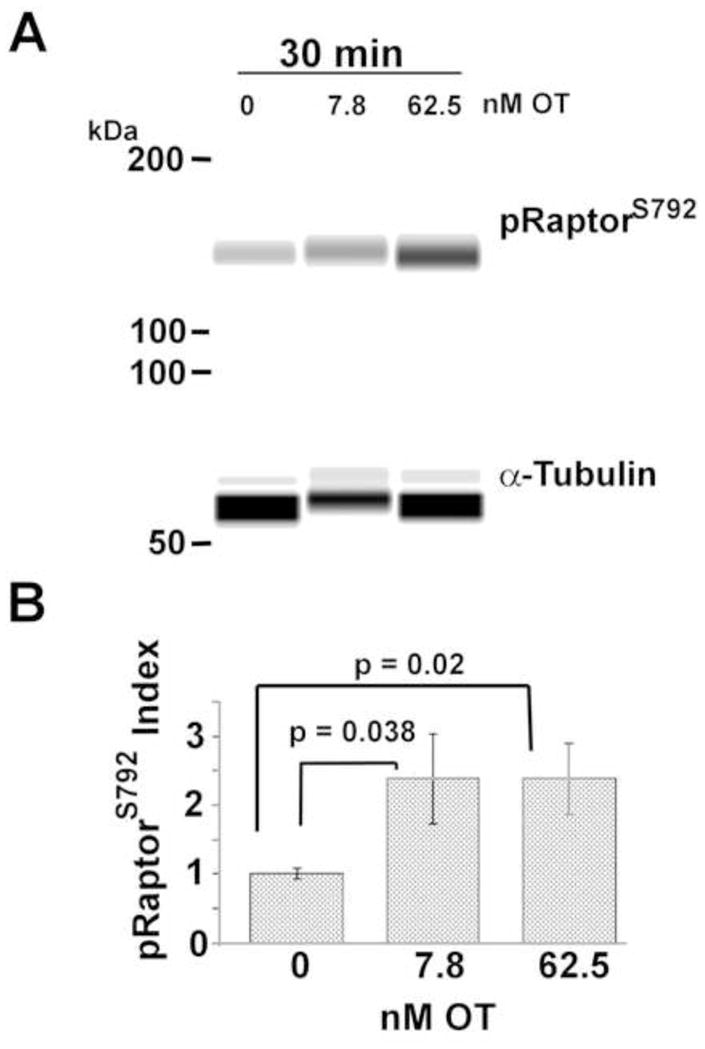
Caco2BB cultures were suspended in FGM with and without low and high OT as indicated. Western blot analysis (A), with quantification of data (B) showed that both high and low OT increased the abundance of pRaptorS792.
DISCUSSION
This investigation is part of a larger group effort to reveal the mechanisms underlying mother-infant nurture [2,21]. As aforementioned, we wish to elucidate the importance of OT in gut function given the exposure of neonates and infants to OT in breast milk, which is tightly correlated with OT and OTR expression in the rat gut pre- and post-weaning, as well as the well-established role of OT in nurture [1,2,22]. Furthermore, because of the newly reported association between mTOR and autism [23,24], demonstrated effectiveness of OT nasal spray in alleviating the symptoms of autism [25], and, gut pathology observed in a subset of autistic children [26], we are particularly interested in the implications of our work for understanding and treating autism. Recently we reported a differential induction of pAkt isoforms in response to high vs low OT in FGM in Caco2BB cells [6]. Additional experiments indicated that OT attenuates the response to FGM alone. The current study extends our previous findings and elucidates possible mechanism(s). Our findings demonstrate that OT, via the OTR, inhibits the mTOR pathway, alters pAkt induction and stimulates RaptorS792 phosphorylation. At the same time, OT reduces phosphorylation and alters the timecourse of induction of mTOR substrates S6K1 and 4E BP1.
We previously described increased pAkt in response to OT concentrations ranging from 7.8 nM (low) to 62.5 nM (high) in Caco2BB cells; notably, the pAktT308 isoform was more abundant than pAktS473 [6]. In the present study we determined that the effect of OT is actually an attenuation of the positive effect of FGM upon pAkt and, which is more pronounced upon pAktT308 than pAktS473. In addition, we found that OT decreased pS6K1 levels in concert with its effects on pAktT308. We infer from this that OT modulates S6K1 activity, since pS6K1 serves as a S6K1 activation marker. We also found that OT induces RaptorS792 phosphorylation, which inhibits mTORC1 [20]. Since S6K1 is a substrate of mTORC1 [27,28], we infer from our RaptorS792 finding that reductions in S6K1 activation evoked by OT are a direct consequence of mTORC1 inhibition. In support, we show that OT also decreased phosphorylation of 4E-BP1S65 (another mTORC1 substrate) in parallel with reductions in S6K1 phosphorylation. Altogether, these results support a role for high OT in negatively regulating the mTORC1 pathway.
Negative regulation of mTORC1 has the potential to slow translation. 4E-BP1 is the binding protein of translation initiation factor 4E (eIF4E), a rate-limiting factor of cap-dependent translation. De-phosphorylation of 4E-BP1S65, like that observed with OT, inhibits translation by permitting 4E-BP1 to bind to eIF4E [29,30,31]. Interestingly, low OT treatment (but not high) of myometrial cells was shown to inhibit phosphorylation of translation elongation factor (eEF2) [32]. This implies OT modulation of eEF2 downstream of rate-limiting, cap-dependent translation initiation by eIF4E (and therefore, eEF2 function in response to OT is negligible). We suggest that OT slows translation initiation by decreasing fully active pAkt (pAktT308-S473) by greater reductions of pAktT308 than pAktS473 and, that this results in less TSC2 inhibition, which reduces Rheb GTPase activity, decreasing the phosphorylation of S6K1 and 4E-BP1 as a result of reduced mTORC1 complex activation.
mTORC1 is actively inhibited by TSC1/TSC2 under basal (non-stimulating) conditions and also inhibited by RaptorS792 phosphorylation [33,34]. While we do not know if the enhanced RaptorS792phosphorylation observed after OT is directly catalyzed by the pAkt/mTORC1 pathway, nutrient insufficiency can activate the energy sensor AMPK (AMP dependent protein kinase). This in turn increases RaptorS792 phosphorylation, downregulates translation, and prepares the cell for autophagy [33,34]. OT may thus be involved in the cellular response to nutrient and energy sufficiency via the Akt/mTORC1/S6K1-4E-BP1 pathway. OT may also affect insulin sensitivity, by downregulation of pS6K1, which upregulates the insulin receptor and its substrate [35,36], and effects inflammation, proliferation and other cellular processes in the gut.
These findings support our prior study showing that OT dampens translation machinery in the PI3K/Akt/mTORC1 pathway in gut cell cultures under certain in vivo-like conditions. Our findings are highly novel and suggest that OT modulates important functional processes in the gut and may extend to other sites of OT signaling, including the brain. Altogether, our work supports the need for physiological assessment of OT function in the gut, which may have compelling implications for interventions that affect OT exposure in neonates and infants and autism treatment.
Highlights.
A role for oxytocin in slowing protein translation in the gut is proposed.
The mechanism relies upon oxytocin eliciting inhibiting markers of mTORC1 pathway.
Oxytocin attenuates Akt phosphorylation in response to growth medium stimulation.
Abundance and phosphorylation of mTORC1 substrates is also reduced with oxytocin.
Oxytocin induces Raptor S792 phosphorylation implying direct mTORC1 inhibition.
Acknowledgments
Chris Heger and Robert Ludwig helped in manuscript preparation. We thank Maurice Manning for supplying the oxytocin antagonist.
Funding: Defense Threat Reduction Agency and the National Center for Advancing Translational Sciences, National Institutes of Health and the Einhorn Family Charitable Trust (UL1 TR000040 (formerly National Center for Research Resources, # UL1 RR024156) and 044824).
Abbreviations
- 4E-BP1
eIF-4E binding protein 1
- Akt
serine/threonine protein kinase (B)
- mTORC1
mammalian target of rapamycin complex 1
- OT
oxytocin
- OTR
oxytocin receptor
- PI3K
phosphoinositide 3-kinase
- pAkt
phosphorylated Akt
- pS6
phosphorylated S6
- pS6K1
phosphorylated S6K1
- PDK1
3-phosphoinositide-dependent kinase 1
- S6
ribosomal S6 protein
- S6K1
P70 ribosomal S6 kinase-1
- TSC1/TSC2
hamartin/tuberin complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takeda S, Kuwabara Y, Mizuno M. Concentrations and origin of oxytocin in breast milk. Endocrinologia japonica. 1986;33:821–826. doi: 10.1507/endocrj1954.33.821. [DOI] [PubMed] [Google Scholar]
- 2.Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. The Journal of comparative neurology. 2009;512:256–270. doi: 10.1002/cne.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch MG, Anwar M, Chang CY, Gross KJ, Ruggiero DA, Tamir H, Gershon MD. Combined administration of secretin and oxytocin inhibits chronic colitis and associated activation of forebrain neurons. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22:654–e202. doi: 10.1111/j.1365-2982.2010.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biyikli NK, Tugtepe H, Sener G, Velioglu-Ogunc A, Cetinel S, Midillioglu S, Gedik N, Yegen BC. Oxytocin alleviates oxidative renal injury in pyelonephritic rats via a neutrophil-dependent mechanism. Peptides. 2006;27:2249–2257. doi: 10.1016/j.peptides.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 2008;295:E1495–1501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein BY, Tamir H, Welch MG. PI3K/Akt responses to oxytocin stimulation in Caco2BB gut cells. Journal of cellular biochemistry. 2011;112:3216–3226. doi: 10.1002/jcb.23243. [DOI] [PubMed] [Google Scholar]
- 7.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21:827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 10.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 11.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 12.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 13.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rong L, Livingstone M, Sukarieh R, Petroulakis E, Gingras AC, Crosby K, Smith B, Polakiewicz RD, Pelletier J, Ferraiuolo MA, Sonenberg N. Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. RNA. 2008;14:1318–1327. doi: 10.1261/rna.950608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedzwiecka A, Marcotrigiano J, Stepinski J, Jankowska-Anyszka M, Wyslouch-Cieszynska A, Dadlez M, Gingras AC, Mak P, Darzynkiewicz E, Sonenberg N, Burley SK, Stolarski R. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J Mol Biol. 2002;319:615–635. doi: 10.1016/S0022-2836(02)00328-5. [DOI] [PubMed] [Google Scholar]
- 19.Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY. Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int J Pept Protein Res. 1995;46:244–252. doi: 10.1111/j.1399-3011.1995.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 20.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shair HN, Smith JA, Welch MG. Cutting the vagus nerve below the diaphragm prevents maternal potentiation of infant rat vocalization. Developmental Psychobiology. 2012;54:70–76. doi: 10.1002/dev.20577. [DOI] [PubMed] [Google Scholar]
- 22.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 23.Angelidou A, Asadi S, Alysandratos KD, Karagkouni A, Kourembanas S, Theoharides TC. Perinatal stress, brain inflammation and risk of autism-review and proposal. BMC pediatrics. 2012;12:89. doi: 10.1186/1471-2431-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen FE. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PloS one. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Progress in brain research. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- 26.Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in children with autistic disorder. The Journal of pediatrics. 1999;135:559–563. doi: 10.1016/s0022-3476(99)70052-1. [DOI] [PubMed] [Google Scholar]
- 27.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. Journal of cellular physiology. 2005;203:144–155. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 28.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 29.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 30.Niedzwiecka A, Stepinski J, Darzynkiewicz E, Sonenberg N, Stolarski R. Positive heat capacity change upon specific binding of translation initiation factor eIF4E to mRNA 5′ cap. Biochemistry. 2002;41:12140–12148. doi: 10.1021/bi0258142. [DOI] [PubMed] [Google Scholar]
- 31.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes & development. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devost D, Carrier ME, Zingg HH. Oxytocin-induced activation of eukaryotic elongation factor 2 in myometrial cells is mediated by protein kinase C. Endocrinology. 2008;149:131–138. doi: 10.1210/en.2007-0548. [DOI] [PubMed] [Google Scholar]
- 33.Dunlop EA, Hunt DK, Acosta-Jaquez HA, Fingar DC, Tee AR. ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy. 2011;7:737–747. doi: 10.4161/auto.7.7.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2011;5(11) doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]


