Summary
Background:
Papillary muscle rupture is one of the catastrophic mechanical complications following myocardial infarction. Rupture leads to acute mitral valve regurgitation, pulmonary edema, and cardiogenic shock. Survival is dependent on prompt recognition and surgical intervention.
Cases Report:
We present two cases where acute myocardial infarction was complicated by papillary muscle rupture resulting in severe mitral regurgitation and cardiogenic shock. In both cases rupture occurred within one week of infarction. Both patients did not receive coronary revascularization; one patient presented late after the onset of chest pain, the other patient percutaneous revascularization attempted and was not successful. Both patients suffered an inferior wall infarction. Echocardiogram demonstrated severe mitral regurgitation with a jet directed posteriorly. In both cases rupture of the posteromedial papillary muscle resulted in flail of the anterior mitral valve leaflet, thus serving as a reminder that both the anterior and the posterior leaflets attach to both papillary muscles.
Conclusions:
While one case had a good outcome, the other reinforces the fact that this is a very serious complication requiring prompt recognition and treatment.
Keywords: acute myocardial infarction complication, mitral valve, papillary muscle rupture, flail anterior leaflet
Background
There are three catastrophic mechanical complications of acute myocardial infarction. These include left ventricular free wall rupture, rupture of the interventricular septum, and papillary muscle rupture. All involve loss of structural integrity of the infarcted tissue and are associated with extraordinarily high mortality rates if not promptly recognized and treated. Fortunately with an increasing emphasis on early revascularization coupled with improved techniques these catastrophic complications are relatively rare, representing 2.3% of acute myocardial infarctions [1].
Papillary muscle rupture (PMR) frequently presents with symptoms ranging from acutely decompensated heart failure to cardiogenic shock. The most frequent scenario involves infarction upstream from the posterior descending artery (i.e. the right coronary artery in right dominant systems or the left circumflex artery in left dominant systems). Valvular competence during ventricular systole is maintained by the actions of two papillary muscles (anterolateral and posteromedial). The anterolateral muscle typically has a dual blood supply while the posteromedial muscle is supplied from only the PDA. Therefore the posteromedial muscle is more susceptible to infarction and rupture.
Treatment often necessitates emergent surgical intervention, with mitral valve repair (if muscle necrosis is limited) or valve replacement [2]. Both immediate and long-term outcomes are improved with concomitant coronary revascularization [3]. Herein we present two cases that demonstrate the typical presentation of acute papillary muscle rupture and remind clinicians that prompt recognition and management are critical in this uncommon but lethal complication.
Case Report
Case 1
A 51 year old male presented to the emergency room complaining of severe shortness of breath. Over the prior 3 days he noticed a dry cough and vague chest discomfort. Approximately four hours prior to presentation the patient acutely developed severe and worsening shortness of breath. Initial vital signs were temperature of 99.2 degrees Fahrenheit, blood pressure 159/98 mmHg, heart rate 153 beats/minute, respiratory rate 30 breaths/minute, and oxygen saturation of 90% on room air. Cardiovascular exam revealed tachycardia with a holosystolic murmur loudest at the apex with radiation to the axilla. Point of maximal impulse was not displaced; there was jugular venous distention to the mandible. Pulmonary exam demonstrated tachypnea with use of accessory muscles, and bibasilar rales extending midway up the lung fields.
Chest X-ray revealed diffuse bilateral hazy opacification without cardiomegaly. Electrocardiogram demonstrated ST segment elevation in the inferior leads (II, III, and aVF). The patient was emergently transferred to the cardiac catheterization laboratory for angiography and possible intervention. Coronary angiogram revealed diffuse, calcified triple vessel coronary artery disease with 40% stenosis of the left main coronary artery, 40% stenosis of the left anterior descending artery, 99% occlusion of the distal left circumflex/posterior descending artery (PDA) with thrombus burden (Figure 1), 80% stenosis of the first obtuse marginal branch, and 80% stenosis of the right coronary artery. There was a left dominant system with the PDA arising from the left circumflex artery. Estimated ejection fraction by ventriculogram was 40%, left ventricular end-diastolic pressure was 34 mmHg, there was evidence of inferior wall hypokinesis, and severe mitral regurgitation. Due to hemodynamic instability and to guide further management a right heart catheterization was performed which demonstrated a mean right atrial pressure of 20 mmHg, right ventricular pressure of 87/24 mmHg, pulmonary artery pressure of 95/34 mmHg, and pulmonary capillary wedge pressure of 60 mmHg. These findings are consistent with acute left heart failure with pulmonary venous hypertension.
Figure 1.
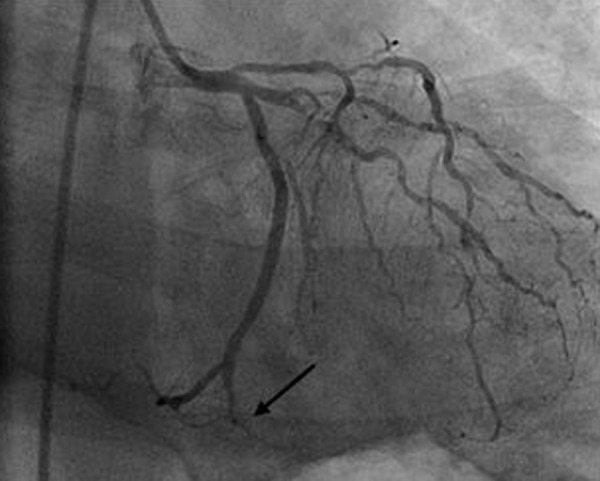
Coronary angiogram demonstrating a 99% occlusion of the distal left circumflex artery/posterior descending artery (arrow).
The patient was then referred for emergent surgical repair with concomitant coronary artery bypass grafting. Prior to surgery a Transesophageal echocardiogram was performed in order to better assess function of the mitral valve. Papillary muscle rupture was identified with resultant flail of the anterior mitral leaflet (Figures 2 and 3). There was severe mitral regurgitation with an eccentric posteriorly directed jet (Figure 4). Upon surgical inspection the posteromedial papillary muscle of the mitral valve was found to be ruptured which resulted in complete flail of the anterior leaflet. It was deemed un-repairable and a porcine bioprosthesis was placed. Bypass vessels were anastomosed to the left anterior descending artery, the first obtuse marginal branch, and the distal right coronary artery.
Figure 2.
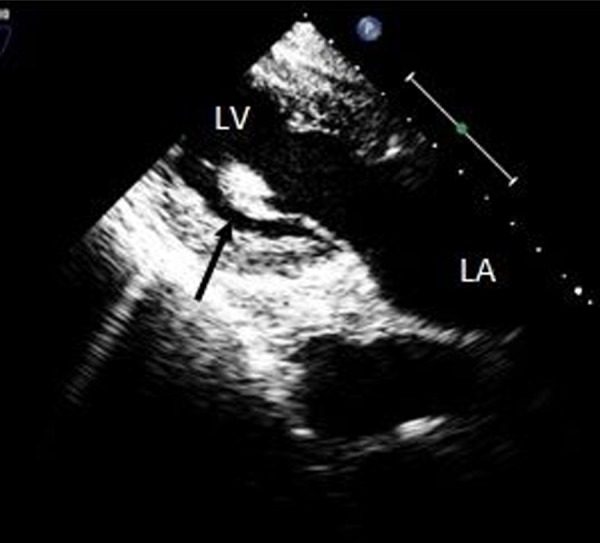
Transesophageal echocardiogram, transgastric long axis view, demonstrating ruptured papillary muscle attached to the anterior leaflet (arrow). LV – Left Ventricle, LA – Left Atrium.
Figure 3.
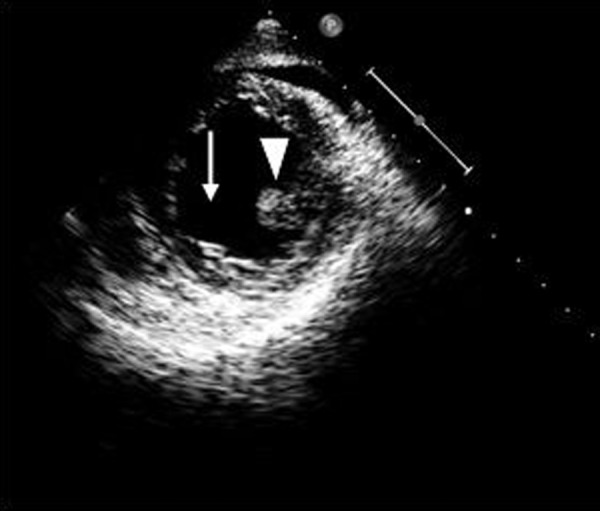
Transesophageal echocardiogram, mid-ventricular short axis view, demonstrating a missing posteromedial papillary muscle (arrow), arrow head indicates anterolateral papillary muscle.
Figure 4.
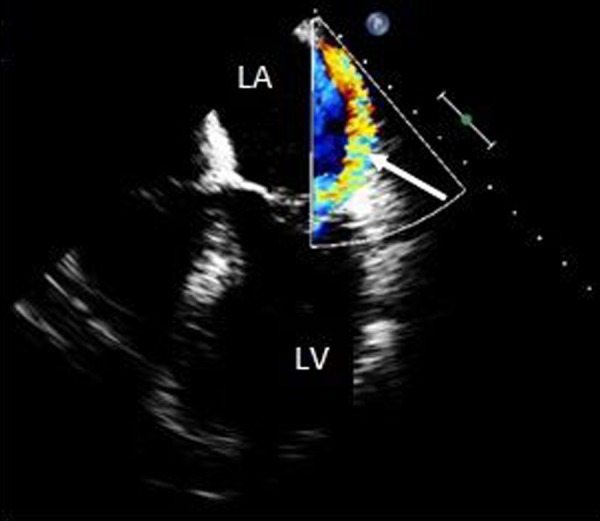
Transesophageal echocardiogram, apical four chamber view, demonstrating a posteriorly directed regurgitant jet by color flow Doppler (arrow). LV – left ventricle, LA – left atrium.
The patient gradually recovered over the next 12 days and was successfully discharged home in good condition. Follow up echocardiogram two weeks after discharge revealed a normal functioning prosthetic mitral valve with normal Doppler flow pattern.
Case 2
A 73 year old male presented to the emergency room complaining of sudden onset chest tightness. He had a known history of coronary artery disease treated surgically by two vessel bypass with left internal mammary artery (LIMA) to left anterior descending artery and a saphenous vein graft to the right coronary artery (RCA). Most recent coronary angiogram revealed patency of the LIMA and 100% occlusion vein graft to the RCA with good collateral flow from the left circumflex artery to the RCA.
Upon presentation his chest discomfort was associated with pain radiating to the back, shortness of breath and diaphoresis. Blood pressure was 92/60 mmHg; heart rate was 100 beats/minute. The patient was not in distress. Cardiovascular exam revealed a regular rhythm without murmur or gallop noted. Point of maximal impulse was not displaced; jugular venous pressure was estimated at 12 cm water. There were no carotid, abdominal, or femoral bruits. The lungs were clear without adventitious sounds. Peripheral pulses were diminished with a weakly palpable but equal posterior tibialis and nonpalpable dorsalis pedis bilaterally. The remainder of the exam was unremarkable.
Electrocardiogram demonstrated inferior Q-waves, prominent R-wave in V2, and ST segment depression in leads II, III, aVF, and V2 through V6. Right sided leads demonstrated ST segment elevation in leads RV5 and RV6, confirming right ventricular infarct. He was emergently transferred to the cardiac catheterization laboratory for possible percutaneous intervention. Coronary angiogram revealed severe stenosis of the ostium of the left circumflex artery suggestive of a ruptured plaque (Figure 5). As stated, he had known total occlusions of his RCA and vein bypass graft to the RCA. The myocardium supplied by the RCA was entirely dependent on collaterals from the left circumflex artery, thus occlusion of the left circumflex artery resulted in infarction of the right ventricle and inferior wall of the left ventricle. Percutaneous angioplasty was attempted but unsuccessful. He was transferred to the coronary care unit for further medical management.
Figure 5.
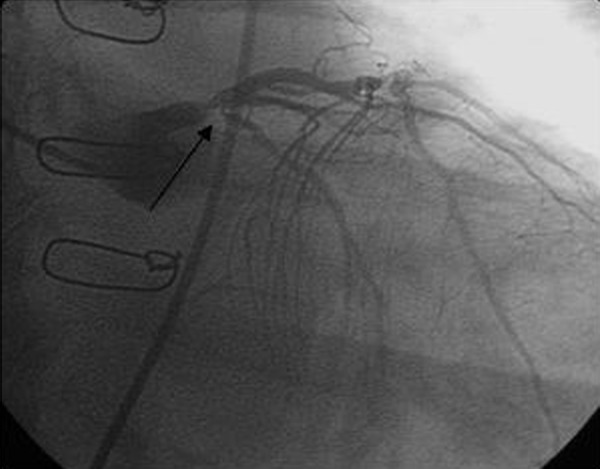
Coronary angiogram demonstrating a high grade lesion at the ostium of the left circumflex artery (arrow).
He was steadily improving until post-infarction day six when he developed sudden onset chest pain, cardiogenic shock, and flash pulmonary edema. Urgent echocardiogram performed at bedside revealed rupture of the posteromedial papillary muscle (Figures 6 and 7) with flailed anterior leaflet causing severe mitral regurgitation with an eccentric posterior directed jet (Figure 8). An intraaortic balloon pump (IABP) was emergently placed for hemodynamic support while preparations were made for emergent surgical intervention. Prior to surgery he suffered a cardiac arrest. Cardiopulmonary resuscitation was attempted but was unsuccessful and the patient expired.
Figure 6.
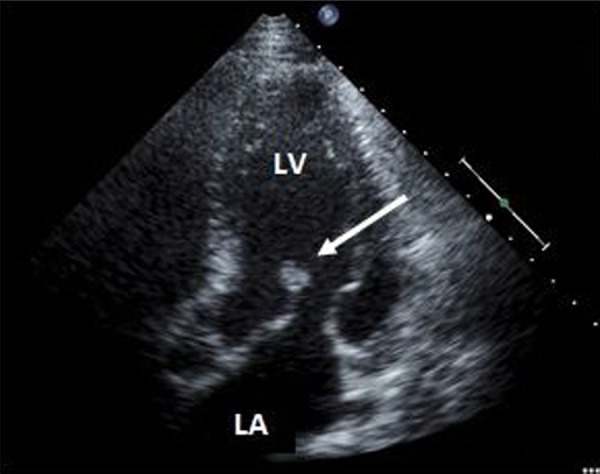
Transthoracic echocardiogram, apical five chamber view, demonstrating ruptured papillary muscle attached to the anterior leaflet (arrow). LV – Left Ventricle, LA – Left Atrium.
Figure 7.
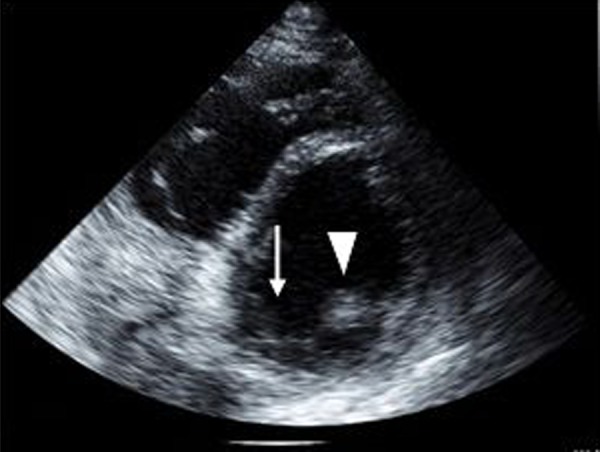
Transthoracic echocardiogram, mid-ventricular short axis view, demonstrating a missing posteromedial papillary muscle (arrow), arrow head indicates anterolateral papillary muscle.
Figure 8.
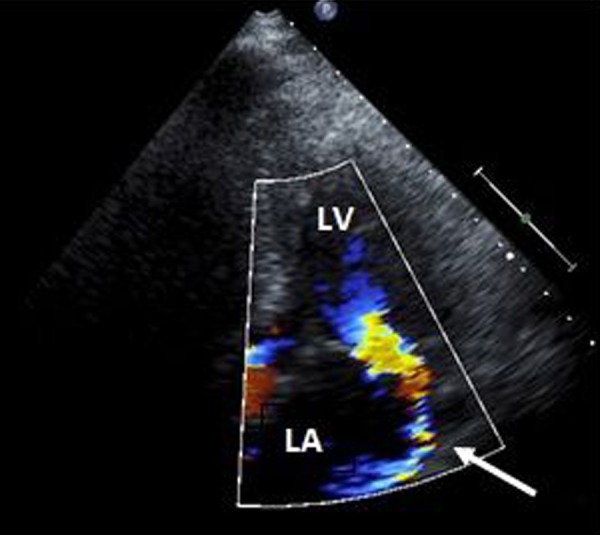
Transthoracic echocardiogram, apical four chamber view, demonstrating a posteriorly directed regurgitant jet by color flow Doppler (arrow). LV – left ventricle, LA – left atrium.
Discussion
Papillary muscle rupture occurs most frequently within two to seven days after a myocardial infarction [4]. During this time period the ventricular muscle is weakened due to infarction and subsequent necrosis and has yet to develop a fibrous scar which provides long-term structural support after MI.
Rupture of the posteromedial papillary muscle occurs much more frequently than rupture of the anterolateral muscle. This is due to differences in blood supply with the posteromedial muscle receiving blood from the posterior descending artery only while the anterolateral muscle receives a duel blood supply from both the left anterior descending and left circumflex arteries. Given the singular blood supply to the posteromedial muscle, about half the cases of rupture occur with relatively small infarcts [5].
Transthoracic echocardiography is useful, and often the initial imaging modality utilized, in the diagnosis of PMR with a sensitivity of 65–85% [6]. The mitral apparatus is a posterior structure; therefore transesophageal echocardiography (which places the ultrasound probe closer to the valve) can offer superior visibility with a diagnostic yield between 95% and 100% [7]. The sensitivity of both echo modalities is improved with the use of color flow Doppler to measure eccentric regurgitant jets [5].
In both cases the PMR occurred within the 2–7 day time period when patients are most susceptible. Case 1 appeared to suffer his myocardial infarction three days prior to PMR, case 2 six days prior. Both patients demonstrated posteriorly directed regurgitant jets indicative of a flail anterior mitral valve leaflet. Rupture of the posteromedial muscle more often affects the posterior valve leaflet. However, as pointed out by Czarnecki et al., both leaflets are attached to both papillary muscles; therefore rupture of either papillary muscle can result in flail of either leaflet [6]. Thus the direction of the regurgitant jet does not reliably predict the papillary muscle involved.
Once identified urgent or emergent surgery is warranted, without surgical repair approximately 90% of patients with PMR will die within one week [9]. The valve should be repaired if possible; however the tissue is often weak and friable making repair difficult or impossible. In such cases the valve should be replaced with prosthesis. Concomitant coronary revascularization (i.e. coronary artery bypass grafting) has been shown to improve both early and long-term survival and should thus be performed [10].
Fortunately, with the increasing use of early and effective revasculation therapies, PMR has become a fairly rare complication. Clinicians should, however, remain aware and be able to diagnose this serious and potentially lethal complication, especially where thrombolytics are still used as a primary treatment for acute coronary syndromes.
Conclusions
Cardiogenic shock developing several days after the onset of angina, an angina- equivalent, or diagnosed infarction may indicate a catastrophic mechanical complication of AMI, such as papillary muscle rupture. Although the incidence appears to be on the decline, clinicians must keep these lethal sequelae in mind when evaluating these unstable patients.
References:
- 1.Yuan S, Jing H, Lavee J. The mechanical complications of acute myocardial infarction: echocardiographic visualizations. Turkish J Thorac Cardiovasc Surg. 2011;19:36. [Google Scholar]
- 2.David TE. Techniques and results of mitral valve repair for ischemic mitral regurgitation. J Card Surg. 1994;9:274. doi: 10.1111/j.1540-8191.1994.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 3.Kishon Y, Oh JK, Schaff HV, et al. Mitral valve operation in postinfarction rupture of a papillary muscle: immediate results and long-term follow-up of 22 patients. Mayo Clin Proc. 1992;67:1023. doi: 10.1016/s0025-6196(12)61116-1. [DOI] [PubMed] [Google Scholar]
- 4.Lavie CJ, Gersh BJ. Mechanical and electrical complications of acute myocardial infarction. Mayo Clin Proc. 1990;65:709. doi: 10.1016/s0025-6196(12)65133-7. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Morrow DA. Braunwald’s Heart Disease – A Textbook of Cardiovascular Medicine. 9th ed. Bonow RO; Saunders, Philadelphia: 2011. Chapter 55- ST-segment elevation myocardial infarction: management; p. 1150. [Google Scholar]
- 6.Czarnecki A, Thakrar A, Fang T, et al. Acute severe mitral regurgitation: consideration of papillary muscle architecture. Cardiovasc Ultrasound. 2008;6:5. doi: 10.1186/1476-7120-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sochowski RA, Chan KL, Ascah KJ, Bedard P. Comparison of accuracy of transesophageal versus transthoracic echocardiography for the detection of mitral valve prolapse with ruptured chordae tendinae (flail mitral leaflet) Am J Cardiol. 1991;67:1251. doi: 10.1016/0002-9149(91)90936-f. [DOI] [PubMed] [Google Scholar]
- 8.Colombo PC, Wu RH, Weiner S, et al. Value of quantitative analysis of mitral regurgitation jet eccentricity by color flow doppler for identification of flail leaflet. Am J Cardiol. 2001;88:534. doi: 10.1016/s0002-9149(01)01733-7. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura RA, Gersh BJ, Schaff HV. The case for an aggressive surgical approach to papillary muscle rupture following myocardial infarction: ‘from paradise lost to paradise regained’. Heart. 2000;83:611. doi: 10.1136/heart.83.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo A, Suri RM, Grigioni F, et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation. 2008;118:1528. doi: 10.1161/CIRCULATIONAHA.107.747949. [DOI] [PubMed] [Google Scholar]


