Abstract
Collaboration between human neuropsychology and comparative neuroscience has generated invaluable contributions to our understanding of human brain evolution and function. Further cross-talk between these disciplines has the potential to continue to revolutionize these fields. Modern neuroimaging methods could be applied in a comparative context, yielding exciting new data with the potential of providing insight into brain evolution. Conversely, incorporating an evolutionary base into the theoretical perspectives from which we approach human neuropsychology could lead to novel hypotheses and testable predictions. In the spirit of these objectives, we present here a new theoretical proposal, the Inferential Brain Hypothesis, whereby the human brain is thought to be characterized by a shift from perceptual processing to inferential computation, particularly within the social realm. This shift is believed to be a driving force for the evolution of the large human cortex.
Keywords: Brain evolution, Neuropsychology, Inference, Olfaction, Comparative neuroanatomy, Primates
INTRODUCTION
The vertebrate brain is highly plastic, supporting vast differences in species-specific behavior while maintaining adaptations necessary for survival. Evolution does not reinvent brains wholesale for each instantiation in each species; common neural features are manipulated by selection to adapt the brain to organismal needs. Indeed, mammalian isocortex (neocortex) allows modular, neural expansion and enhancement of associated cognitive abilities. Through changes in the proportion and organization of isocortex, organisms can allocate processing resources to cognitive and behavioral functions relevant to the organisms' ecological needs. In general, increasing the size of neural regions (relatively or absolutely) enhances associated functional domains. For example, larger olfactory bulbs are associated with better olfactory ability in humans (e.g., Buschhuter et al., 2008) and across species (Barton, 2006; Bhatnagar & Kallen, 1974; Gittleman, 1991), relatively larger hippocampi are associated with spatial proficiency across species (Sherry, Jacobs, & Gaulin, 1992), and visual specialization is associated with visual cortex expansion in primates (Barton, 1998). However, the relationship between size and function is not simplistic—enhanced function may occur through adding new regions, rerouting connections, or changing cell types and distribution (without affecting size). Moreover, enlargements can be pathological, for example, neuronal overgrowth occurs early on in autism (Courchesne, Carper, & Akshoomoff, 2003), and reductions are normal during cortical maturation due to pruning (Jernigan, Trauner, Hesselink, & Tallal, 1991).
Neural tissue is energetically expensive, so we assume that brains provide niche-necessary cognitive resources with little waste. Since brains evolve in a conservative, mosaic manner, systematic changes in a species or clade could point toward adaptive cognitive specializations. Improved understanding of neural evolution can inform the study of human neuropsychology, leading to testable hypotheses of structure-function relationships and constraining biological and psychological frameworks.
Knowledge and theoretical perspectives of neuropsychology and brain evolution are limited by observations, which are in turn limited by the precision of tools and methods. A recent discussion by Bilder (2011) suggests that neuropsychology might benefit from integrating neuroimaging, genomics, and information science—we agree, and would add integration of evolutionary biology, including an understanding of human brain origins and evolution over time to provide a meaningful base upon which to build theoretical principles. In the current essay, we have two objectives: (1) to present a novel hypothesis on human brain evolution, along with a test of some key neurological predictions; and (2) elucidate potential benefits of integrating comparative neuroscience and human neuropsychology.
We hypothesize that a shift from perceptual processing (e.g., chemosensation) to cognitive computation for conspecific evaluation emphasized processing power and drove expansion of the human brain. We call this the Inferential Brain Hypothesis (IBH). Certain neurological predictions of the IBH can be tested using comparative brain data—a particular pattern of expansion and reduction of brain regions across primates and humans. We close with a discussion of the potential for neuropsychological methods to inform the types of comparative brain data we collect. Bridging the gap between animal models and humans has profound implications for our knowledge-base, theoretical views, and translation of scientific results into clinical treatments.
The Inferential Brain Hypothesis
Under the novel framework of the IBH, we hypothesize that human social processing has shifted from a process of perceptual evaluation, whereby evaluations were dependent on intrinsic properties of stimuli, to inferential computation, where information is extracted or inferred from stimuli independent of the intrinsic properties of the stimuli. This placed a premium on cognitive capacity creating a driving force toward larger, more powerful brains. Humans possess many unique cognitive specializations (e.g., symbolic language, innovative tool-use, praxis) that depend on the brain's processing power. We propose the shift to inferential processing is primal compared to other critical transitions in human evolution, as inferential processing is a basic platform on which other transitions build. For example, symbolic language requires the basic assumption that sound strings represent aspects of the environment. This is consistent with our definition of inferential processing, such that the information contained in words and sentences is (in most cases) independent of the sensory qualities of the sounds of words themselves; the same sounds in an incorrect order will be incomprehensible noise.
Chemosignaling serves to attract and evaluate conspecifics for most mammalian species (e.g., as potential mates) (for reviews see Brennan & Keverne, 2004; Halpern & Martinez-Marcos, 2003; Sanchez-Andrade & Kendrick, 2009). The main olfactory system is critical for maternal recognition of lambs and subsequent lamb-ewe bonding (Lévy, Locatelli, Piketty, Tillet, & Poindron, 1995) and for individual identification in rodents (e.g., Matochik, 1988). Primates, in contrast, have evolved conspicuous, non-chemical cues to reproductive fitness and social status. Obvious swellings and coloration changes accompanying ovulation are observed in most primate species and likely serve as visual signals of reproductive susceptibility (Gilad, Wiebe, Przeworski, Lancet, & Pääbo, 2004). Orangutans and humans are noted exceptions and have “concealed” ovulation (referring to the absence of an overt stimulus not a lack of a measurable behavioral effect; for a review see Haselton & Gildersleeve, 2011). Non-human primates have other morphological differences, such as differences in gross, facial morphology between dominant male orangutans and non-dominant, sexually-mature male subordinates, serving as obvious visual cues of social dominance (Kuze, Malim, & Kohshima, 2005). These chemosensory or visual cues provide a direct link between stimulus properties and signal meaning, which require little computation beyond simple association.
Social behavior is fundamental to human survival, success, and personal fulfillment. Despite this, humans lack dramatic demonstrations of chemosensory communication, as in most mammals, and obvious, compensatory visual adaptations as in non-human primates. Useful cues for human social evaluation exist; however, they tend to be highly variable, ambiguous, difficult to detect and interpret, and prone to deception and dissimulation. Moreover, there is no direct relationship between human social signal properties and their intrinsic value. This makes social value difficult to compute, requiring greater cognitive ability to meet the challenges and objectives of social interactions, including reproduction. Importantly, we hypothesize that the brain regions important for conspecific evaluation in mammals remain important for social evaluation in humans. However, the cognitive permutations implemented in these regions are profoundly changed in humans, shifted from sensory identification to inferential computation of social value. We are not suggesting that humans are the sole purveyors of inferential thinking. For example, the ability to use transitive1 inference is common, having been observed in fish (Grosenick, Clement, & Fernald, 2007), birds (e.g., Bond, Kamil, & Balda, 2003; von Fersen, Wynne, Delius, & Staddon, 1991; Weiss, Kehmeier, & Schloegl, 2010), rodents (e.g., Davis, 1992; DeVito, Kanter, & Eichenbaum, 2010), and primates (e.g., Gillan, 1981; MacLean, Merritt, & Brannon, 2008; Treichler & Van Tilburg, 1996). Additionally, orangutans can use inference by exclusion to find food (Marsh & MacDonald, 2011). We propose that humans depend on inferential thought instead of perception, thereby achieving social evaluation goals in a computationally expensive manner.
The IBH, in contrast to other hypotheses that focus on climatological, ecological, or sociological driving forces of human brain enlargement, emphasizes cognition itself as a driving force in brain evolution. This idea may be implicit in other theoretical perspectives, but we suggest that cognition should be recognized as a driving force of brain evolution.
Two theories of brain evolution that are closely related to the IBH warrant mention. The Social Brain Hypothesis suggests that increased sociality is responsible for enhanced cognition and increased brain size (Byrne, 1996; Dunbar, 1998). However, larger group size is not necessarily associated with increased cognitive demand, provided there are mechanisms to facilitate inter-individual information transfer. Massive colonies of social insects succeed because of a sophisticated system of chemical communication. We propose that inferential complexity is a fundamental determining factor in brain evolution. Where for social insects chemical signals are honest2 and absolute, requiring little inferential power, for humans, obvious, honest signaling mechanisms are absent and social value inferences are computationally intense.
The Reinterpretation Hypothesis suggests that hominins3 gained the ability to detect environmental regularities and interpreted these in terms of unobservable causes (Subiaul, Barth, Okamoto-Barth, & Povinelli, 2007). Like our IBH, the Reinterpretation Hypothesis posits that the key factor in human brain evolution was the ability to infer information. The IBH specifically emphasizes the importance of inference in a social context; inferential abilities arose or were enhanced by the need to maintain biologically mandatory social evaluation despite a chemosensory handicap.
In addition to primary olfactory regions (e.g., piriform cortex), the ventromedial prefrontal cortex (vmPFC) and amygdala are both integral components of chemosensory (Gottfried & Zald, 2005) and social processing networks (Adolphs, 1999, 2003; Damasio, 1994). We predict that the vmPFC and amygdala play roles in social processing that reflect their past roles in chemosensation. The vmPFC is involved in processing olfactory valence, whereas the amygdala is involved in processing olfactory intensity (Anderson et al., 2003). The vmPFC may be involved in inferring social values (akin to evaluating the valence value of olfactory stimuli) and the amygdala may be involved in processing the social relevance rather than value per se (akin to evaluating the intensity of olfactory stimuli, insofar as intensity maps on to relevance, where stronger signals are intrinsically more relevant). Damage to either region results in social deficit. VmPFC damage can impair “somatic markers” (including autonomic responses) (Damasio, Tranel, & Damasio, 1990) that normally help bias decisions at the intersection of social and evaluative processing, for example, moral judgment and reasoning (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Koenigs et al., 2007; Young et al., 2010). Amygdala damage results in abnormal sociality, for example, resulting in abnormal interpersonal trust (Koscik & Tranel, 2011) and abnormal deployment of interpersonal space (Kennedy, Glaäscher, Tyszka, & Adolphs, 2009).
The IBH predicts that the human neural homologues to the mammalian chemosensory network will be repurposed to support the shift from perceptual to inferential processing. Specifically, primates will show a decrease in chemosensory abilities associated with reductions in olfactory bulb (OB) volume, and OB reductions in humans will be extreme. This will be decoupled from changes in chemosensory cortical regions. In non-human primates, chemosensory regions will be degraded to a similar extent as the OB, but in humans these reductions will be limited as they are necessary for social inferential functions. The Social Brain and Reinterpretation hypotheses do not predict this mosaic pattern. Those theories present an alternative whereby regional changes in humans will reflect extreme values of a consistent primate trend, for example, both reduced OB and chemosensory cortex. Below we report a qualitative test of these predictions based on comparative brain data on regional brain volumes. Specifically, the null hypothesis is that both chemosensory cortical regions and OB volumes will not deviate from the primate trends, and the IBH presents an alternative hypothesis whereby the chemosensory apparatus, for example, OB volume will follow the primate trend, but chemosensory cortical regions will not.
Comparative Brain Data
Large-scale, multi-species datasets of comparative brain data are relatively few. Data collected by H. Stephan and colleagues (HS dataset; Stephan, Frahm, & Baron, 1981) are widely cited and consist of volumetric measurements of major brain subdivisions from approximately 80 primate and “insectivore” species (also see Baron, Frahm, Bhatnagar, & Stephan, 1983; Baron, Stephan, & Frahm, 1987; Frahm, Stephan, & Stephan, 1982; Stephan, Frahm, & Baron, 1987).
Other datasets exist (e.g., Harvey & Clutton-Brock, 1985; Semendeferi, Damasio, Frank, & Van Hoesen, 1997), but they tend to provide single brain measures (e.g., overall brain weight or volume), comprise fewer species, need further parcellation and measurement to provide regional volumetric information, or focus on specific brain regions.
Using the HS dataset, we grouped available species according to major phylogenetic distinctions varying in phylogenetic distance from humans. These groups include species of non-primate mammals and primates including: strepsirrhines, tarsiers, platyrrhines, cercopithecines, hylobates, panins,4 and humans.5 This grouping scheme categorizes according to higher-order classifications as phylogenetic distance from humans increases.
To measure the potential degradation of chemosensory processes we examined OB volumes and chemosensory cortical regions (piriform cortex, formerly “paleocortex,”6 consisting of: prepiriform cortex, retrobulbar cortex, olfactory tubercles, the lateral olfactory tract, the anterior commissure, and substantia innominata). We also examined differences in overall isocortical volume, hippocampal volume, and amygdala volume. It is inappropriate to compare regional volumes between species as a proportion of overall brain volume, as this would obscure meaningful interspecies variance. Therefore, we calculated the proportion of regional brain tissue as a proportion of medulla volume. Medulla volume is preferred since grade shifts in the relationship between volume and body size are not observed (see Figure 1a) but are observed for overall brain volume (see Figure 1b).
Fig. 1.
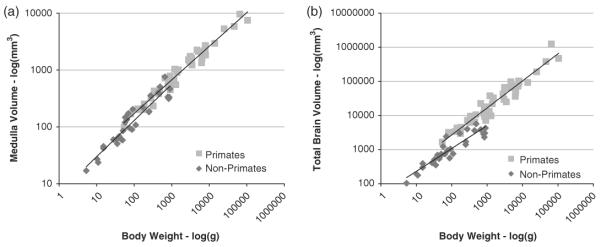
Panel (a) displays the relationship between medulla volume and body weight. Both primates (light gray squares) and non-primates (dark gray diamonds) follow the same relationship, where medulla volume scales with body size. In contrast, total brain volume, Panel (b), does not follow the same relationship between primates and non primates. Instead there is an observable grade shift, such that primates have larger brain volumes by body mass than non-primates. Within groups, total brain volume scales regularly with body size.
Overall, humans have the largest isocortical volumes, and there is a clear trend toward larger isocortical volumes phylogenetically closer to humans (see Figure 2). In contrast, hippocampus and amygdala volumes remain relatively constant across species (see Figure 3a & 3b). This supports the notion that hippocampal memory functions and amygdalar emotional and learning processes are necessary for the success of an organism regardless of ecological niche.
Fig. 2.
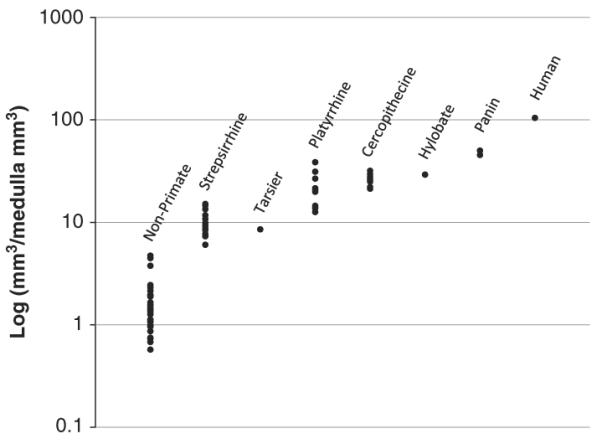
Volume of isocortex proportional to medulla volume. Data are grouped according species groups, progressively more closely related to humans to the right. A clear increase in proportional isocortex volume is visible across species, where the species more closely related to and including humans have the largest volumes.
Fig. 3.
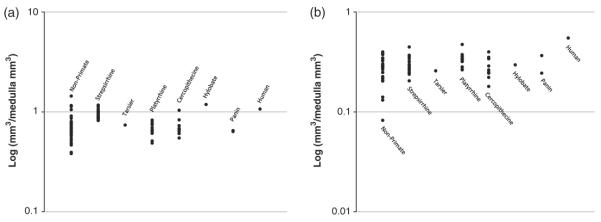
Proportional hippocampal (Panel a) and amygdala volume (Panel b). Volumes of both of these structures appear to maintain their proportions across species.
Olfactory bulbs decrease in volume across groups, most extremely in humans; all primates except strepsirrhines are outside the range of non-primate mammals (see Figure 4a). A decrease in olfactory abilities across primates is well documented, and coupled with an increase in visual abilities, is characteristic of primates (for a review see Preuss, 2007). Indeed, the decline in chemosensory abilities may be a direct result of the evolution of trichromatic vision (Liman & Innan, 2003; Zhang & Webb, 2003).
Fig. 4.
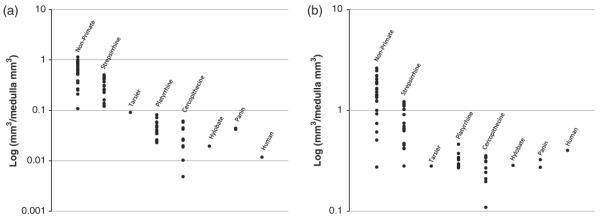
The proportional volume of the olfactory bulbs (Panel a) displays a clear trend of decrease in proportional volume across primate species. Humans display the greatest reduction in olfactory bulb volume. Piriform cortex volume (Panel b) is decreased in most primates compared to non-primates, with limited reductions in strepsirrhines. Humans buck this trend and display piriform volumes larger than the observed range of all but one of the cercopithecines and strepsirrhine primates.
In a similar manner, piriform cortex decreases in volume across primates, however humans are an exception (see Figure 4b). Indeed, human piriform cortex volume is greater than the maximum value for all but one other species of haplorhine7 primate. It is possible that the lack of reduced piriform volume is a by-product of evolution of some other trait. However, it is not obvious that this is the case given that humans are the only species examined in the opposite direction to the primate trend, and humans do so for piriform cortex only. This conspicuous lack of decrease in piriform cortex volume specific to humans may point to adaptations where former chemosensory regions are repurposed for inferential processing as postulated by the IBH. These conclusions are limited since the many species are represented by a single individual, potentially obscuring significant intraspecies variation.
Neuropsychological Methods Applied to Evolutionary Neurobiology
Understanding what is known about human evolution can allow us to make speculations and formulate testable hypotheses in human neuropsychology (such as the IBH). Our theories and hypotheses are ultimately limited by the quality of the data we collect; better predictions can be made with better knowledge, which is attained in turn through better observation. That being said, comparative neuroscience has made revolutionary insights into brain function. Primate studies (especially lesion studies and single cell recording methodologies which are mostly untenable in humans) have provided insight into major cognitive processes and their neuroanatomy, including: dorsolateral prefrontal cortex contributions to working memory (for a review, see Levy & Goldman-Rakic, 2000), the conceptualization of dual “what” and “where” visual processing streams (Mishkin, Ungerleider, & Macko, 1983), the contribution of medial temporal lobe structures to memory (for a review see Squire & Zola-Morgan, 1991), and potential relationships between mirror neuron systems, language evolution, and tool use (Rizzolatti & Arbib, 1998; Rizzolatti, Luppino, & Matelli, 1998, p. 283–296).
Modern neuroimaging techniques could further revolutionize our understanding of the human brain and properly root our knowledge in an evolutionary context. Advances in ultra-high field MRI allow high resolution imaging of micro-structural neural composition. Moreover, modern techniques explore both structural (i.e., diffusion tensor imaging, DTI) and functional (i.e., resting-state functional connectivity MRI, fcMRI) interconnections and how information is routed throughout networks. Discovering how neural connectivity differs across species and how brain networks have evolved would add significantly to our knowledge of human brain evolution and facilitate neuropsychological predictions of human brain function. DTI and fcMRI applied across many species might further revolutionize our understanding of human neuropsychology and brain evolution in general (for detailed discussion, see Preuss, 2010, 2011).
DTI could facilitate cross-species comparisons of connectivity, elucidating how existing connections change in strength and importance and how connections are added, lost or rerouted. For example, the arcuate fasciculus, a prominent, human fronto-temporal pathway, is smaller in chimpanzees and possibly absent in macaques (Rilling et al., 2008). Damage to this pathway in humans leads to “conduction aphasia” (Damasio & Damasio, 1980), suggesting that this connection is a critical component in the evolution of human language (Rilling et al., 2008).
FcMRI could provide task-independent8 measurement of functional relationships (limited by the magnet's bore and animal's size) and could provide a novel means to identify homologous regions by their functional relationships across species. Moreover, since fcMRI can be used with sedation (Kiviniemi et al., 2005; Martuzzi, Ramani, Qiu, Rajeevan, & Constable, 2010; Peltier et al., 2005; Vincent et al., 2007) a large number of species could be easily and safely studied. For example, comparison of humans and macaques demonstrates conserved patterns of precuneus functional connectivity suggesting it should be divided into sensorimotor, cognitive/associative, and visual subregions (Margulies et al., 2009). By extending these methods to the study of a phylogenetically diverse set of species, we can begin to address the evolutionary forces and phylogenetic trends shaping brains over time.
CONCLUSION
The study of brain evolution could benefit greatly from methods and techniques used by cognitive neuroscience and neuropsychology, and in turn, neuropsychology could benefit from incorporating comparative data on human brain evolution. The Inferential Brain Hypothesis is an example of how understanding changes in structure-function relationships of brain regions between humans and our evolutionary relatives can allow us to predict the functions of human brain regions. In general, theoretical perspectives on the functions of particular regions of the human brain can be grounded on a solid, evolutionary base by understanding the driving forces behind evolution across species. A sharing of techniques and expertise would mutually benefit human neuropsychology and comparative neuroscience. This cross-talk could point to new and exciting scientific avenues and could lead to a deeper link between humans and the rest of the animal kingdom.
ACKNOWLEDGMENTS
The information in this manuscript and this manuscript itself have never been published electronically or in print. Several of the key concepts are published in the primary author's doctoral dissertation.
This work was supported by the National Institute of Neurological Disorders and Stroke (D.T., P50 NS19632); National Institute on Drug Abuse (D.T., R01 DA022549); and the Natural Sciences and Engineering Research Council of Canada (T.K., NSERC PGS-D).
Footnotes
Transitive here refers to the logical or mathematical notion that given a relationship between the first and second elements (e.g., A > B) and the second and third elements (e.g., B > C); the relationship necessarily holds between the first and third elements (e.g., A > C).
Honest here refers to the notion that these signals are not susceptible to deception or dissimulation on the part of the signaller and can, thus, be counted on as reliable by the receivers of the signal.
This adaptation is assumed to predate the emergence of Homo sapiens rather is a defining characteristic of the hominin clade.
Non primate mammals include: “insectivores”, 10 species of shrews, Order Soricomorpha; 13 species of tenrecs, Order Afrosoricida; 3 species of hedgehogs, Order Erinaceus; 2 species of elephant shrews, Order Macro-scelidea; and 3 species of treeshrews, Order Scandentia; strepsirrhines include 17 species of lemur, aye-aye, galago, and loris, Suborder Strepsirrhini; tarsiers include 1 species of tarsier, Infraorder Tarsiiformes; platyrrhines include 13 species of New World monkey, Parvorder Platyrrhini; cercopithecines include 10 species of Old World monkey, Family Cercopithecidae; Hylobates include 1 gibbon species, Family Hylobatidae; panins include 2 species of non-human hominids, chimpanzees and gorillas, Family Hominidae; and humans.
The reported values for humans represent a single individual included in the HS dataset (and importantly measured the same way as other species). This individual is well within the normal range of human brain size (1.12–1.88 dm3, including men and women) (Luders, Steinmetz, Jäncke, 2002). The many of species sampled include a single individual, species values represent mean values if more than one individual was measured. See the HS Dataset for more detailed information on sampling.
The term piriform cortex is preferred here as paleocortex may conjure incorrect notions of progression in evolution where differences between clades do not reflect a ranking from lower and older to higher and newer forms.
Haplorhine refers to all non-strepsirrhine primates.
fMRI experiments are limited by the quality of the tasks that can be performed within the confines of an MR scanner. This limitation is compounded in a comparative context by the difficulty of developing tasks that are equivalent across species.
The authors have no financial or conflicting interest affecting this manuscript.
REFERENCES
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3(12):469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2(11):1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Baron G, Frahm HD, Bhatnagar KP, Stephan H. Comparison of brain structure volumes in Insectivora and Primates. III. Main olfactory bulb (MOB) Journal für Hirnforschung. 1983;24(5):551–568. [PubMed] [Google Scholar]
- Baron G, Stephan H, Frahm HD. Comparison of brain structure volumes in Insectivora and primates. VI. Paleocortical components. Journal für Hirnforschung. 1987;28(4):463–477. [PubMed] [Google Scholar]
- Barton RA. Visual specialization and brain evolution in primates. Proceedings of the Royal Society B: Biological Sciences. 1998;265(1409):1933–1937. doi: 10.1098/rspb.1998.0523. doi:10.1098/rspb.1998.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA. Olfactory evolution and behavioral ecology in primates. American Journal of Primatology. 2006;68(6):545–558. doi: 10.1002/ajp.20251. [DOI] [PubMed] [Google Scholar]
- Bhatnagar KP, Kallen FC. Cribriform plate of ethmoid, olfactory bulb and olfactory acuity in forty species of bats. Journal of Morphology. 1974;142(1):71–89. doi: 10.1002/jmor.1051420104. [DOI] [PubMed] [Google Scholar]
- Bilder RM. Neuropsychology 3.0: Evidence-based science and practice. Journal of the International Neuropsychological Society. 2011;17(01):7–13. doi: 10.1017/S1355617710001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Animal Behaviour. 2003;65(3):479–487. [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Current Biology. 2004;14(2):R81–R89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Buschhuter D, Smitka M, Puschmann S, Gerber JC, Witt M, Abolmaali ND, Hummel T. Correlation between olfactory bulb volume and olfactory function. Neuroimage. 2008;42(2):498–502. doi: 10.1016/j.neuroimage.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Byrne RW. Machiavellian intelligence. Evolutionary Anthropology. 1996;5(5):172–180. [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–340. doi: 10.1001/jama.290.3.337. doi:10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error: Emotion, reason, and the human brain. Putnam; New York: 1994. [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social-stimuli. Behavioural Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. The anatomical basis of conduction aphasia. Brain. 1980;103(2):337–350. doi: 10.1093/brain/103.2.337. [DOI] [PubMed] [Google Scholar]
- Davis H. Transitive inference in rats. (Rattus norvegicus) Journal of Comparative Psychology. 1992;106(4):342–349. doi: 10.1037/0735-7036.106.4.342. doi:10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- DeVito LM, Kanter BR, Eichenbaum H. The hippocampus contributes to memory expression during transitive inference in mice. Hippocampus. 2010;20(1):208–217. doi: 10.1002/hipo.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;6(5):178–190. [Google Scholar]
- Frahm HD, Stephan H, Stephan M. Comparison of brain structure volumes in Insectivora and Primates. I. Neocortex. Journal für Hirnforschung. 1982;23(4):375–389. [PubMed] [Google Scholar]
- Gilad Y, Wiebe V, Przeworski M, Lancet D, Pääbo S. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2004;2(1):e5. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan DJ. Reasoning in the chimpanzee: II. Transitive inference. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7(2):150–164. [Google Scholar]
- Gittleman JL. Carnivore olfactory bulb size: Allometry, phylogeny and ecology. Journal of Zoology. 1991;225(2):253–272. [Google Scholar]
- Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates. Brain Research Reviews. 2005;50(2):287–304. doi: 10.1016/j.brainresrev.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445(7126):429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: An update. Progress in Neurobiology. 2003;70(3):245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Clutton-Brock TH. Life history variation in primates. Evolution. 1985;39(3):559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Gildersleeve K. Can men detect ovulation? Current Directions in Psychological Science. 2011;20(2):87–92. doi:10.1177/0963721411402668. [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(5):2037–2049. doi: 10.1093/brain/114.5.2037. doi:10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Gläscher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nature Neuroscience. 2009;12(10):1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi VJ, Haanpää H, Kantola J-H, Jauhiainen J, Vainionpää V, Alahuhta S, Tervonen O. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magnetic Resonance Imaging. 2005;23(4):531–537. doi: 10.1016/j.mri.2005.02.009. doi:10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446(7138):908–911. doi: 10.1038/nature05631. doi:10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. The human amygdala is necessary for developing and expressing normal interpersonal trust. Neuropsychologia. 2011;49(4):602–611. doi: 10.1016/j.neuropsychologia.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuze N, Malim TP, Kohshima S. Developmental changes in the facial morphology of the Borneo orangutan (Pongo pygmaeus): Possible signals in visual communication. American Journal of Primatology. 2005;65(4):353–376. doi: 10.1002/ajp.20121. [DOI] [PubMed] [Google Scholar]
- Lévy F, Locatelli A, Piketty V, Tillet Y, Poindron P. Involvement of the main but not the accessory olfactory system in maternal behavior of primiparous and multiparous ewes. Physiology & Behavior. 1995;57(1):97–104. doi: 10.1016/0031-9384(94)00200-o. doi:10.1016/0031-9384(94)00200-o. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133(1):23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3328–3332. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Steinmetz H, Jäncke L. Brain size and grey matter in the healthy human brain. Neuroreport. 2002;13(17):2371–2374. doi: 10.1097/01.wnr.0000049603.85580.da. [DOI] [PubMed] [Google Scholar]
- MacLean EL, Merritt DJ, Brannon EM. Social complexity predicts transitive reasoning in prosimian primates. Animal Behaviour. 2008;76(2):479–486. doi: 10.1016/j.anbehav.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HL, MacDonald SE. Information seeking by orangutans: A generalized search strategy? Animal Cognition. 2011 doi: 10.1007/s10071-011-0453-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage. 2010;49(1):823–834. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA. Role of the main olfactory system in recognition between individual spiny mice. Physiology & Behavior. 1988;42(3):217–222. doi: 10.1016/0031-9384(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. Trends in Neurosciences. 1983;6(0):414–417. doi:10.1016/0166-2236(83)90190-x. [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16(3):285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas J, editor. Evolution of nervous systems: A comprehensive review. Vol. 4. Elsevier; New York: 2007. [Google Scholar]
- Preuss TM. Reinventing primate neuroscience for the twenty-first century. Primate Neuroethology. 2010;1(9):422–454. [Google Scholar]
- Preuss TM. The human brain: Rewired and running hot. Annals of the New York Academy of Sciences. 2011;1225:E182–E191. doi: 10.1111/j.1749-6632.2011.06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TEJ. The evolution of the arcuate fasciculus revealed with comparative DTI. Nature Neuroscience. 2008;11(4):426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends in Neurosciences. 1998;21(5):188–194. doi: 10.1016/s0166-2236(98)01260-0. doi:10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: New concepts. Electroencephalography and Clinical Neurophysiology. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, Kendrick KM. The main olfactory system and social learning in mammals. Behavioural Brain Research. 2009;200(2):323–335. doi: 10.1016/j.bbr.2008.12.021. doi:10.1016/j.bbr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H, Frank R, Van Hoesen GW. The evolution of the frontal lobes: A volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. Journal of Human Evolution. 1997;32(4):375–388. doi: 10.1006/jhev.1996.0099. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Jacobs LF, Gaulin SJC. Spatial memory and adaptive specialization of the hippocampus. Trends in Neurosciences. 1992;15(8):298–303. doi: 10.1016/0166-2236(92)90080-r. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. doi:10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatologica. 1981;35(1):1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm HD, Baron G. Comparison of brain structure volumes in Insectivora and primates. VII. Amygdaloid components. Journal für Hirnforschung. 1987;28(5):571–584. [PubMed] [Google Scholar]
- Subiaul F, Barth J, Okamoto-Barth S, Povinelli DJ. Human cognitive specializations. In: Kaas J, editor. Evolution of nervous systems: A comprehensive review. Vol. 4. Elsevier; New York: 2007. [Google Scholar]
- Treichler FR, Van Tilburg D. Concurrent conditional discrimination tests of transitive inference by macaque monkeys: List linking. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22(1):105–117. [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- von Fersen L, Wynne CD, Delius JD, Staddon JE. Transitive inference formation in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17(3):334–341. doi: 10.1037//0097-7403.17.3.281. [DOI] [PubMed] [Google Scholar]
- Weiss BM, Kehmeier S, Schloegl C. Transitive inference in free-living greylag geese, Anser anser. Animal Behaviour. 2010;79(6):1277–1283. [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio A. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65(6):845–851. doi: 10.1016/j.neuron.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Webb DM. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8337–8341. doi: 10.1073/pnas.1331721100. [DOI] [PMC free article] [PubMed] [Google Scholar]


