Abstract
Background:
The effect of BRCA1/2 gene test result on anxiety, depression, cancer-related thought intrusion or avoidance and perceived control over cancer risk was assessed in breast cancer (BC) patients, according to their perceived probability of genetic predisposition to cancer.
Methods:
Two hundred and forty-three (89% response rate) women with BC completed questionnaires after an initial genetic counselling visit (T1), of which 180 (66%) completed questionnaires again after receiving the BRCA1/2 results (T2). The discrepancy between women's perceived probability of cancer genetic predisposition at T1 and the geneticist's computed estimates was assessed.
Results:
In all, 74% of women received a negative uninformative (NU), 11% a positive BRCA1/2 and 15% an unclassified variant (UV) result. On hierarchical regression analysis, in women with a positive BRCA1/2 result (vs NU or UV), a lower perceived probability of cancer genetic predisposition than objective estimates at T1 predicted lower levels of anxiety at T2 (β=−0.28; P<0.01), whereas in women receiving a UV result (vs NU or positive BRCA1/2), a lower perceived probability of cancer genetic predisposition than objective estimates at T1 predicted higher levels of anxiety (β=0.20; P<0.01), depression (β=0.19; P<0.05) and intrusion (β=0.18; P<0.05) at T2.
Conclusion:
The type of BRCA1/2 test result differently affects distress according to women's perceived probability of genetic predisposition before testing.
Keywords: BRCA1/2 testing, anxiety, depression, stress reactions, perceived probability of genetic predisposition to cancer
Breast cancer (BC) is the most common cancer in women worldwide and a family history of BC is among the best recognised BC risk factors. In 15–30% of patients from high-risk families, BC is caused by a germline mutation in the BRCA1 or BRCA2 (BRCA1/2) gene (Wevers et al., (2011)). Women with BC who carry a mutation in the BRCA1/2 gene have an increased risk of developing a second primary BC (Kirova et al, 2010). They also present up to 40% lifetime risk of developing ovarian cancer (Chen and Parmigiani, 2007).
BRCA1/2 testing is primarily proposed to women (index cases) in the family who developed BC as the probability of identifying a mutation is highest when testing starts with an affected woman. Identification of a BRCA1/2 mutation in women with BC may inform potential decisions for preventive oophorectomy or mastectomy and can have implications for the relatives' cancer risks and the likelihood that cancer is due to a genetic mutation in the family.
Research on the psychological impact of BRCA1/2 genetic testing initially focused on the response to a positive, compared with a negative, test result mainly in individuals unaffected with cancer (Meiser, 2005). Although women who received a BRCA1/2 negative test result evidence a decrease in distress (Croyle et al, 1997; Schwartz et al, 2002), BRCA1/2 mutation carriers may experience increased distress shortly after test result disclosure (Meiser et al, 2002; Van Roosmalen et al, 2004; Watson et al, 2004), which abates over the following years (Halbert et al, 2011; Graves et al, 2012).
However, in about 80% of cases, a BRCA1/2 mutation in the first person tested in a BC high-risk family is not identified. In an additional 12.5% of cases, unclassified variants (UV), for which the effect on the protein function or the gene is still unknown, are detected (Vink et al, 2005). In BC patient index cases, a negative (i.e., no deleterious mutation found) or UV results are both ‘uninformative', also referred as ‘inconclusive' such results do not significantly decrease the probability of cancer genetic predisposition in families with a high number of breast and ovarian cancer cases; however, no clear consensual risk management guidelines can be proposed (Gadzicki et al, 2011).
An uninformative BRCA1/2 result may lead to potential distress (O'Neill et al, 2009), misunderstanding (Cypowyj et al., 2009), feeling less in control over the stress of cancer risk (Claes et al, 2004) or decisional conflicts (Rini et al, 2009). Previous results indicate lower levels of distress in women receiving an uninformative result compared with those receiving a positive BRCA1/2 test result (Dorval et al, 2005; Beran et al, 2008; Smith et al, 2008), but risk management decisions have shown both no ‘false reassurance' (i.e., women maintain appropriate risk management intentions) (Dorval et al, 2005; van Dijk et al, 2005) and infrequent surveillance (Vos et al, 2012).
Although emotional reactions to a negative uninformative (NU) (as opposed to a true negative where individuals are found non-carriers of their family's mutation) or UV results appears similar (van Dijk et al, 2004; Smith et al, 2008), a UV result has recently predicted genetic testing distress (O'Neill et al, 2009) and, associated with overestimation of cancer risk, has led to high distress and intention to undergo prophylactic interventions similar to that induced by a positive BRCA1/2 test result (Vos et al, 2012), suggesting that the communication of these ambiguous results elicits a ‘false alarm'.
High perceived cancer risks (Hopwood, 2000; Vos et al, 2010, 2012) or heredity likelihood (Vos et al, 2010, 2012), or high expectation of carrying a predisposing mutation (O'Neill et al, 2009), are often associated with high levels of distress. Perceptions of cancer risks or of the probability of genetic predisposition to cancer integrate knowledge (i.e., the recall of information gathered, e.g., from health-care professionals, relationships or the media) as well as feelings and subjective interpretations (van Dooren et al, 2004; Vos et al, 2012).
To our knowledge, only two studies prospectively addressed the emotional impact of BRCA1/2 testing in BC patients considering separately a NU or UV result (O'Neill et al, 2009; Vos et al, 2012). So, this study was designed firstly to address the specific effect of the type of BRCA1/2 test result received (positive vs NU or UV vs NU) on BC patients in terms of anxiety, depression, cancer-related thought intrusion or avoidance and perceived personal control.
Secondly, the BRCA1/2 test result has been shown to affect distress via the perception of cancer risks (i.e., the recall of factual information associated with the test result and its interpretation; Vos et al, 2012). In the present study, we wanted to address of patients' risk perception before disclosure of test result. This perception may be related to genetic counselling, which may vary across cultures. In BC patients, cancer (recurrence) risk perception may be experienced not solely in relation to the genetic predisposition to cancer, so we only focused on the perceived probability of genetic predisposition to cancer as a moderator of psychological outcomes. We appraised how far BC patients' perception of the probability of genetic predisposition to cancer was from objective estimates.
We paid particular attention to the perceived probability of genetic predisposition to cancer because of the potential influence of inadequate expectations on emotional responses (Phelps et al, 2008; Hilgart et al, 2010) and the potential implication of this relationship on improvement of cancer genetic counselling communication.
To focus on the BRCA1/2 test result and the discrepancy between perceived probability of carrying a predisposing mutation and objective estimates, we controlled for other potential factors of distress, including higher initial distress (Meiser, 2005; Smith et al, 2008), age (Schlich-Bakker et al, 2006), children (Meiser, 2005), recency of BC diagnosis (van Roosmalen et al, 2004), and high family pedigree-based risk (Meiser, 2005; van Dijk et al, 2006; O'Neill et al, 2009), being young when a parent was affected (van Oostrom et al, 2006).
Patients and methods
This study protocol was approved by the Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé (CCTIRS: Consultative committee for information management in health research – DGRI CCTIRS MG/CP°08.42) and by the Commission Nationale Informatique et Libertés (CNIL: French Information Technology and Privacy Commission). All recruited women provided written informed consent.
Participants and procedure
From November 2008 to December 2009, women aged >18 years, eligible for BRCA1/2 testing and the first woman to be tested in the family (index case) with a personal history of BC were consecutively recruited at the cancer genetic counselling unit of the Ensemble Hospitalier Institut Curie, Institut Gustave Roussy (June-December 2009) and Tenon Hospital (March–September 2009) in the Paris region (France). A minimum sample size of 200 was required to allow for comparisons between BRCA1/2 deleterious vs NU or UV vs NU test results. Women with a personal history of ovarian cancer or a major psychiatric disorder were not included.
The study objectives were explained to the women on the day of the initial cancer genetic counselling visit (T1) and, when they agreed to participate, they were given questionnaires to fill in at home and return within 2 weeks. At the BRCA1/2 test result notification visit (T2), they received another set of questionnaires to be filled in at home and returned within 2 weeks. When necessary, a reminder call was made at both the time points. Questionnaires not received within 21 days of the genetic visit were considered to be missing.
Genetic counselling
In our centres, at the initial genetic consultation with a geneticist or a genetic counsellor, patients are informed about hereditary cancer risks and the genetic testing process. The hereditary risk is evaluated and, if testing is indicated, patients are provided with further medical information. Although practice may vary across clinicians, information most systematically provided at that time comprises the risk of genetic predisposition to cancer, cancer risks (breast or ovarian) and the probability that an index case may be a carrier of a BRCA1/2 mutation. The probability of genetic predisposition to cancer is provided in terms of ‘moderate', ‘high' or ‘very high'. No figure is provided and patients are not informed of the possibility of receiving a UV.
At disclosure of the BRCA1/2 test result, women receiving an uninformative BRCA1/2 test result are generally told that no deleterious gene mutation was found, which does not preclude a possible yet unknown genetic predisposition explaining the family history of cancer. When a UV is identified, women are told that this result does not allow for concluding that a causal mutation was found; however, clinicians will be kept informed whether this mutation is reclassified as deleterious in the future.
Instruments
Outcome measures
General distress (i.e., anxiety and depression) was measured by the Hospital Anxiety and Depression French version, anxiety (HADS-Anxiety) and depression (HADS-Depression) subscales (Zigmond and Snaith, 1983; Razavi et al, 1990).
The Impact of Event Scale (IES) (Horowitz et al, 1979) measuring psychological reactions (i.e., thoughts or feelings of intrusion or avoidance) to a stressful event (i.e., in this case cancer risks) has been validated in the setting of hereditary BC risk to address cancer-specific distress (Thewes et al, 2001). Items of the HADS-Anxiety and -Depression and IES-Intrusion and Avoidance scales both concern frequency of thoughts or feelings during the previous week.
The Perceived Personal Control (PPC) scale consists of a measure of the genetic counselling impact (Berkenstadt et al, 1999). This concept is central to coping with health threats and refers to the ‘beliefs that one has at one's disposal a response that can influence the aversiveness of an event' (Thompson, 1981). Recent validation studies recommend a one-dimension scale (Smets et al, 2006; McAllister et al., (2012)). A PPC French version measuring perceived personal control over the genetic risk of cancer was produced following a forward–backward translation process.
All these outcome measures, completed at both T1 and T2, presented adequate internal consistencies with Cronbach's alpha >0.70.
Predictor measures
Perceived probability of genetic predisposition to cancer assessed at T1 and perceived risks of breast or ovarian cancer assessed at T2 were each measured by two items; the first in terms of absolute figure using a visual analogue scale ranging from 0% (‘no risk') to 100% (‘maximum risk') and the second in terms of comparative figure to age-matched general population women, using a five-level categorical scale with responses ranging from ‘much lower' to ‘much higher'. As the absolute and comparative evaluations for each perceived probability or risks assessed were strongly correlated (r=0.41 for perceived probability of genetic predisposition to cancer; r=0.50 for BC risk perception, r=0.53 for ovarian cancer risk perception), scores of the absolute and comparative evaluation items were standardised and their sum was averaged providing a total score based on a two-item scale.
Objective estimates of cancer genetic predisposition probability were expressed as a percentage, computed at T1 by the genetic counsellor. The model used is derived from the results of the segregation analysis published by Claus et al (1991) and is based on the LINKAGE programme developed by Lathrop and Lalouel (1984). The use of the LINKAGE programme allows taking into account the number of breast and ovarian cancers in the family, their distribution among relatives and the age at onset of BCs and thus estimating the probability that a dominant, highly penetrant allele is running in the family whatever the gene involved. These values represented reference points according to which the woman's perceived probability of genetic predisposition to cancer was compared and the degree of discrepancy of their perception with objective figures estimated.
Statistical analysis
For each multi-item scale, missing data were replaced by the mean value of the scale when at least half of the items on that scale had been completed. All items of psychometric instruments presented <1% of missing data; however, individual risk or probability perception items comprised 1–27% of missing responses.
The discrepancy between objective estimates and perceived probability of genetic predisposition to cancer was computed using standardized residuals of the objective cancer genetic predisposition probability variable regressed on the perceived probability of genetic predisposition to cancer-dependent variable. Mean (s.d.) of this variable was 0.00 (1.00) ranging from −2.34 to 2.06; a positive value indicates lower perceived probability of genetic predisposition to cancer than objective estimates.
Multiple regression analyses were performed on the dependent outcome variables that is, HADS-Anxiety, HADS-Depression, IES-Intrusion, IES-Avoidance and PPC at T2. For each regression model, we controlled for sociodemographic (age, education level above secondary school or not, having a partner or not, daughters or not), clinical data, time interval between initial genetic counselling visit and BRCA1/2 test result notification, breast and ovarian cancer risk perception at T2, perceived cancer genetic predisposition probability and its difference with respect to objective estimates, as well as for T1 scores of the outcome variable. Due to our sample characteristics, recency of the BC diagnosis was measured using the dichotomised ‘undergoing treatment' or ‘in remission' variable. Family cancer history and being young when a parent was affected was assessed by the number of relatives diagnosed with breast or ovarian cancer before 50 years of age. We also assessed the number of deceased relatives in different regression models and also tested regression models without all risk perception variables as covariates. This led to similar statistical results (not reported). Hierarchical multiple regressions were performed (Tabachnick and Fidell, 2007) in which these covariates were introduced into a first block, the BRCA1/2 result into a second block and the interaction between the degree of discrepancy between the perceived probability of genetic predisposition to cancer and objective estimates and the BRCA1/2 result into a third block.
Interaction graphs represent the relationship, for the different BRCA1/2 results, between the degree of discrepancy between the perceived probability of genetic predisposition to cancer and objective estimates assessed at T1 and the variables at T2 representing anxiety, depression, intrusion or avoidance and perceived personal control over cancer risk, in the multiple regression models, setting continuous covariates (e.g., age, number of affected relatives) at the mean and dichotomous covariates at the mode (e.g., having a partner).
Statistical analyses were performed with SPSS software version 18.0 (IBM, Somers, NY, USA) and interaction graphs were drawn and their estimates computed using R 2.15.0.
Results
Sample characteristics
Two hundred and seventy-three women were recruited. Of these, 30 (11%) at T1 and, of the 243 respondents at T1, 63 (23%) at T2 did not provide evaluable data for the following motives: unwilling to participate in the study (13 women), not returning or completing questionnaires within the protocol time frame (74), declining to attend the second cancer genetic counselling visit (3) or deceased (3). Respondents and non-respondents at T1 differed significantly only in terms of medical status (P=0.006) (Table 1).
Table 1. Descriptive characteristics at initial consultation (T1).
| Respondents (N=243) | Non-respondents (N=30) | |
|---|---|---|
|
Age (years) | ||
| Mean (s.d.) |
47.3 (11.4) |
49 (13.5) |
|
Education level (N/%) | ||
| < high school | 84 (35) | |
| ⩾ high school | 156 (65) | — |
| Missing data |
3 |
|
|
Family status (N/%) | ||
| Living with a partner (N/%) | 193 (79) | 12 (71) |
| Missing data |
— |
13 |
| Having children (N/%) | 184 (76) | 25 (86) |
| Missing data |
— |
1 |
| Having daughters (N/%) |
148 (61) |
19 (65) |
|
Medical status (N/%)a | ||
| Under treatment | 127 (52) | 14 (87) |
| In remission | 116 (48) | 2 (13) |
| Missing data |
— |
14 |
|
Number of first-degree relatives with cancer | ||
| Mean (s.d.) |
1.2 (1.0) |
1.4 (1.2) |
|
Number of sceond-degree relatives with cancer | ||
| Mean (s.d.) |
1.8 (1.4) |
1.6 (1.3) |
|
Objective estimate of cancer genetic predisposition risk (N/%)b | ||
| (Mean/s.d.) | 42.5 (29.2) | 49.0 (31.8) |
| ⩽ 20% | 73 (32) | 10 (36) |
| > 20% to ⩽40% | 45 (20) | 2 (7) |
| > 40% to ⩽80% | 71 (31) | 10 (36) |
| > 80% | 37 (16) | 6 (21) |
| Missing data | 17 | 2 |
Significant difference between respondents and non-respondents at P<0.01.
According to Claus model.
Non-respondents at T2 did not differ in terms of treatment status, type of BRCA1/2 test result or psychological assessment at T1, except for a tendency to a higher mean HADS-Depression score (P=0.07).
Mean age (s.d.) of the respondent sample was 47.3 (11.4); 65% exhibited an education level above high school and 52% were undergoing treatment. The mean (s.d.) clinical objective estimate of the probability of a genetic predisposition to cancer on the 0–100% scale was: 42.5 (29.2) with women spread over each probability category from low (⩽20) (32%) to moderate (>20–⩽40) (20%), high (>40–⩽80) (31%) and very high (>80) (16%) (Table 1).
Among respondents at T2 (66% response rate), 133 (74%), 20 (11%) and 27 (15%) received a negative, deleterious mutation or UV result at a mean (s.d.) time of 11 (3) months after their initial cancer genetic counselling visit (Table 2).
Table 2. Descriptive characteristics after BRCA1/2 test result notification consultation (T2).
| Respondents (N=180) | Non-respondents (N=63) | |
|---|---|---|
|
BRCA test result (N/%) | ||
| Negative uninformative (NU) | 133 (74) | 42 (84) |
| Positive BRCA1/2 | 20 (11) | 1 (2) |
| Unclassified variant (UV) |
27 (15) |
7 (14) |
|
Length of time between initial and BRCA1/2 test result notification consultation (days) | ||
| Mean (s.d.) | 321.3 (85.9) | 328.2 (108.6) |
BRCA1/2 test results and objective probability of genetic predisposition to cancer were strongly correlated (P=0.001). Mean objective probabilities (s.ds.) were 40.7 (28.6), 70.2 (24.4) and 38.5 (28.1) (F(2,214)=10.26, P<0.001) for negative, positive and UV results, respectively
Psychological assessment
As shown in Table 3, mean scores for anxiety (7.8 and 8.2), depression (3.6 and 3.7), intrusions (7.2 and 9.1) and avoidance (9.9 and 10.9) at T1 and at T2, respectively, were low to moderate and only significantly different for intrusions (P=0.002); the mean score was moderate for perceived personal control (11.9 and 11.8); 24, 3, 8 and 14% of women at T1 and 31, 2, 11 and 18% of women at T2 presented a level of anxiety, depression, intrusion or avoidance requiring psychology professional attention, respectively.
Table 3. Psychological assessment at initial (T1) and after BRCA1/2 test result notification (T2).
| T1 (N=243) | T2 (N=180) | T test (P) | |
|---|---|---|---|
|
Perceived probability of genetic predisposition to cancer | |||
| Two-item standardised scale score (−2.8 to 1.61] | — | — | |
| Mean (s.d.) | −0.02 (0.08) | ||
| Missing data |
7 |
|
|
|
Perceived probability of genetic predisposition to cancer | |||
| Absolute figure (0–100%) (N=180)a | — | — | |
| Mean (s.d.) | 48.3 (25.6) | ||
| N/% higher perceived probability/objective estimatesb | 34 (23.6) | ||
| N/% lower perceived probability/objective estimatesb | 34 (23.7) | ||
| Missing data |
36 |
|
|
|
HADS-Anxiety score (0–21) | |||
| Mean (s.d.) | 7.8 (4.3) | 8.2 (4.5) | −1.81 (0.07) |
| Percentage of clinical case (score >10)c | 24 | 31 | |
| Missing data |
— |
1 |
|
|
HADS-Depression score (0–21) | |||
| Mean (s.d.) | 3.6 (3.2) | 3.7 (3.1) | −1.27 (0.20) |
| Percentage of clinical case (score >10)c | 3 | 2 | |
| Missing data |
— |
1 |
|
|
IES (risk of cancer)-Intrusion score (0–35) | |||
| Mean (s.d.) | 7.2 (7.7) | 9.1 (8.5) | −3.15 (0.002) |
| Percentage of clinical case (score >20)d | 8 | 11 | |
| Missing data |
2 |
2 |
|
|
IES (risk of cancer)-Avoidance score (0–40) | |||
| Mean (s.d.) | 9.9 (9.3) | 10.9 (9.6) | −0.93 (0.35) |
| Percentage of clinical case (score >21)d | 14 | 18 | |
| Missing data |
2 |
2 |
|
|
PPC scale total score (0–18) | |||
| Mean (s.d.) | 11.9 (3.8) | 11.8 (4.0) | 0.99 (0.32) |
| Missing data | — | 1 | |
Computed only for respondents at both T1 and T2.
Figures provided by women falls outside a range of ±25%.
Distress threshold from Hopwood et al (1991).
Distress threshold from Horowitz et al (1979).
For respondents at both T1 and T2 (n=180), mean (s.d.) of absolute perceived probability of genetic predisposition to cancer assessed at T1 was 48.3 (25.6); 23.6% and 23.7% of women presented a higher or lower perceived probability of genetic predisposition to cancer than objective estimate by ⩾25% on the 0–100% scale, respectively (Table 3).
As shown in Table 4, for the perceived probability of genetic predisposition to cancer assessed at T1 on the 0–100% one-item absolute scale, means were significantly different across the clinician-computed estimate categories (respectively: F(3,192)=8.0, P<0.001, η2=0.11). The concordance correlation coefficient (Lin, 1989) between objective estimate of predisposition probability and the two-item standardised scale predisposition probability perception was .31 (P<0.001).
Table 4. Mean (s.d.) absolute perceived probability of genetic predisposition to cancer by Claus model-based objective estimates categories computed at T1.
| Objective estimates of cancer genetic predisposition categories | Perceived probability of genetic predisposition to cancer absolute scale (0-100%)a |
|---|---|
| ⩽20% |
37.9 (27.6) |
| >20% to ⩽40% |
48.4 (23.2) |
| >40% to ⩽80% |
50.0 (24.4) |
| >80% | 63.2 (22.0) |
Means are significantly different: F(3,192)=8.0, P<.001, η2 =0.11.
Due to missing data on the one-item absolute figure scale, N=196.
Hierarchical regression analyses
In hierarchical regression analysis (Table 5), the percentage of explained variance (adjusted R2) for anxiety, depression, intrusions, avoidance and perceived personal control at T2 ranged from 23–30% (all P<0.001). Among covariates, anxiety was also predicted by being on treatment rather than in remission (β=0.17, P<0.05) and higher ovarian cancer risk perception (β=0.16, P<0.05); avoidance was predicted by an increased time interval since the initial cancer genetic counselling visit (β=0.21, P<0.01); and perceived personal control was predicted by lower ovarian cancer risk perception (β=−0.17, P<0.05).
Table 5. Predictors of anxiety, depression, intrusion, avoidance or perceived personal control at BRCA1/2 test result notification consultation (final model β).
| HADS-Anxiety | HADS-Depression | IES-Intrusion | IES-Avoidance | Perceived Personal Control | |
|---|---|---|---|---|---|
| Score at T1 |
0.46*** |
0.50*** |
0.41*** |
0.43*** |
0.45*** |
| Age |
−0.00 |
−0.11 |
−0.05 |
−0.08 |
0.09 |
| Education |
0.06 |
0.07 |
−0.10 |
−0.05 |
−0.04 |
| Having a partner |
0.03 |
−0.02 |
0.03 |
0.01 |
−0.06 |
| Having daughters |
0.11 |
0.07 |
0.00 |
−0.10 |
−0.04 |
| Under treatment (vs in remission) |
0.17* |
0.02 |
0.13 |
0.13 |
0.04 |
| Relatives diagnosed of breast or ovarian cancer <50 years old |
−0.00 |
0.03 |
−0.05 |
−0.08 |
0.05 |
| Lapse of time between initial and second genetic consultation |
0.09 |
−0.02 |
0.13 |
0.21** |
0.03 |
| BC risk perception at T2 |
0.04 |
0.07 |
−0.11 |
−0.11 |
−0.04 |
| OC risk perception at T2 |
0.16* |
0.12 |
0.16 |
0.16 |
−0.17* |
| Perceived probability of genetic predisposition |
−0.01 |
−0.03 |
0.04 |
0.00 |
−0.08 |
| Objective estimates vs perceived probability of genetic predisposition difference |
−0.05 |
−0.12 |
0.01 |
0.14 |
0.13 |
| Block 1 (control variables): F(df); R2; Adjusted R2 |
5.06 (148,12)***0.29; 0.23 |
5.22 (148,12)***0.30; 0.24 |
4.18 (148,12)***0.25; 0.19 |
5.26 (148,12)***0.30; 0.24 |
5.60 (148,12)***0.31; 0.26 |
| Positive BRCA (vs NU) | 0.31** | 0.14 | 0.21* | 0.02 | 0.05 |
| UV (vs NU) |
0.03 |
−0.00 |
0.14* |
0.05 |
0.03 |
| Block 2 (+ BRCA1/2 test result): F change(df); R2change; Adjusted R2 |
1.26 (146,2);0.01; 0.24 |
0.32 (146,2);0.003; 0.23 |
2.68 (146,2);0.03; 0.21 |
0.33 (146,2);0.003; 0.24 |
1.30 (146,2);0.01; 0.26 |
| Objective vs perceived probability of genetic predisposition difference × positive BRCA | −0.28** | −0.09 | −0.10 | −0.04 | 0.12 |
| Objective vs perceived probability of genetic predisposition difference × UV |
0.20** |
0.19* |
0.18* |
−0.05 |
0.02 |
| Block 3 (+ interactions): F change(df); R2change; Adjusted R2 | 8.18 (144,2)*** 0.07; 0.30 | 3.64 (144,2)*0.03; 0.26 | 3.2 (144,2)*0.03; 0.23 | 0.21 (144,2);0.002; 0.23 | 0.68 (144,2);0.01; 0.26 |
Abbreviations: BC=breast cancer; HADS=Hospital Anxiety and Depression Scale; IES=Impact of Event Scale; OC=ovarian cancer; UV=unclassified variant. *P<0.05; **P<0.01; ***P<0.001.
Adding the BRCA1/2 result to the model did not significantly improve the predictions. However, addition in a third block of the combined effect of the degree of discrepancy between the perceived probability of genetic predisposition to cancer and objective estimate, and test result was significant for HADS-Anxiety (F(144, 2) change=8.18, P<0.001), HADS-Depression (F(144, 2) change=3.64, P<0.05) and IES-Intrusion (F(144, 2) change=3.2, P<0.05).
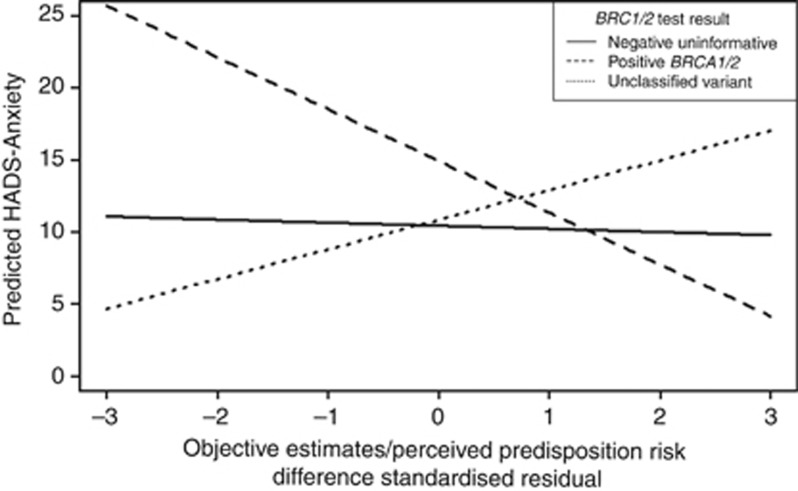
A higher perceived probability of genetic predisposition to cancer compared with objective estimate predicted higher levels of anxiety in women with a positive BRCA1/2 compared with women receiving a NU or UV result, and a lower perceived probability of genetic predisposition to cancer compared with objective estimate predicted lower levels of anxiety (β=−0.28, P<0.01) (Table 5; Figure 1). On the contrary, compared with women receiving a NU or positive BRCA1/2 test result, a higher perceived probability of genetic predisposition to cancer compared with objective estimate in women with an UV result predicted lower levels of anxiety (β=0.20, P<0.01) (Table 5; Figure 1), depression (β=0.19, P<0.05) and thought intrusion (β=0.18, P<0.05), whereas a lower perceived probability of genetic predisposition to cancer compared with objective estimates predicted higher levels of these latter outcomes. The degree of discrepancy between perceived probability of genetic predisposition to cancer and objective estimates by BRCA1/2 test results had no effect on IES-Avoidance and PPC (see Supplementary Figures 1–4 and Table 5).
Figure 1.
HADS-Anxiety level at T2 according to discrepancy between perceived probability of genetic predisposition to cancer and objective probability estimates for positive BRCA1/2, NU and UV test result. Note: a negative or positive value in abscise reflect an over or under estimation of predisposition risk.
Discussion
This prospective study addressed the effect of BRCA1/2 testing on anxiety, depression and cancer-specific distress and perceived personal control in BC patients, considering the type of BRCA1/2 result received and the degree of discrepancy between their perceived probability of genetic predisposition to cancer and objective estimates before test disclosure. After controlling for covariates, the effect of receiving a positive BRCA1/2 or a UV result compared with a NU result did not predict higher levels of general or specific distress and did not affect perceived personal control over cancer risk. Increased distress has been evidenced in carriers after BRCA1/2 testing (van Dijk et al, 2004, 2006; Dorval et al, 2005; Beran et al, 2008; Smith et al, 2008; Hamilton et al, 2009), whereas decrease in distress was observed in women receiving an uninformative result (Hamilton et al, 2009), with comparable emotional reactions to receipt of a NU and UV result (van Dijk et al, 2004; Smith et al, 2008) but higher distress in UV (O'Neill et al, 2009). However, the lack of association between the type of BRCA1/2 test result and distress is in line with Bennett et al (2008) and Vos et al (2010), who also found no relation between assigned risk status and emotional distress underlining the major role of the woman's interpretations of the genetic test result meaning.
An effect of the BRCA1/2 test result became apparent when taking into account the perception of the probability of genetic predisposition to cancer relative to objective estimates before test disclosure. In women with a positive BRCA1/2 result compared with those with a NU or UV result, a higher perceived probability of genetic predisposition to cancer compared with objective estimates before testing predicted higher levels of anxiety after notification of a BRCA1/2 test result. These women, who presented higher appraisals of their probability of genetic predisposition to cancer, were probably already anxious and, as they were anticipating a positive BRCA1/2, their worries were confirmed when they were notified about this result. On the contrary, women who received a positive BRCA1/2 test result but who presented a lower perceived probability of genetic predisposition to cancer compared with objective estimates displayed lower levels of anxiety than those receiving a UV or NU test result, suggesting possible minimisation or denial of thoughts related to cancer genetic risks in these women.
In women receiving an UV test result, a higher perceived probability of genetic predisposition to cancer compared with objective estimates predicted lower levels of anxiety after notification of BRCA1/2 test results compared with women receiving a positive BRCA1/2 or NU test result. This result may reflect the relief of these women when they were informed that their cancer risk was lower than if they carried a BRCA1/2 mutation. However, in women receiving a UV test result compared with those informed of a positive BRCA1/2 or NU test result, a lower perceived probability of genetic predisposition to cancer compared with objective estimates before test result notification predicted higher levels of anxiety, depression and intrusion. In these women who approached cancer genetic testing with confidence, the uncertainty raised by this ambiguous result may have elicited increased distress. As suggested by Vos et al (2010), a UV, although indicating the presence of a gene modification of unknown clinical significance, may have been interpreted as harmful. The effect of receiving a UV result rather than a positive BRCA1/2 result on depression may be related to the lack of clearly defined risk management recommendations leaving women uncertain about the actions to be taken to cope with increased cancer-related anxiety.
During the initial cancer genetic consultation, most women were informed of their cancer genetic predisposition probability using the verbal categories ‘low', ‘moderate', ‘high' or ‘very high'. So, we expected a difference between objective risk estimates and patients' perception of their probability of genetic predisposition to cancer in addition to the deviance between objective information and perception resulting from psychological processes (Pilarski, 2009). About one half of this sample of women either presented a higher (24%) or a lower (24%) perceived probability of genetic predisposition to cancer compared with the clinician's objective probability estimates, which is in line with a recent review of genetic risk perception accuracy (Smerecnik et al, 2009). Our results highlight the role of woman's inappropriate expectations combined with the BRCA1/2 test result on emotional reactions. Women were not informed of the possibility of receiving a UV result, so they only expected to receive either a positive or a negative result but not a positive-UV, which may have been more puzzling than receiving a clear positive or negative (even uninformative) result.
At the BRCA1/2 test result disclosure, both women who received a NU or a UV result were similarly warned that possible development in genetics could explain women's family history of cancer in the future and so it seems that evocation of a ‘mutation' convey a worrisome connotation.
A level of anxiety and avoidance requiring psychological care was observed in, respectively, 24% and 14% of the women after the initial cancer genetic counselling visit and in 31% and 18% after notification of BRCA1/2 test results, indicating psychological strain during this genetic testing process in a significant number of these women either on BC treatment or survivors. These figures are in line with those published in the literature showing that about one quarter of subjects undergoing genetic risk assessment will experience emotional distress at some time (Bennett et al, 2008; Power et al, 2011). As we assessed distress after the initial cancer genetic counselling visit and after communication of the BRCA1/2 test results, we only report the short-term emotional response during the genetic testing process. However, it is important to consider that some of these women may want to decide on their cancer risk management at that time and may therefore potentially make their decisions in a troubled emotional state.
BRCA1/2 testing is increasingly proposed to BC patients at the time of BC diagnosis (Meiser et al, 2008) or treatment (Schlich-Bakker et al, 2008). However, these circumstances may encompass additional stress. In addition, BC patients invited to undergo BRCA1/2 testing may be less well prepared for the test results than women who decide by themselves to attend cancer genetic counselling clinics for BRCA1/2 genetic testing. Although no effect of a BC diagnosis (Schlich-Bakker et al, 2006; Smith et al, 2008) or lower distress in women unaffected with BC, especially those receiving an uninformative test result (Hamilton et al, 2009) has been evidenced, in this study, women on BC treatment presented higher anxiety than survivors, which is in line with studies showing a risk of psychological distress in the case of a more recent BC diagnosis (Bonadona et al, 2002; Van Roosmalen et al, 2004).
BRCA1/2 test results, either alone or combined with perceived probability of genetic predisposition to cancer, had no effect on perceived personal control over cancer risk. This may be related to the limited efficacy of breast and especially ovarian cancer surveillance and the difficult decision-making regarding prophylactic interventions. Information on cancer risk management alternatives may help to increase the degree of control (Bennett et al, 2008). By contrast, as expected in view of the clinical features of ovarian cancer, a higher perception of ovarian cancer risk also lowered perceived personal control.
The generalisability of these results is limited for the following reasons. First, they reflect reactions of women with BC attending French cancer genetic services whereas genetic testing delivery and counselling may vary cross-culturally. Second, inclusion criteria planned for a homogeneous clinical sample in order to limit medical factors could explain variation in distress, but in consequence this study results cannot represent the psychological reactions of other populations such as healthy women or those with ovarian cancer or more advanced cancer. Third, among eligible patients, comparisons between respondents and non-respondents at T1 demonstrated significant differences on medical status (i.e., women currently on BC treatment were less likely to answer the questionnaires) and the effect of BRCA1/2 result could only be assessed on the 66% of the eligible patients who provided questionnaire responses at T2. Finally, this study should be replicated given its small sample size relative to the number of predictors assessed and the number of women provided with a positive BRCA1/2 or a UV test result.
Conclusions
The results of this study indicate that women affected with BC undergoing BRCA1/2 gene testing who presented a higher perceived probability of genetic predisposition to cancer than objective estimates and who received a positive BRCA1/2 test result presented higher levels of anxiety than those receiving a NU or UV result, probably because their worries were confirmed. In addition, women who presented a lower perceived probability of genetic predisposition to cancer than objective estimates and who received a positive BRCA1/2 test result evidenced lower levels of anxiety than those receiving a NU or UV result, which could suspect denial and possibly risk of surveillance neglect requiring closer attention by clinicians.
These findings also highlight that, in women receiving an UV test result, a higher perceived probability of genetic predisposition to cancer compared with objective estimates predicted lower levels of anxiety, suggesting relief in these women. However, those who presented a lower perceived probability of genetic predisposition to cancer than objective estimates and who received a UV test result were more anxious than those receiving a NU or positive BRCA1/2 test result, suggesting that they misinterpret this gene modification as being harmful, especially as they did not expect such result.
During the initial cancer genetic counselling, patients should be informed of the different possible BRCA1/2 test results. Particular attention should be paid to exploring the women's perception of their probability of genetic predisposition to cancer by facilitative communication skills so as to correct misperception and facilitate emotional adjustment at BRCA1/2 test result disclosure.
Acknowledgments
This work was conducted by means of a grant from the Canceropôle Ile-de-France and was partly financed, at Institut Curie, within the designated integrated cancer research site (SiRIC). We acknowledge the help of Claire Guillemeau, Tatiana Kogut-Kubiak, Jean-Louis Van Autreve and Mathilde Warcoin for providing family genealogical trees. We are grateful to all women who accepted to take part in this study and who provided their time to complete the questionnaires.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Bennett P, Wilkinson C, Turner J, Brain K, Edwards RT, Griffith G, France B, Gray J. Psychological factors associated with emotional responses to receiving genetic risk information. J Genet Couns. 2008;17:234–241. doi: 10.1007/s10897-007-9136-x. [DOI] [PubMed] [Google Scholar]
- Beran TM, Stanton AL, Kwan L, Seldon J, Bower JE, Vodermaier A, Ganz PA. The trajectory of psychological impact in BRCA1/2 genetic testing: does time heal. Ann Behav Med. 2008;36:107–116. doi: 10.1007/s12160-008-9060-9. [DOI] [PubMed] [Google Scholar]
- Berkenstadt M, Shiloh S, Barkai G, Katznelson MB, Goldman B. Perceived Personal Control (PPC): a new concept in measuring outcome of genetic counseling. Am J Med Genet. 1999;82 (1:53–59. doi: 10.1002/(sici)1096-8628(19990101)82:1<53::aid-ajmg11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bonadona V, Saltel P, Desseigne F, Mignotte H, Saurin JC, Wang Q, Sinilnikova O, Giraud S, Freyer G, Plauchu H, Puisieux A, Lasset C. Cancer patients who experienced diagnostic genetic testing for cancer susceptibility: reactions and behavior after the disclosure of a positive test result. Cancer Epidemiol Biomarkers Prev. 2002;11:97–104. [PubMed] [Google Scholar]
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Diagnostic genetic testing for hereditary breast and ovarian cancer in cancer patients: women's looking back on the pre-test period and a psychological evaluation. Genet Test. 2004;8:13–21. doi: 10.1089/109065704323015996. [DOI] [PubMed] [Google Scholar]
- Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991;48 (2:232–242. [PMC free article] [PubMed] [Google Scholar]
- Croyle RT, Smith KR, Botkin JR, Baty B, Nash J. Psychological responses to BRCA1 mutation testing: preliminary findings. Health Psychol. 1997;16:63–72. doi: 10.1037//0278-6133.16.1.63. [DOI] [PubMed] [Google Scholar]
- Cypowyj C, Eisinger F, Huiart L, Sobol H, Morin M, Julian-Reynier C. Subjective interpretation of inconclusive BRCA1/2 cancer genetic test results and transmission of information to the relatives. Psychooncology. 2009;18:209–215. doi: 10.1002/pon.1407. [DOI] [PubMed] [Google Scholar]
- Dorval M, Gauthier G, Maunsell E, Dugas MJ, Rouleau I, Chiquette J, Plante M, Laframboise R, Gaudet M, Bridge PJ, Simard J. No evidence of false reassurance among women with an inconclusive BRCA1/2 genetic test result. Cancer Epidemiol Biomarkers Prev. 2005;14:2862–2867. doi: 10.1158/1055-9965.EPI-05-0512. [DOI] [PubMed] [Google Scholar]
- Gadzicki D, Evans DG, Harris H, Julian-Reynier C, Nippert I, Schmidtke J, Tibben A, van Asperen CJ, Schlegelberger B. Genetic testing for familial/hereditary breast cancer-comparison of guidelines and recommendations from the UK, France, the Netherlands and Germany. J Community Genet. 2011;2:53–69. doi: 10.1007/s12687-011-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves KD, Vegella P, Poggi EA, Peshkin BN, Tong A, Isaacs C, Finch C, Kelly S, Taylor KL, Luta G, Schwartz MD. Long-term psychosocial outcomes of BRCA1/BRCA2 testing: differences across affected status and risk-reducing surgery choice. Cancer Epidemiol Biomarkers Prev. 2012;21 (3:445–455. doi: 10.1158/1055-9965.EPI-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CH, Stopfer JE, McDonald J, Weathers B, Collier A, Troxel AB, Domchek S. Long-term reactions to genetic testing for BRCA1 and BRCA2 mutations: does time heal women's concerns. J Clin Oncol. 2011;29:4302–4306. doi: 10.1200/JCO.2010.33.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol. 2009;28:510–518. doi: 10.1037/a0014778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgart J, Phelps C, Bennett P, Hood K, Brain K, Murray A. ‘I have always believed I was at high risk...' The role of expectation in emotional responses to the receipt of an average, moderate or high cancer genetic risk assessment result: a thematic analysis of free-text questionnaire comments. Fam Cancer. 2010;9:469–477. doi: 10.1007/s10689-010-9324-y. [DOI] [PubMed] [Google Scholar]
- Hopwood P. Breast cancer risk perception: what do we know and understand. Breast Cancer Res. 2000;2:387–391. doi: 10.1186/bcr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P, Howell A, Maguire P. Screening for psychiatric morbidity in patients with advanced breast cancer: validation of two self-report questionnaires. Br J Cancer. 1991;64 (2:353–356. doi: 10.1038/bjc.1991.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Wilner M, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Kirova YM, Savignoni A, Sigal-Zafrani B, de La Rochefordiere A, Salmon RJ, This P, Asselain B, Stoppa-Lyonnet D, Fourquet A. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat. 2010;120 (1:119–126. doi: 10.1007/s10549-009-0685-6. [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984;36 (2:460–465. [PMC free article] [PubMed] [Google Scholar]
- Lin L. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- McAllister M, Wood AM, Dunn G, Shiloh S, Todd C. The Perceived Personal Control (PPC) Questionnaire: reliability and validity in a sample from the United Kingdom. Am J Med Genet A. 2012;158A:367–372. doi: 10.1002/ajmg.a.34374. [DOI] [PubMed] [Google Scholar]
- Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psychooncology. 2005;14 (12:1060–1074. doi: 10.1002/pon.933. [DOI] [PubMed] [Google Scholar]
- Meiser B, Butow P, Friedlander M, Barratt A, Schnieden V, Watson M, Brown J, Tucker K. Psychological impact of genetic testing in women from high-risk breast cancer families. Eur J Cancer. 2002;38:2025–2031. doi: 10.1016/s0959-8049(02)00264-2. [DOI] [PubMed] [Google Scholar]
- Meiser B, Tucker K, Friedlander M, Barlow-Stewart K, Lobb E, Saunders C, Mitchell G. Genetic counselling and testing for inherited gene mutations in newly diagnosed patients with breast cancer: a review of the existing literature and a proposed research agenda. Breast Cancer Res. 2008;10:216. doi: 10.1186/bcr2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SC, Rini C, Goldsmith RE, Valdimarsdottir H, Cohen LH, Schwartz MD. Distress among women receiving uninformative BRCA1/2 results: 12-month outcomes. Psychooncology. 2009;18:1088–1096. doi: 10.1002/pon.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps C, Bennett P, Brain K. Understanding emotional responses to breast/ovarian cancer genetic risk assessment: an applied test of a cognitive theory of emotion. Psychol Health Med. 2008;13:545–558. doi: 10.1080/13548500701767346. [DOI] [PubMed] [Google Scholar]
- Pilarski R. Risk perception among women at risk for hereditary breast and ovarian cancer. J Genet Couns. 2009;18:303–312. doi: 10.1007/s10897-009-9227-y. [DOI] [PubMed] [Google Scholar]
- Power TE, Robinson JW, Bridge P, Bernier FP, Gilchrist DM. Distress and psychosocial needs of a heterogeneous high risk familial cancer population. J Genet Couns. 2011;20:249–269. doi: 10.1007/s10897-010-9344-7. [DOI] [PubMed] [Google Scholar]
- Razavi D, Delvaux N, Farvacques C, Robaye E. Screening for adjustment disorders and major depressive disorders in cancer in-patients. Br J Psychiatry. 1990;156:79–83. doi: 10.1192/bjp.156.1.79. [DOI] [PubMed] [Google Scholar]
- Rini C, O'Neill SC, Valdimarsdottir H, Goldsmith RE, Jandorf L, Brown K, DeMarco TA, Peshkin BN, Schwartz MD. Cognitive and emotional factors predicting decisional conflict among high-risk breast cancer survivors who receive uninformative BRCA1/2 results. Health Psychol. 2009;28:569–578. doi: 10.1037/a0015205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing: Vienna, Austria; 2011. ISBN 3-900051-07-0, URL: http://www.R-project.org/ [Google Scholar]
- Schlich-Bakker KJ, Warlam-Rodenhuis CC, van Echtelt J, van den Bout J, Ausems MG, ten Kroode HF. Short term psychological distress in patients actively approached for genetic counselling after diagnosis of breast cancer. Eur J Cancer. 2006;42:2722–2728. doi: 10.1016/j.ejca.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Schlich-Bakker KJ, Ausems MG, Schipper M, Ten Kroode HF, Warlam-Rodenhuis CC, van den Bout J. BRCA1/2 mutation testing in breast cancer patients: a prospective study of the long-term psychological impact of approach during adjuvant radiotherapy. Breast Cancer Res Treat. 2008;109:507–514. doi: 10.1007/s10549-007-9680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Peshkin BN, Hughes C, Main D, Isaacs C, Lerman C. Impact of BRCA1/BRCA2 mutation testing on psychologic distress in a clinic-based sample. J Clin Oncol. 2002;20:514–520. doi: 10.1200/JCO.2002.20.2.514. [DOI] [PubMed] [Google Scholar]
- Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. J Genet Couns. 2009;18:217–228. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EM, Pieterse AH, Aalfs CM, Ausems MG, van Dulmen AM. The Perceived Personal Control (PPC) Questionnaire as an outcome of genetic counseling: reliability and validity of the instrument. Am J Med Genet A. 2006;140 (8:843–850. doi: 10.1002/ajmg.a.31185. [DOI] [PubMed] [Google Scholar]
- Smith AW, Dougall AL, Posluszny DM, Somers TJ, Rubinstein WS, Baum A. Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psychooncology. 2008;17:767–773. doi: 10.1002/pon.1291. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS.2007Using Multivariate Statistics5th ednPearson International Edition: Boston, MA, USA [Google Scholar]
- Thewes B, Meiser B, Hickie IB. Psychometric properties of the Impact of Event Scale amongst women at increased risk for hereditary breast cancer. Psycho-Oncol. 2001;10 (6:459–468. doi: 10.1002/pon.533. [DOI] [PubMed] [Google Scholar]
- Thompson SC. Will it hurt less if I can control it? A complex answer to a simple question. Psychol Bull. 1981;90 (1:89–101. [PubMed] [Google Scholar]
- van Dijk S, Otten W, Timmermans DR, an Asperen CJ, Meijers-Heijboer H, Tibben A, Breuning MH, Kievit J. What's the message? Interpretation of an uninformative BRCA1/2 test result for women at risk of familial breast cancer. Genet Med. 2005;7:239–245. doi: 10.1097/01.gim.0000159902.34833.26. [DOI] [PubMed] [Google Scholar]
- van Dijk S, Otten W, van Asperen CJ, Timmermans DR, Tibben A, Zoeteweij MW, Silberg S, Breuning MH, Kievit J. Feeling at risk: how women interpret their familial breast cancer risk. Am J Med Genet A. 2004;131:42–49. doi: 10.1002/ajmg.a.30322. [DOI] [PubMed] [Google Scholar]
- van Dijk S, Timmermans DR, Meijers-Heijboer H, Tibben A, van Asperen CJ, Otten W. Clinical characteristics affect the impact of an uninformative DNA test result: the course of worry and distress experienced by women who apply for genetic testing for breast cancer. J Clin Oncol. 2006;24:3672–3677. doi: 10.1200/JCO.2005.03.7259. [DOI] [PubMed] [Google Scholar]
- van Dooren S, Rijnsburger AJ, Seynaeve C, Duivenvoorden HJ, Essink-Bot ML, Tilanus-Linthorst MM, de Koning HJ, Tibben A. Psychological distress in women at increased risk for breast cancer: the role of risk perception. Eur J Cancer. 2004;40:2056–2063. doi: 10.1016/j.ejca.2004.05.004. [DOI] [PubMed] [Google Scholar]
- van Oostrom I, Meijers-Heijboer H, Duivenvoorden HJ, Brocker-Vriends AH, van Asperen CJ, Sijmons RH, Seynaeve C, Van Gool AR, Klijn JG, Tibben A. Experience of parental cancer in childhood is a risk factor for psychological distress during genetic cancer susceptibility testing. Ann Oncol. 2006;17:1090–1095. doi: 10.1093/annonc/mdl069. [DOI] [PubMed] [Google Scholar]
- van Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra-Weebers JE, Oosterwijk JC, Hoogerbrugge N, Moog U, van Daal WA. Impact of BRCA1/2 testing and disclosure of a positive test result on women affected and unaffected with breast or ovarian cancer. Am J Med Genet A. 2004;124A:346–355. doi: 10.1002/ajmg.a.20374. [DOI] [PubMed] [Google Scholar]
- Vink GR, van Asperen CJ, Devilee P, Breuning MH, Bakker E. Unclassified variants in disease-causing genes: nonuniformity of genetic testing and counselling, a proposal for guidelines. Eur J Hum Genet. 2005;13 (5:525–527. doi: 10.1038/sj.ejhg.5201379. [DOI] [PubMed] [Google Scholar]
- Vos J, Gómez-García E, Oosterwijk JC, Menko FH, Stoel RD, van Asperen CJ, Jansen AM, Stiggelbout AM, Tibben A. Opening the psychological black box in genetic counseling. The psychological impact of DNA testing is predicted by the counselees' perception, the medical impact by the pathogenic or uninformative BRCA1/2-result. Psychooncology. 2010;21 (1:29–42. doi: 10.1002/pon.1864. [DOI] [PubMed] [Google Scholar]
- Vos J, Oosterwijk JC, Gomez-Garcia E, Menko FH, Collee MJ, van Asperen CJ, Jansen AM, Stiggelbout AM, Tibben A. Exploring the short-term impact of DNA-testing in breast cancer patients: the counselees' perception matters, but the actual BRCA1/2 result does not. Patient Educ Couns. 2012;86 (2:239–251. doi: 10.1016/j.pec.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Watson M, Foster C, Eeles R, Eccles D, Ashley S, Davidson R, Mackay J, Morrison PJ, Hopwood P, Evans DG. Psychosocial impact of breast/ovarian (BRCA1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Br J Cancer. 2004;91:1787–1794. doi: 10.1038/sj.bjc.6602207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers MR, Ausems MG, Verhoef S, Bleiker EM, Hahn DE, Hogervorst FB, van der Luijt RB, Valdimarsdottir HB, van Hillegersberg R, Rutgers EJ, Aaronson NK. Behavioral and psychosocial effects of rapid genetic counseling and testing in newly diagnosed breast cancer patients: design of a multicenter randomized clinical trial. BMC Cancer. 2011;11:6. doi: 10.1186/1471-2407-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.