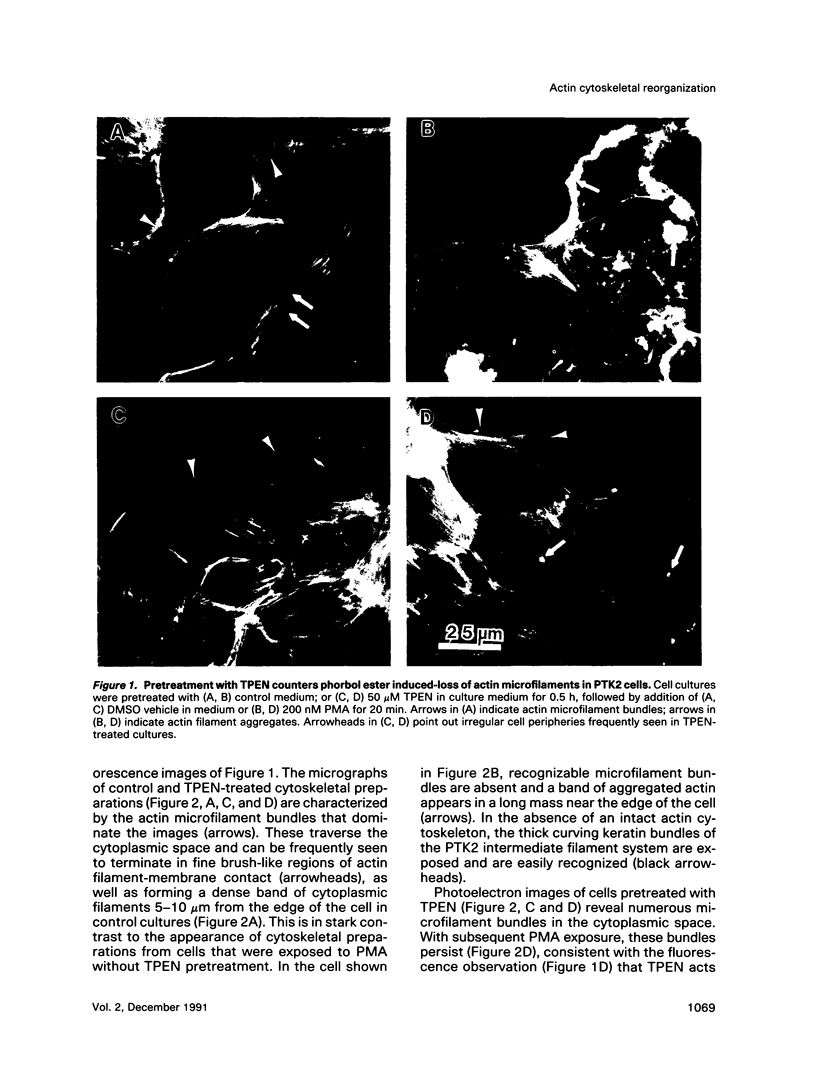
Abstract
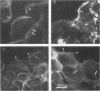
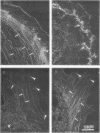
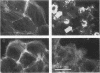
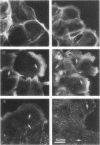
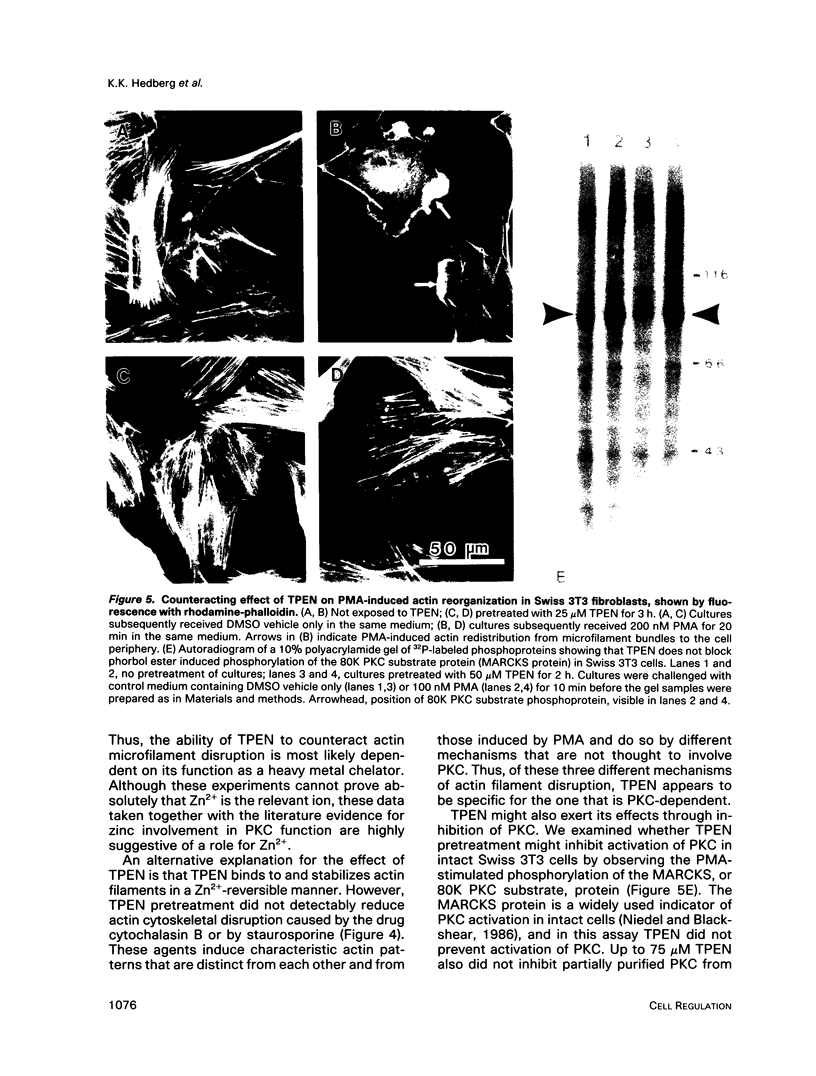
The cell-permeant heavy metal chelator N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine(TPEN) was found to counteract phorbol ester-induced actin reorganization in PTK2 and Swiss 3T3 cells. By using fluorescence and the higher resolution technique of photoelectron microscopy to monitor actin patterns, 15-min pretreatment with 25-50 microM TPEN was found to dramatically reduce actin alterations resulting from subsequent phorbol ester treatment in PTK2 cells. Similar results were obtained with Swiss 3T3 cells using 50 microM TPEN for 1.5 h. Phorbol ester-induced actin alterations are thought to depend on activation of protein kinase C (PKC). In contrast to the phorbol ester effect, the PKC-independent actin cytoskeletal disruption caused by staurosporine and cytochalasin B was unaffected by TPEN pretreatment. TPEN did not block phorbol ester-induced activation of PKC in Swiss 3T3 cells, as observed by the phosphorylation of the 80K PKC substrate protein (MARCKS protein). TPEN also did not inhibit partially purified PKC from Swiss 3T3 cells in an in vitro PKC-specific commercial assay. To establish that the effect of TPEN is the removal of metal ions and not some other nonspecific effect of TPEN, a series of transition metal ions was added at the end of the TPEN pretreatment. The results indicate that the transient but dramatic phorbol ester-induced reorganization of the actin cytoskeleton in cultured cells depends on an interaction of PKC with a heavy metal, probably zinc.
Full text
PDF

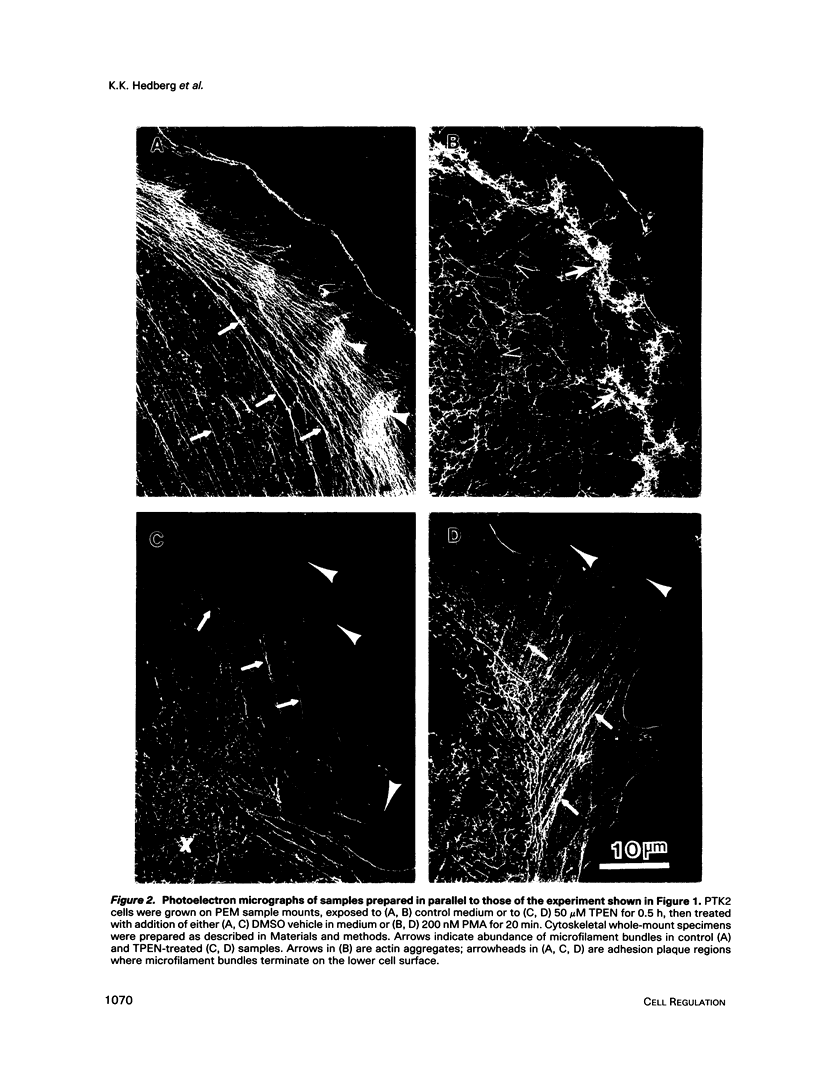
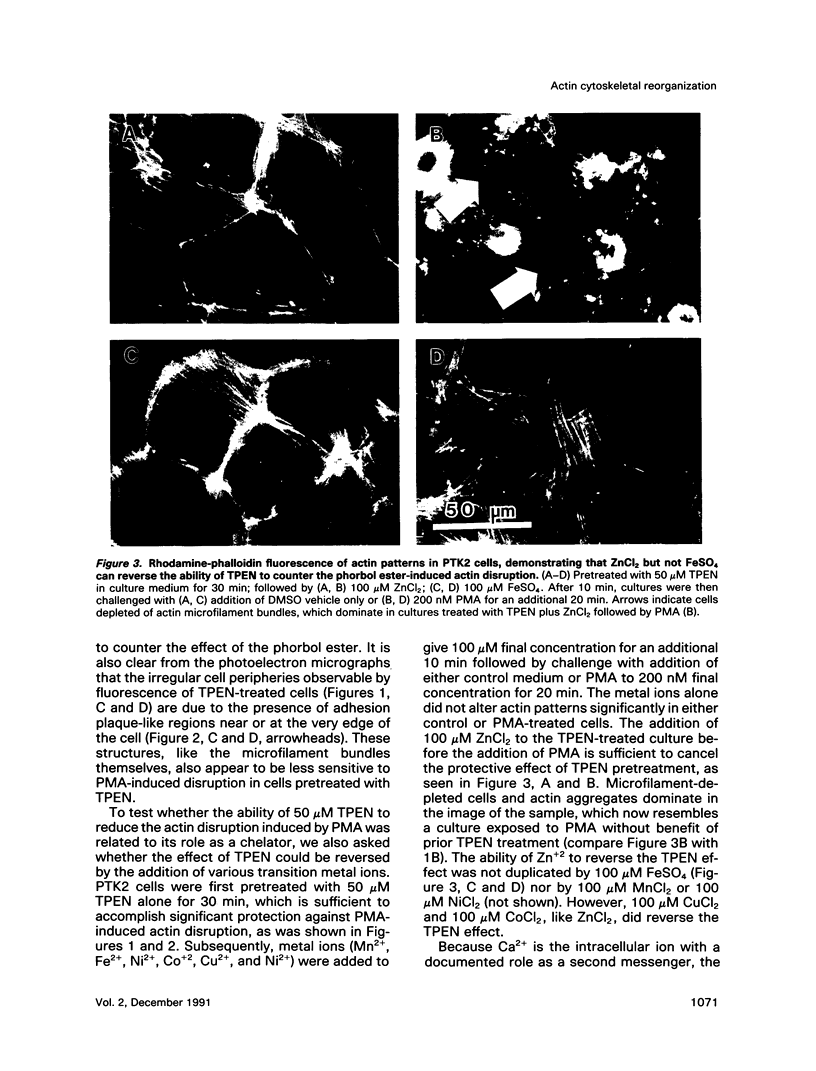


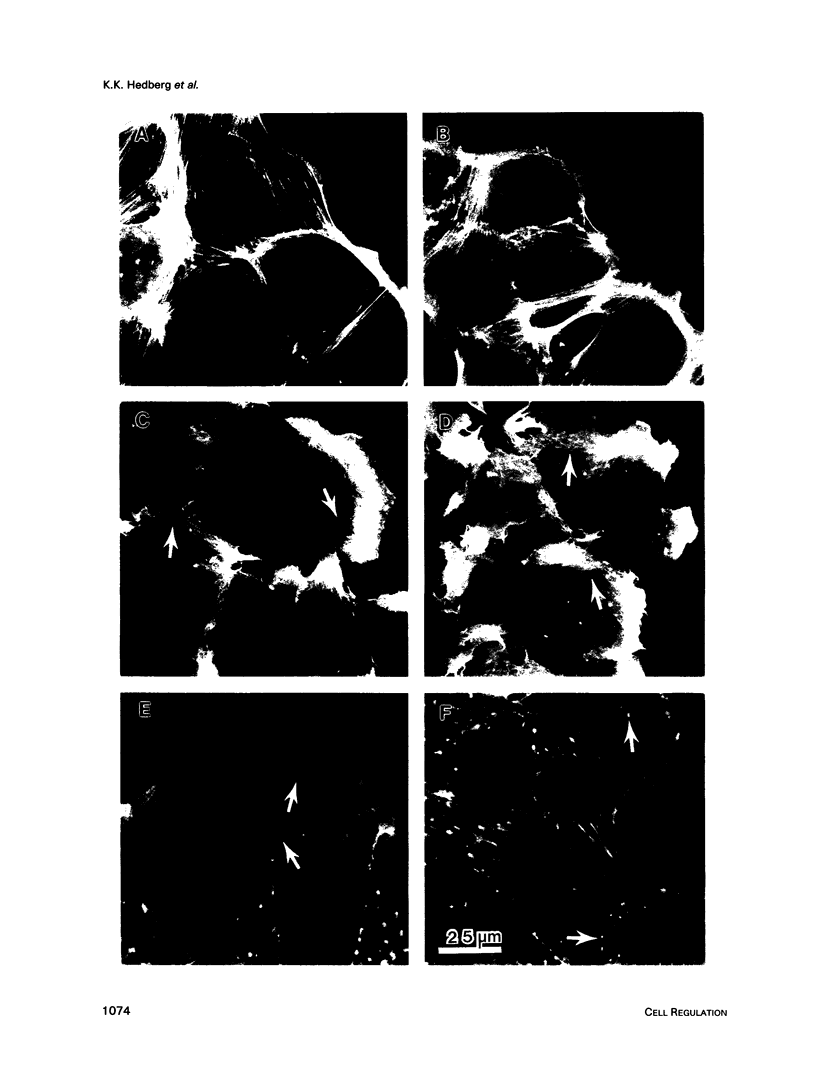




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Bell P. B., Jr The application of scanning electron microscopy to the study of the cytoskeleton of cells in culture. Scan Electron Microsc. 1981;(Pt 2):139–157. [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Bershadsky A. D., Ivanova O. Y., Lyass L. A., Pletyushkina O. Y., Vasiliev J. M., Gelfand I. M. Cytoskeletal reorganizations responsible for the phorbol ester-induced formation of cytoplasmic processes: possible involvement of intermediate filaments. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1884–1888. doi: 10.1073/pnas.87.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell G. B., Hedberg K. K., Habliston D. L., Griffith O. H. Protein kinase C inhibitor H-7 alters the actin cytoskeleton of cultured cells. J Cell Physiol. 1989 Oct;141(1):74–84. doi: 10.1002/jcp.1041410112. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Csermely P., Szamel M., Resch K., Somogyi J. Zinc can increase the activity of protein kinase C and contributes to its binding to plasma membranes in T lymphocytes. J Biol Chem. 1988 May 15;263(14):6487–6490. [PubMed] [Google Scholar]
- Danowski B. A., Harris A. K. Changes in fibroblast contractility, morphology, and adhesion in response to a phorbol ester tumor promoter. Exp Cell Res. 1988 Jul;177(1):47–59. doi: 10.1016/0014-4827(88)90024-9. [DOI] [PubMed] [Google Scholar]
- Delescluse C., Bernard B. A., Fürstenberger G., Sorg B., Marks F., Bailly J., Darmon M. Effect of diterpene esters on actin cytoskeleton of SV40-transformed keratinocytes is not reproduced by diacylglycerols. Carcinogenesis. 1988 Feb;9(2):333–334. doi: 10.1093/carcin/9.2.333. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D., Giannakis C., Betts W. H. Zinc induces specific association of PKC with membrane cytoskeleton. Biochem Int. 1990 Nov;22(4):741–748. [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D., Giannakis C., Petkoff H. S., Cowled P. A. Interaction between protein kinase C and regulatory ligand is enhanced by a chelatable pool of cellular zinc. Biochim Biophys Acta. 1990 Jul 12;1053(2-3):113–117. doi: 10.1016/0167-4889(90)90001-t. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W., Marks F. Protein kinase C activation by phorbol esters: do cysteine-rich regions and pseudosubstrate motifs play a role? Trends Biochem Sci. 1991 May;16(5):167–169. doi: 10.1016/0968-0004(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Habliston D. L., Baker B., Griffith O. H., Skoczylas W. P. A computer-aided control, design and image-processing system for electron microscopes. Ultramicroscopy. 1991 May;36(1-3):222–234. doi: 10.1016/0304-3991(91)90152-v. [DOI] [PubMed] [Google Scholar]
- Harrison B. C., Mobley P. L. Phorbol ester-induced change in astrocyte morphology: correlation with protein kinase C activation and protein phosphorylation. J Neurosci Res. 1990 Jan;25(1):71–80. doi: 10.1002/jnr.490250109. [DOI] [PubMed] [Google Scholar]
- Hedberg K. K., Birrell G. B., Habliston D. L., Griffith O. H. Staurosporine induces dissolution of microfilament bundles by a protein kinase C-independent pathway. Exp Cell Res. 1990 Jun;188(2):199–208. doi: 10.1016/0014-4827(90)90160-c. [DOI] [PubMed] [Google Scholar]
- Hedberg K. K., Griffith O. H. Immunophotoelectron microscopy of the cell surface and cytoskeleton. Ann N Y Acad Sci. 1986;483:372–386. doi: 10.1111/j.1749-6632.1986.tb34545.x. [DOI] [PubMed] [Google Scholar]
- Huang K. P. The mechanism of protein kinase C activation. Trends Neurosci. 1989 Nov;12(11):425–432. doi: 10.1016/0166-2236(89)90091-x. [DOI] [PubMed] [Google Scholar]
- Härd T., Kellenbach E., Boelens R., Maler B. A., Dahlman K., Freedman L. P., Carlstedt-Duke J., Yamamoto K. R., Gustafsson J. A., Kaptein R. Solution structure of the glucocorticoid receptor DNA-binding domain. Science. 1990 Jul 13;249(4965):157–160. doi: 10.1126/science.2115209. [DOI] [PubMed] [Google Scholar]
- Jaken S., Leach K., Klauck T. Association of type 3 protein kinase C with focal contacts in rat embryo fibroblasts. J Cell Biol. 1989 Aug;109(2):697–704. doi: 10.1083/jcb.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellie S., Holme T. C., Bissell M. J. Interaction of tumour promoters with epithelial cells in culture. An immunofluorescence study. Exp Cell Res. 1985 Oct;160(2):259–274. doi: 10.1016/0014-4827(85)90174-0. [DOI] [PubMed] [Google Scholar]
- Kiley S. C., Jaken S. Activation of alpha-protein kinase C leads to association with detergent-insoluble components of GH4C1 cells. Mol Endocrinol. 1990 Jan;4(1):59–68. doi: 10.1210/mend-4-1-59. [DOI] [PubMed] [Google Scholar]
- McCaffrey P. G., Rosner M. R., Kikkawa U., Sekiguchi K., Ogita K., Ase K., Nishizuka Y. Characterization of protein kinase C from normal and transformed cultured murine fibroblasts. Biochem Biophys Res Commun. 1987 Jul 15;146(1):140–146. doi: 10.1016/0006-291x(87)90702-9. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Henrich C. J., Cheever L., Khaner H., Simpson P. C. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul. 1990 Aug;1(9):693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozier N. M., Walsh M. P., Pearson J. D. Characterization of a novel zinc binding site of protein kinase C inhibitor-1. FEBS Lett. 1991 Feb 11;279(1):14–18. doi: 10.1016/0014-5793(91)80238-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Hall P. F. Isolation and characterization of protein kinase C from Y-1 adrenal cell cytoskeleton. J Cell Biol. 1989 Feb;108(2):553–567. doi: 10.1083/jcb.108.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Charest D. L., Howard S. L., Paddon H. B., Salari H. Protein kinase C activation by platelet-activating factor is independent of enzyme translocation. Biochim Biophys Acta. 1990 Jan 23;1051(1):100–107. doi: 10.1016/0167-4889(90)90179-h. [DOI] [PubMed] [Google Scholar]
- Rempfer G. F., Skoczylas W. P., Griffith O. H. Design and performance of a high-resolution photoelectron microscope. Ultramicroscopy. 1991 May;36(1-3):196–221. doi: 10.1016/0304-3991(91)90151-u. [DOI] [PubMed] [Google Scholar]
- Rodland K. D., Muldoon L. L., Lenormand P., Magun B. E. Modulation of RNA expression by intracellular calcium. Existence of a threshold calcium concentration for induction of VL30 RNA by epidermal growth factor, endothelin, and protein kinase C. J Biol Chem. 1990 Jul 5;265(19):11000–11007. [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Serum, like phorbol esters, rapidly activates protein kinase C in intact quiescent fibroblasts. EMBO J. 1985 Jan;4(1):71–76. doi: 10.1002/j.1460-2075.1985.tb02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastrodihardjo S., Sasaki Y., Shiba Y., Kanno Y. Possible involvement of reorganization of actin filaments, induced by tumor-promoting phorbol esters, in changes in colony shape and enhancement of proliferation of cultured epithelial cells. J Cell Physiol. 1987 Jul;132(1):49–56. doi: 10.1002/jcp.1041320107. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y., Sasaki Y., Kanno Y. 12-O-tetradecanoylphorbol-13-acetate disrupts actin filaments and focal contacts and enhances binding of fibronectin-coated latex beads to 3T3-L1 cells. Exp Cell Res. 1988 Oct;178(2):233–241. doi: 10.1016/0014-4827(88)90394-1. [DOI] [PubMed] [Google Scholar]
- Sobue K., Fujio Y., Kanda K. Tumor promoter induces reorganization of actin filaments and calspectin (fodrin or nonerythroid spectrin) in 3T3 cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):482–486. doi: 10.1073/pnas.85.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speizer L. A., Watson M. J., Kanter J. R., Brunton L. L. Inhibition of phorbol ester binding and protein kinase C activity by heavy metals. J Biol Chem. 1989 Apr 5;264(10):5581–5585. [PubMed] [Google Scholar]
- Trilivas I., McDonough P. M., Brown J. H. Dissociation of protein kinase C redistribution from the phosphorylation of its substrates. J Biol Chem. 1991 May 5;266(13):8431–8438. [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Turner C. E., Pavalko F. M., Burridge K. The role of phosphorylation and limited proteolytic cleavage of talin and vinculin in the disruption of focal adhesion integrity. J Biol Chem. 1989 Jul 15;264(20):11938–11944. [PubMed] [Google Scholar]
- Wong P. Y., Fritze K. Determination by neutron activation of copper, manganese, and zinc in the pineal body and other areas of brain tissue. J Neurochem. 1969 Aug;16(8):1231–1234. doi: 10.1111/j.1471-4159.1969.tb05970.x. [DOI] [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J., Giannakis C., Cowled P. A., Betts W. H. Synergy between zinc and phorbol ester in translocation of protein kinase C to cytoskeleton. FEBS Lett. 1990 Oct 29;273(1-2):131–134. doi: 10.1016/0014-5793(90)81067-x. [DOI] [PubMed] [Google Scholar]