Abstract
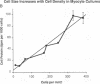
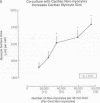
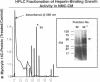
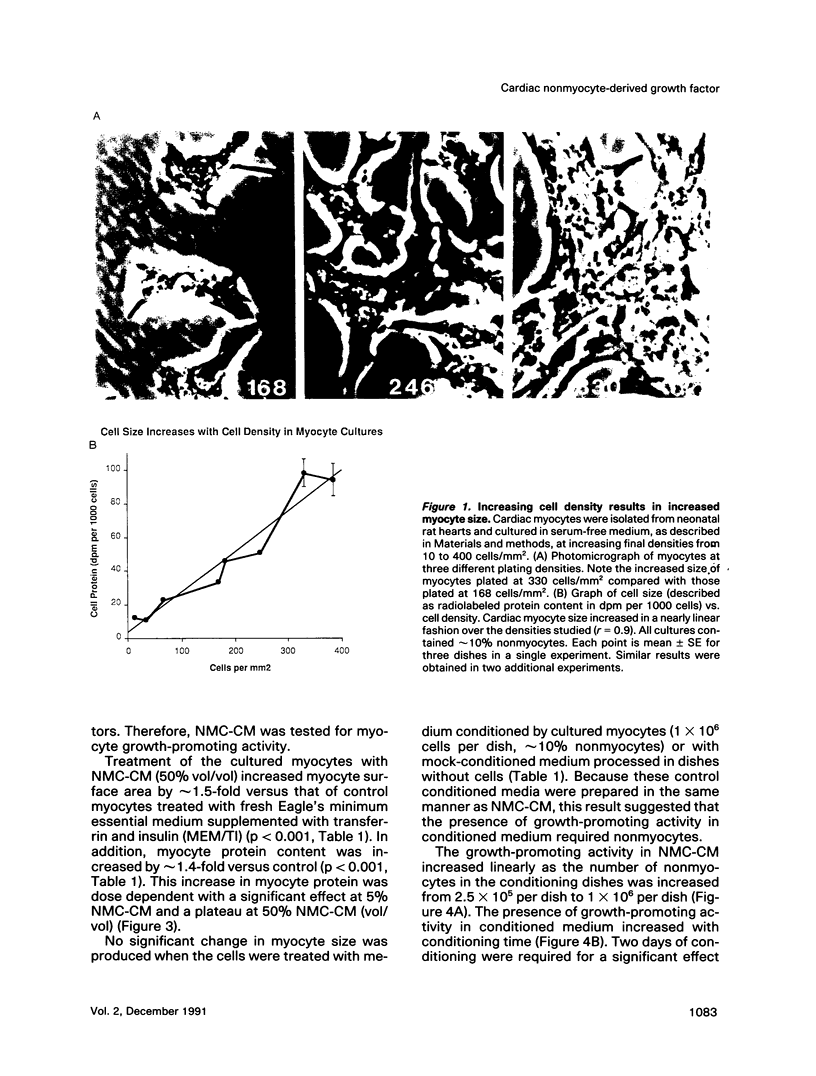
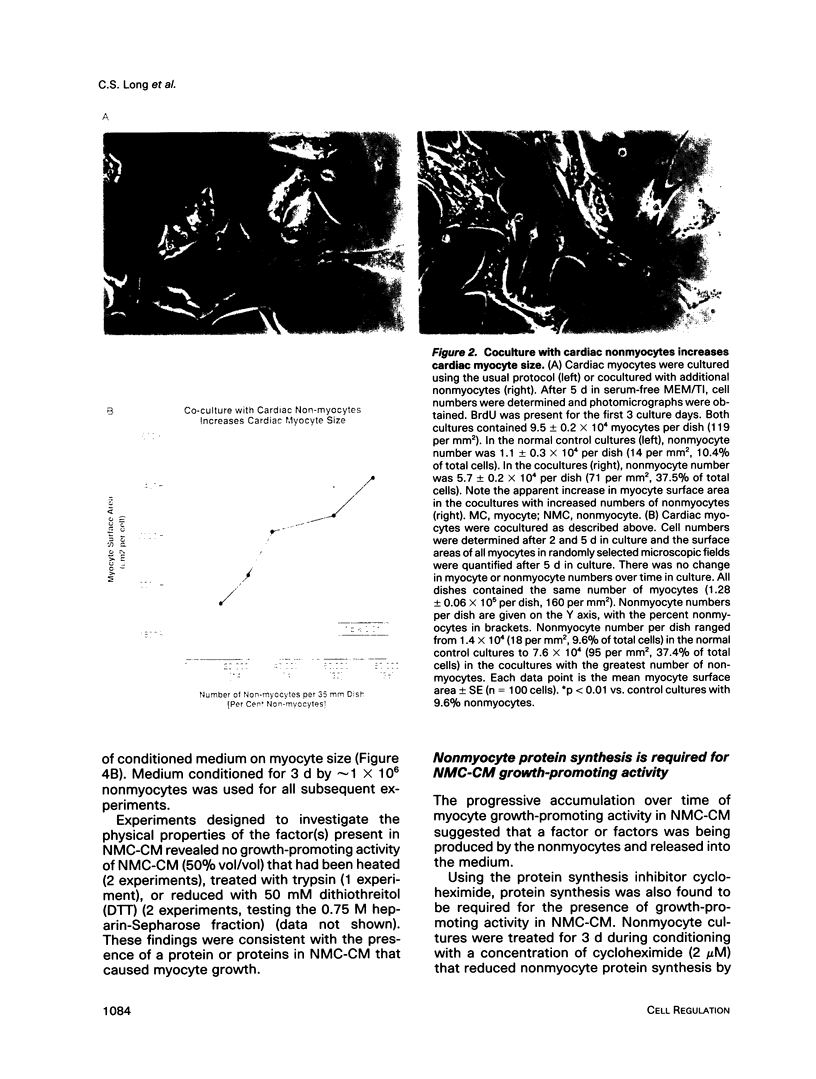
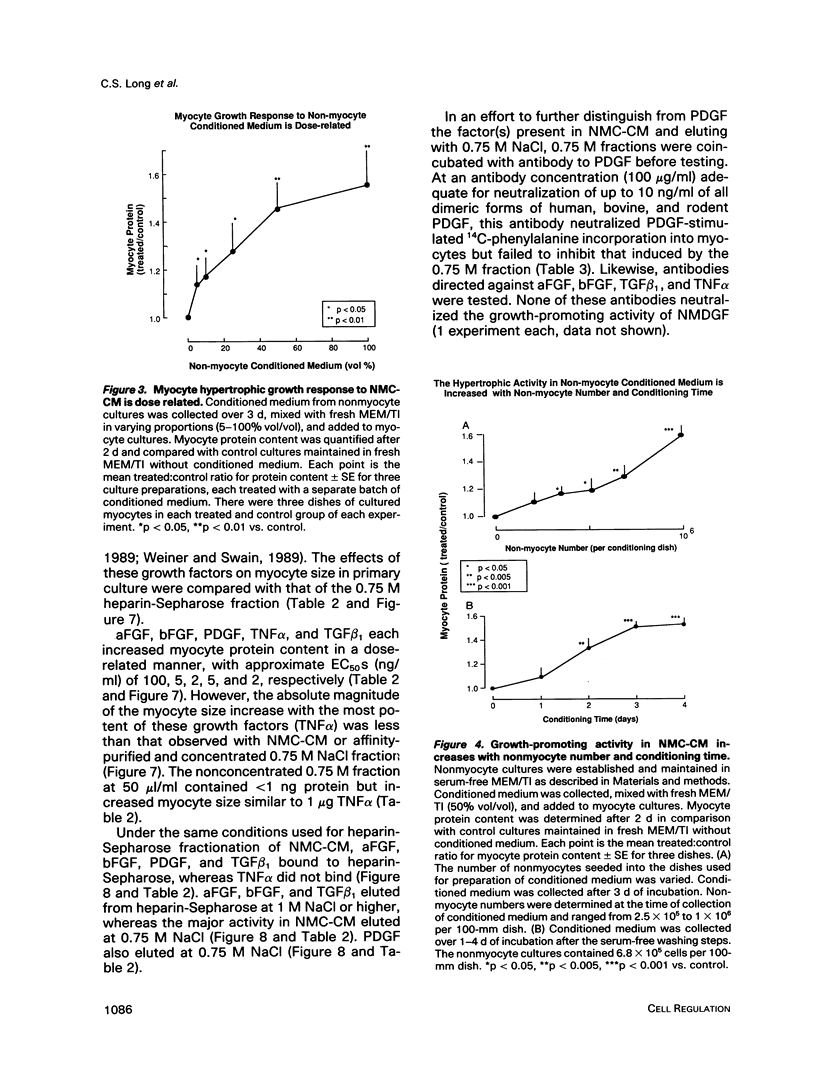
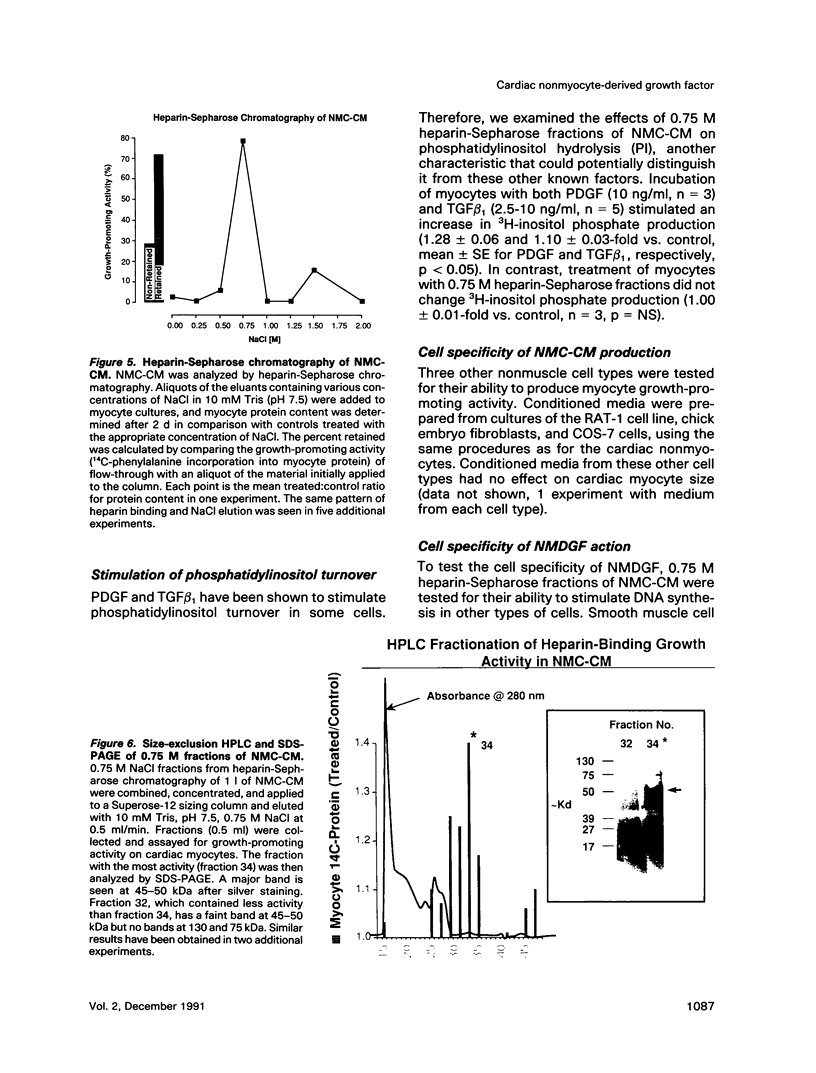
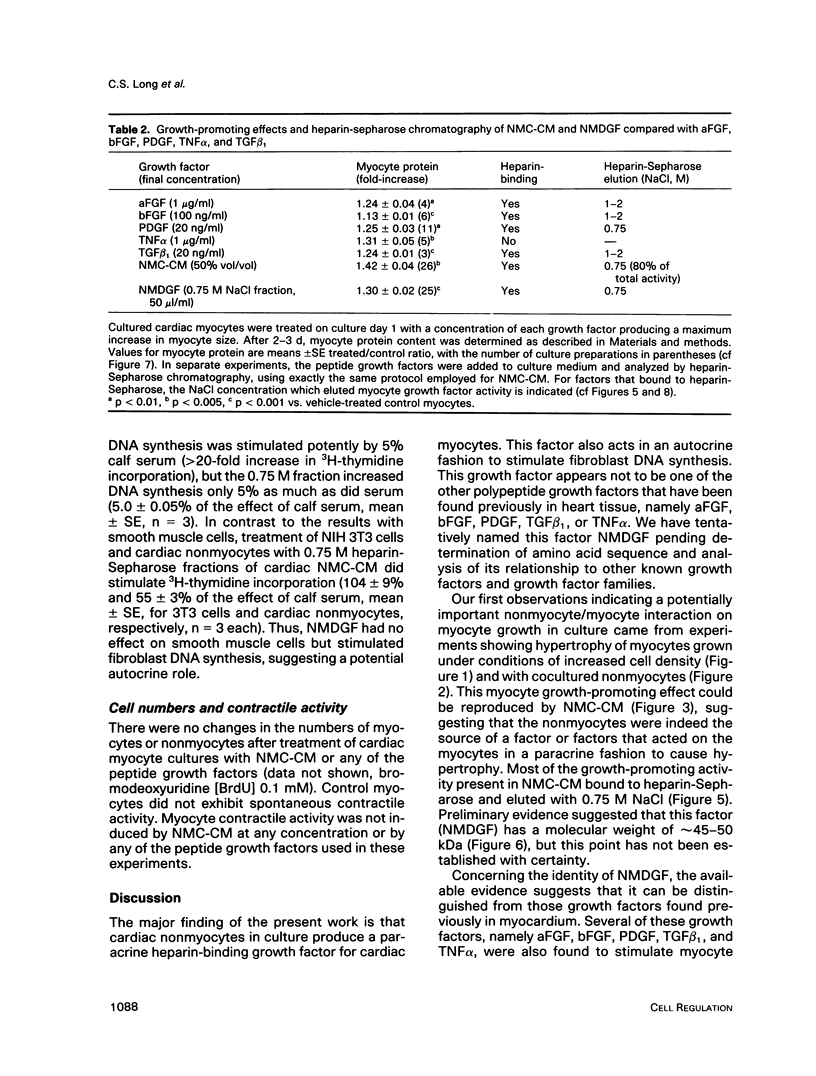
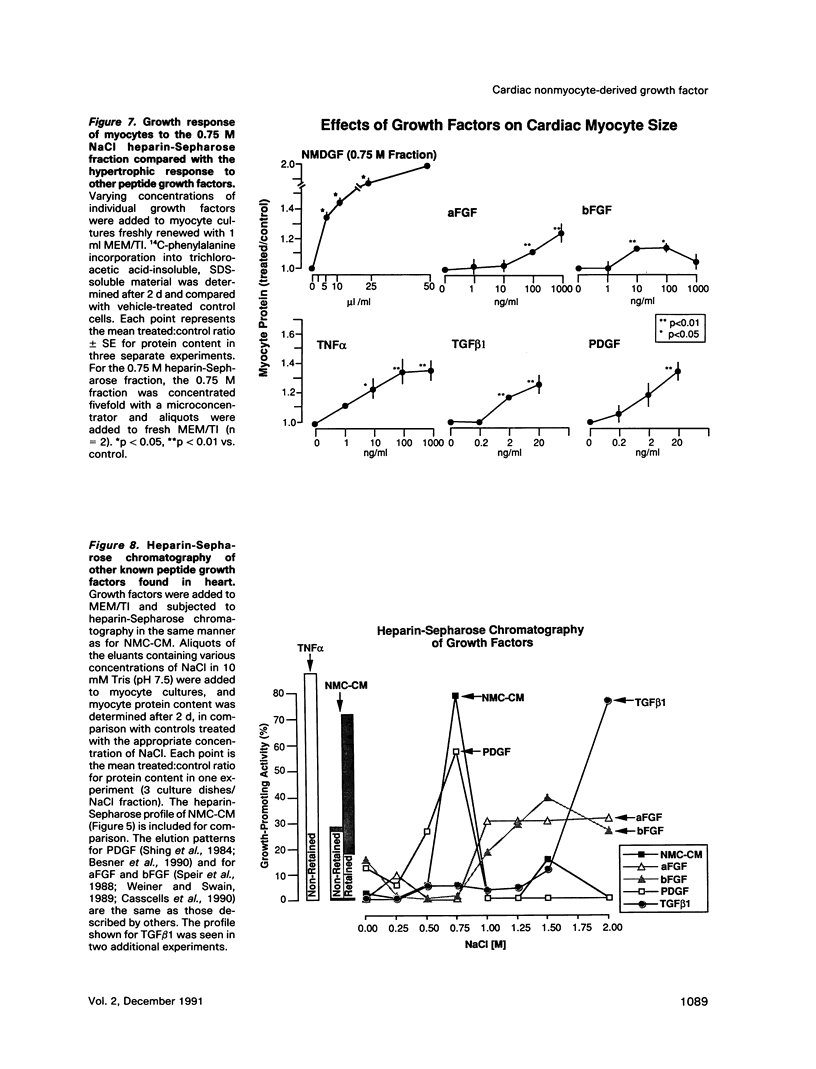
Cardiac nonmyocytes, primarily fibroblasts, surround cardiac myocytes in vivo. We examined whether nonmyocytes could modulate myocyte growth by production of one or more growth factors. Cardiac myocyte hypertrophic growth was stimulated in cultures with increasing numbers of cardiac nonmyocytes. This effect of nonmyocytes on myocyte size was reproduced by serum-free medium conditioned by the cardiac nonmyocytes. The majority of the nonmyocyte-derived myocyte growth-promoting activity bound to heparin-Sepharose and was eluted with 0.75 M NaCl. Several known polypeptide growth factors found recently in cardiac tissue, namely acidic fibroblast growth factor (aFGF), basic FGF (bFGF), platelet-derived growth factor (PDGF), tumor necrosis factor alpha (TNF alpha), and transforming growth factor beta 1 (TGF beta 1), also caused hypertrophy of cardiac myocytes in a dose-dependent manner. However, the nonmyocyte-derived growth factor (tentatively named NMDGF) could be distinguished from these other growth factors by different heparin-Sepharose binding profiles (TNF alpha, aFGF, bFGF, and TGF beta 1) by neutralizing growth factor-specific antisera (PDGF, TNF alpha, aFGF, bFGF, and TGF beta 1), by the failure of NMDGF to stimulate phosphatidylinositol hydrolysis (PDGF and TGF beta 1), and, finally, by the apparent molecular weight of NMDGF (45-50 kDa). This nonmyocyte-derived heparin-binding growth factor may represent a novel paracrine growth mechanism in myocardium.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay E. J., Raines E. W., Seifert R. A., Bowen-Pope D. F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990 Nov 2;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Besner G., Higashiyama S., Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990 Oct;1(11):811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilla C. G., Janicki J. S., Weber K. T. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ Res. 1991 Jul;69(1):107–115. doi: 10.1161/01.res.69.1.107. [DOI] [PubMed] [Google Scholar]
- Carroll E. P., Janicki J. S., Pick R., Weber K. T. Myocardial stiffness and reparative fibrosis following coronary embolisation in the rat. Cardiovasc Res. 1989 Aug;23(8):655–661. doi: 10.1093/cvr/23.8.655. [DOI] [PubMed] [Google Scholar]
- Casscells W., Speir E., Sasse J., Klagsbrun M., Allen P., Lee M., Calvo B., Chiba M., Haggroth L., Folkman J. Isolation, characterization, and localization of heparin-binding growth factors in the heart. J Clin Invest. 1990 Feb;85(2):433–441. doi: 10.1172/JCI114456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Weber K. T., Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990 Oct;67(4):787–794. doi: 10.1161/01.res.67.4.787. [DOI] [PubMed] [Google Scholar]
- Delli-Bovi P., Curatola A. M., Newman K. M., Sato Y., Moscatelli D., Hewick R. M., Rifkin D. B., Basilico C. Processing, secretion, and biological properties of a novel growth factor of the fibroblast growth factor family with oncogenic potential. Mol Cell Biol. 1988 Jul;8(7):2933–2941. doi: 10.1128/mcb.8.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E. Cultured endothelial cells produce multiple growth factors for connective tissue cells. Exp Cell Res. 1984 Jul;153(1):167–172. doi: 10.1016/0014-4827(84)90458-0. [DOI] [PubMed] [Google Scholar]
- Eghbali M. Cellular origin and distribution of transforming growth factor-beta in the normal rat myocardium. Cell Tissue Res. 1989 Jun;256(3):553–558. doi: 10.1007/BF00225603. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Henzel W. J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Friedman G., Gallily R., Chajek-Shaul T., Stein O., Shiloni E., Etienne J., Stein Y. Lipoprotein lipase in heart cell cultures is suppressed by bacterial lipopolysaccharide: an effect mediated by production of tumor necrosis factor. Biochim Biophys Acta. 1988 May 22;960(2):220–228. doi: 10.1016/0005-2760(88)90067-7. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross W. O. Fibroblast-myocyte interactions in in vitro cardiomyogenesis. Exp Cell Res. 1982 Dec;142(2):341–356. doi: 10.1016/0014-4827(82)90376-7. [DOI] [PubMed] [Google Scholar]
- Hamet P., Hadrava V., Kruppa U., Tremblay J. Transforming growth factor beta 1 expression and effect in aortic smooth muscle cells from spontaneously hypertensive rats. Hypertension. 1991 Jun;17(6 Pt 2):896–901. doi: 10.1161/01.hyp.17.6.896. [DOI] [PubMed] [Google Scholar]
- Huysman J. A., Vliegen H. W., Van der Laarse A., Eulderink F. Changes in nonmyocyte tissue composition associated with pressure overload of hypertrophic human hearts. Pathol Res Pract. 1989 Jun;184(6):577–581. doi: 10.1016/S0344-0338(89)80162-1. [DOI] [PubMed] [Google Scholar]
- Kardami E., Fandrich R. R. Basic fibroblast growth factor in atria and ventricles of the vertebrate heart. J Cell Biol. 1989 Oct;109(4 Pt 1):1865–1875. doi: 10.1083/jcb.109.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karliner J. S., Kagiya T., Simpson P. C. Effects of pertussis toxin on alpha 1-agonist-mediated phosphatidylinositide turnover and myocardial cell hypertrophy in neonatal rat ventricular myocytes. Experientia. 1990 Jan 15;46(1):81–84. doi: 10.1007/BF01955423. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libby P. Long-term culture of contractile mammalian heart cells in a defined serum-free medium that limits non-muscle cell proliferation. J Mol Cell Cardiol. 1984 Sep;16(9):803–811. doi: 10.1016/s0022-2828(84)80004-8. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Henrich C. J., Cheever L., Khaner H., Simpson P. C. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul. 1990 Aug;1(9):693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkin E., Ashford T. P. Myocardial DNA synthesis in experimental cardiac hypertrophy. Am J Physiol. 1968 Dec;215(6):1409–1413. doi: 10.1152/ajplegacy.1968.215.6.1409. [DOI] [PubMed] [Google Scholar]
- Nag A. C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28(109):41–61. [PubMed] [Google Scholar]
- Parker T. G., Packer S. E., Schneider M. D. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990 Feb;85(2):507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. D., Dagle J. M., Walder J. A., Weeks D. L., Runyan R. B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. D., Runyan R. B. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989 Aug;134(2):392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Sarzani R., Arnaldi G., Chobanian A. V. Hypertension-induced changes of platelet-derived growth factor receptor expression in rat aorta and heart. Hypertension. 1991 Jun;17(6 Pt 2):888–895. doi: 10.1161/01.hyp.17.6.888. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Simpson P. C. Proto-oncogenes and cardiac hypertrophy. Annu Rev Physiol. 1989;51:189–202. doi: 10.1146/annurev.ph.51.030189.001201. [DOI] [PubMed] [Google Scholar]
- Simpson P., McGrath A., Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982 Dec;51(6):787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- Simpson P., Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982 Jan;50(1):101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985 Jun;56(6):884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- Tischer E., Gospodarowicz D., Mitchell R., Silva M., Schilling J., Lau K., Crisp T., Fiddes J. C., Abraham J. A. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1198–1206. doi: 10.1016/0006-291x(89)92729-0. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Jalil J. E., Janicki J. S., Pick R. Myocardial collagen remodeling in pressure overload hypertrophy. A case for interstitial heart disease. Am J Hypertens. 1989 Dec;2(12 Pt 1):931–940. doi: 10.1093/ajh/2.12.931. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Swain J. L. Acidic fibroblast growth factor mRNA is expressed by cardiac myocytes in culture and the protein is localized to the extracellular matrix. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2683–2687. doi: 10.1073/pnas.86.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak R. Cell proliferation during cardiac growth. Am J Cardiol. 1973 Feb;31(2):211–219. doi: 10.1016/0002-9149(73)91034-5. [DOI] [PubMed] [Google Scholar]