Abstract
Nucleos(t)ide analogues (NAs) lead to viral suppression and undetectable hepatitis B virus (HBV) DNA in some individuals infected with HBV, but the rate of virological rebound has been unknown in such patients. We examined the prevalence of virological rebound of HBV DNA among NA-treated patients with undetectable HBV DNA. We retrospectively analyzed 303 consecutive patients [158 entecavir (ETV)- and 145 lamivudine (LAM)-treated] who achieved HBV DNA negativity, defined as HBV DNA < 3.7 log IU/mL for at least 3 months. They were followed up and their features, including their rates of viral breakthrough, were determined. Viral rebound after HBV DNA negativity was not observed in the ETV-group. Viral rebound after HBV DNA negativity occurred in 38.7% of 62 HBe antigen-positive patients in the LAM-group. On multivariate analysis, age was an independent factor for viral breakthrough among these patients (P = 0.035). Viral rebound after HBV DNA negativity occurred in 29.1% of 79 HBe antigen-negative patients in the LAM-group. Differently from LAM, ETV could inhibit HBV replication once HBV DNA negativity was achieved. In contrast, LAM could not inhibit HBV replication even if HBV negativity was achieved in the early phase. Attention should be paid to these features in clinical practice.
Keywords: Entecavir, HBeAg, HBV DNA, Lamivudine, Virological rebound.
INTRODUCTION
Hepatitis B virus (HBV) infection remains a major health problem and one of the risk factors for the development of hepatocellular carcinoma (HCC) worldwide 1,2. Chronic HBV infection has been linked epidemiologically to the development of HCC for more than 30 years 3. To date, the mechanism of HBV-related hepatocarcinogenesis is not clear. Although effective vaccine exists for preventing HBV infection 4, acute liver failure due to HBV or acute exacerbation of chronic hepatitis B is also a life-threatening disease 5,6.
Positivity for hepatitis B e antigen (HBeAg), which in serum indicates active viral replication in hepatocytes, is associated with an increased risk of HCC 7. Chronic HBV carriers with high-titer viremia are also at increased risk for HCC 8. The risk for cirrhosis and that for HCC increase significantly with increasing HBV DNA levels 9, 10. Thus, it cannot be overstated that HBV DNA should be directly suppressed to prevent the development of HCC.
There are several nucleos(t)ide analogues (NAs) for the treatment of chronic hepatitis B 11. Currently, the Japanese national health insurance system approves lamivudine (LAM) and entecavir (ETV) as first-line therapy for treatment-naïve patients with chronic hepatitis B, although some patients are treated with standard interferon-alfa or peginterferon-alfa-2a 6,12. In general, LAM, the first oral NA available for the treatment of chronic hepatitis B, is associated with high rates of drug-resistance, with ~76% after 8 years of treatment 13,14. ETV is found to be superior to LAM from the point of view that ETV is stronger than LAM and that resistance to ETV is rare, about 1.2% after 5 years of ETV treatment 14,15.
The aim of this study was to determine the efficacy and the rates of virological rebound after achieving HBV DNA negativity in the use of ETV or LAM in clinical practice. Our study showed that ETV could inhibit HBV replication if HBV DNA negativity had been achieved, but LAM was unable to inhibit HBV replication even if HBV negativity was achieved in the early phase.
MATERIALS AND METHODS
Patients and Study Design
This was a retrospective analysis comparing the rates of virological rebound in patients treated with ETV versus those in patients treated with LAM. A total of 303 patients were examined from Chiba University Hospital, Chiba, Japan, and 4 affiliated hospitals between the period of January 2000 and December 2011. NAs-naïve chronic hepatitis B patients daily receiving 0.5 mg of ETV (ETV group, N=158) or receiving 100 mg of LAM (LAM group, N=145) with undetectable HBV DNA (< 3.7 log IU/mL) for three months were enrolled. Some of the included patients had been previously reported 12, 16. All patients had serum hepatitis B surface antigen (HBsAg) detectable for at least 6 months, regardless of their HBeAg status. They were negative for hepatitis C virus and human immunodeficiency virus antibodies. This study was approved by the Ethics Committee of Chiba University, Graduate School of Medicine (No. 977).
Definition of Virological Rebound of HBV
We defined virological rebound as ≥ 3.7 log IU/mL for at least 3 months after achieving undetectable HBV DNA.
Monitoring of HBV DNA, Serum Liver Function Tests and Hematological Tests
The primary outcome of this study was the virological rebound. Patients were followed up at least every 3 months to examine physical status and to monitor liver biochemistry and virology. All clinical laboratory tests including hematological data, biochemical data, and HBV serologies were performed at the Central Laboratory of Chiba University Hospital. HBsAg, HBeAg and anti-HBe antibody were determined by ELISA (Abbott, Chicago, IL, USA) or CLEIA (Fujirebio, Tokyo, Japan) 17. HBV genotype was determined from patients' sera by ELISA (Institute of Immunology, Tokyo, Japan) as reported by Usuda et al 18. HBV DNA was measured by transcription-mediated amplification (TMA) assay, COBAS Amplicor HBV Monitor assay, or COBAS TaqMan (Roche Diagnostics, Branchburg, NJ, USA). The clinical efficacy of NAs was assessed as the proportion of patients achieving HBV DNA negativity, defined as an HBV DNA level of < 3.7 log IU/mL.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Differences were evaluated by Student's t-test, chi-square test, or Fisher's exact test. P < 0.05 was considered statistically significant. Variables with P < 0.05 at univariate analysis were retained for multivariate logistic-regression analysis. For all tests, two-sided P-values were calculated and the results were considered statistically significant at P < 0.05. Statistical analysis was performed using the Excelstatistics program for Windows, version 7 (SSRI, Tokyo, Japan).
RESULTS
A total 303 patients were recruited into either the ETV group (n = 158) or the LAM group (n = 145), with a follow-up period of 33.7 ± 11.3 months (28.6 ± 11.3 months or 39.3 ± 31.4 months, respectively). Baseline demographic and laboratory data are summarized in Table 1. There were no differences in age, gender, HBV DNA, alanine aminotransferase (ALT) levels, ultrasound findings/presence of cirrhosis, and periods from the initial administration of ETV or LAM to undetectable HBV DNA, between the ETV and LAM groups, although the proportion of HBeAg-positive patients in the ETV group (55%) tended to be higher than that in the LAM group (44%).
Table 1.
Baseline characteristics of patients treated with entecavir (ETV) or lamivudine (LAM).
| Total | ETV group | LAM group | P-values | |
|---|---|---|---|---|
| Number | 303 | 158 | 145 | |
| Age (years) | 51 ± 12 | 51 ± 12 | 50 ± 12 | N.S. |
| Gender (male) | 205 | 101 | 104 | N.S. |
| HBeAg (+) | 146 | 84 | 62 | 0.079 |
| HBV DNA (log IU/mL) | 6.5 ± 1.5 | 6.6 ± 1.7 | 6.4 ± 1.3 | N.S. |
| ALT (IU/L) | 203 ± 280 | 187 ± 290 | 220 ± 266 | N.S. |
| US: Cirrhosis (+) | 113 | 56 | 57 | N.S. |
| Periods to undetectable HBV DNA (months) | 10.0 ± 18.2 | 8.5 ± 11.9 | 11.8 ± 23.3 | N.S. |
Data are expressed as mean ± SD. ETV group, patients receiving 0.5 mg of ETV daily; LAM group, patients receiving 100 mg of LAM daily; P-values, P-values between ETV and LAM groups; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; US, ultrasound findings; N.S., no statistically significant difference.
Virological Rebound
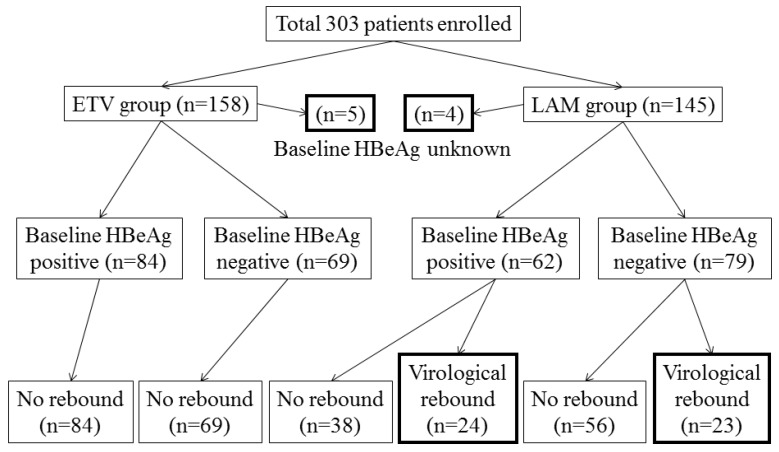
The patient flow and outcome are summarized in Figure 1. We excluded 9 patients, whose HBeAg status at baseline was unknown, from this analysis. When comparing the baseline characteristics of patients according to HBeAg status, HBeAg-positive patients were younger, had higher ALT levels and HBV DNA levels, and less cirrhotic findings by ultrasound than HBeAg-negative patients (Table 2). The period from the initial administration of ETV or LAM to the determination of undetectable HBV DNA in the HBeAg-negative group tended to be shorter than that in the HBeAg-positive group (Table 2).
Figure 1.
Study design and patient flow for both groups.
Table 2.
Baseline characteristics of patients according to HBeAg status.
| HBeAg | Positive group | Negative group | P-values |
|---|---|---|---|
| Number | 146 | 148 | |
| Age (years) | 46 ± 12 | 55 ± 11 | < 0.001 |
| Gender (male) | 101 | 97 | N.S. |
| HBV DNA (log IU/mL) | 7.2 ± 1.1 | 5.8 ± 1.4 | < 0.001 |
| ALT (IU/L) | 257 ± 332 | 156 ± 211 | 0.002 |
| US: Cirrhosis (+) | 41 | 70 | < 0.001 |
| Periods to undetectable HBV DNA (months) | 11.0 ± 18.1 | 7.4 ± 14.4 | 0.063 |
Data are expressed as mean ± SD. P-values, P-values between HBeAg-positive and HBeAg-negative groups; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; US, ultrasound findings; N.S., no statistically significant difference.
In the ETV group, none of the patients had virological rebound during the follow-up periods. In the LAM group, 24 and 23 patients of 62 HBeAg-positive and 79 HBeAg-negative patients at baseline, respectively, developed evidence of virological rebound. In the 24 HBeAg-positive patients at baseline with virological rebound, 9, 8, 3, 1, 2, and 1 had virological rebound at ≤ 1, 1 ~ ≤ 2, 2 ~ ≤ 3, 3 ~ ≤ 4, 4 ~ ≤ 5, and details unknown, respectively. In the 23 HBeAg-negative patients at baseline with virological rebound, 10, 8, 3, 0, 1, and 1 had virological rebound at ≤ 1, 1 ~ ≤ 2, 2 ~ ≤ 3, 3 ~ ≤ 4, 4 ~ ≤ 5 and details unknown, respectively. Baseline characteristics of patients treated with ETV or LAM according to HBeAg status are shown in Table 3. In the ETV group, the period from the initial administration of ETV to the determination of undetectable HBV DNA in the HBeAg-negative group was the same as that in the HBeAg-positive group (Table 3). In the LAM group, the period from the initial administration of LAM to undetectable HBV DNA in the HBeAg-negative group was shorter than that in the HBeAg-positive group (Table 3). In the HBeAg-positive patients, the period from the initial administration to undetectable HBV DNA in the ETV group was shorter than that in the LAM group (Table 3).
Table 3.
Baseline characteristics of patients treated with entecavir (ETV) or lamivudine (LAM) according to HBeAg status.
| ETV group | LAM group | |||
|---|---|---|---|---|
| HBeAg | Positive | Negative | Positive | Negative |
| Number | 84 | 69 | 62 | 79 |
| Age (years) | 48 ± 12 | 56 ± 11* | 44 ± 11 ## | 54 ± 11** |
| Gender (male) | 53 | 45 | 48 | 52** |
| HBV DNA (log IU/mL) | 7.5 ± 1.1 | 5.7 ± 1.5* | 6.9 ± 1.1$ | 5.9 ± 1.3** |
| ALT (IU/L) | 219 ± 325 | 159 ± 246 | 309 ± 334 | 154 ± 174** |
| US: Cirrhosis (+) | 25 | 29 | 16 | 41 |
| Periods to undetectable HBV DNA (months) | 8.3 ± 10.5 | 7.3 ± 11.0 | 15.0 ± 24.7$$ | 7.5 ± 16.9# |
Data are expressed as mean ± SD. HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; US, ultrasound findings; *P < 0.001, compared to HBeAg-positive of ETV group; **P < 0.001 and #P = 0.034, compared to HBeAg-positive of LAM group; ##P = 0.041, $P = 0.001 and $$P = 0.027, compared to HBeAg-positive of ETV group.
Predictors of Virological Rebound in Patients treated with LAM
To clarify the predictors of virological rebound in patients treated with LAM, we compared the pretreatment factors between patients with and without virological rebound according to HBeAg status (Table 4A & 4B). Univariative analysis showed that age, HBV DNA, ALT levels and the period from the initial administration of LAM to the determination of undetectable HBV DNA in HBeAg-positive patients contributed to the occurrence of virological rebound (Table 4A). Factors significantly associated with virological rebound in HBeAg-positive patients treated with LAM by univariate analysis were also analyzed by multivariate logistic regression analysis. Virological rebound was attained independently of age in HBeAg-positive patients treated with LAM (Table 4C). In HBeAg-negative patients, no significant factors contributing to virological rebound could be found (Table 4B).
Table 4A.
Predictors of virological rebound in patients treated with lamivudine (LAM). (A) Comparison of HBeAg-positive patients with or without virological rebound by univariate analysis.
| Virological rebound | No | Yes | P-values |
|---|---|---|---|
| Number | 38 | 23 | |
| Age (years) | 42 ± 11 | 49 ± 11 | 0.019 |
| Gender (male) | 30 | 17 | N.S. |
| HBV DNA (log IU/mL) | 6.9 ± 1.2 | 6.8 ± 0.9 | N.S. |
| ALT (IU/L) | 379 ± 377 | 196 ± 205 | 0.037 |
| US: Cirrhosis (+) | 7 | 9 | N.S. |
| Periods to undetectable HBV DNA (months) | 20.6 ± 29.1 | 4.1 ± 3.1 | 0.009 |
Data are expressed as mean ± SD. P-values, P-values between patients with or without virological rebound groups; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; US, ultrasound findings; N.S., no statistically significant difference.
Table 4B.
(B) Comparison of HBeAg-negative patients with or without virological rebound by univariate analysis.
| Virological rebound | No | Yes | P-values |
|---|---|---|---|
| Number | 56 | 22 | |
| Age (years) | 54 ± 11 | 54 ± 10 | N.S. |
| Gender (male) | 40 | 12 | N.S. |
| HBV DNA (log IU/mL) | 5.9 ± 1.4 | 5.9 ± 1.0 | N.S. |
| ALT (IU/L) | 163 ± 179 | 137 ± 163 | N.S. |
| US: Cirrhosis (+) | 30 | 11 | N.S. |
| Periods to undetectable HBV DNA (months) | 7.3 ± 14.8 | 3.1 ± 2.1 | N.S. |
Data are expressed as mean ± SD. P-values, P-values between patients with or without virological rebound groups; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; US, ultrasound findings; N.S., no statistically significant difference.
Table 4C.
(C) Factor associated with virological rebound among HBeAg-positive patients treated with LAM by multivariate analysis.
| Factor | Category | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|
| Age ≤ 44.5 (years) | (+/-) | 0.222 | 0.0547-0.9023 | 0.0354 |
DISCUSSION
To date, there is not much data regarding virological rebound after achieving HBV DNA negativity in the use of ETV or LAM. A recent report supported the merit of the change from LAM to ETV 14. This study concluded that prior optimal viral suppression with ETV did not confer any significant advantage for patients who switched to LAM.
The present study revealed that ETV could suppress HBV replication after achieving HBV DNA negativity, although additional longer follow-up studies will be needed. On the other hand, LAM could not suppress HBV replication even after achieving HBV DNA negativity (Figure 1), although most cases with virological rebound were observed within 2 years of the start of LAM medication. We could not check the emergence of YMDD motif mutations 19 in all of the cases because the present study was performed as part of regular clinical practice. Of 2 of the HBeAg-positive patients at baseline with virological rebound, one showed YVDD motif (50%). In 4 of the HBeAg-negative patients at baseline with virological rebound, one YVDD motif (25%) and three YIDD motifs (75%) were seen. Virological rebound may not mean the emergence of NA-resistance mutations 12.
We do not know the reason why virological rebound was attained independently of age in HBeAg-positive patients treated with LAM. HBeAg to anti-HBe antibody seroconversions were found in 20 and 11 patients with and without virological rebound, that is, the HBeAg to anti-HBe antibody seroconversion rates were similar in the two groups (data not shown), although the number of study patients seemed small in the present study. Further studies might be needed. In any event, it might be important to consider the LAM-to-ETV switch in HBeAg-positive patients treated with LAM, although some of our patients in the LAM group remained HBV-negative throughout the observation period.
In the present study, 95.3% (122 of 128), 82.3% (14 of 17) and 89.2% (25 of 28) had an adherence rate >90% 16 in ETV-treated, LAM-treated with virological rebound and LAM-treated patients without virological rebound, respectively. These results supported our previous study that viral breakthrough associated with poor adherence could be a more important issue in the treatment with especially stronger NAs, such as ETV 12,16, although we cannot ensure durable HBV negativity after NAs are discontinued. We and others reported that HBeAg could impair both innate and adaptive immune responses to promote chronic HBV infection 16,20,21. Of interest, the virological rebound with the use of LAM seemed unrelated to the HBeAg status, suggesting that it was dependent on resistant mutation.
Recently, other effective antiviral therapies such as peginterferon 22,23 and tenofovir 24,25 were reported to be useful for the control of HBV infection. These drugs might also be candidates for treating virological rebound. Fung et al. 14 reported that prior optimal viral suppression with ETV did not confer any significant advantage for patients who switched to LAM. Our results also supported the previous studies that ETV was much more efficient than LAM 26-29. In conclusion, ETV could inhibit HBV replication if HBV DNA negativity had been achieved. In contrast, LAM could not inhibit HBV replication even if HBV negativity was achieved in the early phase. Attention should be paid to these features in clinical practice.
Acknowledgments
We thank all our colleagues at the liver units of our hospitals who cared for the patients described herein.
ABBREVIATIONS
- ALT
alanine aminotransferase
- ETV
Entecavir
- HBeAg
Hepatitis B e antigen
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- LAM
lamivudine
- NA
nucleos(t)ide analogue.
References
- 1.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012;61:i6–i17. doi: 10.1136/gutjnl-2012-302056. [DOI] [PubMed] [Google Scholar]
- 2.Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49(5 Suppl):S56–S60. doi: 10.1002/hep.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley RP, Hwang LY, Lin CC. et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 4.Lavanchy D. Viral hepatitis: global goals for vaccination. J Clin Virol. 2012;55:296–302. doi: 10.1016/j.jcv.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Imamura T, Yokosuka O, Kurihara T. et al. Distribution of hepatitis B virus genotypes and mutations in the core promoter and precore regions in acute form of liver disease in patients from Chiba, Japan. Gut. 2003;52:1630–1637. doi: 10.1136/gut.52.11.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanda T, Shinozaki M, Kamezaki H. et al. Efficacy of lamivudine or entecavir on acute exacerbation of chronic hepatitis B. Int J Med Sci. 2012;9:27–32. doi: 10.7150/ijms.9.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HI, Lu SN, Liaw YF. et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 8.Harris RA, Chen G, Lin WY. et al. Spontaneous clearance of high-titer serum HBV DNA and risk of hepatocellular carcinoma in a Chinese population. Cancer Causes Control. 2003;14:995–1000. doi: 10.1023/b:caco.0000007984.79987.ec. [DOI] [PubMed] [Google Scholar]
- 9.Chen CJ, Yang HI, Su J. et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Iloeje UH, Yang HI, Su J. et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, Kao JH, Piratvisuth T. et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 12.Kamezaki H, Kanda T, Wu S. et al. Emergence of entecavir-resistant mutations in nucleos(t)ide-naïve Japanese patients infected with hepatitis B virus: virological breakthrough is also dependent on adherence to medication. Scand J Gastroenterol. 2011;46:1111–1117. doi: 10.3109/00365521.2011.584898. [DOI] [PubMed] [Google Scholar]
- 13.Yuen MF, Seto WK, Chow DH. et al. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295–1303. [PubMed] [Google Scholar]
- 14.Fung J, Lai CL, Yuen J. et al. Randomized trial of lamivudine versus entecavir in Entecavir-treated patients with undetectable hepatitis B virus DNA: outcome at 2 years. Hepatology. 2011;53:1148–1153. doi: 10.1002/hep.24192. [DOI] [PubMed] [Google Scholar]
- 15.Tenney DJ, Rose RE, Baldick CJ. et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 16.Kamezaki H, Kanda T, Makoto A. et al. Adherence to medication is a more important contributor to viral breakthrough in chronic hepatitis B patients treated with entecavir than in those with lamivudine. Int J Med Sci. 2013;10:567–574. doi: 10.7150/ijms.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Kanda T, Imazeki F. et al. Hepatitis B virus e antigen downregulates cytokine production in human hepatoma cell lines. Viral Immunol. 2010;23:467–476. doi: 10.1089/vim.2010.0042. [DOI] [PubMed] [Google Scholar]
- 18.Usuda S, Okamoto H, Iwanari H. et al. Serological detection of hepatitis B virus genotypes by ELISA with monoclonal antibodies to type-specific epitopes in the preS2-region product. J Virol Methods. 1999;80:97–112. doi: 10.1016/s0166-0934(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 19.Seta T, Yokosuka O, Imazeki F. et al. Emergence of YMDD motief mutations of hepatitis B virus during lamivudine treatment of immunocompetent type B hepatitis patients. J Med Virol. 2000;60:8–16. doi: 10.1002/(sici)1096-9071(200001)60:1<8::aid-jmv2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Sallberg M, Hughes J. et al. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol. 2005;79:3016–3027. doi: 10.1128/JVI.79.5.3016-3027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Kanda T, Imazeki F. et al. Hepatitis B virus e antigen physically associates with receptor-interacting serine/threonine protein kinase 2 and requires IL-6 gene expression. J Infect Dis. 2012;206:415–420. doi: 10.1093/infdis/jis363. [DOI] [PubMed] [Google Scholar]
- 22.Marcellin P, Lau GK, Bonino F. et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 23.Lau GK, Piratvisuth T, Luo KX. et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 24.Schildgen O, Sirma H, Funk A. et al. Variant of hepatitis B virus with primary resistance to adefovir. N Engl J Med. 2006;354:1807–1812. doi: 10.1056/NEJMoa051214. [DOI] [PubMed] [Google Scholar]
- 25.Marcellin P, Heathcote EJ, Buti M. et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 26.Chang TT, Gish RG, de Man R. et al. A comparison of Entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 27.Lai CC, Shouval D, Lok AS. et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 28.Veenstra DL, Sullivan SD, Clarke L. et al. Cost effectiveness of Entecavir versus lamivudine with adefovir salvage in HBe-positive chronic hepatitis B. Pharmacoeconomics. 2007;25:963–977. doi: 10.2165/00019053-200725110-00006. [DOI] [PubMed] [Google Scholar]
- 29.Lacey L, Chien RN, Chuang WL. et al. Economic evaluation of chronic hepatitis B treatments in Taiwan. J Gastroenterol Hepatol. 2008;23:571–579. doi: 10.1111/j.1440-1746.2008.05360.x. [DOI] [PubMed] [Google Scholar]