Abstract
Significance: Pulmonary hypertension is a devastating disorder without any available treatment strategies that satisfactorily promote the survival of patients. The identification of new therapeutic strategies to treat patients with pulmonary hypertension is warranted. Recent Advances: Human studies have provided evidence that there is increased oxidative stress (lipid peroxidation, protein oxidation, DNA oxidation, and the depletion of small-molecule antioxidants) in patients with pulmonary hypertension. A variety of compounds with antioxidant properties have been shown to have beneficial therapeutic effects in animal models of pulmonary hypertension, possibly supporting the hypothesis that reactive oxygen species (ROS) are involved in the progression of pulmonary hypertension. Thus, understanding the molecular mechanisms of ROS actions could contribute to the development of optimal, antioxidant-based therapy for human pulmonary hypertension. One such mechanism includes action as a second messenger during cell-signaling events, leading to the growth of pulmonary vascular cells and right ventricular cells. Critical Issues: The molecular mechanisms behind promotion of cell signaling for pulmonary vascular cell growth and right ventricular hypertrophy by ROS are not well understood. Evidence suggests that iron-catalyzed protein carbonylation may be involved. Future Directions: Understanding precise mechanisms of ROS actions should be useful for designing preclinical animal experiments and human clinical trials of the use of antioxidants and/or other redox compounds in the treatment of pulmonary hypertension. Antioxid. Redox Signal. 18, 1789–1796.
Introduction
Pulmonary hypertension is a disorder of the pulmonary vasculature that results in an elevated mean pulmonary arterial pressure of ≥25 mmHg (12). Pulmonary arterial hypertension is one class of this disorder and includes idiopathic pulmonary arterial hypertension, heritable pulmonary arterial hypertension, pulmonary arterial hypertension induced by drugs and toxins, persistent pulmonary hypertension of the newborn, and pulmonary arterial hypertension associated with various diseases such as connective tissue diseases, infection with human immunodeficiency virus, portal hypertension, congenital heart disease, schisostomiasis, and chronic hemolytic anemia. Pulmonary hypertension is also associated with hypoxic lung diseases such as chronic obstructive pulmonary disease and pulmonary fibrosis, often worsening the prognosis of these underlying diseases. Other classes of pulmonary hypertension comprise pulmonary hypertension due to left heart dysfunction, chronic thromboembolic pulmonary hypertension, pulmonary capillary hemangiomatosis, venooclusive disease, and diverse forms of pulmonary hypertension with unclear and/or multifactorial etiologies.
Pathologic characteristics of pulmonary hypertension include vasoconstriction within the pulmonary vasculature as well as histological abnormalities of the vascular wall, with the latter including medial hypertrophy, intimal proliferation, fibrosis, adventitial thickening, thrombotic lesions, and inflammatory infiltrates. In advanced stages of pulmonary arterial hypertension, all of these characteristics are present in the plexiform lesions. The pathogenic mechanism of pulmonary hypertension involves various pathways that lead to vasoconstriction of pulmonary arteries and increase in pulmonary vascular resistance due in part to the abnormal production of vasoactive compounds caused by endothelial dysfunction. Increased vasoconstriction is also related to the abnormal expression and activity of potassium channels in pulmonary vascular smooth muscle cells. Endothelial dysfunction increases vasoconstrictive and proliferative substances such as endothelin-1 and thromboxane A2. Also, levels of factors with vasodilatory and antiproliferative properties, such as nitric oxide and prostacyclin, are decreased. These abnormalities increase the vascular muscle tone and promote vascular thickening. The increased pulmonary arterial pressure and resistance strains the right ventricle, leading to right ventricular hypertrophy and ultimately to heart failure and death.
Evidence for the Occurrence of Oxidative Stress in Human Pulmonary Hypertension
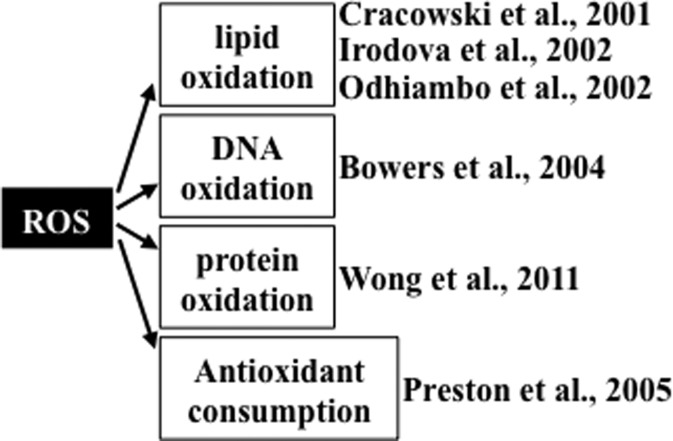
Studies providing evidence of increased oxidative status in patients with pulmonary hypertension are summarized in Figure 1. Cracowski et al. (6) reported that urine F2 isoprostane levels were 2.3 times higher in patients with pulmonary hypertension compared to healthy control subjects, measured using gas chromatography/mass spectrometry. Of patients in this study, 48% had idiopathic pulmonary arterial hypertension and 52% had secondary pulmonary hypertension. Similarly, Irodova et al. (18) reported higher concentrations of plasma malondialdehyde in patients with idiopathic pulmonary arterial hypertension than in healthy volunteers. In addition, a mass spectrometry study revealed that, compared to individuals without pulmonary hypertension, plasmas from patients with idiopathic pulmonary arterial hypertension and patients with sickle cell anemia who had pulmonary hypertension contained more pronounced malondialdehyde-modified albumin at the amino acid residue lysine 159 (31). These studies collectively demonstrate that pulmonary hypertension is associated with increased lipid peroxidation. In addition to lipid peroxidation, oxidation states of other biological molecules are reportedly higher in patients with pulmonary hypertension. Immunohistochemical studies by Bowers et al. (3) demonstrate that lungs from patients with idiopathic pulmonary arterial hypertension had intense 8-hydroxyguanosine staining in the plexiform lesions and in the luminal endothelial cells of concentric intima fibrosis lesions, indicating the occurrence of DNA oxidation. These authors also reported that lungs from patients with pulmonary hypertension had positive staining for nitrotyrosine. The occurrence of protein oxidation was also demonstrated in our laboratory by measuring the plasma protein carbonyl content using an immunological technique. We find that patients with idiopathic pulmonary arterial hypertension have a higher plasma protein carbonyl content compared to healthy subjects (51). Using high-performance liquid chromatography, our study also shows that, compared to control subjects, patients with idiopathic pulmonary arterial hypertension have reduced levels of lipophilic antioxidants in plasma, including α-tocopherol and β-carotene (33).
FIG. 1.
Evidence for the occurrence of oxidative stress in human pulmonary hypertension.
Evidence for Beneficial Effects of Antioxidants in Animal Models of Pulmonary Hypertension
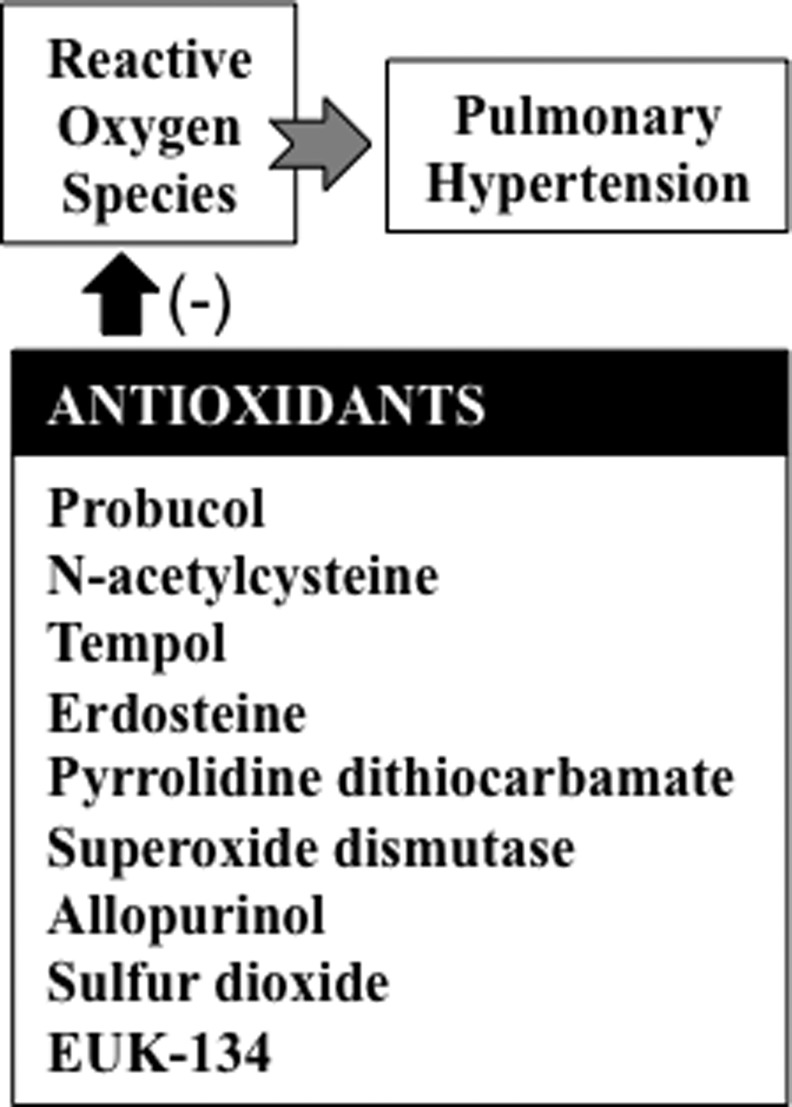
In animal models, various compounds with antioxidant properties have been shown to suppress the progression of pulmonary hypertension (Fig. 2). In rats, compounds with antioxidant activities have been found to inhibit pulmonary hypertension and/or right ventricular dysfunction. Such compounds include probucol (19), N-acetylcysteine (16, 23), tempol (9, 20), erdosteine (46), allicin (43), pyrrolidine dithiocarbamate (17, 39), superoxide dismutase (22), allopurinol (20), sulfur dioxide (21), resveratrol (7, 52), and EUK-134 (35). In neonatal lambs with persistent pulmonary hypertension, superoxide dismutase was found to improve oxygenation, reduce oxidation (24), restore endothelial nitric oxide synthase expression and function (10), and normalize phosphodiesterase 5 (11).
FIG. 2.
Antioxidants that have been shown to inhibit pulmonary hypertension in experimental models.
Role of Reactive Oxygen Species in Cell Signaling for the Pathogenesis of Pulmonary Hypertension
Accumulating evidence suggests that reactive oxygen species (ROS) can regulate cell signaling, with NAD(P)H oxidase serving as the major source of ROS in such events (44). The role of NAD(P)H oxidase in the pathogenesis of pulmonary hypertension has been documented in animal models of pulmonary hypertension, including fetal lambs (4, 13), newborn piglets (8), and mice (30). These results suggest that NAD(P)H oxidase and components of downstream ROS signaling are possible therapeutic targets for the treatment of pulmonary hypertension.
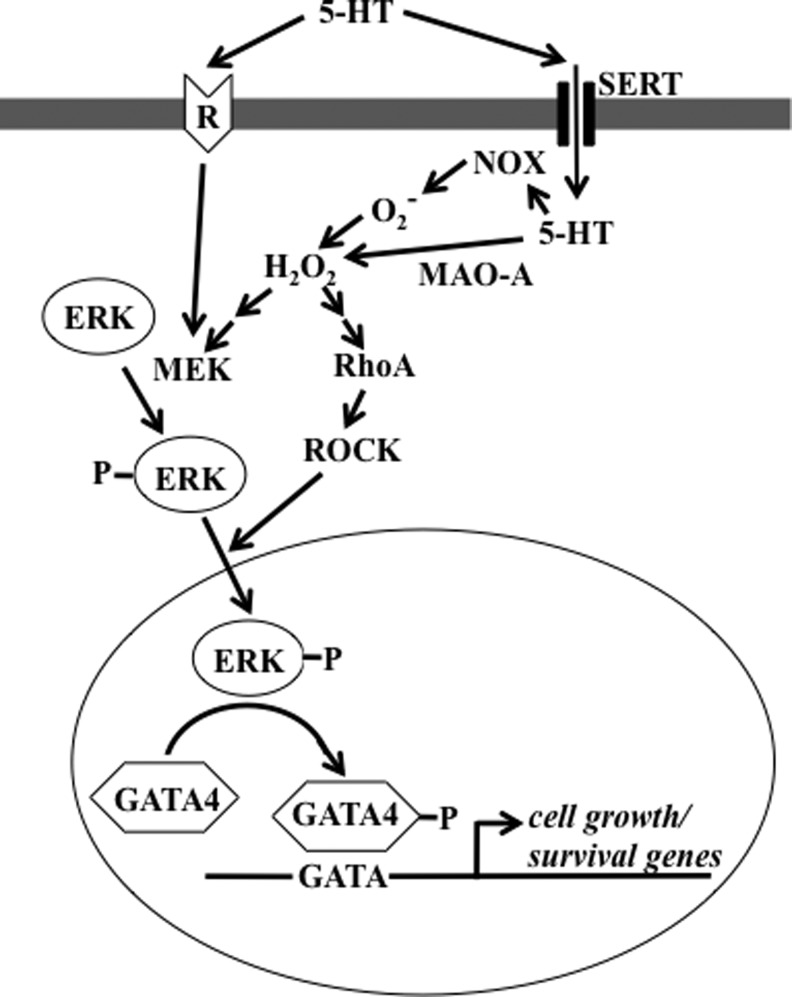
In pulmonary artery smooth muscle cells, serotonin (26) and endothelin-1 (47) activate the production of ROS via NAD(P)H oxidase. The GATA4 transcription factor plays an important role in the regulation of growth of pulmonary artery smooth muscle cells, and antioxidants inhibit serotonin-induced GATA4 phosphorylation and activation (45). The serotonin signal for the nuclear translocation of extracellular signal-regulated kinase (ERK) and subsequent GATA4 phosphorylation is dependent on the activation of RhoA and Rho kinase (27). In response to serotonin, ERK has also been shown to activate GATA4 via monoamine oxidase-A-dependent production of hydrogen peroxide (H2O2), which promotes the translocation of phosphorylated ERK to the nucleus (25). This could operate through RhoA signaling, as our laboratory recently found that Rho guanine nucleotide dissociation inhibitor (RhoGDI), which functions to prevent the exchange of guanosine-5′-triphosphate (GTP) for guanosine-5′-diphosphate on the Rho-GTPases by guanine nucleotide exchange factors, is carbonylated in response to serotonin (Wong & Suzuki, unpublished results). Thus, carbonylation of RhoGDI may trigger RhoA/Rho kinase activation, leading to the nuclear translocation of ERK and GATA4 phosphorylation. Carbonylation of RhoGDI may also be a mechanism for ROS-dependent stimulation of RhoA as described by Resta and coworkers (5). Based on these studies, Figure 3 summarizes proposed ROS-dependent cell-signaling pathways for GATA4 activation by serotonin in pulmonary artery smooth muscle cells. Endothelin-1 also produces ROS in pulmonary artery smooth muscle cells via NAD(P)H oxidase, and antioxidants block endothelin-1-induced cell proliferation (47).
FIG. 3.
Proposed reactive oxygen species (ROS)-dependent signaling pathways for serotonin (5-HT)-induced GATA4 activation in pulmonary artery smooth muscle cells. 5-HT has been shown to produce hydrogen peroxide (H2O2) via 5-HT transporter (SERT) either by activating NAD(P)H oxidase (NOX) or by activating monoamine oxidase-A (MAO-A). H2O2, in turn, (i) activates MEK to phosphorylate ERK and/or (ii) activates RhoA/Rho kinase (ROCK) pathway, facilitating ERK nuclear translocation. In the nucleus, ERK then phosphorylates and activates GATA4 (25–27, 45).
Mechanisms of ROS Signaling
While, as described above, the roles of NAD(P)H oxidase and ROS in the development of pulmonary hypertension have been supported by a number of published studies, the exact mechanisms of ROS actions are not well understood. Our laboratory provided a series of evidence that iron-dependent hydroxyl radical (HO•) formation and resultant protein carbonylation may be involved (49).
Despite the major and active role played by HO• in oxidative stress, its role in ROS signaling has not been well described. It is unlikely that HO•, which has a diffusion distance of only 1–20 nm, serves as a diffusible second messenger as does H2O2, which has a diffusion distance of 1–1600 μm (29, 48). However, as Stadtman and Berlett (42) state, “the metal ion-catalyzed oxidation of amino acids is likely a caged process, since the oxidation is not inhibited by hydroxyl radical scavengers.” Thus, the production of HO• from H2O2 at localized sites may confer a cell-signaling mechanism. HO• scavengers, such as mannitol, dimethylsulfoxide, and tetramethylurea, consistently display no inhibitory effects on tumor necrosis factor-induced nuclear factor-κB activation, while deferoxamine (iron chelator) and orthophenanthroline (copper chelator) show inhibitory effects (40), indicating that metal-catalyzed oxidation may play a role in cell signaling through caged HO• formation.
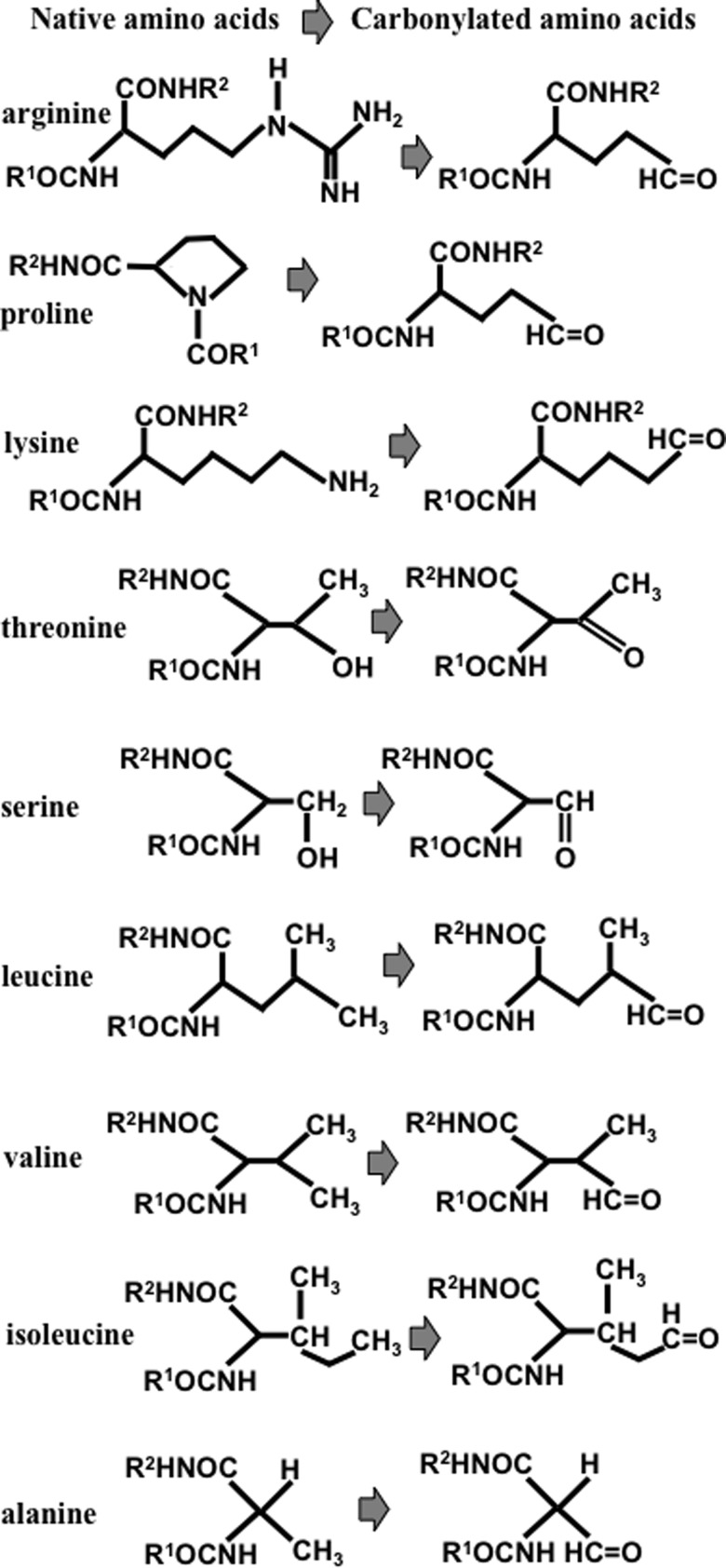
Protein carbonylation is a process in which reactive aldehydes or ketones are introduced into proteins by iron-dependent biological oxidation; the resultant product can react with 2,4-dinitrophenylhydrazine or other hydrazine derivatives to form hydrazones. Protein carbonylation has been described as occurring largely on four susceptible amino-acid residues: arginine, proline, lysine, and threonine (2). The oxidation of proline or arginine side chains produces glutamic semialdehyde, and lysine oxidation forms aminoadipic semialdehyde, introducing carbonyl groups into the protein structure (36). Oxidation also converts the hydroxyl group of threonine side chains to a carbonyl group, 2-amino-3-ketobutyric acid (Fig. 4). Reportedly, these amino acids are susceptible to metal-catalyzed oxidation based on the characterizations of isolated proteins and of amino acid homopolymers that are subjected to oxidative stress (1). In addition, other amino acid residues, such as serine, leucine, valine, isoleucine, and alanine, are also capable of being carbonylated as shown in Figure 4 (14, 42).
FIG. 4.
Chemical structures of native and carbonylated forms of protein amino acids that can be carbonylated.
We find that ligand-receptor stimulation in response to the treatment of pulmonary artery smooth muscle cells with endothelin-1 or serotonin, which are important mediators of pulmonary hypertension and pulmonary vascular remodeling, promotes carbonylation of various proteins (49). Two-dimensional gel electrophoresis and mass spectrometry identified that the carbonylated proteins include annexin A1, which functions as an anti-inflammatory, antiproliferative, and proapoptotic factor. We also find that annexin A1 is degraded by proteasomes subsequent to being carbonylated. Interestingly, in contrast to the situation of strenuous oxidative stress, in which most or all of carbonylated proteins are degraded, under the oxidant signaling condition, only small portions of carbonylated proteins are degraded (49). Thus, we propose that oxidant signaling triggers specific proteolysis of carbonylated proteins and that this defines the specificity and selectivity of signal transduction. Annexin A1 is one such protein that is specifically degraded under oxidant signaling conditions, so the degradation of annexin A1 should promote proinflammatory, proliferative, and antiapoptotic signaling. This “carbonylation-degradation pathway of signal transduction” may underlie the molecular mechanism of ROS signaling for the growth of pulmonary artery smooth muscle cells and pulmonary vascular remodeling (49). Thus, the inhibition of protein carbonylation may be a novel therapeutic strategy for preventing the development and progression of pulmonary hypertension.
ROS Signaling for Right Ventricular Hypertrophy
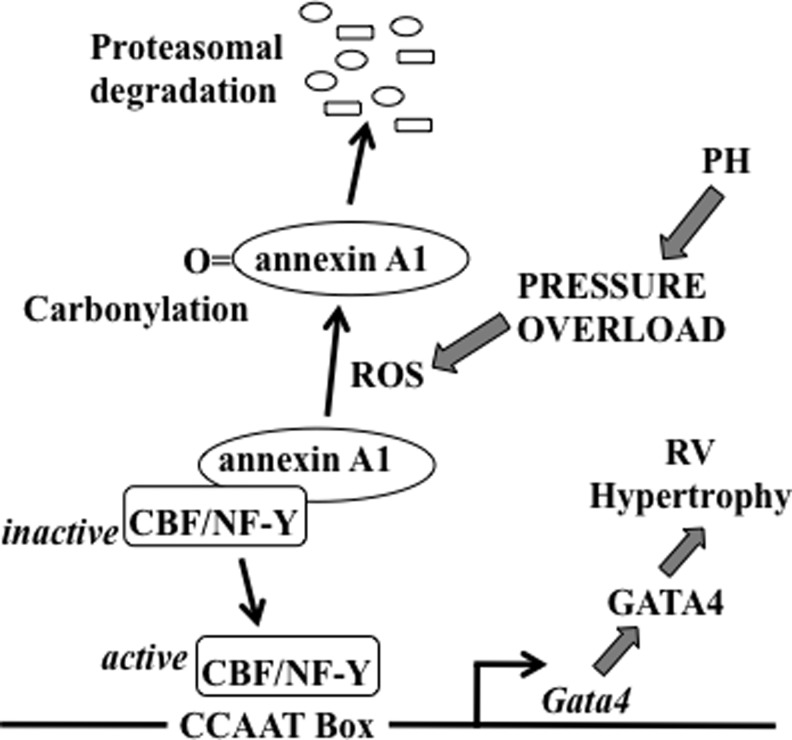
Pulmonary hypertension strains the right ventricle, initially causing compensatory concentric right ventricular hypertrophy, which in turn leads to right-sided heart failure and death. The transcription factor GATA4 plays an important role in the development of cardiac hypertrophy (32). Pressure overload exerted on the right ventricle by pulmonary artery banding or hypoxic pulmonary hypertension can increase GATA4 gene expression. Our laboratory identified the transcriptional start site of the Gata4 gene, finding that the 250-bp region that is proximal to the transcriptional start site is important for regulating the gene transcription of Gata4. This region contains the functionally important CCAAT box, where the CCAAT-binding factor/nuclear factor-Y (CBF/NF-Y) transcription factor binds (32). In the left ventricle, pressure overload by aortic banding activates GATA4 via post-translational modifications, while pulmonary artery banding increases GATA4 gene expression in the right ventricle, suggesting that the right ventricle specifically possesses a transcriptional mechanism for GATA4 activation (32). Using the hypoxic pulmonary hypertension model of right ventricular hypertrophy, we find that CBF/NF-Y activation occurs through the liberation from a complex with the annexin A1 protein via ROS-mediated protein carbonylation and proteasomal degradation of annexin A1, as summarized in Figure 5 (32). We find that the right ventricle has a higher ratio of CBF/NF-Y to annexin A1, compared to the left ventricle, making for more efficient CBF/NF-Y activation upon annexin A1 degradation (32).
FIG. 5.
Proposed ROS signaling mechanism for GATA4 activation in the right ventricle (RV) in response to pulmonary hypertension (PH). In right ventricular myocytes, CCAAT box plays an important role in the transcription of the Gata4 gene and is regulated by CCAAT-binding factor/nuclear factor-Y (CBF/NF-Y) transcription factor. CBF/NF-Y is inactive when annexin A1 is bound. Signaling stimuli in response to PH and pressure overload liberate CBF/NF-Y as an active form via the production of ROS, which in turn carbonylate annexin A1. Carbonylated annexin A1 gets degraded by proteasomes. Active CBF/NF-Y binds to CCAAT box and promotes gene transcription and activation of GATA4, leading to right ventricular hypertrophy. Adapted from Park et al. (32).
We also reported that treating perfused isolated rat hearts with serotonin promoted protein carbonylation, specifically in the right ventricle, but not in the left ventricle (28). We propose that these differential responses to serotonin between the right and left ventricles are defined by the low expression of monoamine oxidase A in the right ventricle compared to the left ventricle, which preserves the cytosolic level of serotonin and the ability of serotonin to produce superoxide via NAD(P)H oxidase, as described previously by Fanburg and coworkers (26). Since patients with pulmonary arterial hypertension have high levels of plasma serotonin (15), the ROS signaling mechanism triggered by serotonin may also play a role in the development of right ventricular hypertrophy.
These results reveal that ROS signaling regulates pulmonary hypertension-induced right ventricular hypertrophy and right heart failure. Consistent with this idea, right-sided heart failure due to monocrotaline-induced pulmonary hypertension was found to have increased ROS production (34), and the treatment of rats with the antioxidant EUK-134 attenuated pulmonary hypertension-induced right heart failure (35). Thus, antioxidant-based therapies to prevent and/or treat pulmonary hypertension-induced heart failure may be possible. A more precise understanding of the mechanisms of ROS actions should help to optimize such therapeutic strategies.
Role of Iron in Pulmonary Hypertension
As described above, accumulating evidence suggests that ROS plays a role in the progression of pulmonary hypertension. Since iron plays a vital role in ROS biology, iron chelation may inhibit pulmonary hypertension. This concept is supported by experiments from our laboratory demonstrating that deferoxamine (an iron chelator) can inhibit pulmonary vascular remodeling induced by chronic hypoxia (50). However, recent human studies reported that 43%–63% of patients with idiopathic pulmonary arterial hypertension are iron-deficient (37, 38, 41). Soon et al. (41) reported that, in their sample, 50% of premenopausal women with idiopathic pulmonary arterial hypertension were iron-deficient, while only 8% of premonopausal women with chronic thromboembolic pulmonary hypertension, 14% of postmenopausal women with idiopathic pulmonary arterial hypertension, and 28% of men with idiopathic pulmonary arterial hypertension were iron-deficient. Since these patients already had developed pulmonary hypertension, iron deficiency may not be related to the proposed roles played by iron and ROS in the mechanisms of the development and progression of the disease. However, it is unclear how lipid peroxidation could be increased (as described above) if these patients have reduced iron levels. Further work is needed to clarify this issue, which makes the design of antioxidant-based therapies less than straightforward.
Conclusions
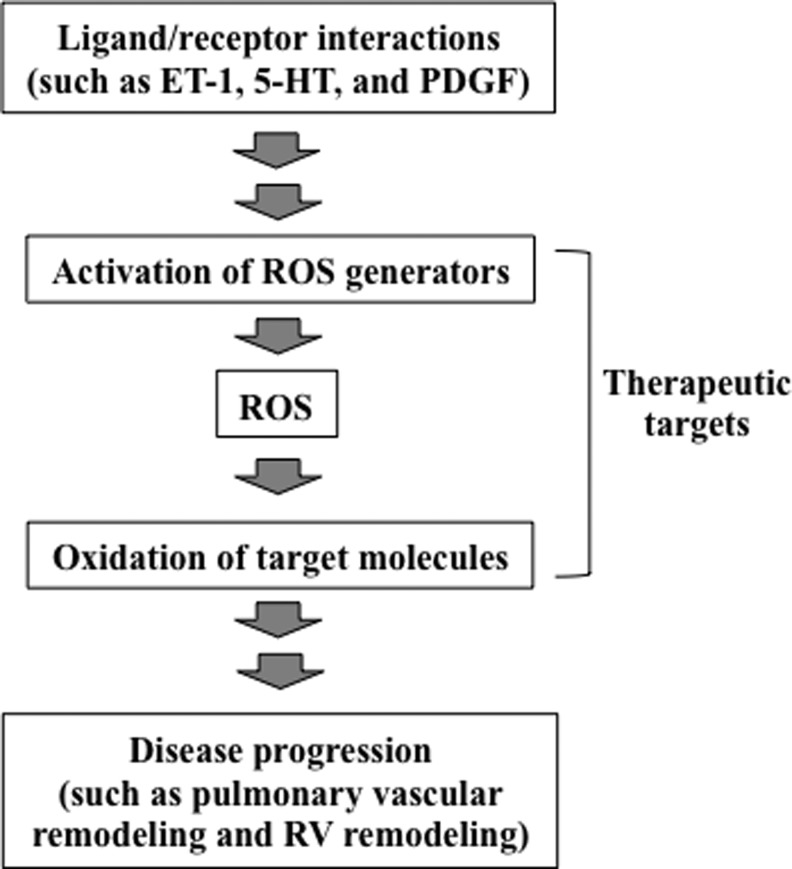
Using various experimental systems, including animal models of chronic hypoxia-induced pulmonary hypertension, many studies have shown that ROS are involved in the development and progression of pulmonary hypertension. ROS have also been implicated in the promotion of right ventricular hypertrophy and right heart failure in response to pulmonary hypertension. Human studies demonstrate that patients with idiopathic pulmonary arterial hypertension have increased oxidation status. Therefore, at least Group 1 (pulmonary arterial hypertension) and Group 3 (pulmonary hypertension due to lung diseases and/or hypoxemia) patients (12) may benefit from antioxidant-based therapies. However, it is unclear whether patients with pulmonary hypertension should receive antioxidants. For many diseases, clinical studies do not support the effectiveness of either pharmacological or dietary antioxidants in humans, despite the existence of ample evidence in experimental models for the importance of ROS. It is likely that we still do not understand the precise mechanisms of ROS actions; thus, we are not utilizing specific therapeutic strategies involving antioxidants that would eliminate unwanted ROS actions, while preserving the necessary functions of ROS. Understanding the molecular mechanisms of oxidant signaling should enable the specific, targeted use of antioxidants to effectively prevent and/or treat diseases, as depicted in Figure 6. In pulmonary hypertension, evidence suggests that ROS are second messengers for the growth of pulmonary artery smooth muscle cells during cell-signaling events that are triggered by mediators of pulmonary vascular remodeling and also for the development of right ventricular hypertrophy. NAD(P)H oxidase and monoamine oxidase-A have been shown to be generators of ROS during these cell-signaling processes. ROS likely react with protein thiols to elicit subsequent events. In addition, ROS may promote iron-dependent protein carbonylation as a means to send intracellular signals. The consequences of protein carbonylation may include the direct activation or inhibition of the activities of target molecules as well as the selective degradation by proteasomes. These studies of cell signaling also suggest that conditions of oxidative stress and ROS signaling may differ in terms of how biological processes operate and function. However, recent reports of some patients with idiopathic pulmonary arterial hypertension, particularly in premenopausal women (37, 38, 41), complicate the interpretation of the role of iron and ROS in pulmonary hypertension. Further study of the molecular mechanisms of ROS actions should contribute to the development of therapeutic strategies to treat pulmonary hypertension and right heart failure.
FIG. 6.
A proposed mechanism involving oxidant signaling for disease progression and possible therapeutic strategies. Receptor interactions with various ligands such as endothelin-1 (ET-1), 5-HT, and platelet-derived growth factor (PDGF) activate the generation of ROS, which in turn promote oxidation of target signaling molecules, leading to the progression of various diseases, including pulmonary vascular and right ventricular remodeling. Selective inhibition of these oxidant-signaling components may allow for optimal antioxidant-based therapeutic strategies, which specifically eliminate unwanted actions of ROS.
Abbreviations Used
- 5-HT
serotonin
- CBF/NF-Y
CCAAT-binding factor/nuclear factor-Y
- ERK
extracellular signal-regulated kinase
- ET-1
endothelin-1
- GTP
guanosine-5′-triphosphate
- H2O2
hydrogen peroxide
- HO•
hydroxyl radical
- NOX
NAD(P)H oxidase
- PDGF
platelet-derived growth factor
- PH
pulmonary hypertension
- RhoGDI
Rho guanine nucleotide dissociation inhibitor
- ROS
reactive oxygen species
- RV
right ventricle
Acknowledgment
This work was supported in part by grants from National Institutes of Health (R01HL72844 and R01HL97514) to Y.J.S.
References
- 1.Amici A. Levine RL. Tsai L. Stadtman ER. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989;264:3341–3346. [PubMed] [Google Scholar]
- 2.Berlett BS. Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 3.Bowers R. Cool C. Murphy RC. Tuder RM. Hopken MW. Flores SC. Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 4.Brennan LA. Steinhorn RH. Wedgwood S. Mata-Greenwood E. Roark EA. Russell JA. Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 5.Broughton BR. Jernigan NL. Norton CE. Walker BR. Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol. 2010;298:L232–L242. doi: 10.1152/ajplung.00276.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cracowski JL. Cracowski C. Bessard G. Pepin JL. Bessard J. Schwebel C. Stanke-Labesque F. Pison C. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:1038–1042. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A. Labinskyy N. Olson S. Pinto JT. Gupte S. Wu JM. Hu F. Ballabh P. Podlutsky A. Losonczy G. de Cabo R. Mathew R. Wolin MS. Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis KE. Aschner JL. Milatovic D. Schmidt JW. Aschner M. Kaplowitz MR. Zhang Y. Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L596–L607. doi: 10.1152/ajplung.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmedal B. de Dam MY. Mulvany MJ. Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br J Pharmacol. 2004;141:105–113. doi: 10.1038/sj.bjp.0705580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrow KN. Lakshminrusimha S. Reda WJ. Wedgwood S. Czech L. Gugino SF. Davis JM. Russell JA. Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L979–L987. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrow KN. Lakshminrusimha S. Czech L. Groh BS. Gugino SF. Davis JM. Russell JA. Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L109–L116. doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galiè N. Hoeper MM. Humbert M. Torbicki A. Vachiery JL. Barbera JA. Beghetti M. Corris P. Gaine S. Gibbs JS. Gomez-Sanchez MA. Jondeau G. Klepetko W. Opitz C. Peacock A. Rubin L. Zellweger M. Simonneau G ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 13.Grobe AC. Wells SM. Benavidez E. Oishi P. Azakie A. Fineman JR. Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 14.Headlam HA. Davies MJ. Beta-scission of side-chain alkoxyl radicals on peptides and proteins results in the loss of side-chains as aldehydes and ketones. Free Radic Biol Med. 2002;32:1171–1184. doi: 10.1016/s0891-5849(02)00814-6. [DOI] [PubMed] [Google Scholar]
- 15.Herve P. Launay JM. Scrobohaci ML. Brenot F. Simonneau G. Petitpretz P. Poubeau P. Cerrina J. Duroux P. Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 16.Hoshikawa Y. Ono S. Suzuki S. Tanita T. Chida M. Song C. Noda M. Tabata T. Voelkel NF. Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 17.Huang J. Kaminski PM. Edwards JG. Yeh A. Wolin MS. Frishman WH. Gewitz MH. Mathew R. Pyrrolidine dithiocarbamate restores endothelial cell membrane integrity and attenuates monocrotaline-induced pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1250–L1259. doi: 10.1152/ajplung.00069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irodova NL. Lankin VZ. Konovalova GK. Kochetov AG. Chazova IE. Oxidative stress in patients with primary pulmonary hypertension. Bull Exp Biol Med. 2002;133:580–582. doi: 10.1023/a:1020238026534. [DOI] [PubMed] [Google Scholar]
- 19.Irukayama-Tomobe Y. Sakai S. Miyauchi T. Chronic treatment with probucol effectively inhibits progression of pulmonary hypertension in rats. Life Sci. 2000;67:2017–2023. doi: 10.1016/s0024-3205(00)00780-3. [DOI] [PubMed] [Google Scholar]
- 20.Jankov RP. Kantores C. Pan J. Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2008;294:L233–L245. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 21.Jin HF. Du SX. Zhao X. Wei HL. Wang YF. Liang YF. Tang CS. Du JB. Effects of endogenous sulfur dioxide on monocrotaline-induced pulmonary hypertension in rats. Acta Pharmacol Sin. 2008;29:1157–1166. doi: 10.1111/j.1745-7254.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamezaki F. Tasaki H. Yamashita K. Tsutsui M. Koide S. Nakata S. Tanimoto A. Okazaki M. Sasaguri Y. Adachi T. Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 23.Lachmanová V. Hnilicková O. Povýsilová V. Hampl V. Herget J. N-acetylcysteine inhibits hypoxic pulmonary hypertension most effectively in the initial phase of chronic hypoxia. Life Sci. 2005;77:175–182. doi: 10.1016/j.lfs.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Lakshminrusimha S. Russell JA. Wedgwood S. Gugino SF. Kazzaz JA. Davis JM. Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrie A. Spiekerkoetter E. Martinez EC. Ambartsumian N. Sheward WJ. MacLean MR. Harmar AJ. Schmidt AM. Lukanidin E. Rabinovitch M. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res. 2005;97:227–235. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- 26.Lee SL. Wang WW. Fanburg BL. Superoxide as an intermediate signal for serotonin-induced mitogenesis. Free Radic Biol Med. 1998;24:855–858. doi: 10.1016/s0891-5849(97)00359-6. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y. Suzuki YJ. Day RM. Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res. 2004;95:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- 28.Liu L. Marcocci L. Wong CM. Park AM. Suzuki YJ. Serotonin-mediated protein carbonylation in the right heart. Free Radic Biol Med. 2008;45:847–854. doi: 10.1016/j.freeradbiomed.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Møller IM. Jensen PE. Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- 30.Nisbet RE. Graves AS. Kleinhenz DJ. Rupnow HL. Reed AL. Fan TH. Mitchell PO. Sutliff RL. Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odhiambo A. Perlman DH. Huang H. Costello CE. Farber HW. Steinberg MH. McComb ME. Klings ES. Identification of oxidative post-translational modification of serum albumin in patients with idiopathic pulmonary arterial hypertension and pulmonary hypertension of sickle cell anemia. Rapid Commun Mass Spectrom. 2007;21:2195–2203. doi: 10.1002/rcm.3074. [DOI] [PubMed] [Google Scholar]
- 32.Park AM. Wong CM. Jelinkova L. Liu L. Nagase H. Suzuki YJ. Pulmonary hypertension-induced GATA4 activation in the right ventricle. Hypertension. 2010;56:1145–1151. doi: 10.1161/HYPERTENSIONAHA.110.160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston IR. Tang G. Tilan JU. Hill NS. Suzuki YJ. Retinoids and pulmonary hypertension. Circulation. 2005;111:782–790. doi: 10.1161/01.CIR.0000155254.86840.47. [DOI] [PubMed] [Google Scholar]
- 34.Redout EM. Wagner MJ. Zuidwijk MJ. Boer C. Musters RJ. van Hardeveld C. Paulus WJ. Simonides WS. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–781. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Redout EM. van der Toorn A. Zuidwijk MJ. van de Kolk CW. van Echteld CJ. Musters RJ. van Hardeveld C. Paulus WJ. Simonides WS. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1038–H1047. doi: 10.1152/ajpheart.00097.2009. [DOI] [PubMed] [Google Scholar]
- 36.Requena JR. Chao C-C. Levine RL. Stadtman ER. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Natl Acad Sci U S A. 2001;98:69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes CJ. Howard LS. Busbridge M. Ashby D. Kondili E. Gibbs JS. Wharton J. Wilkins MR. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58:300–309. doi: 10.1016/j.jacc.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 38.Ruiter G. Lankhorst S. Boonstra A. Postmus PE. Zweegman S. Westerhof N. van der Laarse WJ. Vonk-Noordegraaf A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37:1386–1391. doi: 10.1183/09031936.00100510. [DOI] [PubMed] [Google Scholar]
- 39.Sawada H. Mitani Y. Maruyama J. Jiang BH. Ikeyama Y. Dida FA. Yamamoto H. Imanaka-Yoshida K. Shimpo H. Mizoguchi A. Maruyama K. Komada Y. A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest. 2007;132:1265–1274. doi: 10.1378/chest.06-2243. [DOI] [PubMed] [Google Scholar]
- 40.Schreck R. Albermann K. Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 41.Soon E. Treacy CM. Toshner MR. MacKenzie-Ross R. Manglam V. Busbridge M. Sinclair-McGarvie M. Arnold J. Sheares KK. Morrell NW. Pepke-Zaba J. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax. 2011;66:326–332. doi: 10.1136/thx.2010.147272. [DOI] [PubMed] [Google Scholar]
- 42.Stadtman ER. Berlett BS. Fenton chemistry. Amino acid oxidation. J Biol Chem. 1991;266:17201–17211. [PubMed] [Google Scholar]
- 43.Sun X. Ku DD. Allicin in garlic protects against coronary endothelial dysfunction and right heart hypertrophy in pulmonary hypertensive rats. Am J Physiol Heart Circ Physiol. 2006;291:H2431–H2438. doi: 10.1152/ajpheart.00384.2006. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki YJ. Forman HJ. Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki YJ. Day RM. Tan CC. Sandven TH. Liang Q. Molkentin JD. Fanburg BL. Activation of GATA-4 by serotonin in pulmonary artery smooth muscle cells. J Biol Chem. 2003;278:17525–17531. doi: 10.1074/jbc.M210465200. [DOI] [PubMed] [Google Scholar]
- 46.Uzun O. Balbay O. Comunoğlu NU. Yavuz O. Nihat Annakkaya A. Güler S. Silan C. Erbaş M. Arbak P. Hypobaric-hypoxia-induced pulmonary damage in rats ameliorated by antioxidant erdosteine. Acta Histochem. 2006;108:59–68. doi: 10.1016/j.acthis.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Wedgwood S. Dettman RW. Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol. 2001;281:L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 48.Winterbourn CC. Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Wong CM. Cheema AK. Zhang L. Suzuki YJ. Protein carbonylation as a novel mechanism in redox signaling. Circ Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 50.Wong CM. Suzuki YJ. Metal chelators inhibit pulmonary artery smooth muscle cell growth and vascular thickening. Am J Resp Crit Care Med. 2010;181:A1182. (abstract) [Google Scholar]
- 51.Wong CM. Preston IR. Hill NS. Suzuki YJ. Protein carbonylation in human pulmonary hypertension. Am J Resp Crit Care Med. 2011;183 (abstract). [Google Scholar]
- 52.Yang DL. Zhang HG. Xu YL. Gao YH. Yang XJ. Hao XQ. Li XH. Resveratrol inhibits right ventricular hypertrophy induced by monocrotaline in rats. Clin Exp Pharmacol Physiol. 2010;37:150–155. doi: 10.1111/j.1440-1681.2009.05231.x. [DOI] [PubMed] [Google Scholar]