Abstract
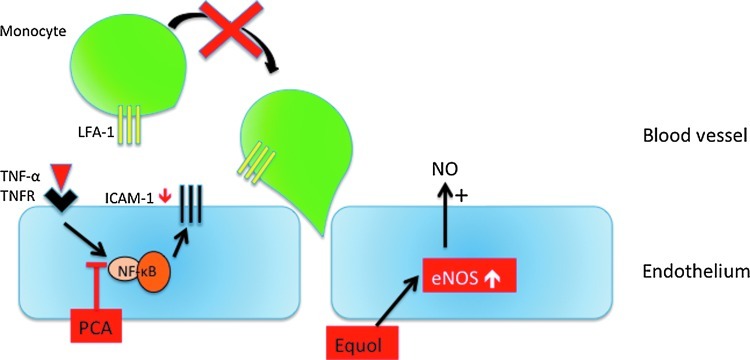
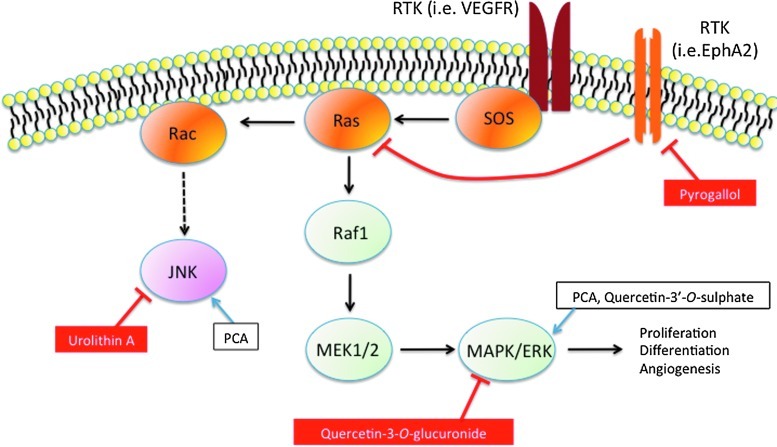
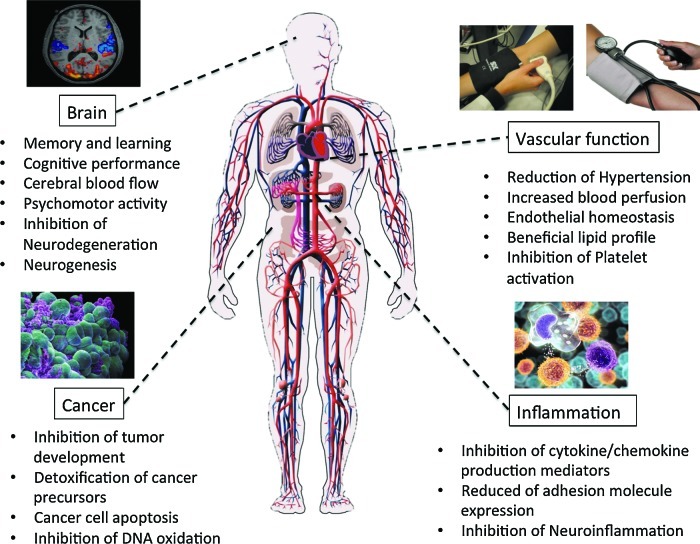
Human intervention trials have provided evidence for protective effects of various (poly)phenol-rich foods against chronic disease, including cardiovascular disease, neurodegeneration, and cancer. While there are considerable data suggesting benefits of (poly)phenol intake, conclusions regarding their preventive potential remain unresolved due to several limitations in existing studies. Bioactivity investigations using cell lines have made an extensive use of both (poly)phenolic aglycones and sugar conjugates, these being the typical forms that exist in planta, at concentrations in the low-μM-to-mM range. However, after ingestion, dietary (poly)phenolics appear in the circulatory system not as the parent compounds, but as phase II metabolites, and their presence in plasma after dietary intake rarely exceeds nM concentrations. Substantial quantities of both the parent compounds and their metabolites pass to the colon where they are degraded by the action of the local microbiota, giving rise principally to small phenolic acid and aromatic catabolites that are absorbed into the circulatory system. This comprehensive review describes the different groups of compounds that have been reported to be involved in human nutrition, their fate in the body as they pass through the gastrointestinal tract and are absorbed into the circulatory system, the evidence of their impact on human chronic diseases, and the possible mechanisms of action through which (poly)phenol metabolites and catabolites may exert these protective actions. It is concluded that better performed in vivo intervention and in vitro mechanistic studies are needed to fully understand how these molecules interact with human physiological and pathological processes. Antioxid. Redox Signal. 18, 1818–1892.
I. Introduction
While not essential for the successful growth and development of most plants, flavonoids and related phenolic compounds* can occur in high concentrations in some species and are referred to as secondary metabolites. They are structurally diverse with in excess of 8000 structures having been reported (447), and many are found in only a limited number of species. In planta, they have various functions, including protecting plants from herbivores and microbial infection, as attractants for pollinators and seed-dispersing animals, as allelopathic agents, UV protectants, and signal molecules in the formation of nitrogen-fixing root nodules (95, 218).
The role of flavonoids and related compounds, as components responsible, in part, for the protective effects of a fruit- and vegetable-rich diet has become an increasingly important area of human nutrition research. Unlike the traditional vitamins, they are not essential for short-term well being, but there is increasing evidence that modest long-term intakes can have favorable effects on the incidence of cancers and chronic diseases, including cardiovascular disease (CVD), type II diabetes, and impaired cognitive function, which are occurring with increasing frequency in Western populations (433). This review will summarize the different groups of compounds that are involved, their fate in the body after ingestion as they pass through the gastrointestinal tract (GIT) and are absorbed into the circulatory system, the evidence of their protective impact on human health, and the possible mechanisms of action through which their metabolites may exert such effects.
II. Classification of Phenolic Compounds
Phenolic compounds have at least one aromatic ring with one or more hydroxyl groups attached and are classified as flavonoids and nonflavonoids. Processed foods and beverages, such as black tea, matured red wine, coffee, and cocoa, may contain phenolic transformation products that are best described as derived polyphenols (95).
A. Flavonoids
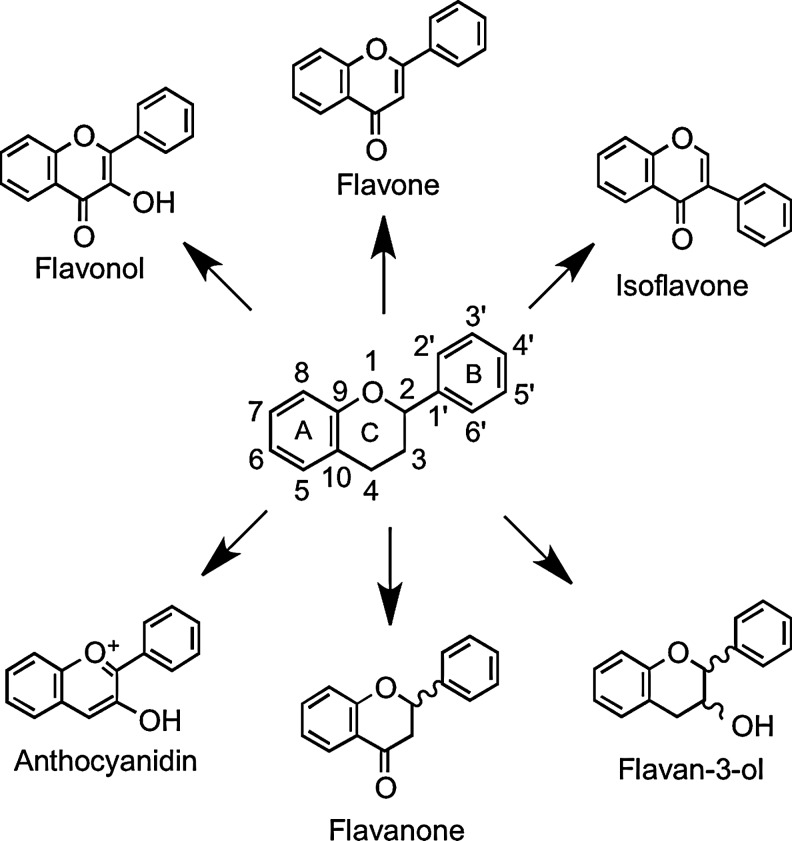
Flavonoids are polyphenolic compounds comprising 15 carbons with two aromatic rings connected by a three-carbon bridge (Fig. 1). The main subclasses of these C6–C3–C6 compounds are the flavones, flavonols, flavan-3-ols, isoflavones, flavanones, and anthocyanidins. Other flavonoid groups that are more minor dietary components are the chalcones, dihydrochalcones, dihydroflavonols, flavan-3,4-diols, coumarins, and aurones. The basic flavonoid skeleton can have numerous substituents. The majority of flavonoids occur naturally as glycosides rather than aglycones.
FIG. 1.
Structure of the flavonoid skeleton.
1. Flavonols
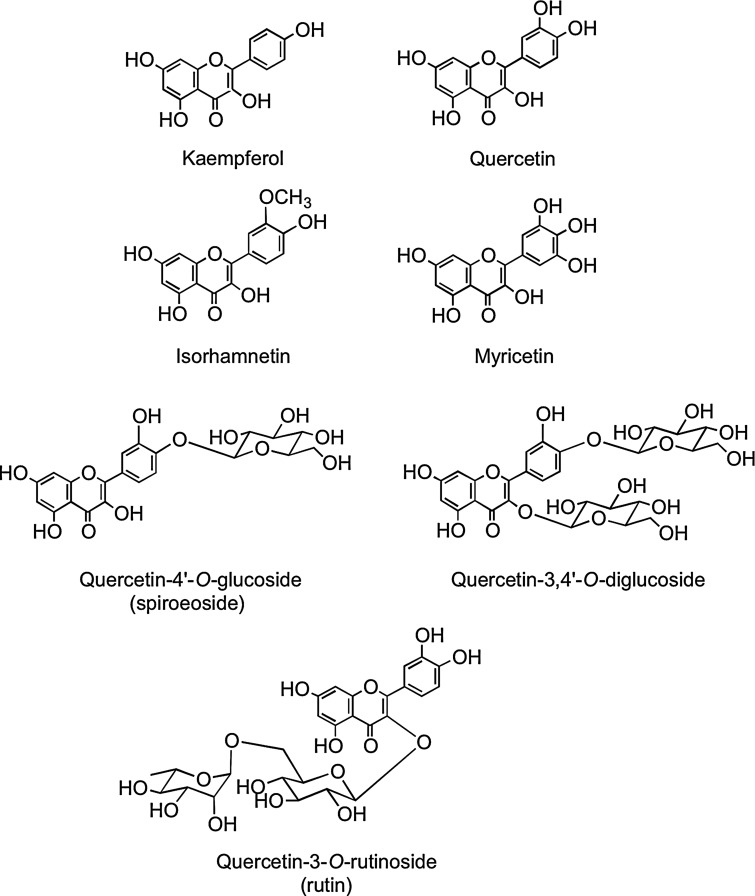
Flavonols occur widely throughout the plant kingdom with the exception of fungi and algae. The most common flavonols, kaempferol, quercetin, isorhamnetin, and myricetin (Fig. 2), are typically found as glycosides with conjugation occurring at the 5, 7, 3′, 4′, and 5′ positions. Although the number of aglycones is limited, there are more than 200 sugar conjugates of kaempferol (447). There is information on the flavonol content of commonly consumed fruits, vegetables, and beverages with sizable differences in the amounts found in seemingly similar produce, possibly due to local growing conditions, seasonal changes, and varietal differences (94, 194, 195, 298). Yellow and red onions (Allium cepa) are especially rich source of flavonols containing high concentrations of quercetin-4′-O-glucoside and quercetin-3,4′-O-diglucoside. The disaccharide quercetin-3-O-rutinoside (Fig. 2) is a common dietary component.
FIG. 2.
Structures of the flavonol aglycones kaempferol, quercetin, isorhamnetin, and myricetin and three common quercetin-O-glycosides.
2. Flavones
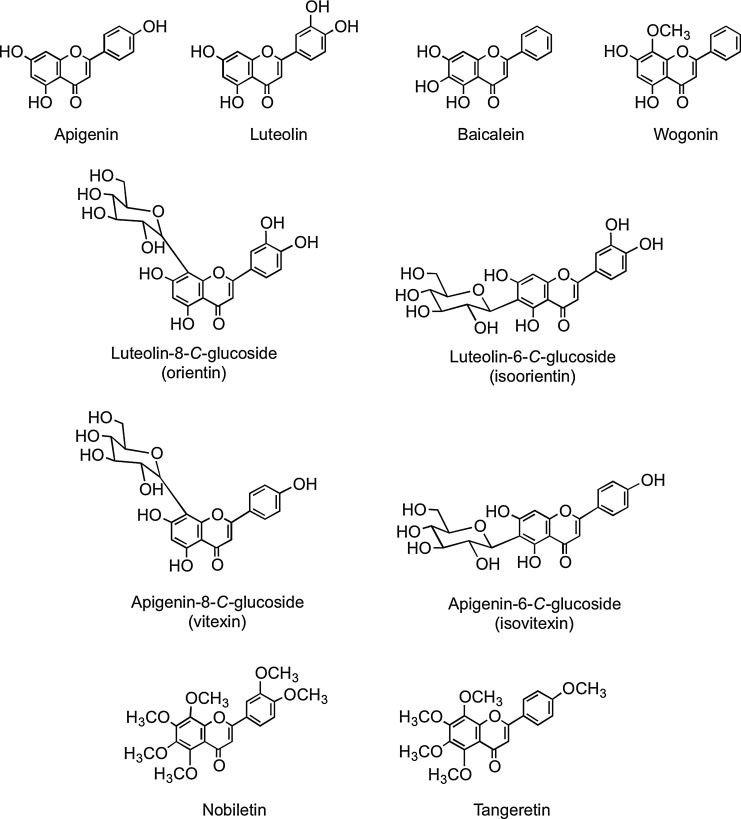
Flavones, such as apigenin, luteolin, wogonin, and baicalein, are similar structurally to flavonols, except they lack oxygenation at C-3 (Fig. 3). A wide range of substitutions is possible with flavones, including hydroxylation, methylation, O- and C-glycosylation, and alkylation. In general, flavones are not distributed widely, although substantial amounts have been detected in celery (Apium graveolens), parsley (Petroselinum hortense), and some herbs. Many flavones occur as 7-O-glycosides, although rooibos tea, a caffeine-free beverage prepared from leaves of the South African shrub Aspalathus linearis, contains small amounts of apigenin-8-C-glucoside (vitexin), apigenin-6-C-glucoside (isovitexin), luteolin-8-C-glucoside (orientin), and luteolin-6-C-glucoside (iso-orientin) (80, 437). Polymethoxylated flavones, such as nobiletin and tangeretin (Fig. 3), occur in citrus species.
FIG. 3.
The flavone aglycones apigenin, luteolin, baicalein, and wogonin; the flavone C-glycosides orientin, isoorientin, vitexin, and isovitexin; and the polymethoxylated flavones nobiletin and tangeretin.
3. Isoflavones
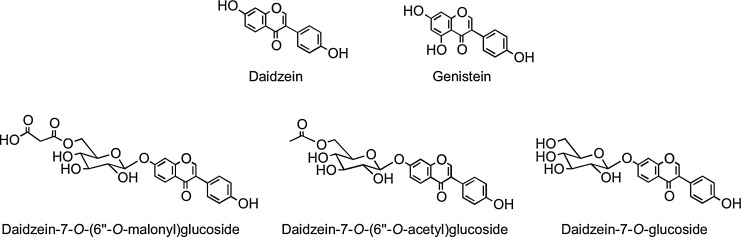
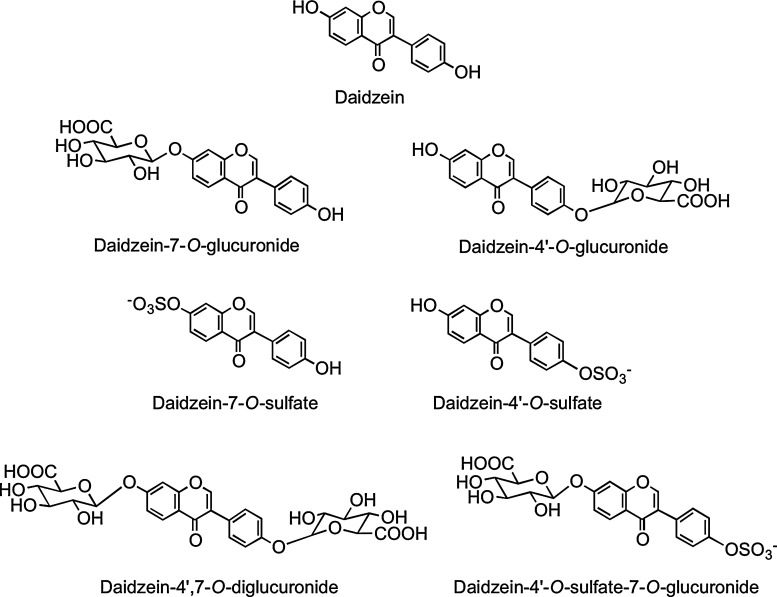
Isoflavones have the B-ring attached at C-3 rather than at the C-2 position (Fig. 1). They are found almost exclusively in leguminous plants with substantial quantities of daidzein and genistein occurring in soybean (Glycine max) principally as 7-O-(6"-O-malonyl)glucosides with lower amounts of the corresponding 7-O-(6"-O-acetyl)glucosides, 7-O-glucosides, and the aglycones (Fig. 4). Fermented soy products can be rich in the aglycones as a result of hydrolysis of the glycosides, whereas products whose manufacture involves heating, such as soy milk and tofu, contain reduced quantities of isoflavones, mainly in the form of the daidzein and genistein glucosides, which form as a result of degradation of malonyl- and acetylglucosides (88).
FIG. 4.
The isoflavone aglycones daidzein and genistein and O-glycosides of daidzein, which along with similar genistein conjugates, are found in soy products.
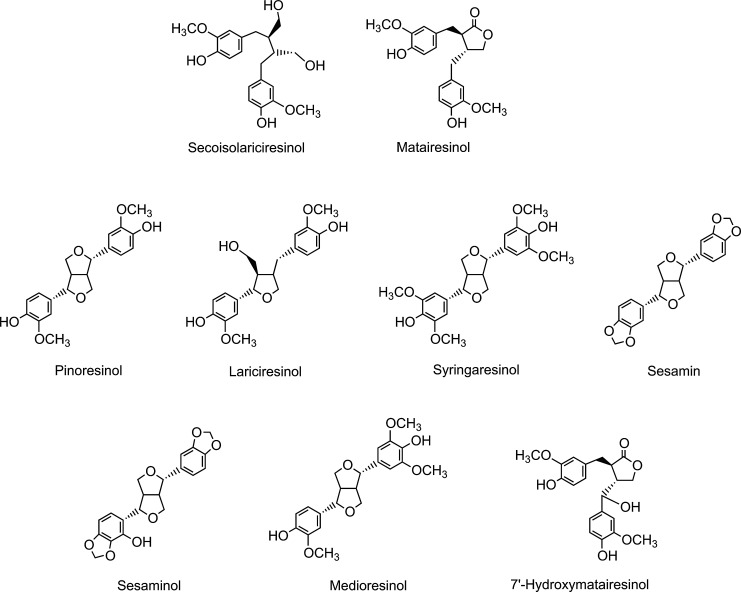
Because of their structural similarity to estrogen, isoflavones are classified as phytoestrogens, as are the nonflavonoid lignans, which are a diverse group of compounds that occur in high concentrations principally in cereal grains (Fig. 5).
FIG. 5.
Structures of plant lignans present in cereal grains and sesame seeds.
4. Flavanones
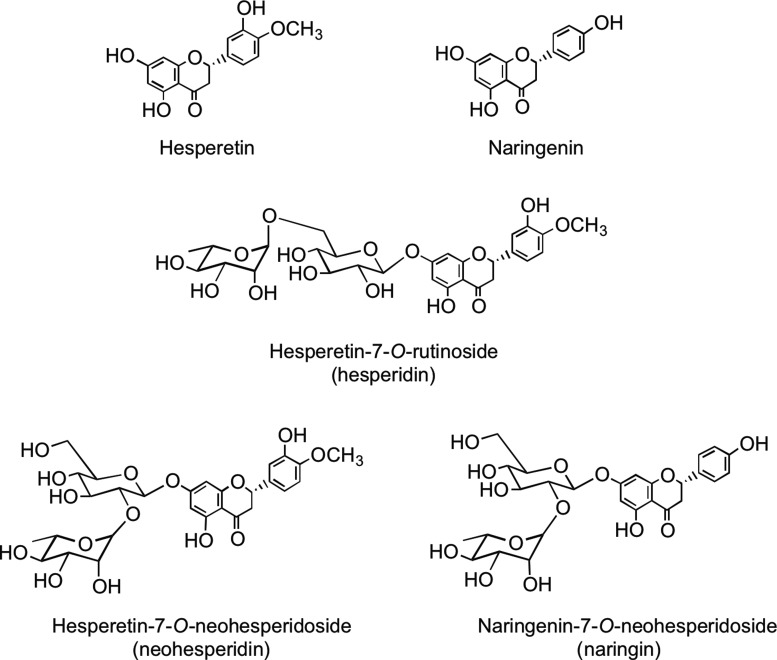
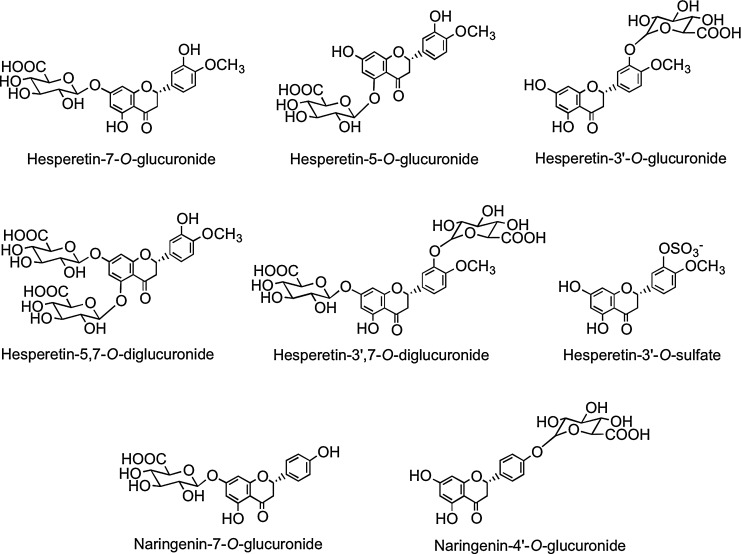
Flavanones such as naringenin and hesperetin are characterized by the absence of Δ2,3 double bond and the presence of a chiral center at C-2 (Fig. 1). In planta flavanones occur predominantly as the S- or (−)-enantiomer with the C-ring attached to the B-ring at C-2 in the α-configuration (93). Flavanones occur as hydroxyl, glycosylated, and O-methylated derivatives (Fig. 6). They are present in especially high amounts in flavedo of citrus fruits. The most common flavanone glycoside is hesperetin-7-O-rutinoside (hesperidin). Flavanone rutinosides are tasteless, in contrast to flavanone neohesperidoside conjugates, such as hesperetin-7-O-neohesperidoside (neohesperidin) from bitter oranges (Citrus aurantium) and naringenin-7-O-neohesperidoside (naringin) from grapefruit (Citrus paradise), which have an intense bitter taste.
FIG. 6.
The S-enantiomers of the flavanone aglycones naringenin and hesperetin together with some of their naturally occurring glycosides.
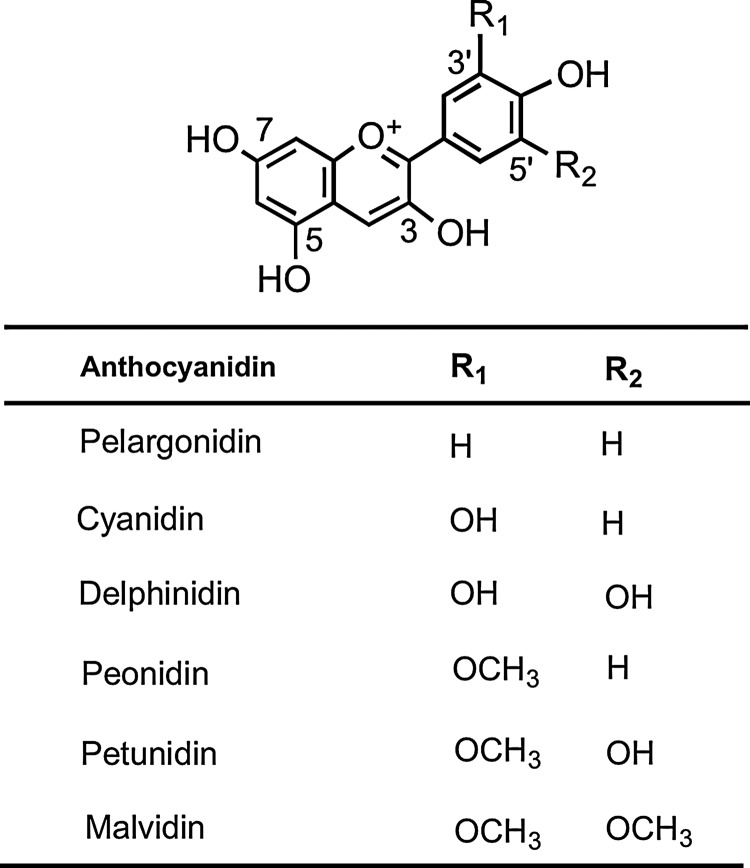
5. Anthocyanidins
The most common anthocyanidin aglycones are pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin (Fig. 7), which form conjugates with sugars and organic acids to generate a multitude of anthocyanins of differing colors, ranging from orange and red to blue and purple, and as a consequence, they are readily visible in fruits and flowers (219, 338).
FIG. 7.
Structures of the major anthocyanidins.
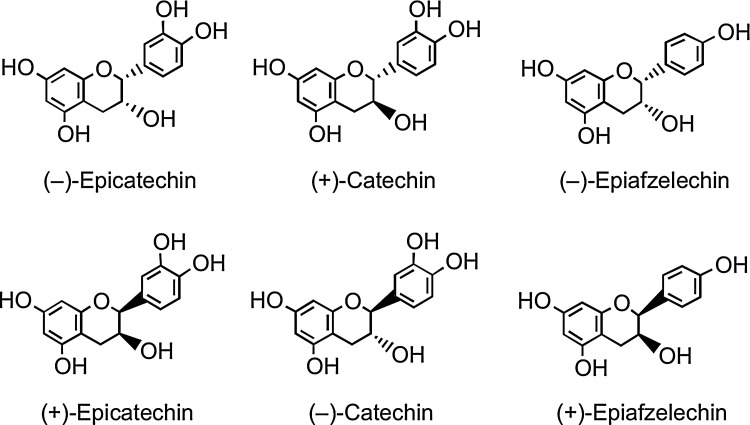
6. Flavan-3-ols
Flavan-3-ols, unusually, do not exist in planta predominantly as glycosides. They are the most complex subclass of flavonoids, ranging from the simple monomers to the oligomeric and polymeric proanthocyanidins, which are also known as condensed tannins. The two chiral centers at C2 and C3 of the monomeric flavan-3-ol (Fig. 1) produce four isomers for each level of B-ring hydroxylation, two of which, (+)-catechin and (−)-epicatechin, are widespread in nature, while others such as (−)-epiafzelechin have a more limited distribution (Fig. 8) (12, 93). Pairs of enantiomers can be resolved by chiral chromatography, but not with the more commonly used reversed-phase high-performance liquid chromatography (HPLC), and as a consequence, they are easily overlooked.
FIG. 8.
Structures of monomeric flavan-3-ol enantiomers.
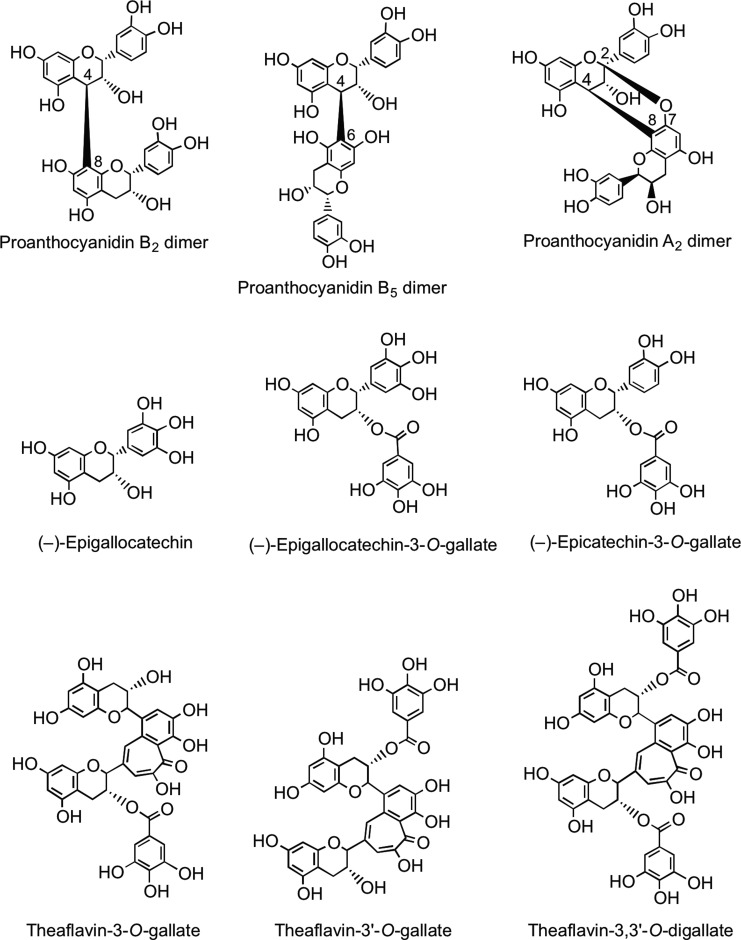
Oligomeric and polymeric proanthocyanidins have an additional chiral center at C4 in the upper and lower units. Type B proanthocyanidins are formed from (+)-catechin and (−)-epicatechin by oxidative coupling between the C-4 of the upper monomer and the C-6 or C-8 of the adjacent lower or extension unit to create oligomers or polymers. Type A proanthocyanidins have an additional ether bond between C-2 in the B-ring of one monomer and C-7 in the A-ring of the other monomer (Fig. 9). Proanthocyanidins can occur as polymers of up to 50 units. Proanthocyanidins that consist exclusively of (epi)catechin units are called procyanidins, and are the most abundant type of proanthocyanidins in plants. The less-common proanthocyanidins containing (epi)afzelechin or (epi)gallocatechin subunits are propelargonidins and prodelphinidins, respectively (80).
FIG. 9.
Flavan-3-ol structures.
Green tea (Camellia sinensis) contains very high levels of flavan-3-ol monomers with the main components being (−)-epigallocatechin, (−)-epigallocatechin-3-O-gallate, and (−)-epicatechin-3-O-gallate (Fig. 9). The levels of these flavan-3-ols decline during fermentation of the green leaves to produce black tea, principally as a result of the action of polyphenol oxidase, and there is a concomitant accumulation of theaflavins and thearubigins (110). Theaflavin, theaflavin-3-O-gallate, theaflavin-3′-O-gallate, and theaflavin-3,3′-O-digallate are dimer-like structures (Fig. 9) that contribute to the quality of the black tea beverage. The brownish, water-soluble, high-molecular-weight thearubigins are the major phenolic fraction in black tea. Recent pioneering studies have established that black teas contain on average 5000 thearubigin components in the mass range of 1000–2100 amu (261, 262). A typical cup of black tea contains ∼100 mg of thearubigins (164, 499).
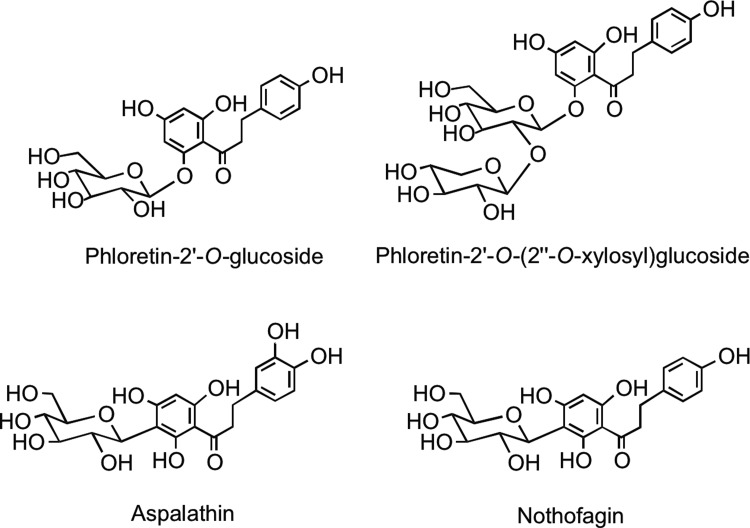
7. Dihydrochalcones
This minor group of flavonoids, which have an open C-ring structure, is of limited dietary significance, restricted to the presence of phloretin-2′-O-glucoside (phloridzin) and phloretin-2′-O-(2′′-O-xylosyl)glucoside in apples (Malus domestica) and 2′,3,4,4′,6′-pentahydroxydihydrochalcone-3′-C-glucoside (aspalathin) and 2′,4,4′,6′-tetrahydroxydihydrochalcone-3′-C-glucoside (nothofagin) (Fig. 10) in rooibos tea (254, 437).
FIG. 10.
Naturally occurring dihydrochalcone glycosides.
B. Nonflavonoids
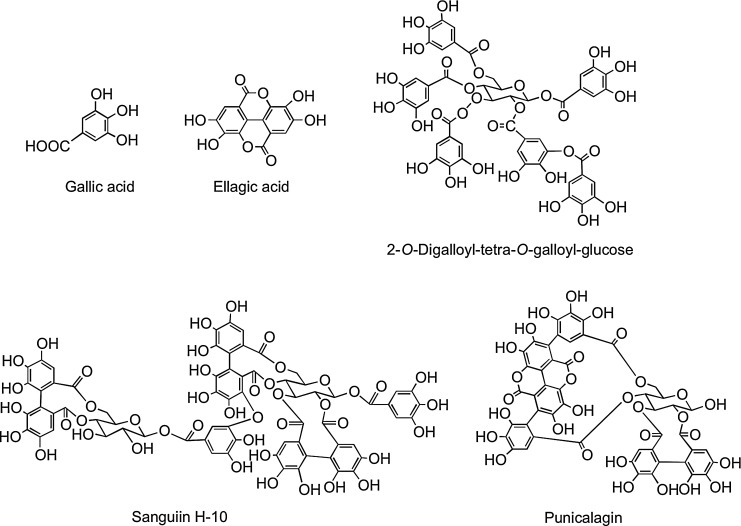
Among the nonflavonoids of dietary significance are the C6–C1 phenolic acids. Gallic acid is the commonest phenolic acid, and occurs widely as complex sugar esters in gallotannins, such as 2-O-digalloyl-tetra-O-galloyl-glucose (Fig. 11), which are minor dietary components. The related ellagic acid-based ellagitannins, such as sanguiin H-6 and punicalagin (Fig. 11), are found in a diversity of food, including raspberries (Rubus idaeus), strawberries (Fragaria ananassa), blackberries (Rubus spp.), and many other fruits, including pomegranate (Punica granatum) and persimmon (Diospyros kaki), as well as walnuts (Juglans regia), hazelnuts (Corylus avellana), and oak-aged wines where they are leached from the oak during maturation of the wines (267). The ellagitannin content of some food products can be high with a glass of pomegranate juice and a 100 g serving of raspberries providing ∼300 mg, and four walnuts ∼400 mg (269)
FIG. 11.
Gallic acid, ellagic acid, the gallotannin 2-O-digalloyl-tetra-O-galloyl-glucose, and the ellagitannins sanguiin H-6, and punicalagin.
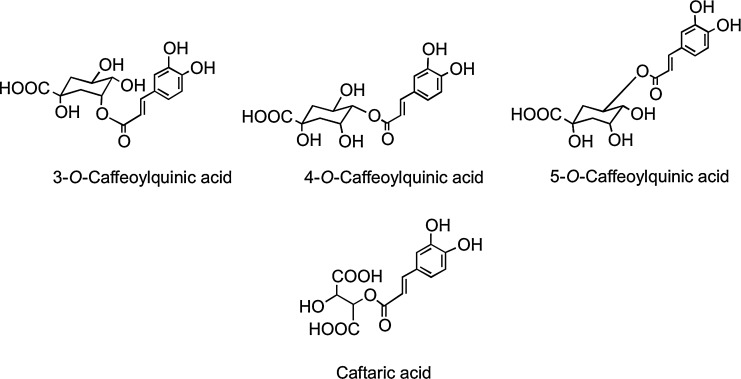
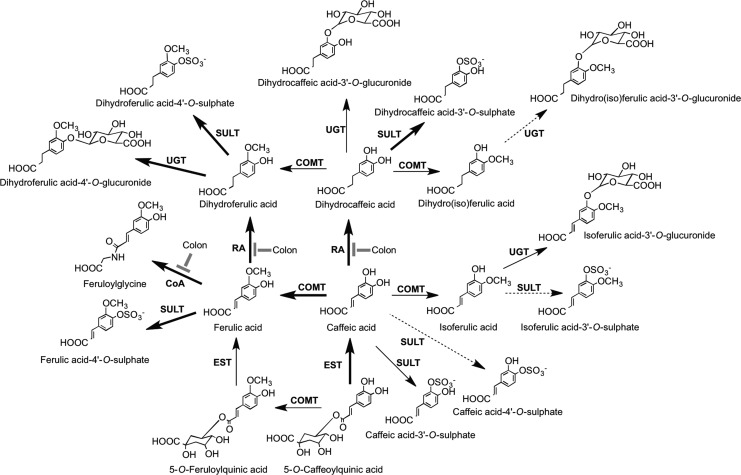
The C6–C3 hydroxycinnamates occur mainly as conjugates, for example, with tartaric acid or quinic acid, and collectively are referred to as chlorogenic acids (Fig. 12). Chlorogenic acids, principally 3-O-, 4-O-, and 5-O-caffeoylquinic acids, form ∼10% of green robusta coffee beans (Coffea canephora). Regular consumers of coffee may provide a daily intake in excess of 1 g of chlorogenic acids, and these for many people will be the major dietary phenolics. Accumulating in the flesh of grapes, caftaric acid is the main hydroxycinnamate in both red and white wines produced from Vitis vinifera and well as Concord grape juice, which is a product of grapes of Vitis lambrusca (435).
FIG. 12.
The conjugated hydroxycinnamates 3-O-, 4-O-, and 5-O-caffeoylquinic acids and caftaric acid.
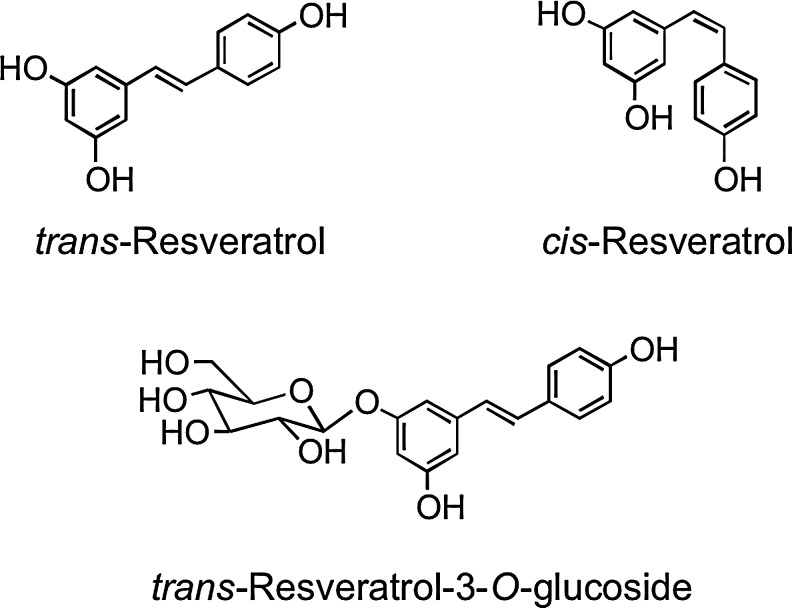
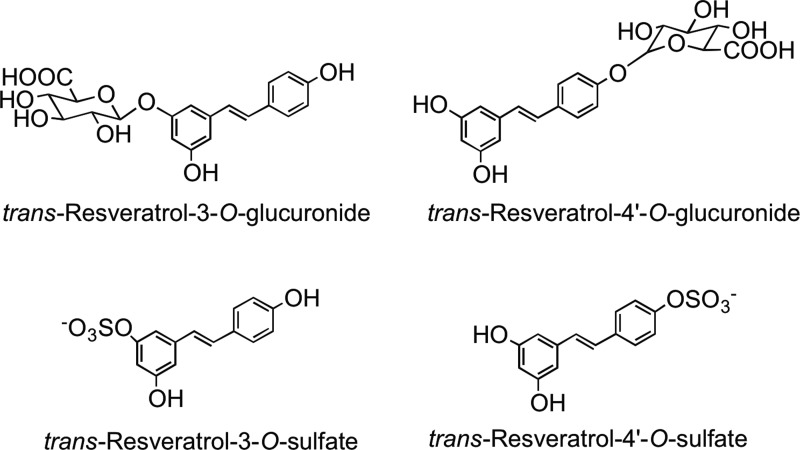
Stilbenes have a C6–C2–C6 structure and are phytoalexins produced by plants in response to disease, injury, and stress (268). Although only extremely minor dietary components, the main stilbene is resveratrol (3,5,4′-trihdroxystilbene), which occurs as cis and trans isomers as well as conjugated derivatives, including trans-resveratrol-3-O-glucoside (trans-piceid) (Fig. 13). The woody root of the noxious weed Polygonum cuspidatum (Japanese knotweed or Mexican bamboo) contains unusually high levels of trans-resveratrol and its glucoside with concentrations of up to 377 mg/100 g dry weight (474). Red wines contain a diversity of stilbene derivatives, but invariably in very low concentrations compared to the levels of other (poly)phenolic components (92).
FIG. 13.
Common stilbene structures.
III. Bioavailability of Flavonoids and Related Compounds
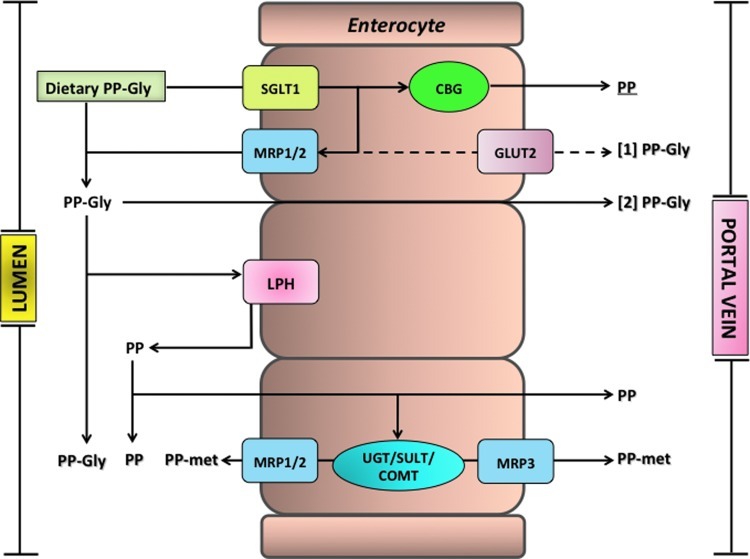
The absorption, distribution, metabolism, and excretion of flavonoids and related phenolics after dietary intake have been topics of increasing research in recent years. After the acute ingestion, absorption of some, but by no means all, components into the circulatory system occurs in the small intestine. Typically, the absorption of flavonoid glycosides, as illustrated in Figure 14, is associated with cleavage and release of the aglycone as a result of the action of lactase phloridzin hydrolase (LPH) in the brush border of the small intestine epithelial cells. LPH exhibits broad substrate specificity for flavonoid-O-β-D-glucosides, and the released aglycone may then enter the epithelial cells by passive diffusion as a result of its increased lipophilicity and its proximity to the cellular membrane (104). An alternative hydrolytic step is mediated by a cytosolic β-glucosidase (CBG) within the epithelial cells. For CBG-catalyzed hydrolysis to occur, the polar glucosides must be transported into the epithelial cells, possibly with the involvement of the active sodium-dependent glucose transporter 1 (SGLT1) (151). Thus, there are two possible routes by which the glycoside conjugates are hydrolyzed, and the resultant aglycones appear in the epithelial cells, namely LPH/diffusion and transport/CBG (Fig. 14). However, an investigation in which SGLT1 was expressed in Xenopus laevis oocytes has shown that at least in this model system, SLGT1 does not transport flavonoids, and that glycosylated flavonoids and some aglycones have the capability to inhibit the glucose transporter (253). Using Caco-2 cells, Johnson et al. (228) found that glucose uptake into cells under sodium-dependent conditions was inhibited by flavonoid glycosides and nonglycosylated polyphenols, whereas aglycones and phenolic acids were without effect.
FIG. 14.
Proposed mechanisms for the absorption and metabolism of (poly)phenolic compounds in the small intestine. CBG, cytosolic β-glucosidase; COMT, catechol-O-methyl transferase; GLUT2, glucose transporter; LPH, lactase phloridzin hydrolase; MRP1-2–3, multidrug-resistant proteins; PP, (poly)phenol aglycone; PP-gly, (poly)phenol glycoside, PP-met, polyphenol sulfate/glucuronide/methyl metabolites; SGLT1, sodium-dependent glucose transporter; SULT, sulfotransferase; UGT, uridine-5′-diphosphate glucuronosyltransferase. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Before passage into the blood stream, the aglycones undergo some degree of phase II metabolism forming sulfate, glucuronide, and/or methylated metabolites through the respective action of sulfotransferases (SULTs), uridine-5′-diphosphate glucuronosyltransferases (UGT), and catechol-O-methyltransferases (COMTs). There is also efflux of some of the metabolites back into the lumen of the small intestine, and this is thought to involve members of the adenosine triphosphate-binding cassette (ABC) family of transporters, including multidrug resistance protein (MRP) and P-glycoprotein (Fig. 14). MRP-3 and the glucose transporter GLUT2 have also been implicated in the efflux of metabolites from the basolateral membrane of the enterocytes (293, 468). Once in the portal bloodstream, metabolites rapidly reach the liver, where they can be subjected to further phase II metabolism, and enterohepatic recirculation may result in some recycling back to the small intestine through bile excretion (118).
(Poly)phenol conjugates with sugar moieties that are resistant to the action of LPH/CBG (104) are not absorbed in the small intestine to any degree and pass to the colon. Analysis of ileal fluid collected from ileostomists after the ingestion of various foodstuffs has shown that even when dietary (poly)phenols are absorbed in the proximal GIT, substantial quantities nonetheless pass from the small to the large intestine (220, 235, 236, 294), where the colonic microbiota cleave conjugating moieties, and the resultant aglycones undergo ring fission leading to the production of smaller molecules, including phenolic acids and hydroxycinnamates. These can be absorbed and may be subjected to metabolism in the liver before being excreted in urine in amounts that, in most instances, are well in excess of the metabolites that enter the circulatory system via the small intestine (220, 387, 438, 439).
As a result of more recent studies on the bioavailability of dietary (poly)phenolic compounds [see (91, 433)], there is a growing realization that (poly)phenolic glucuronide, methyl, and sulfate conjugates are treated by the body as xenobiotics, and instead of accumulating in the circulatory system, they are rapidly turned over and removed by excretion via the kidneys. As a consequence, although plasma pharmacokinetics of these metabolites provides useful information, estimates such as of area-under-the curve values do not necessarily yield accurate quantitative data on absorption. In the circumstances, urinary excretion provides a more realistic assessment.† However, as this does not include the possibility of sequestration in body tissues, this too is theoretically an underestimate of absorption, but to what degree remains undetermined. However, the fact that with few exceptions, tissue sequestration on any scale has yet to be convincingly demonstrated suggests that it can only be at low levels, if at all (see Section IV).
A. Flavonols and flavanones
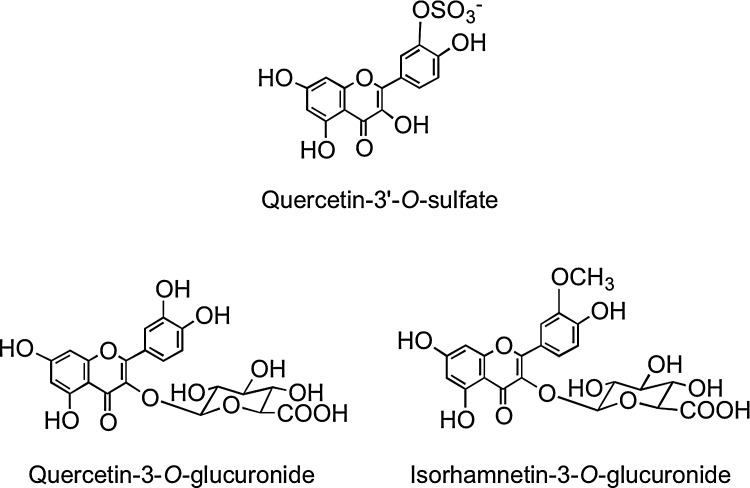
A human feeding study with lightly fried onions containing a total of 275 μmol of flavonol glucosides, principally in the form of quercetin-4′-O-glucoside and quercetin-3,4′-O-diglucoside (Fig. 2), resulted in the appearance of quercetin-3′-O-sulfate, quercetin-3-O-glucuronide, isorhamnetin-3-O-glucuronide (Fig. 15), and two partially identified metabolites, a quercetin-O-diglucuronide and a quercetin-O-glucuronide-O-sulfate, in the circulatory system within 30 min of ingestion (321). This is indicative of cleavage of the conjugating glucose moieties in the proximal GIT and metabolism of the released aglycone by SULTs, UGTs, and COMTs in the enterocyte before entry of the metabolites into circulatory system. Sub-μM peak plasma concentrations (Cmax) were attained in <1 h (Tmax), in the case of the majority of the metabolites, most of which had a relative short elimination half-life (T½) as they were rapidly removed from the bloodstream (Table 1). A more complex array of metabolites was detected in urine, indicating substantial phase II metabolism of the quercetin derivatives before excretion. Most of the metabolites were excreted within the initial 12 h after ingestion of the onions, and over a 0–24-h collection period, a total of 12.9 μmol of metabolites was excreted, which corresponds to 4.7% of intake.
FIG. 15.
Structures of the quercetin metabolites quercetin-3′-O-sulfate, quercetin-3-O-glucuronide, and isorhamnetin-3-O-glucuronide (105, 321).
Table 1.
Pharmacokinetic Analysis of Quercetin Metabolites in the Plasma of Volunteers After the Consumption of 270 g of Fried Onions Containing 275 μmol of Flavonol Glucosides
| Metabolites | Cmax (nM) | Tmax (h) | T½ (h) |
|---|---|---|---|
| Quercetin-3′-O-sulfate | 665±82 | 0.75±0.12 | 1.71 |
| Quercetin-3-O-glucuronide | 351±27 | 0.60±0.10 | 2.33 |
| Isorhamnetin-3-O-glucuronide | 112±18 | 0.60±0.10 | 5.34 |
| Quercetin-O-diglucuronide | 62±12 | 0.80±0.12 | 1.76 |
| Quercetin-O-glucuronide-O-sulfate | 123±26 | 2.5±0.22 | 4.54 |
Data presented as mean values±standard error (n=6).
Adapted from Mullen et al. (321).
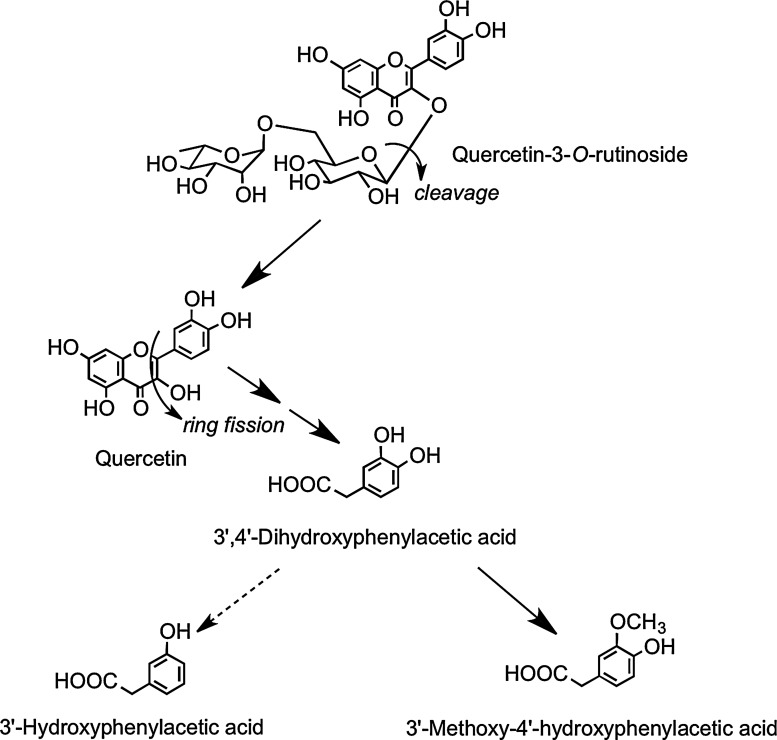
After drinking tomato juice containing 176 μmol of quercetin-3-O-rutinoside, quercetin-3-O-glucuronide and isorhamnetin-3-O-glucuronide were detected in plasma (220). Their Cmax values were ∼25-fold lower than in the onion study, and the Tmax times were extended to ∼5 h (Table 2), which is characteristic of absorption in the large rather than small intestine. Absorption in the large intestine was confirmed when the tomato juice was fed to ileostomists, as neither plasma nor urinary metabolites of quercetin were detected, and ileal fluid collected post-tomato juice consumption contained 86% of the ingested quercetin-3-O-rutinoside. This is in keeping with the report of Day et al. (104) that the flavonol disaccharide is not cleaved by LPH. In healthy subjects, quercetin-3-O-rutinoside will pass from the small to large intestine, where cleavage of the sugar moiety occurs as a result of the action of the colonic microbiota, and the released aglycone undergoes low-level methylation and glucuronidation before absorption into the portal vein. Most of the quercetin, however, is subject to ring fission, resulting in the production of hydroxyphenylacetic acid catabolites (Fig. 16) in amounts equivalent to ∼22% of intake of the rutinoside. These catabolites were not excreted by ileostomists after ingestion of tomato juice containing quercetin-3-O-rutinoside (220).
Table 2.
Pharmacokinetic Analysis of Quercetin Metabolites in the Plasma of Volunteers After the Consumption of 250 ml of Tomato Juice Containing 176 μmol of Quercetin-3-O-Rutinoside
| Metabolites | Cmax (nM) | Tmax (h) |
|---|---|---|
| Quercetin-3-O-glucuronide | 12±2 | 4.7±0.3 |
| Isorhamnetin-3-O-glucuronide | 4.3±1.5 | 5.4±0.2 |
Data presented as mean values±standard error (n=6).
Adapted from Jaganath et al. (220).
FIG. 16.
Proposed pathway for colonic bacterium-mediated catabolism of quercetin-3-O-rutinoside in the human large intestine resulting in the production of 3′,4′-dihydroxyphenylacetic acid and smaller quantities of 3′-hydroxyphenylacetic acid with subsequent hepatic conversion of 3′,4′-dihydroxyphenylacetic acid to 3′-methyoxy-4′-hydroxyphenylacetic acid before urinary excretion. Adapted from Jaganath et al. (220).
It is of interest to note that quercetin released in the small intestine by cleavage of quercetin glucosides is converted to glucuronide, sulfate, and methylated metabolites (321), whereas quercetin produced in the colon from quercetin-3-O-rutinoside is metabolized to methyl and glucuronide derivatives, but not sulfate metabolites (220). This suggests that sulfation of quercetin is a feature of SULTs in the wall of the small intestine rather than the colon or the liver.
Flavanone bioavailability studies with healthy human subjects have been carried out using a 250-ml supplement of orange (Citrus sinensis) juice containing 168 μmol of hesperetin-7-O-rutinoside and 12 μmol of naringenin-7-O-rutinoside (319). The hesperetin-7-O-rutinoside dose was therefore very similar to that of the quercetin-3-O-rutinoside in the tomato juice study (220). Plasma contained hesperetin-7-O-glucuronide (Fig. 17) and a second unassigned hesperetin-O-glucuronide, and the combined Cmax for the metabolites was 922 nM at a Tmax of 4.4 h. The two hesperetin metabolites were also excreted in urine along with a third hesperetin-O-glucuronide, two hesperetin-O-glucuronide-O-sulfates, and a hesperetin-O-diglucuronide.‡ These marked differences in the plasma and urinary hesperetin metabolite profiles demonstrate that substantial postabsorption phase II metabolism is occurring. The quantities of metabolites excreted 0–24 h after ingestion corresponded to 6.5% of hesperetin-7-O-rutinoside intake. Although no naringenin metabolites were detected in plasma, urine contained naringenin-7-O-glucuronide, narigenin-4′-O-glucuronide (Fig. 17), and a naringenin-O-diglucuronide in amounts equivalent to 17.3% of the ingested naringenin-7-O-rutinoside. The differing levels of excretion of hesperetin and naringenin metabolites, relative to the amounts ingested, is a trend that has been observed in some, but not all, flavanone-feeding studies (291). While it could be a dose effect reflecting the higher intake of the hesperetin conjugate, it is more likely to be due to naringenin-7-O-rutinoside being more bioavailable than hesperetin-7-O-rutinoside, indicating that the 3′ and 4′ substituents impact on absorption.
FIG. 17.
Although both are absorbed in the large intestine, the 922 nM Cmax of the hesperetin-O-glucuronides is more than 50-fold higher than that of the quercetin-3-O-rutinoside metabolites despite the amounts ingested being similar. This, coupled with the higher level of excretion of the orange juice metabolites, indicates that hesperetin-7-O-rutinoside metabolites are absorbed from the large intestine much more effectively than those of quercetin-3-O-rutinoside. This may be a consequence of the hesperetin-7-O-rutinoside being converted to glucuronides in the large intestine more efficiently than quercetin-3-O-rutinoside, perhaps because it is less prone to degradation by colonic bacteria. Among the urinary hesperetin metabolites were two O-glucuronide-O-sulfates. This contrasts with the absence of both sulfated naringenin metabolites and sulfated quercetin metabolites derived from large intestine absorption of quercetin-3-O-rutinoside in the tomato juice feed (Section III.A). Thus, there appear to be clear differences in the substrate specificity of flavonoid SULTs in the large intestine and/or the liver.
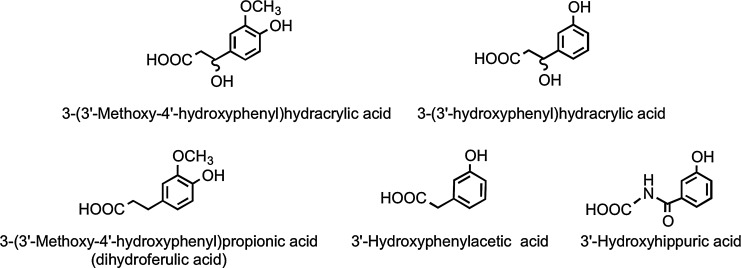
Analysis of phenolic acids and aromatic compounds excreted in urine after the ingestion of orange juice indicates that the hesperetin, released through colonic bacterium-mediated deglycosylation, as well as being glucuronidated, undergoes ring fission and is catabolized, producing 3(3′-hydroxyphenyl)hydracrylic acid, 3(3′-methoxy-4′-hydroxyphenyl)hydracrylic acid, 3(3′-methoxy-4′-hydroxyphenyl)propionic acid (dihydroferulic acid) 3′-hydroxyphenylacetic acid, and 3′-hydroxyhippuric acid (Fig. 18) (386). The overall level of the five phenolic acids excreted 0–24 h after drinking water was 6.7 μmol, and this rose to 62 μmol, equivalent to 37% of the ingested flavanones, after orange juice consumption.
FIG. 18.
Colonic phenolic acid catabolites of hesperetin.
B. Anthocyanins
Anthocyanins can be major dietary components with intakes in excess of 1 g being feasible for those who routinely eat berries and drink red wine. Unlike other flavonoids that are absorbed and excreted, they do not appear to undergo extensive metabolism to glucuronide and sulfate derivatives. In feeding studies with humans, typical recoveries of anthocyanins in urine are <0.1% of intake (109). For instance, Wu et al. (504) report a urinary recovery of blueberry (Vaccinium corymbosum) anthocyanins fed to elderly women of 0.004%. To some degree, these low recoveries could be a consequence of anthocyanins undergoing structural rearrangements in response to pH. The red flavylium cation predominates at pH 1–3, but as the pH increases to 4 and above, the colorless carbinol pseudobase becomes the major component along with smaller amounts of a colorless chalcone pseudobase and a blue quinoidal base (78). Such changes are likely to occur in vivo as anthocyanins pass from a low pH in the stomach to the more-basic conditions of the small intestine.
The available evidence implies that the limited absorption and excretion of anthocyanins that does take place are influenced by the nature of both the sugar moiety and the anthocyanidin structure (299, 361, 505). There are much complex data on the limited bioavailability of anthocyanins, which is, in part, due to the use of supplements that contain several structurally diverse anthocyanins. For instance, raspberries (Rubies idaeus) contain 10 or more anthocyanins as cyanidin- and pelargonidin-3-O-glycosides ranging from mono- to trisaccharides, while more than 14 anthocyanins comprising mainly 3-O-glucoside, galactosides, and arabinosides of cyanidin, delphinidin, petunidin, and malvidin occur in blueberries (41). This makes the trace levels of complex anthocyanins in plasma and urine exceedingly difficult, if not impossible, to interpret in terms of absorption, metabolism, excretion, and potential phase I and phase II metabolism. Especially so when 3′-O-methylation can convert cyanidin to peonidin, and delphinidin to petunidin and 5′-O-methylation results in the metabolism of petunidin to malvidin.
Comparatively, simple anthocyanin profiles are found in strawberries and blackberries (Rubus fruticosa), as they contain one predominant anthocyanin, pelargonidin-3-O-glucoside, in the former and cyanidin-3-O-glucoside in the latter. After feeding strawberries to volunteers, the main component in plasma was a pelargonidin-O-glucuronide, with plasma Cmax of 274 nM and a Tmax of 1.1 h, indicating absorption from the small intestine, whereas urinary excretion of the glucuronide corresponded to 0.75% of pelargonidin-3-O-glucoside intake (322). In other strawberry feeding studies, urinary excretion of ∼1.8% of the ingested pelargonidin-3-O-glucoside was obtained (62, 139). After feeding blackberries, 12 anthocyanins were detected in urine, including unmetabolized cyanidin-3-O-glucoside, a cyanidin-O-glucuronide, and a peonidin-O-glucuronide, in quantities equivalent to ∼0.18% of intake. It, therefore, appears that the 3′-hydroxyanthocyanin, pelargonidin-3-O-glucoside, is metabolized to fewer products and may be absorbed more readily that its 3′,4′-dihydroxy analog, cyanidin-3-O-glucoside. This is in keeping with the evidence obtained with other flavonoids that the presence of a substituent group at the 3′- and 4′-positions can influence absorption. For instance, kaempferol with a 4′-hydroxyl group is more bioavailable than quercetin, which has a 3′,4′-dihydroxy structure (Fig. 2) (38, 125). Also, as noted above, naringenin with a 4′-hydroxyl group is absorbed more readily than hesperetin, which has 3′-hydroxyl and 4′-methoxy groups (Fig. 6).
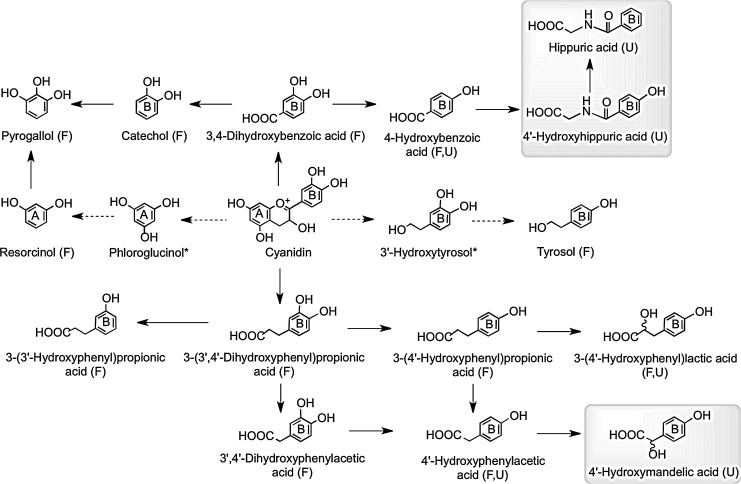
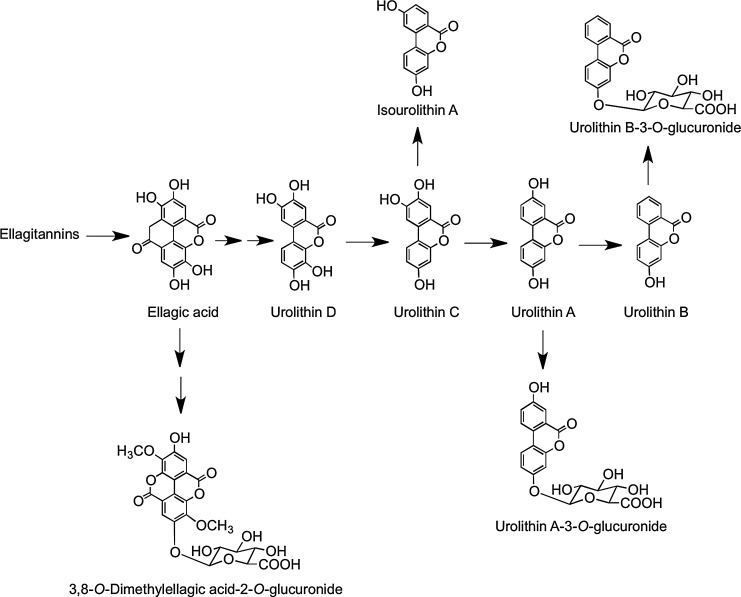
Consumption of raspberries by ileostomists has shown that 40% of anthocyanin intake remained in the ileal fluid, although the recovery of individual compounds varied greatly ranging from 5.9% for cyanidin-3-O-glucoside to 93% for cyanidin-3-O-(2′′-O-xylosyl)rutinoside (158). Analysis of urine collected from healthy volunteers after raspberry consumption and incubation of raspberry anthocyanins in fecal slurries under anaerobic conditions indicate that in vivo, the cyanidin-based anthocyanins are degraded by the colonic microbiota to a diversity of phenolic and aromatic catabolites by the pathways illustrated in Figure 19 (159). Vitaglione et al. have reported that protocatechuic acid (3,4-dihydroxybenzoic acid) is the principal catabolite of cyanidin-3-O-glucoside in humans (478).
FIG. 19.
Proposed pathways for the conversion of cyanidin-based red raspberry anthocyanins to phenolic acids and aromatic compounds. After consumption of 300 g of raspberries ∼40% of the ingested anthocyanins, principally cyanidin-O-glycosides, pass into the large intestine. When raspberry anthocyanins are incubated with fecal suspensions under anaerobic conditions, the cyanidin aglycone is released and catabolized by the colonic microflora undergoing C-ring fission, releasing phenolic acids, originating from both the A- and B-rings, which are metabolized via the pathways illustrated. Analysis of urine collected after raspberry consumption indicates that some of the colonic catabolites enter the circulatory and undergo further metabolism before being excreted in urine. These catabolites are thus detected in urine, but not fecal suspensions. F, catabolites detected in fecal suspensions; U, catabolites detected in urine (also highlighted in gray); *potential intermediates that did not accumulate in detectable quantities. Adapted from Gonzalez-Barrio et al. (159).
Further complicating matters, anthocyanins also breakdown to phenolic acid and aldehyde constituents when subjected to simulated physiological conditions and during sample processing before analysis. In this regard, experiments with pelargonidin, cyanidin, and delphinidin showed that increased B-ring hydroxylation is associated with decreased in vitro stability (500).
C. Flavones
Compared to other flavonoids, there have been relatively few human feeding studies involving naturally occurring flavones. After feeding an infusion of the Chinese medicinal herb medicine Scutellariae Radix, which contains the flavones baicalein and wogonin (Fig. 3) and their 7-O-glucuronides, 36-h urinary excretion of baicalein metabolites was 4.0% of intake compared to 7.1% for wogonin metabolites (265). This implies that the presence of a methoxy group at C-8 reduces flavone absorption by ∼50%. After ingestion of unfermented and fermented rooibos teas, which contain low levels of the flavone C-linked conjugates, vitexin, isovitexin, orientin, and iso-orientin (Fig. 3), no flavones or flavone metabolites were detected in either plasma or urine (437). This is probably a reflection of these compounds not being absorbed in either the small or the large intestine, and that the colonic microbiota were unable to cleave the C-linked sugar moiety.
D. Isoflavones
After consumption of soy milk containing mainly the 7-O-glucosides of daidzein and genistein (Fig. 4), Setchell et al. (410) detected urinary isoflavones as deglycosylated sulfates and glucuronides, implying the involvement of intestinal metabolism in their bioavailability. In a subsequent study, the same group fed several doses of [4-13C]daidzein and [4-13C]genistein to premenopausal women (411). Metabolites of both [13C]isoflavones began appearing in plasma within 1 h of feeding, and sub–μM Cmax values were associated with Tmax times of 5.5 h for genistein and 7.4 h for daidzein metabolites, and T½ times of ∼7.7 h. This suggests that the isoflavone metabolites are being absorbed into the circulatory system from both the small and large intestine. Urinary recoveries were 31% for [13C]daidzein and 9.0% for [13C]genistein, demonstrating the reduced bioavailability of the 5-hydroxylated isoflavone.
In a more recent investigation, Hosoda et al. (213) fed kinako, baked soybean flour, containing daidzein-7-O-glucoside (13.2 μmol), daidzein (23.9 μmol), genistein-7-O-glucoside (21.2 μmol), and genistein (41.0 μmol) to human volunteers. Plasma and urine collected over a 48-h postingestion period contained the aglycone daidzein, daidzein-4′-O-glucuronide, daidzein-7-O-glucuronide, daidzein-4′,7-O-diglucuronide, daidzein-4′-O-sulfate, daidzein-7-O-sulfate, and daidzein-4′-O-sulfate-7-O-glucuronide (Fig. 20) together with a similarly comprehensive spectrum of genistein metabolites. Plasma Tmax times ranged from 1.6 to 17.1 h, indicating that absorption seemingly takes place in both the proximal and distal GIT. Although no individual component attained a μM Cmax, urinary excretion of genistein metabolites was 29.5% of intake and that of daidzein derivatives 72.1% (Table 3), confirming that isoflavones, especially daidzein, are highly bioavailable compared to most other (poly)phenols.
FIG. 20.
Daidzein metabolites detected in plasma and urine after acute supplementation with baked soy flour in milk. A similar array of genistein metabolites was also detected. Adapted from Hosoda, et al. (213).
Table 3.
Pharmacokinetic Analysis of Isoflavone Metabolites in the Plasma of Volunteers After the Consumption of 10 g of Baked Soybean Flour Containing 275 μmol of Daidzein, Genistein, and Their 7-O-Glucosides
| Isoflavones | Tmax (h) | Cmax (μM) | Excretion (μmol) |
|---|---|---|---|
| Daidzein | 2.3±0.5 | 0.01±0.01 | 0.39±0.01 |
| Daidzein-4′-O-glucuronide | 4.5±0.8 | 0.10±0.02 | 4.65±0.55 |
| Daidzein-7-O-glucuronide | 6.1±0.8 | 0.09±0.01 | 12.86±0.99 |
| Daidzein-4′,7-O-diglucuronide | 2.9±0.5 | 0.05±0.00 | 3.26±0.81 |
| Daidzein-4′-O-sulfate | 4.1±1.0 | 0.04±0.00 | 0.57±0.1 |
| Daidzein-7-O-sulfate | 1.6±0.4 | 0.09±0.01 | 0.70±0.13 |
| Daidzein-4′-O-sulfate-7-O-glucuronide | 7.4±0.6 | 0.34±0.30 | 4.30±0.99 |
| Total/mean | 4.7 | 0.64±0.06 | 26.73±1.92 (72.1%) |
| Genistein | 8.0±2.8 | 0.01±0.01 | 0.11±0.03 |
| Genistein-4′-O-glucuronide | 3.9±0.8 | 0.14±0.02 | 4.95±1.02 |
| Genistein-7-O-glucuronide-4′-O-glucuronide | 5.0±0.5 | 0.11±0.01 | 5.23±0.98 |
| Genistein-4′,7-O-diglucuronide-7-O-glucuronide | 6.8±0.9 | 0.45±0.08 | 3.66±0.74 |
| Genistein-4′-O-sulfate-4′,7-O-diglucuronide | 17.1±5.1 | 0.02±0.04 | 0.34±0.05 |
| Genistein-7-O-sulfate-4′-O-sulfate | 1.7±0.4 | 0.18±0.03 | 1.09±0.41 |
| Genistein-4′-O-sulfate-7-O-glucuronide-7-O-sulfate | 6.4±0.6 | 0.85±0.12 | 2.96±0.79 |
| Total/mean | 5.4 | 1.58±0.16 | 18.34±2.67 (29.5%) |
Data presented as mean values±standard error (n=10). Italicized figures in parentheses represent urinary recoveries as a percentage of intake.
Adapted from Hosoda et al. (213).
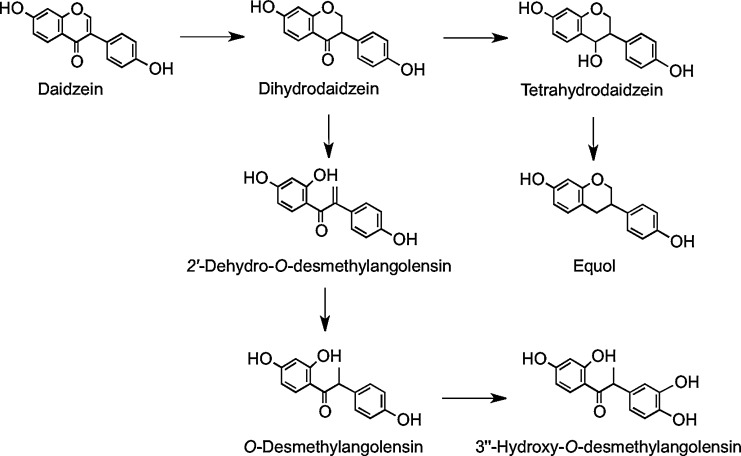
In vitro anaerobic incubations with human feces have shown two routes of metabolism for daidzein, depending on subjects and their gut microbiota. Some subjects produce equol via dihydrodaidzein and tetrahydrodaidzein, and others produce O-desmethylangolensin via 2′-dehydro-O-desmethylangolensin (Fig. 21). Thus, there are two subpopulations: those who have the microbiota capable of synthesizing equol, as it S-enantiomer, and those who lack the microbes to do so (225, 409).
FIG. 21.
Microbial metabolism of the isoflavonoid genistein. Adapted from Joannou et al. (225) and Setchell et al. (409).
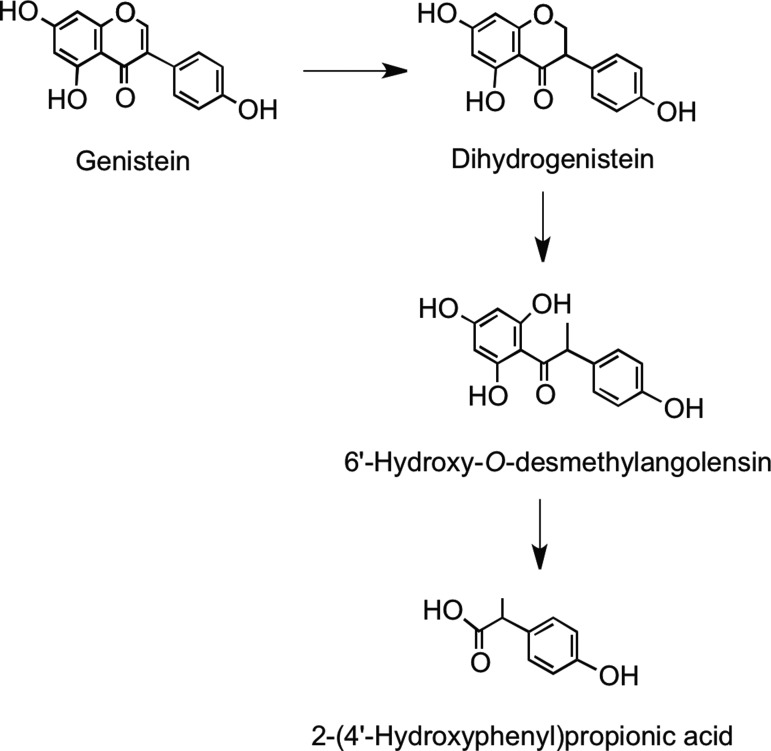
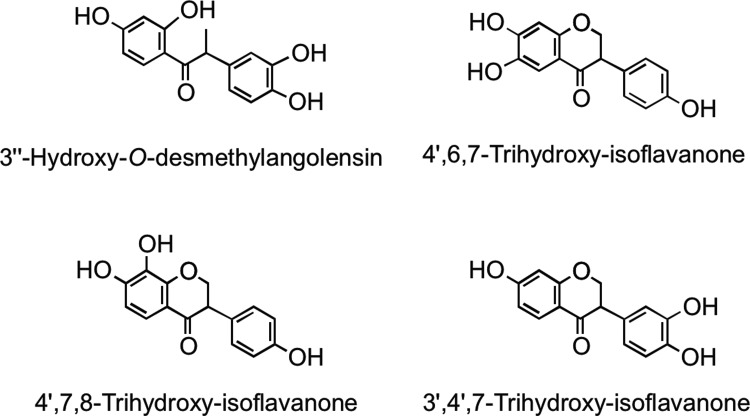
Microbial metabolism of genistein is different to that of daidzein. Genistein is reduced to dihydrogenistein, which is further metabolized to 6′-hydroxy-O-desmethylangolensin (225). In vitro incubations with human fecal and rat cecal microbiota revealed formation of 2-(4′-hydroxyphenyl)propionic acid from 6′-hydroxy-O-desmethylangolensin, indicating C-ring fission (81) (Fig. 22). Heinonen et al. (186) identified four novel isoflavonoid metabolites from human urine after soy supplementation: 3′′-hydroxy-O-desmethylangolensin, 3′,4′,7-trihydroxyisoflavanone, 4′,7,8-trihydroxyisoflavanone, and 4′,6,7-trihydroxyisoflavanone (Fig. 23).
FIG. 22.
Microbial metabolism of the isoflavonoid daidzein. Adapted from Coldham et al. (81) and Joannou et al. (225).
FIG. 23.
Human urinary metabolites excreted after the consumption of soy. Adapted from Heinonen et al. (186)
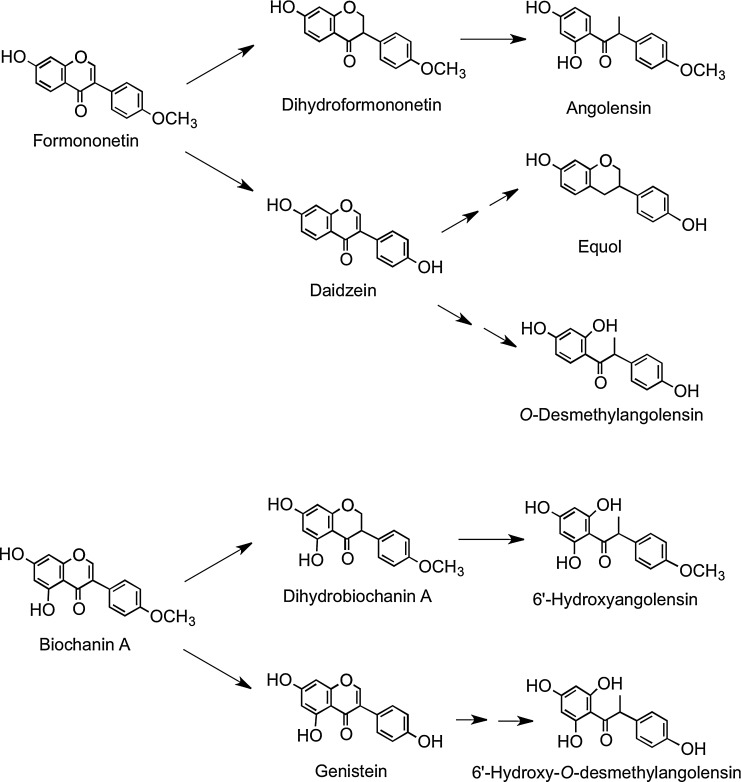
Red clover (Trifolium pratense), which contains the isoflavones biochanin A and formononetin, is consumed by cows in Finland, and as a consequence, high levels of equol have been detected in Finnish milk that is sold for human consumption (208). In humans, urinary excretion indicates that formononetin is readily metabolized to daidzein, which can be converted to O-desmethylangolensin and equol, as well as being metabolized to dihydroformononetin and angolensin. Biochanin A is metabolized to dihydrobiochanin A and 6′-hydroxyangolensin and is also converted, via genistein, to 6′-hydroxy-O-desmethylangolensin (Fig. 24) (185). This explains the similarity of these microbial metabolites to those of daidzein and genistein (15).
FIG. 24.
Human metabolism of the red clover isoflavones formononetin and biochanin A. Adapted from Heinonen et al. (185).
E. Flavan-3-ols
Bioavailability studies with flavan-3-ols typically involve acute supplementation with either green tea or cocoa-based products. In a recent study, Ottaviani et al. (336) investigated the bioavailability of different enantiomeric forms of flavan-3-ol monomers in a study in which adult human males consumed equal quantities of (−)-epicatechin, (−)-catechin, (+)-epicatechin, and (+)-catechin (see Fig. 8) in a cocoa drink. Based on plasma concentrations and urinary excretion, the bioavailability of the stereoisomers was ranked as (−)-epicatechin >(+)-epicatechin=(+)-catechin >(−)-catechin. There were also differences in the metabolic fate of the catechin and epicatechin epimers as reflected in the ratios of their 3′- and 4′-O-methylated metabolites. In addition, the levels of nonmethylated metabolites of (−)- and (+)-epicatechin in plasma and urine differed, demonstrating that flavan-3-ol stereochemistry also affects metabolic pathways other than O-methylation. The samples were analyzed as aglycones released by glucuronidase/sulfatase treatment, so it was not possible to determine in any detail to what degree this impacted on the production of glucuronide and sulfate metabolites. As the individual flavan-3-ol stereoisomers in cocoa products and green teas used in feeding studies are usually not determined, this finding raises the possibility that varying stereochemical ratios could be a contributing factor in the different (epi)catechin metabolite profiles reported in the literature, with the stereochemical variation reflecting differences in product processing.
1. Cocoa flavan-3-ol monomers
Because the stereochemistry of the flavan-3-ols consumed in cocoa as well as the stereochemistry of the flavan-3-ol metabolites observed in plasma and urine have rarely been characterized to the enantiomer level, this uncertainty will be made clear by using, for example, (epi)catechin and (epi)gallocatechin rather than the apparently more-precise terms used in almost all the original publications.
Early human studies on the postingestion fate of cocoa flavan-3-ols treated plasma and urine samples with β-glucuronidase/sulfatase and analyzed the released (epi)catechin monomers by reverse-phase HPLC (199, 372, 376, 482). Richelle et al. (376) showed that after the consumption of 40 g of dark chocolate containing 282 μmol of (−)-epicatechin, the (epi)catechin metabolite levels in plasma rose rapidly and reached a Cmax of 355 nM with a Tmax of 2.0 h, characteristic of absorption in the upper GIT rather than the large intestine. With double the chocolate intake, the Cmax increased to 676 nM while the Tmax was extended to 2.6 h, which was attributed to the ad libitum consumption of bread by the volunteers rather than the increased intake of chocolate. Wang et al. (482) also carried out a dose study in which varying amounts of chocolate were served with 40 g of bread. The data, which are summarized in Table 4, show a positive relationship between intake and (epi)catechin plasma concentrations.
Table 4.
Concentration of (Epi)catechin Metabolites in Plasma of Volunteers 0, 2, and 6 h After the Ingestion of Chocolate Containing 159, 312, and 417 μmol of (−)-Epicatechin
| (−)-Epicatechin intake (μmol) | 0 h | 2 h | 6 h |
|---|---|---|---|
| 0 | 1±1a | 19±14a | 1±1a |
| 159 | 2±2a | 133±27c | 26±8b |
| 312 | 4±2a | 258±29c | 66±8b |
| 471 | 4±3a | 355±49c | 103±16b |
Data expressed as mean values in nM±standard error (n=9–13). Mean values with a different superscript are significantly different (p<0.05).
Adapted from Wang et al. (482).
In a further study, Baba et al. (20) fed a chocolate containing 760 μmol of (−)-epicatechin and 214 μmol of catechin, most probably the (−)-isomer, to human subjects and collected plasma and urine over the ensuing 24-h period. The combined Cmax of the metabolites was 4.8±0.9 μM, and the Tmax was 2 h. The total 0–24-h urinary excretion of the (epi)catechin and methyl-(epi)catechin metabolites was 227±39 μmol, which corresponds to 29.9% of the ingested (−)-epicatechin. As will be discussed later, this high level of excretion of (epi)catechin metabolites has been confirmed in a number of subsequent feeding studies with both cocoa and green tea.
More recent cocoa flavan-3-ol bioavailability studies have analyzed plasma and urine samples using the HPLC-MS2 methodology. In one such study by Mullen et al. (320), volunteers drank a cocoa beverage containing 22.3 μmol of catechin, almost all of it as the less-bioavailable (−)-isomer, and 23.0 μmol of (−)-epicatechin. Two flavan-3-ol metabolites were detected in plasma, an O-methyl-(epi)catechin-O-sulfate and an (epi)catechin-O-sulfate. Both had a Cmax below 100 nM and a Tmax of <1.5 h, and after 8 h, only trace levels remained in the circulatory system. The two sulfated flavan-3-ols were also the main metabolites in urine which, in addition, contained an (epi)catechin-O-glucuronide, and a further (epi)catechin-O-sulfate. The amount of flavan-3-ol metabolites excreted in urine over the 0–24-h collection period was 7.32±0.82 μmol, which is equivalent to 16.3%±1.8% of intake. Considering that half of the flavan-3-ol monomer content of the cocoa was (−)-catechin, which has reduced bioavailability, the real figure for (−)-epicatechin absorption was probably nearer 30%, and as such is comparable with urinary (epi)catechin excretion levels reported by Baba et al. (20).
2. Green tea flavan-3-ol monomers
Green tea contains high concentrations of flavan-3-ol monomers. In addition to (−)-epicatechin and (+)-catechin, (epi)gallocatechins and 3-O-galloylated flavan-3-ols are present, components that do not occur in cocoa. Typically, (−)-epigallocatechin-3-O-gallate, (−)-epigallocatechin, and (−)-epicatechin (Figs. 8 and 9) predominate (110).
In an acute feeding study, healthy human subjects consumed 500 ml of green tea containing 648 μmol of flavan-3-ols, after which plasma and urine collected over a 24-h period were analyzed by HPLC-MS3 (440). The plasma contained a total of 12 metabolites in the form of O-methylated, sulfated, and glucuronide conjugates of (epi)catechin and (epi)gallocatechin along with the native green tea flavan-3-ols (−)-epigallocatechin-3-O-gallate and (−)-epicatechin-3-O-gallate. An analysis of the pharmacokinetic profiles of these compounds is presented in Table 5. None of the flavan-3-ols were present in the circulatory system at 0 h, but they did appear in detectable quantities 30 min after green tea consumption. The main component that accumulated was an (epi)gallocatechin-O-glucuronide, with a Cmax of 126 nM and a Tmax of 2.2 h, whereas an (epi)catechin-O-glucuronide attained a Cmax of 29 nM with a 1.7 h Tmax. The unmetabolized flavan-3-ols (−)-epigallocatechin-3-O-gallate and (−)-epicatechin-3-O-gallate had Cmax values of 55 and 25 nM after 1.6 and 2.3 h, respectively. The Tmax durations ranged from 1.6 to 2.3 h (Table 5); all the flavan-3-ols and their metabolites were present in only trace quantities after 8 h, and were not detected in the 24-h plasma. These Tmax values and the pharmacokinetic profiles are indicative of absorption in the small intestine.
Table 5.
Pharmacokinetic Analysis of Flavan-3-ols and Their Metabolites Detected in Plasma of Volunteers Following the Ingestion of 500 ml of Green Tea
| Flavan-3-ols (number of isomers) | Cmax (nM) | Tmax (h) |
|---|---|---|
| (Epi)catechin-O-glucuronide (1) | 29±4.7 | 1.7±0.2 |
| (Epi)catechin-O-sulfates (2) | 89±15 | 1.6±0.2 |
| O-Methyl-(epi)catechin-O-sulfates (5) | 90±15 | 1.7±0.2 |
| (Epi)gallocatechin-O-glucuronide (1) | 126±19 | 2.2±0.2 |
| 4′-O-Methyl-(epi)gallocatechin-O-glucuronide (1) | 46±6.3 | 2.3±0.3 |
| 4′-O-Methyl-(epi)gallocatechin-O-sulfates (2) | 79±12 | 2.2±0.2 |
| (−)-Epigallocatechin-3-O-gallate (1) | 55±12 | 1.9±0.1 |
| (−)-Epicatechin-3-O-gallate (1) | 25±3.0 | 1.6±0.2 |
Data expressed as mean values±standard error (n=10).
Adapted from Stalmach et al. (440).
The appearance of unmetabolized flavonoids in plasma is unusual. The passage of (−)-epicatechin-3-O-gallate and (−)-epigallocatechin-3-O-gallate through the wall of the small intestine into the circulatory system without metabolism could be a consequence of the presence of the 3-O-galloyl moiety interfering with phase II metabolism. Gallic acid per se is readily absorbed with a reported urinary excretion of 37% of intake (412, 413), and so the gallate ester might exhibit improved absorption.
Urine collected 0–24 h after green tea ingestion contained flavan-3-ol metabolites similar to those detected in plasma, except for the presence of minor amounts of three additional (epi)gallocatechin-O-sulfates and the absence of (−)-epicatechin-3-O-gallate and (−)-epigallocatechin-3-O-gallate (Table 6) (440). There were, however, differences in the relative amount of individual metabolites in plasma and urine. For instance, an (epi)gallocatechin-O-glucuronide was the main metabolite in plasma (Table 5), but not in urine (Table 6). In total, 52.4 μmol of metabolites was excreted, which was equivalent to 8.1% of the ingested green tea flavan-3-ols. When the urinary (epi)gallocatechin and (epi)catechin metabolites were considered separately, a somewhat different picture emerged. The 33.3 μmol excretion of (epi)gallocatechin metabolites was 11.4% of the ingested (−)-epigallocatechin and (+)-gallocatechin, while the 19.1±2.2 μmol recovery of (epi)catechin represented 28.5% of intake (Table 6). This is in keeping with high urinary recoveries of (epi)catechin metabolites obtained in the studies with cocoa products (see Section III.E.1), indicating that (−)-epicatechin is highly bioavailable and is absorbed and excreted to a much greater extent than other flavonoids, with the possible exception of isoflavones (see Section III.D).
Table 6.
Quantification of the Major Groups of Flavan-3-ol Metabolites Excreted in Urine 0–24 h After the Ingestion of 500 ml of Green Tea by Volunteers
| Flavan-3-ol metabolites (number of isomers) | 0–24 h excretion (μmol) |
|---|---|
| (Epi)gallocatechin-O-glucuronide (1) | 6.5±1.2 |
| 4′-O-Methyl-(epi)gallocatechin-O-glucuronide (1) | 4.4±1.5 |
| 4′-O-Methyl-(epi)gallocatechin-O-sulfates (2) | 19.8±0.3 |
| (Epi)gallocatechin-O-sulfates (3) | 2.6±3.0 |
| Total (epi)gallocatechin metabolites | 33.3 (11.4%) |
| (Epi)catechin-O-glucuronide (1) | 1.5±0.3 |
| (Epi)catechin-O-sulfates (2) | 6.7±0.7 |
| O-Methyl-(epi)catechin-O-sulfates (5) | 10.9±1.2 |
| Total (epi)catechin metabolites | 19.1 (28.5%) |
| Total flavan-3-ol metabolites | 52.4 (8.1%) |
Data expressed as mean value±standard error (n=10). Italicized figures in parentheses indicate the amount excreted as a percentage of intake.
Adapted from Stalmach et al. (440).
The absence of detectable amounts of (−)-epigallocatechin-3-O-gallate in urine, despite its presence in plasma, an event observed by several investigators (75, 192, 464), is difficult to explain. It is possible that the kidneys are unable to remove (−)-epigallocatechin-3-O-gallate from the bloodstream, but if this is the case, there must be other mechanisms that result in its rapid decline after reaching Cmax. Studies with rats have led to speculation that (−)-epigallocatechin-3-O-gallate may be removed from the bloodstream in the liver and returned to the small intestine in the bile (247, 252). To what extent enterohepatic recirculation of (−)-epigallocatechin-3-O-gallate, and also (−)-epicatechin-3-O-gallate, occurs in humans remains to be established. Quite possibly, these bile-excreted flavan-3-ols would be degallated by the gut microbiota, and if not more extensively degraded, would be excreted in urine as (epi)catechin and (epi)gallocatechin metabolites. It is of note that feeding studies with [2-14C]resveratrol have provided evidence that metabolites of the stilbene do undergo enterohepatic recycling in humans (480).
a. Dose effects
Auger et al. (14) fed ileostomists increasing doses of Polyphenon E, a green tea extract containing a characteristic array of flavan-3-ols; urinary excretion of metabolites was used as a measure of absorption in the small intestine. The data obtained with (epi)gallocatechin and (epi)catechin metabolites are summarized in Table 7. At a dose of 22 μmol, the 0–24-h excretion of (epi)gallocatechin metabolites was 5.7±1.9 μmol, and this figure did not increase significantly with intakes of 55 and 165 μmol. There is, therefore, a strict limit on the extent to which (epi)gallocatechins can be absorbed. After the ingestion of 77 μmol of (epi)catechins, 36±9 μmol was excreted, and with doses of 192 and 577 μmol, and urinary excretion increased significantly to 107±27 and 262±26 μmol. Thus, even the highest dose of (epi)catechins, unlike (epi)gallocatechins, is still readily absorbed. The addition of a 5′-hydroxyl group to (epi)catechin, therefore, markedly reduces the extent to which the molecule can enter the circulatory system from the small intestine. It is also of note that at the three doses that were administered, the ratio of the urinary glucuronide, sulfate, and methylated (epi)catechin metabolites changed little (Table 7), implying that even at the highest intake, the UGT, SULT and COMT enzymes involved in the formation of the (epi)catechin metabolites do not become saturated and limit conversions.
Table 7.
Urinary Excretion of Flavan-3-ol Metabolites After the Ingestion of Increasing Doses of (−)-Epicatechin and (−)-Epigallocatechin in Polyphenon E by Humans with an Ileostomy
| 22 μmol dose | 55 μmol dose | 165 μmol dose | |
|---|---|---|---|
| Glucuronides | 1.8 (17%) | 1.0 (18%) | 1.7 (19%) |
| Sulfates | 3.9 (37%) | 2.0 (36%) | 3.6 (40%) |
| Methylated | 4.8 (46%) | 2.6 (46%) | 4.7 (51%) |
| Total (epi)gallocatechin metabolites | 5.7±1.9a | 3.0±0.8a | 5.3±1.2a |
| 77 μmol dose | 192 μmol dose | 577 μmol dose | |
| Glucuronides | 3.4 (7%) | 14 (9%) | 38 (10%) |
| Sulfates | 33 (64%) | 93 (58%) | 224 (59%) |
| Methylated | 15 (29%) | 53 (33%) | 120 (31%) |
| Total (epi)catechin metabolites | 36±9a | 107±27b | 262±26c |
Values for total (epi)gallocatechin and (epi)catechin metabolites with different superscripts are significantly different (p<0.05).
Metabolites excreted over a 24-h period after ingestion expressed as μmol, and italicized figures in parentheses represent the percentage of glucuronide, sulfate, and methylated metabolites.
Adapted from Auger et al. (14).
b. Colonic catabolism of green tea flavan-3-ols
Stalmach et al. (438) carried out an acute green tea feeding study with ileostomists. The volunteers drank tea containing 634 μmol of flavan-3-ols, a very similar intake to that used in their studies with healthy subjects (440). The plasma flavan-3-ol pharmacokinetics were similar to those presented in Table 5, which were obtained from healthy subjects with an intact colon. Urinary excretion by the ileostomists was 8.0% of intake for (epi)gallocatechin metabolites and 27.4% of (epi)catechin metabolites, values that were similar to those observed with healthy subjects (see Table 6). This confirms that the flavan-3-ol monomers are absorbed in the upper part of the GIT, and suggests that absorption of (epi)catechin and (epi)gallocatechin after degallation of flavan-3-ol gallates excreted in bile does not contribute significantly to bioavailability of the intact flavan-3-ols in consumers with an intact colon. Despite the substantial absorption of green tea flavan-3-ols in the upper GIT, Stalmach et al. (438) found that 69% of intake was recovered in 0–24-h ileal fluid as a mixture of native compounds and metabolites. Thus, in volunteers with a colon, most of the ingested flavan-3-ols will pass from the small to large intestine, where their fate is a key part of the overall bioavailability equation.
To mimic events taking place in the large intestine, two sets of experiments were carried out by Roowi et al. (387). First, 50 μmol of (−)-epicatechin, (−)-epigallocatechin, and (−)-epigallocatechin-3-O-gallate was incubated with fecal suspensions in vitro under anaerobic conditions and their degradation to phenolic acid and aromatic catabolites by the microbiota monitored. Secondly, to complement the in vitro incubations, catabolites excreted in urine 0–24 h after (i) the ingestion of green tea and water by healthy subjects in a crossover study and (ii) the consumption of green tea by ileostomists were also investigated (387).
The data obtained in these studies provided the basis for the operation of the proposed catabolic pathways illustrated in Figure 25. Some of these catabolites, such as 4′-hydroxyphenylacetic acid and hippuric acid (N-benzoylglycine), were detected in urine from subjects with an ileostomy, indicating that they are produced in the body by additional routes unrelated to colonic degradation of flavan-3-ols. It is, for instance, well known that there are pathways to hippuric acid from compounds such as benzoic acid, quinic acid (79), tryptophan, tyrosine, and phenylalanine (50, 171, 408). Nonetheless, the elevated urinary excretion of hippuric acid and 4′-hydroxyphenylacetic acid occurring after green tea consumption is likely to be partially derived from flavan-3-ol degradation.
FIG. 25.
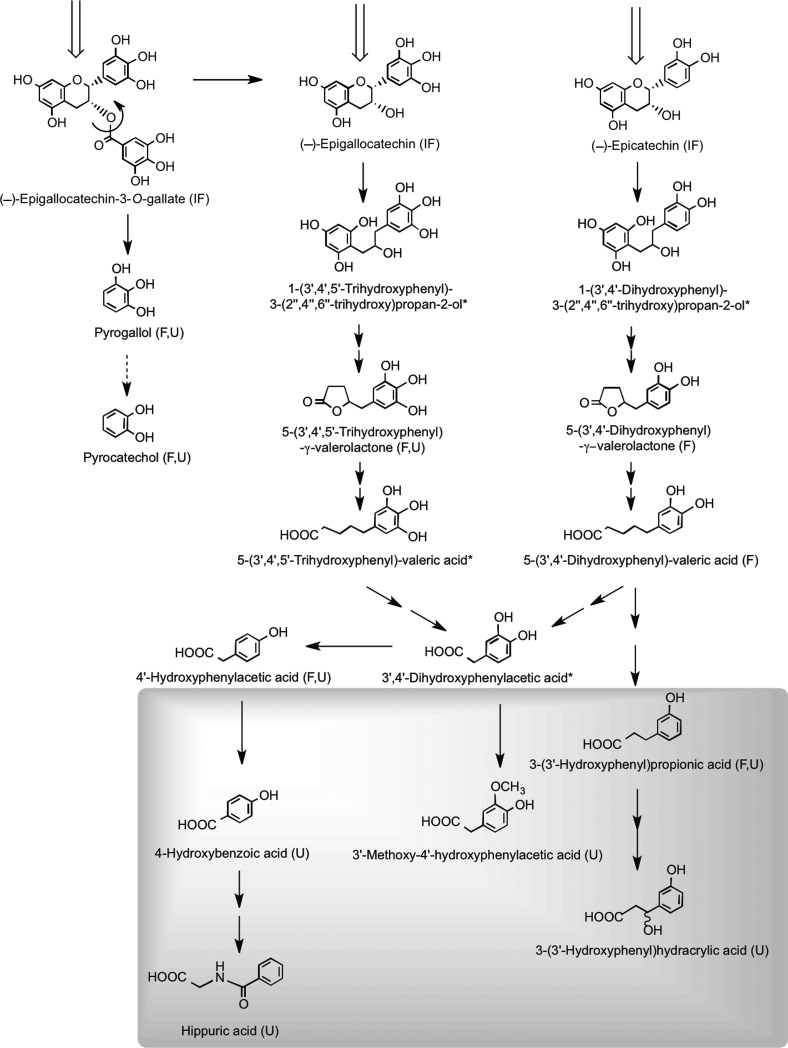
Proposed pathways involved in the colonic catabolism and urinary excretion of green tea flavan-3-ols. After consumption of green tea, more than 50% of the ingested flavan-3-ols (gray structures) pass into the large intestine. When incubated with fecal slurries, these compounds are catabolized by the colonic microflora, probably via the proposed pathways illustrated. Analysis of urine after green tea consumption indicates that some of the colonic catabolites enter the circulation and undergo further phase II metabolism before being excreted in urine. Structures in the gray box indicate such catabolites that are detected in urine, but not produced by fecal fermentation of (−)-epicatechin, (−)-epigallocatechin, or (−)-epigallocatechin-3-O-gallate. The dotted arrow between pyrogallol and pyrocatechol indicates that this is a minor conversion. Double arrows indicate conversions where the intermediates did not accumulate and are unknown. Compounds detected in ileal fluid after green tea consumption (IF); catabolites detected in fecal slurries (F) and in urine (U); potential intermediates that did not accumulate in detectable quantities in fecal slurries (*). Adapted from Roowi et al. (387).
Quantitative estimates of the extent of ring fission of the flavan-3-ol skeleton are difficult to assess, because as noted above, the production of some of the urinary catabolites is not exclusive to colonic degradation of flavan-3-ols. If these compounds are excluded along with pyrogallol and pyrocatechol, which are derived from cleavage of the gallate moiety from (−)-epigallocatechin-3-O-gallate rather than ring fission, the excretion of the remaining urinary phenolic acids, namely 4-hydroxybenzoic acid, 3′-methoxy-4′-hydroxyphenylacetic acid, 3-(3′-hydroxyphenyl)hydracrylic acid, and 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone, after ingestion of green tea was 210 μmol compared to 38 μmol after drinking water (387). The 172-μmol difference between these figures corresponds to 27% of the 634 μmol of flavan-3-ols present in the ingested green tea (438). Added to this is the ∼8% excretion of glucuronide, sulfate, and methylated flavan-3-ols originating from absorption in the small intestine. This estimate of a 35% recovery is nonetheless a minimum value, because with the analytical methodology used, some urinary catabolites will have escaped detection (387). This will include glucuronide and sulfate metabolites of 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone, 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone, and 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone, which were detected after green tea consumption with a cumulative 0–24-h excretion corresponding to 16% of flavan-3-ol intake (277, 303, 400). More recently, in a similar study in which urine was collected for 24 h after green tea intake, valerolactone metabolites were excreted in quantities equivalent to 36% of intake (108). When added to the 35% recovery of Roowi et al. (387), this gives a total of 71% of intake. While this figure is obviously an approximation because of factors such as different volunteers, flavan-3-ol intakes, and analytical methodologies, it does demonstrate that despite substantial modification as they pass through the body, there is a very high urinary recovery of flavan-3-ols, principally in the form of colon-derived catabolites. Moreover, the excretion of colonic valerolactones has been shown to continue far beyond 24 h after ingestion, as some of these metabolites have been detected in urine after 54 h (108). This observation is consistent with a possible underestimation of the real bioavailability of flavan-3-ols, at least when green tea is consumed. In this context, the study of Calani et al. (60), who monitored urinary excretion of (epi)catechin catabolites by 20 healthy volunteers for 48 h after ingestion of green tea, is of interest. On a mole-for-mole basis, some volunteers exhibited a 100% recovery of flavan-3-ols in urine, whereas with others, excretion was <30% of intake. Arguably, this suggests that colonic ring-fission catabolism could be a key factor in the bioactivity of green tea flavan-3-ols.
c. Identification and quantification of flavan-3-ol metabolites
Rather different (epi)catechin metabolite profiles have been obtained by different research groups who in the absence of standards used HPLC-MS3 to partially identify metabolites that were then quantified by reference to (−)-epicatechin. In the Mullen et al. (320) cocoa investigation, an (epi)catechin-O-sulfate and an O-methyl-(epi)catechin-O-sulfate were detected in plasma. These sulfates were also the main metabolites in urine, which also contained an additional (epi)catechin-O-sulfate and smaller amounts of an (epi)catechin-O-glucuronide. As outlined above, Auger et al. (14) and Stalmach et al. (438, 440), using an identical analytical methodology, detected a similar spectrum of (epi)catechin metabolites in plasma and urine collected after ingestion of green tea flavan-3-ols. However, in cocoa studies by other groups in which plasma and urine samples were analyzed by HPLC-MS3, an (epi)catechin-O-glucuronide was the main metabolite, and sulfates were either absent or minor components (390–393, 458). The reason for these varying metabolite profiles, especially the dominance of glucuronides in some studies and sulfates in others, could be due to losses of (epi)catechin-O-sulfates that are known to be unstable during sample processing before analysis (106). A further factor influencing quantitative estimates is that the response of the mass spectrometers used by the different groups may vary to an extent, which in the absence of reference compounds cannot be determined. Thus, it is possible that the Mullen et al. (320), Auger et al. (14), and Stalmach et al. (438, 440) studies, and others by this group (42, 435), may have used instruments that are more sensitive to sulfates than to glucuronides, and vice versa for other investigators. One way around this is to treat samples with sulfatase/glucuronidase to release the aglycones, but this too has its drawbacks, as different batches of the enzyme preparations vary in the effectiveness with which they hydrolyze flavan-3-ol sulfate metabolites (337).
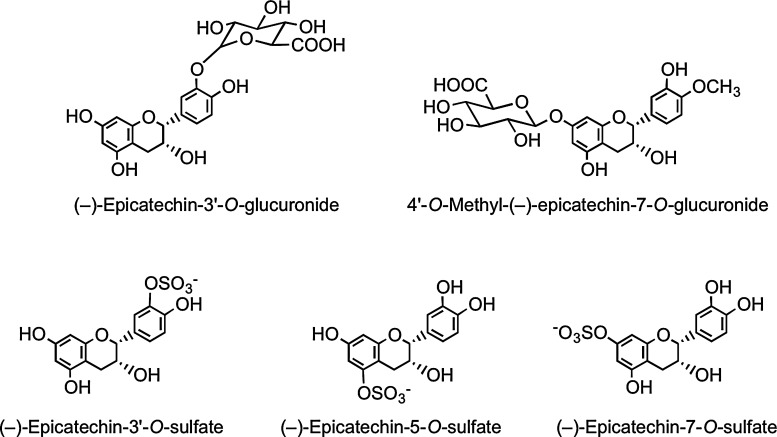
In a recent human cocoa feeding study in which (i) authentic flavan-3-ol metabolites were available to assist analysis, and (ii) the (−)-epicatechin intake was 6.2 mmol/kg body weight, the following metabolites were identified in plasma with a Tmax of 2 h: (−)-epicatechin-3′-O-glucuronide (Cmax, 589±85 nM), 4′-O-methyl-(−)-epicatechin-7-O-glucuronide (Cmax, ∼20 nM), (−)-epicatechin-3′-O-sulfate (Cmax, 331±26 nM), (−)-epicatechin-5-O-sulfate (Cmax, 37±3 nM), and (−)-epicatechin-7-O-sulfate (Cmax, 12±1 nM) (Fig. 26). Also present in trace amounts were four distinct 3′- and 4′-O-methyl-(−)-epicatechin-O-sulfates, each of which was sulfated at the either C-5 or C-7 position (337). This establishes that the main (−)-epicatechin metabolite in humans is (−)-epicatechin-3′-O-glucuronide, and that sulfate metabolites accumulate in plasma at slightly lower concentrations.
FIG. 26.
Human (−)-epicatechin plasma metabolites detected in plasma after the consumption of an–(−)-epicatechin-rich cocoa drink. Adapted from Ottaviani et al. (337).
3. Black tea theaflavins and thearubigins
Although consumed far more extensively in Europe than green tea, the absorption of flavan-3-ols from black tea, their gut microbial catabolism, and human metabolism have been investigated less extensively, and such studies as have been performed focused on the absorption of flavan-3-ol monomers (192). The appearance of the gallic acid metabolites 3-O-methylgallic acid, 4-O-methylgallic acid, and 3,4-di-O-methylgallic acid in urine (Fig. 27) has also been reported and used as an index of black tea consumption (201, 202), although these metabolites are also to be expected after not only green and black tea consumption but also the ingestion of certain fruits, such as grapes, and the associated wines. The absorption and metabolism of flavan-3-ol monomers from black tea are not obviously different from those observed after green tea consumption, although pro rata, the dose of flavan-3-ols is much reduced.
FIG. 27.
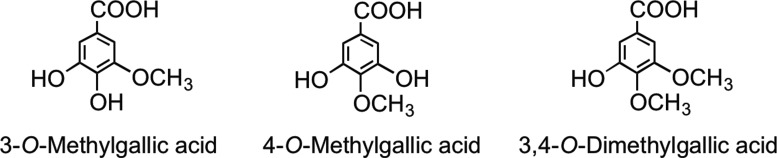
Methylated gallic acid derivatives excreted after the consumption of black tea.
To date, only one study has investigated the absorption of mixed theaflavins (theaflavin 17.7%, theaflavin-3-O-gallate 31.8%, theaflavin-3′-O-gallate 16.7%, and theaflavin-3,3′-digallate 31.4%) (Fig. 9) (318). An extremely high-dose 700 mg mixed theaflavins, equivalent to about 30 cups of black tea, was given to two healthy volunteers, one male and one female. Plasma and urine concentrations were analyzed by HPLC-MS2 after enzymatic deconjugation with β-glucuronidase and sulfatase. Only theaflavin was detected because the enzyme treatment also removed ester gallate. Maximum theaflavin concentrations detected in the plasma of the female and male volunteers were 1.8 and 0.9 fM, respectively, and maximum urine concentrations were 1.1 and 7.4 fM, respectively, all at 2 h. These values should be doubled to correct for the relatively poor recovery observed with standard theaflavin, but even so, the total amount of theaflavin excreted was considerably <0.001% of the very large dose consumed (318).
Feeding studies have demonstrated that the consumption of black tea beverage results in a substantially increased excretion of hippuric acid relative to baseline, suggesting that a combination of gut microbial catabolism and postabsorption metabolism results in a significant production of benzoic acid (79, 107, 317). The yield of benzoic acid excreted as hippuric acid is such that it points to thearubigins and theaflavins serving as substrates in vivo, and being degraded to aromatic acids. The yield of hippuric acid was not significantly affected by the presence of caffeine in the black tea or by the addition of milk, but varied by ∼4-fold between individuals for a given intake of black tea (79).
F. Proanthocyanidins
There have been numerous feeding studies with animals and humans, indicating that the oligomeric and polymeric proanthocyanidins are not absorbed to any degree. A study in which ileostomists consumed apple juice indicates that most pass unaltered to the large intestine (233), where they are catabolized by the colonic microbiota, yielding a diversity of phenolic acids and aromatic components (133, 291), including 3-(3′-hydroxyphenyl)propionic acid and 4-O-methyl-gallic acid (111, 156), which are absorbed into the circulatory system and excreted in the urine. There is a report based on data obtained from an in vitro model of gastrointestinal conditions that procyanidins degrade, yielding more readily absorbable flavan-3-ol monomers (234). However, in vivo studies (119, 380, 462), including feeds to ileostomists (233), have not supported this conclusion, suggesting that no more than ∼10% of procyanidin dimers are converted to monomers in this way (10, 445).
There is a report of minor quantities of procyanidin B2 being detected in enzyme-treated human plasma collected after the consumption of cocoa (210). The Tmax and pharmacokinetic profile of the B2 dimer were similar to those of flavan-3-ol monomers, but the Cmax was ∼100-fold lower. Urpi-Sarda et al. (465) also detected and quantified procyanidin B2 in human and rat urine after cocoa intake.
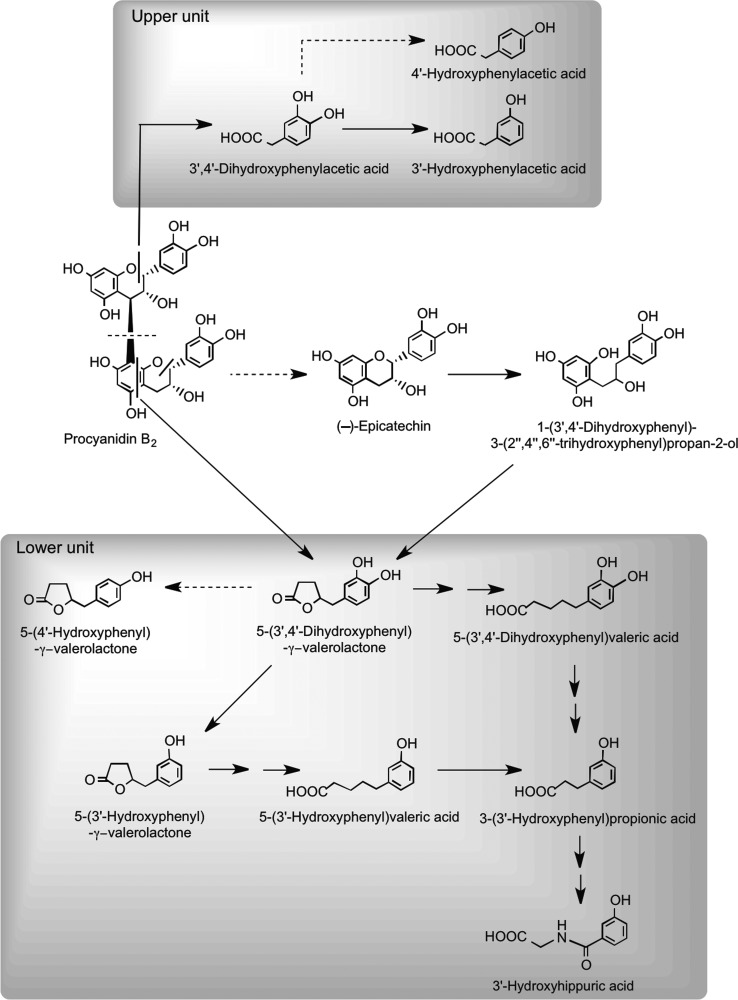
Recent studies using procyanidin B2 and [14C]procyanidin B2 have provided information on their in vitro catabolism by the gut microbiota (10, 444, 445) and rodent pharmacokinetics (446). After oral dosing of rats with a [14C]procyanidin B2 dimer, ∼60% of the radioactivity was excreted in urine after 96 h with the vast majority in a form very different from the intact procyanidin dosed (446). This observation is consistent with the in vitro studies that show extensive catabolism by the gut microbiota. The scission of the interflavan bond represents a minor route, and the dominant products are a series of phenolic acids having one or two phenolic hydroxyls and between one and five aliphatic carbons in the side chain (10, 444, 445). There were, in addition, some C6–C5 catabolites with a side chain hydroxyl group and associated lactones and several diaryl-propan-2-ols, most of which are also produced from the flavan-3-ol monomers via the proposed routes illustrated in Figure 25, whereas 3′,4′-dihydroxyphenylacetic acid is derived from cleavage of the C-ring of the upper flavan-3-ol unit. However, the findings of Stoupi et al. (445) also indicate that a feature of flavan-3-ol catabolism is the conversion to C6–C5 valerolactones and the progressive β-oxidation to C6–C3 and C6–C1 products, broadly in keeping with the (−)-epicatechin catabolism routes illustrated in Figure 28.
FIG. 28.
Proposed pathways for human microbial degradation of procyanidin B1 dimer. Main routes are indicated with solid arrows, and minor pathways with dotted arrows. Metabolites derived from upper and lower units are grouped within the shaded rectangles. Adapted from Appeldoorn et al. (10) and Stouppi et al. (444, 445).
The potential biological effects of procyanidins are generally attributed to their more readily absorbed colonic breakdown products, the phenolic acids and valerolactones, although there is a lack of detailed investigation in this area. There is, however, a dissenting view as trace levels of procyanidins, in contrast to (−)-catechin and (+)-epicatechin, inhibit platelet aggregation in vitro and suppress the synthesis of the vasoconstricting peptide endothelin-1 by cultured endothelial cells (84). Supporting this view is a study in which individual procyanidins were fed to rats after which dimers through to pentamers were detected in the plasma that was extracted with 8 M urea, rather than the more traditional methanol/acetonitrile, which was proposed to have prevented or reversed the binding of procyanidins to plasma proteins (419). The procyanidins were, however, administered by gavage at an extremely high dose, 1 g/kg body weight, and it remains to be determined if procyanidins can be similarly detected in urea-extracted plasma after the ingestion by humans of more dietary-relevant quantities in cocoa or chocolate products.
G. Dihydrochalcones
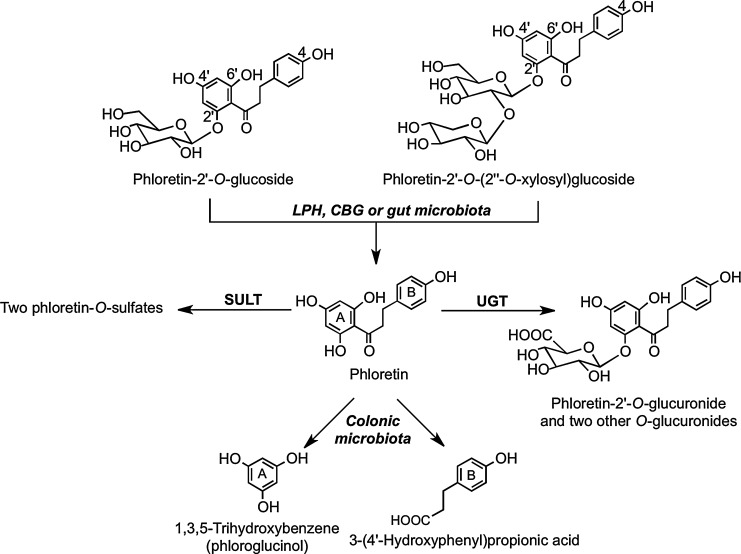
Human bioavailability studies with dihydrochalcones have been restricted to feeds with apples, apple cider, and rooibos tea (377). After ingestion, the principal components in apples and cider, phloretin glucosides (Fig. 10), undergo cleavage in the small intestine with the released phloretin being subject to glucuronidation before appearing rapidly in the circulatory system as phloretin-2′-O-glucuronide with a Tmax of <1 h (Table 8). The short duration of the Tmax values and the similar plasma Cmax of the glucuronide in healthy subjects and ileostomists after apple cider consumption (294) are all indicative of absorption in the proximal GIT. Overall recoveries of phloretin derivatives in ileal fluid have varied from 47.3% (175) to 38.5% (294) and 24.8% (233). Ileal fluid contained phloretin-2′-O-(2′′-O-xylosyl)glucoside, but none of the ingested phloretin-2′-O-glucoside. This implies that the glucoside is more readily cleaved than the xylosyl-glucoside by LPH in the brush border of the small intestine or by CBG in the epithelial cells. Also detected in ileal fluid were the aglycone phloretin, two further phloretin-O-glucuronides, and two phloretin-O-sulfates (294).
Table 8.
Plasma Pharmacokinetic Data for Phloretin-2′-O-Glucuronide After the Consumption of Apple Cider and a Polyphenol-Rich Drink by Healthy Volunteers and Subjects with an Ileostomy
In feeds with apple cider and a polyphenol-rich drink, the main dihydrochalcones in urine was phloretin-2′-O-glucuronide with smaller amounts of two additional phloretin-O-glucuronides and a phloretin-O-glucuronide-O-sulfate. The overall excretion of these metabolites was ∼5.0% of intake in both healthy subjects and volunteers with an ileostomy (Table 9), providing further evidence of the absorption of these compounds in the small intestine. Nonetheless, the data obtained with ileal fluid reveal that in subjects with a colon, substantial amounts of ingested phloretin derivatives reach the colon (42, 294).
Table 9.
Urinary Excretion of Phloretin Metabolites Over a 24-h Period After the Consumption of Apple Cider and a Polyphenol-Rich Drink by Healthy Volunteers and Subjects with an Ileostomy
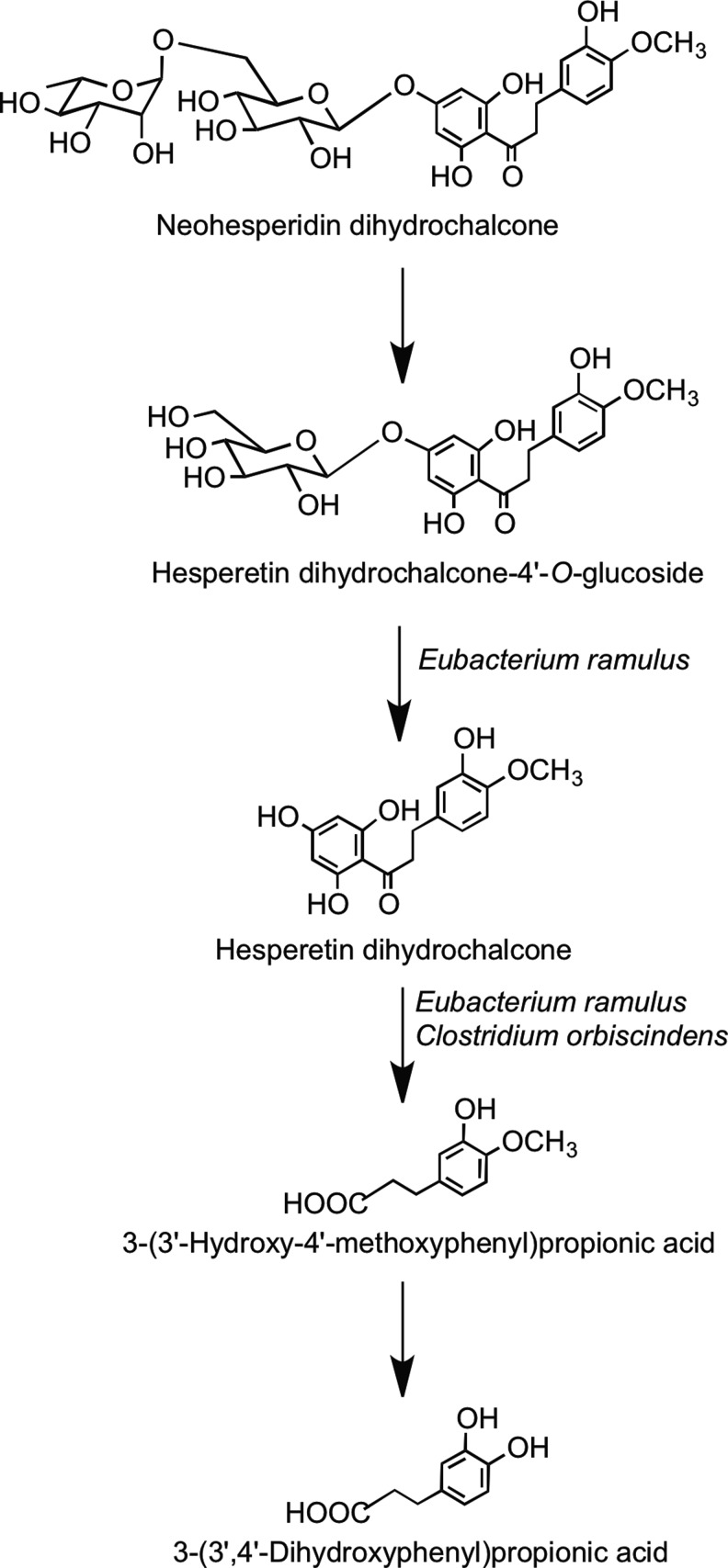
There is only limited information on the fate of dihydrochalcones when they come into contact with the colonic microbiota. Rats fed phloretin excrete the parent dihydrochalcone along with 3-(4′-hydroxyphenyl)propionic acid and 1,3,5-trihydroxybenzene (phloroglucinol). The metabolism and degradation of phloretin-O-glycosides after ingestion, therefore, probably follow the pathways outlined in Figure 29 (314). In vitro fecal bacteria convert the synthetic sweetener neohesperidin dihydrochalcone to 3-(3′-dihydroxy-4′-methoxyphenyl)propionic acid and 3-(3′, 4′-hydroxyphenyl)propionic acid with the intermediates hesperetin dihydrochalcone-4′-O-glucoside and hesperetin dihydrochalcone also being detected (Fig. 30) (45).
FIG. 29.
Metabolism of phloretin-O-glycosides after ingestion. Adapted from Richling (377).
FIG. 30.
Catabolism of neohesperidin dihydrochalcone to 3-(3′,4′-dihydroxyphenyl)propionic acid by human fecal slurries. Steps catalyzed by the colonic bacteria Eubacterium ramulus and Clostridium orbiscindens are indicated. Adapted from Braune et al. (45).
As noted in Section II.A.7, the principal components in unfermented rooibos tea are the dihydrochalcone C-glucosides aspalathin and nothofagin (Fig. 10) with smaller amounts of flavanone and flavone C-glucosides and flavonol O-glycosides. Fermentation results in a decline in the dihydrochalcones and an increase in the flavanone C-glucosides, while the other flavonoids remained largely unchanged (437).
Five-hundred-milliliter volumes of the unfermented and fermented rooibos teas that were analyzed by Stalmach et al. (437) were, in the same study, also fed to volunteers, after which plasma and urine collected at intervals over a 24-h period were analyzed by HPLC-MS3. This was the first detailed study in which the bioavailability of flavonoid C-glycosides, as opposed to O-glycosides, was investigated in humans. Presumably, because of their low concentration in the teas and/or their low bioavailability, no dihydrochalcones were detected in plasma, although aspalathin metabolites were present in urine in detectable quantities.
Urine contained glucuronides, sulfates, methyl, methyl sulfates, and methyl glucuronide metabolites of aspalathin. In contrast, no nothofagin metabolites were detected. Most urinary excretion of the aspalathin metabolites occurred during the first 5 h after intake (80%–90% of the total amounts excreted), indicative of small intestine absorption. In total, 316 nmol of aspalathin metabolites was excreted after an intake of 90 μmol with unfermented tea. This represents a recovery of 0.35% (Table 10). After the ingestion of 8 μmol of aspalathin with the fermented tea, there was urinary recovery of 14.3 nmol of metabolites corresponding to 0.18% of intake. Aspalathin, therefore, has very limited bioavailability.
Table 10.
Summary of the Urinary Excretion of Aspalathin Metabolites in Urine Collected from 10 Volunteers After Drinking 500 ml of Unfermented Rooibos Tea Containing 90 μmol of Aspalathin
| |
Quantity excreted in urine (nmol) |
|||
|---|---|---|---|---|
| Metabolites | 0–5 h | 5–12 h | 12–24 h | 0–24 h |
| Aspalathin-O-glucuronide | 6.2±1.1 | 5.0±1.1 | 1.3±0.6 | 12±1.6 |
| O-Methyl-aspalathin-O-glucuronide | 180±22 | 42±9.1 | 7.2±2.5 | 229±26 |
| Aspalathin-O-sulfate | 40±6.6 | 11±2.3 | 1.3±1.1 | 52±6.8 |
| O-Methyl-aspalathin-O-sulfate | 20±3.0 | 2.7±0.9 | n.d. | 23±3.2 |
| Total aspalathin metabolites (% intake) | 0.35% | |||
Data expressed as mean values in nmol±standard error (n=10). Italicized figure in parentheses indicates 0–24-h excretion as a percentage of intake.
Adapted from Stalmach et al. (437).
n.d., not detected.
Incubation of aspalathin with artificial gastric juice for up to 2 h led to a recovery of ∼100%, suggesting that the dihydrochalcone-C-glucoside is not subject to degradation in the stomach. The very low urinary recoveries of aspalathin metabolites and their failure to accumulate in detectable quantities in plasma are probably consequences of the C-glucoside moiety not being readily cleaved by either LPH or CBG as the dihydrochalcone passes through the small intestine (437).
Kreuz et al. (256) reported urinary excretion of aspalathin in the form of glucuronidated, methyl glucuronidated, methylated, and free aspalathin, in pigs, after intakes of 15.3 g of aspalathin for 11 days—a daily intake equivalent to ∼400 times that given as a single dose by Stalmach et al. (437). They reported urinary excretion levels ranging from 0.16% to 0.87% in three pigs after 7 days of treatment, which is in broad agreement with the observed level of human urinary excretion. This suggests that limited absorption occurs regardless of the dose ingested.
H. Ellagitannins
Most studies on the bioavailability of ellagitannins have involved feeding humans pomegranate juice, which contains punicalin and punicalagin (Fig. 11) (68, 407). However, the bioavailability of ellagitannins in raspberries (158), strawberries, walnuts, and oak-aged wines has also been investigated (69).
After drinking pomegranate juice containing 318 mg of punicalagins, ellagic acid was detected in plasma with Cmax of 60 nM at a Tmax of 0.98 h, suggesting acid hydrolysis of at least some of the ellagitannins releasing free ellagic acid, which is absorbed directly from the stomach or the proximal small intestine (407). Also detected in the plasma of some, but not all, volunteers, mainly 6 h after supplementation, were urolithin A, urolithin A-3-O-glucuronide, urolithin B, and methylated urolithin B. Urinary metabolites that began to appear after 12 h included urolithin A-3-O-glucuronide, urolithin B-3-O-glucuronide, and 3,8-O-dimethylellagic acid-2-O-glucuronide (see Fig. 31), and excretion continued for up to further 36 h. None of these compounds were quantified, and there was much subject-to-subject variation in the spectrum of metabolites produced. This implies that when the ellagitannins and/or ellagic acid reaches the distal part of the small intestine and the colon, they are metabolized by the gut microbiota, producing urolithins A and B, which are then absorbed along with ellagic acid and subjected to the action of phase II UGTs and/or COMTs before being excreted in urine (407).
FIG. 31.
Potential pathways for the conversion of ellagitannins to urolithins.
In a further study in which volunteers ingested 1 liter of pomegranate juice containing 4.37 g of punicalagins on a daily basis for 5 days, circulating urolithin levels reached a concentration of 18.6 μM (68). Feeding human subjects a single dose of strawberries, raspberries, walnuts, and oak-aged red wine, all of which contain ellagitannins, resulted in excretion of urolithin A 3-O-glucuronide in quantities equivalent to 2.8% (strawberries), 3.4% (raspberries), 6.5% (oak-aged red wine), and 16.6% (walnuts) of intake (69).
After the ingestion of a raspberry supplement, containing 123 μmol of ellagitannins, mainly as sanguiin H-6, by healthy subjects, urolithin A-3-O-glucuronide, two of its isomers, and urolithin B-O-glucuronide were excreted in 7–48-h urine (158). There was a marked variation in the urolithin profile of individual volunteers with recoveries relative to the ingested ellagitannins ranging from 0% to 8.2%, indicative of differences in the colonic microbiota responsible for ellagitannin degradation.
In vitro anaerobic incubations of punicalagin with fecal slurries resulted in the appearance of ellagic acid, urolithin A, isourolithin A, and urolithin B (Fig. 32) (159). There were substantial qualitative and quantitative differences in the profile of metabolites obtained with residual punicalagin concentrations at the end of the 72-h incubation ranging from 1.3 to 27.7 μM and the final concentration of urolithins varying from 0 to 27.4 μM. Once again, this is almost certainly a consequence of person-to-person variations in bacterial composition of the colonic micobiota. Although there was again much sample-to-sample variation, fecal incubations converted ellagic acid to urolithins much more efficiently than punicalagin (Fig. 33). Thus, in vivo, the anaerobic bacterial conversion of ellagitannins to ellagic acid appears to be the rate-limiting step in the production of urolithins in the colon. Furthermore, the fecal incubations did not yield urolithin glucuronides that are excreted in urine after in vivo feeding studies. This points to glucuronidation of urolithins occurring in the wall of the large intestine and/or postabsorption in the liver.
FIG. 32.
Anaerobic metabolism of punicalagins to ellagic acid and urolithins in fecal suspensions. Bars represent standard errors (n=5). The initial concentration of punicalagin was 100 μM. Adapted from Gonzalez-Barrio et al. (159).
FIG. 33.
Anaerobic metabolism of ellagic acid to urolithins in fecal suspensions. Bars represent standard errors (n=4). The initial concentration of ellagic acid was 50 μM. Adapted from Gonzalez-Barrio et al. (159).
The most detailed study on ellagitannins to date has been carried out with Iberian pigs, which in their natural habit feed on oak acorns, a rich source of ellagitannins (134). The pigs consumed an average of 4.04 kg of acorns on a daily basis for 117 days after which tissues and body fluids were processed and analyzed by HPLC-MS3. A total of 31 ellagitannin-derived metabolites were detected, including 25 urolithin and six ellagic acid derivatives. A summary of the complex picture that emerges is that in the jejunum, the acorn ellagitannins release ellagic acid (which is metabolized sequentially by intestinal microbiota), producing urolithin D, urolithin C, urolithin A, and urolithin B, as illustrated in Figure 31. These urolithins are absorbed preferentially as their lipophilicity increases with plasma containing mainly urolithin A-3-O-glucuronide and urolithin B-3-O-glucuronide with traces of a urolithin C-O-glucuronide and 3,8-O-dimethylellagic acid-2-O-glucuronide. The urolithin A and B glucuronides were the major components in urine. Among the 26 conjugated metabolites were detected in bile were glucuronides and methyl glucuronides of ellagic acid and sizable quantities of urolithin A, C, and D derivatives. This indicates substantial hepatic metabolism and active enterohepatic circulation, and also explains the persistence of urinary urolithin metabolites observed in the human studies. No ellagitannins or their metabolites were detected in body tissues of the Iberian pigs outside the GIT (134). However, after consumption of pomegranate juice and walnuts, urolithin A-O-3- glucuronide and traces of urolithin B have been detected in human prostate tissue (162).
I. Chlorogenic acids
Coffee beans as noted in Section II.B is a rich source of chlorogenic acids, mainly 3-O-, 4-O-, and 5-O-caffeoylquinic acids, and smaller amounts of the equivalent feruloylquinic acids, which as a result of their ready extraction by hot water can be consumed in a quantity by regular coffee drinkers (96). The most detailed research on the fate of chlorogenic acids after ingestion of coffee is that of Stalmach et al's. (436, 439) in studies with healthy humans and ileostomists, in which it was possible to distinguish between events occurring in the proximal and distal GIT and elucidate the metabolic pathways illustrated in Figure 34. Ileostomists consumed coffee containing 385 μmol of chlorogenic acids of which 274 μmol was recovered as both the parent compounds and sulfate and glucuronide conjugates in a 0–24-h ileal fluid. Thus, ∼30% of intake is absorbed in the small intestine, and in healthy subjects with an intact colon, 70% of the ingested chlorogenic acids pass from the small to large intestine, where they are subject to the action of the colonic microbiota. Urinary excretion, principally of metabolites, by healthy subjects was equivalent to 29.2% of intake, but only 8.0% with ileostomists, demonstrating the role of the colon in the bioavailability of chlorogenic acids. The study also showed that urinary dihydrocaffeic acid-3′-O-sulfate and feruloylglycine serve as sensitive biomarkers for the consumption of relatively small amounts of coffee.
FIG. 34.
Proposed metabolism of chlorogenic acids following the ingestion of coffee by volunteers. 5-Caffeoylquinic acid and 5-feruloylquinic acid are illustrated structures, but their respective 3- and 4-isomers would be metabolized in a similar manner. Co-A, Co-enzyme A; EST, esterase; RA, reductase. Bold arrows indicate major routes, and dotted arrows minor pathways. Steps blocked in subjects with an ileostomy, and hence occurring in the colon are indicated. Adapted from Stalmach et al. (436, 439).
Concord grape juice from grapes of V. lambrusca contains sizable amounts of hydroxycinnamate tartarate esters with the main component being caftaric acid (Fig. 12). After ingestion, esterases cleave the conjugating tartartic acid, and the released caffeic acid is metabolized and absorbed in a manner similar, but not identical, to that of caffeic acid cleaved from caffeoylquinic acids in coffee (434, 435).
J. Resveratrol
trans-Resveratrol is widely credited with being responsible for the protective effects of red wine in both the press (405) and the scientific literature (76, 399). This is almost certainly not true, as the level of the stilbenes in red wines is so low that >60 liters would have to be consumed on a daily basis by humans for intake to reach the amounts that are required to increase longevity and provide protective effects in model animal systems (85). Resveratrol is an extremely minor component in the human diet, and as such, its potential use is as a therapeutic agent at pharmacological doses.
Bioavailability studies in humans with trans-resveratrol have shown absorption and a rapid metabolism, but relatively low excretion in urine and feces. Gastric absorption explains the peak of free resveratrol in the plasma 30 min after ingestion (8, 39, 475, 479). However, the Cmax of free resveratrol is typically extremely low: <37 nM after oral ingestion at physiological doses ranging from 1.1 to 110 μmol (154, 479, 480). In acute feeds with doses of 0.44–22 mmol, the higher trans-resveratrol dosage increased the Cmax to ∼280 nM (8, 39, 475). The general consensus is that resveratrol-O-glucuronides and sulfates are the major plasma and urine metabolites, with the sulfates being predominant (39, 40, 57, 467).
Resveratrol-3-O-glucuronide, resveratrol-4′-O-glucuronide, resveratrol-3-O-sulfate, resveratrol-4′-O-sulfate (Fig. 35), a resveratrol-O-disulfate, and an O-glucuronide-O-sulfate were found in the plasma of both healthy humans and colorectal cancer patients ingesting 2.2 and 4.4 mmol of trans-resveratrol (40, 348). In addition to these metabolites, two novel trans-resveratrol C/O-conjugated diglucuronides were detected in plasma and urine of subjects who ingested 128 μmol of trans-piceid (57).
FIG. 35.
Human metabolites of trans-resveratrol. Adapted from Boocock et al. (40) and Patel et al. (348).
K. Plant lignans
A diverse range of plant lignans, including secoisolariciresinol, matairesinol, medioresinol, pinoresinol, and lariciresinol (Fig. 5), are all converted to enterodiol and enterolactone (Fig. 36) by human gut microbiota (15, 128, 184, 308, 353, 354). Enterolactone production in vitro is slow compared with synthesis of enterodiol (16, 128). The ratio of enterolactone- and enterodiol-producing bacteria is 1:2000 as shown by culture-based and 16S rRNA-targeted molecular methods. These observations indicate that enterodiol-synthesizing bacteria are dominant, while enterolactone-forming microbiota are a minor population (77). Several research groups have shown a negative association between frequent bowel movements and enterolactone levels (227, 249). Human subjects with more rapid colonic transit may have less time to convert plant lignans to enterolactone than subjects whose colon motility is slow and the subsequent transit time of the colonic contents is more prolong.
FIG. 36.
The mammalian lignans enterodiol and enterolactone.
IV. Evidence for the Accumulation of (Poly)phenol Metabolites in Body Tissues
As will be discussed in Section VI, there is evidence from a number of sources that consumption of berry extracts and fruit juices can delay the decline of various aspects of cognitive function in elderly rats and humans (359). There is, however, contradictory evidence as to whether flavonoids themselves cross the blood–brain barrier.
In one of the early studies, Andres-Lacueva et al. (9) detected trace levels of several anthocyanins in brains of rats that had received a diet supplemented with a blueberry extract, containing unspecified amounts of anthocyanins, for a period of 10 weeks. In a further study, 18 h after feeding pelargonidin to rats by gavage at a dose of 184 μmol/kg, the unmetabolized anthocyanidin was detected in brains at a concentration of ca. 0.2 nmol/g (fresh weight) (129). In contrast, anthocyanins did not accumulate in detectable amounts in the brains of rats obtained up to 24 h after acute supplementation by gavage with 2.8 ml of raspberry juice, which is a nutritional-relevant dose equivalent to a 70-kg human drinking 700 ml of juice (43). In contrast, after a 4-week supplementation of pigs with a blueberry extract, containing undefined amounts of anthocyanins, 300 pg of anthocyanins/g was detected in cerebellum tissue and 700 pg/g in eye tissue (237). In another study, oral ingestion of 100 mg/kg of blackcurrant anthocyanins by rats resulted in a plasma anthocyanin Cmax of 1.9 μg/ml 30 min after ingestion and a maximum concentration of anthocyanins in the whole eye of 115 ng/g, also after 30 min (295). However, in a study with male mice, feeding a bilberry (Vaccinium myrtillus) extract for 2 weeks resulted in the accumulation of anthocyanins in detectable amounts in plasma, the liver, kidney, testes, and lungs, but not in other tissues, including the brain and eyes (398). One of the possible reasons for the seemingly contradictory data obtained in these studies could be the use of extracts containing very high amounts of anthocyanins well in excess of what could be ingested as part of a normal berry-based diet.
As far as localization of other flavonoids within the body is concerned, acute supplementation of rats with [2-14C]quercetin-4′-O-glucoside at a dose of 4 mg/kg body weight is a study of relevance (323). The bulk of the ingested radiolabeled flavonol reached the cecum and colon, and it was rapidly degraded to phenolic acids, principally 3-hydroxyphenylacetic acid and benzoic acid. Most of radioactivity, over a 72-h period, was rapidly excreted in urine without any noticeable buildup in the circulatory system or body tissues, including the brain. In contrast, feeds of unlabeled quercetin to rats at substantially higher doses of 50 and 200 mg/kg, corresponding to intakes of 271 and 1084 mg for a 70-kg human, did result in the detection of trace levels of quercetin in perfused rat brain tissues (216). In a human study in which volunteers drank 250 ml of green tea containing 505 μmol of flavan-3-ol monomers, typical (epi)catechin and (epi)gallocatechin metabolites were detected in plasma 1 h after ingestion, but they were not present in the cerebrospinal fluid collected 1 h later (513). In contrast, small amounts of radioactivity were detected in the brain of rats 6 and 24 h after feeding [3H]-(−)-epigallocatechin-3-O-gallate by gavage (449). Radioactivity was also detected in the brain of rats after feeding mixtures of 14C-labeled grape (poly)phenols (222). However, in neither of these studies was the identity of radiolabeled compounds in brain tissues determined.
There is a report on the detection of flavan-3-ols in extracts of glucuronidase/sulfatase-treated prostate biopsy tissue obtained from subjects who had consumed 1.42 L of either green or black tea for a period of 5 days (192), and as noted earlier, after consumption of pomegranate juice and walnuts, urolithin A glucuronide and traces of urolithin B have also been detected in human prostate tissue (162). In a further study, glucuronide metabolites of epicatechin and methyl-epicatechin were detected in the thymus, testes, and liver, with lower amounts in the lymph nodes and spleen, of rats that had been fed a 10% cocoa diet for a period of 15 days (466). In addition, after the ingestion of isoflavones, the presence of daidzein and genistein and their metabolites equol, enterolactone, and enterodiol has also been detected in human prostate tissue, whereas after ingesting ∼300 μmol of daidzein, concentrations up to 6 μM of equol accumulated in the breast tissue [see(93)].
Immunohistochemical studies have established that quercetin-3-O-glucuronide (Fig. 15), one of the main quercetin metabolites in the circulatory system (105), accumulates in macrophage-derived foam cells of human atherosclerotic lesions, but not in the normal aorta (243). In vitro experiments with murine macrophage cell lines showed that quercetin-3-O-glucuronide was taken up and deconjugated to its more bioactive aglycone quercetin, which was in turn partially converted to a methylated metabolite. In addition, mRNA expression of the class A scavenger receptor and CD36, which play key roles in the formation of foam cells, was suppressed by treatment with quercetin-3-O-glucuronide (243). Likewise, immunohistochemical studies have also shown that (−)-epicatechin-3-O-gallate, a constituent of green tea that appears in plasma in trace amounts after ingestion (438), is also specifically localized in macrophage-derived foam cells and similarly suppresses gene expression of CD36 (244).
V. In Vitro Biological Activity and Mode of Action
Bioactivity investigations using human or animal cell lines have made extensive use of both (poly)phenol aglycones and their sugar conjugates, the latter being the typical form in which they exist in planta. The concentrations at which these compounds have been assayed have usually been in the low-μM-to-mM range (340, 385, 502). However, as outlined in previous sections of this review, after ingestion, with few exceptions, dietary (poly)phenolics appear in the circulatory system not as the parent compounds, but as glucuronide, methyl, and sulfate metabolites, and their presence in plasma after normal dietary intake rarely exceeds nM concentrations, even at Cmax (93, 109). Moreover, substantial quantities of (poly)phenols are not absorbed in the small intestine, but pass to the colon where they are degraded by the action of the microbiota, to give rise to a plethora of small phenolic acid and aromatic catabolites that are absorbed into the circulatory system before being excreted in quantities that vastly exceed those of metabolites absorbed in the small intestine (108). Therefore, the focus of this section of the review will be on the in vitro bioactivity of in vivo metabolites and catabolites formed within body after intake of dietary-relevant doses of (poly)phenol-rich foods.
A. Anthocyanins
Protocatechuic acid (3,4-dihydroxybenzoic acid) is one of the catabolites derived from colonic microbial degradation of cyanidin-O-glucosides (159, 478) (see Fig. 19), and its bioactivity in vitro has been investigated in some detail, with its influence in cell proliferation and death being among the areas of interest. It has apoptotic effects on human gastric adenocarcinoma cells in a time- and dose-dependent manner (280). This study also showed that the effect of protocatechuic acid on an adenocarcinoma cell line might be mediated via sustained phosphorylation and activation of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), but not extracellular-signal-regulated kinases (ERK), thus defining a very specific mechanism of action. This is in good agreement with Yip et al. (508), who reported cytotoxicity of protocatechuic acid against HepG2 hepatocellular carcinoma cells exerted by stimulating JNK and p38 subgroups of the MAPK family. In contrast to these proapoptotic effects, protocatechuic acid was able to increase the viability of cultured neural stem cells and to stimulate cell proliferation. The reduced level of apoptosis of neural stem cells was probably brought about by lowering the level of reactive oxygen species (ROS) and decreasing the activity of caspase-3 (172). This indicates that protocatechuic acid can act in varying ways in different environments, acting in a proapoptotic manner in actively proliferating cells, while protecting normal cells from apoptosis. This antiapoptotic ability was seemingly confirmed in a study in which treatment of cultured rat adrenal gland pheochromocytoma (PC12) cells with protocatechuic acid prevented 1-methyl-4-phenylpyridinium ion (MPP+)-induced loss of mitochondrial membrane potential, formation of ROS, glutathione depletion, activation of caspase-3, and downregulation of Bcl-2 (173). However, the concentration of protocatechuic acid used to treat cells in these studies ranged from 0.06 to 8 mM, which vastly exceeds the levels likely to be attained in vivo (478), making the reported effects of this anthocyanin catabolite of questionable physiological relevance, in this instance.
Consumption of fruits and vegetables and related products, rich in anthocyanins and other (poly)phenols, is thought to reduce inflammatory stress, and as a consequence, provides some degree of protection against the development of chronic diseases, including cancer, cardiovascular, and neurodegenerative pathologies (4, 150, 402). From the inflammation perspective, protocatechuic acid has been reported to inhibit monocyte adhesion to tumour necrosis factor-α (TNF-α)-activated mouse aortic endothelial cells, and also to reduce vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1) expression, the nuclear content of p65, and nuclear factor κB (NF-κB)-binding activity (481). In the same study, protocatechuic acid administered to apolipoprotein E (ApoE)-deficient mice reduced aortic VCAM-1 and ICAM-1 expression, NF-κB activity, and plasma-soluble VCAM-1 and ICAM-1 levels, thus inhibiting the development of atherosclerosis. These results suggest that protocatechuic acid could be considered as at least partially antiatherogenic because of its vascular anti-inflammatory activity.
Verzelloni et al. (476) observed that 3′-hydroxyphenylacetic acid, 3′,4′-dihydroxyphenylacetic acid, and 3′-methoxy-4′-hydroxyphenylacetic acid, known anthocyanin microbial catabolites (493), inhibited the formation of advanced glycation end products (AGEs) when applied as a mixture at a combined concentration of 2.0 μM. The same set of catabolites was effective in preserving cultivated neuron cells from death due to oxidative stress at a concentration of 1.5 μM. Additional experiments were carried out with the AGE model that pointed to the glucose-mediated pathway as the likely site of action of the catabolites. In another study by this group, hydroxycinnamate catabolites, derived from caffeoylquinic acids in coffee, were able to inhibit protein glycation, which was related to a reduction of Amadori products, suggesting that these compounds could act as pre-Amadori inhibitors of protein glycation (477). Since phenolic compounds have the ability to bind to proteins, it was hypothesized that the catabolites may protect albumin from glycation by a mechanism involving some form of specific chemical binding. These studies are good examples of testing the right compounds, as the phenolic catabolites known to be generated in vivo were able, at physiological concentrations, to counteract two key features of diabetic complications, at least in vitro, namely protein glycation and neurodegeneration.
Evidence exists for an inverse relationship between moderate intake of red wine and the incidence of rectal cancer (351), and this could be attributed to the colonic metabolites of malvidin-3-O-glucoside and other anthocyanins, which along with procyanidins are major components in many red wines. Forester and Waterhouse (144) tested the anthocyanin microbial catabolites gallic acid, 3-O-methyl-gallic acid, protocatechuic acid, vanillic acid (3-methoxy-4-hydroxybenzoic acid), syringic acid (3,5-dimethoxy-4-hydroxybenzoic acid), and 2,4,6-trihydroxybenzaldehyde for their ability to induce apoptosis and inhibit cell proliferation in a colon cancer model using Caco-2 cells. Gallic acid, 3-O-methyl-gallic acid, and 2,4,6-trihydroxybenzaldehyde all reduced cell proliferation without being cytotoxic and were more effective than the parent anthocyanins. The catabolites degraded rapidly in the culture medium, leaving open the possible involvement of subsequent breakdown products in the reduction in cell viability.
Russell et al. (396) compared the anti-inflammatory properties of blueberry microbial catabolites, mostly derivatives of benzoic acid, phenylacetic acid, and phenylpropionic acid, obtained by fermenting a blueberry extract with fecal slurries from two volunteers. This was done by assessing the ability of the catabolites to modulate prostanoid production in a cell system in which the inflammatory pathways were upregulated by application of a cytokine-induced stimulation. After interleukin-1 beta (IL-1β) treatment of these cells, the catabolites generated by the fecal material from volunteer 1 decreased the amount of prostanoids produced, whereas the aromatic compounds produced by slurries from volunteer 2 had the opposite effect and increased prostanoid production. Although the number of volunteers used in this study makes any conclusions extremely tentative, the results suggest that the anti-inflammatory effect of blueberry (poly)phenolics in the colon may be dependent upon microbial catabolism which, as noted reviously, can vary somewhat as a consequence of person-to-person diversity in the composition of the colonic microbiota.
B. Chlorogenic acids
Colonic metabolites of chlorogenic acids such as m-coumaric acid and dihydroferulic acid [3-(3′-methoxy-4′-hydroxyphenyl)propionic acid] showed high antioxidant activity in a study carried out by Gómez-Ruiz et al. (155). However, despite these antioxidant properties, they were not able to protect human low-density lipoprotein (LDL) oxidation induced by copper and 2,2′-azobis(2-amidinopropane) dihydrochloride.
Platelet activation and subsequent aggregation play a major role in the pathogenesis of thromboembolic diseases such as myocardial infarction and ischemic heart disease. Hyperactive platelets with a high predisposition for activation are known to be apparent in conditions such as diabetes and heart disease (197). Dihydroferulic acid and 3-(3′-hydroxyphenyl)propionic acid, chlorogenic acid, and anthocyanin colonic catabolites (371, 436) partly reversed hyper-reactivity of platelets induced by oxidative stress, and could also counteract the negative effects of hormonal stress-induced platelet hyper-reactivity (370).
The results regarding the other main chlorogenic acid catabolite, dihydrocaffeic acid [3-(3′, 4′-dihydroxyphenyl)propionic acid], suggested that this compound is able to scavenge intracellular ROS (215). In the same study, dihydrocaffeic acid also increased nitric oxide (NO) synthase activity in a dose-dependent manner in cultured cells, with a comparable increase in endothelial NO synthase protein. Although the concentrations of the hydroxycinnamate required to achieve this effect were higher (>50 μM) than those observed in plasma after ingestion of a cup of coffee (436), these results do indicate that dihydrocaffeic acid may function as an intracellular antioxidant.
The antioxidant character of dihydrocaffeic acid was confirmed in another study in which it reduced cytotoxicity and proinflammatory cytokine production (IL-6 and IL-8) in HaCaT cells, a keratinocyte model, after UV radiation. However, these effects may be attributed to direct radical scavenging of the ROS formed, and to reinforcement of the internal antioxidant defenses of the keratinocytes, as well as to a direct interference with the pathway involved in cytokine stimulation (358).
The fact that dihydrocaffeic acid is not just an antioxidant was convincingly established by Miene et al. (307), who demonstrated that it was able to upregulate glutathione S-transferase T2 (GSTT2) and decrease cyclooxygenase-2 (COX-2) expression in human adenoma cells LT97 at concentrations ranging from 5 to 25 μM. Cumene hydroperoxide-induced DNA damage was significantly reduced by the catabolite. It was proposed that an upregulation of GSTT2 with a concomitant downregulation of COX-2 may contribute to the chemopreventive potential of (poly)phenols after degradation in the distal GIT.
Recently, chlorogenic acid-derived catabolites (dihydrocaffeic acid, dihydroferulic acid, and feruloylglycine) (436) (Fig. 34), when tested in combination at physiological concentrations, were shown to be effective in protecting human neuronal cells from oxidative insults in an in vitro experimental model (476). However, despite the fact that the induced cellular stress was of oxidative origin (2 μM 2,3-dimethoxy-1,4-naphthoquinone), the combination of chlorogenic acid-derived catabolites was the most effective, and independent of the antioxidant activity of the individual components, indicating that the protective mechanisms should not be attributed to the mere ability to prevent oxidation. Another ferulic acid catabolite, ferulaldehyde (aka coniferaldehyde) (Fig. 37), had been previously tested in a murine lipopolysaccharide (LPS)-induced septic shock model (367). It was found that intraperitoneally administered ferulaldehyde (6 mg/kg every 12 h) prolonged the lifespan of LPS-treated mice, decreased the inflammatory response, decreased early proinflammatory cytokines (TNF-α and IL-1β), increased anti-inflammatory IL-10 levels in serum, and inhibited LPS-induced activation of NF-κB in the liver of the mice.
FIG. 37.
Ferulaldehyde.
C. Ellagitannins
There is a rapidly growing body of literature describing the biological activities of ellagitannin-derived urolithins. The antioxidant activity of seven urolithins was evaluated in a cell-based assay (31), and the antioxidant activity was correlated with the number of hydroxyl groups and the lipophilicity of the urolithin molecule. The most potent antioxidants were urolithins D and C, with four and three hydroxyl groups, respectively, whereas the dihydroxylated urolithin A exhibited only weak activity. However, all these urolithins possessed less antioxidant activity than their parent molecules, ellagic acid and punicalagins. Contrary to what was observed in this study, Ito (217) reported that urolithins exhibited more potent antioxidant activities in the oxygen-radical absorbance capacity (ORAC) assay than intact ellagitannins, with urolithin A being among the most active.
However, as discussed with other components, antioxidant properties are not central and other more specific and biologically relevant activities are beginning to emerge. In a human colon cancer cell line, urolithin A and urolithin B, at concentrations achievable in the lumen from a dietary intake, influenced the expression levels of both signaling genes, such as growth factor receptors, oncogenes, and tumour suppressors, and genes involved in the cell cycle. These effects can be linked to cancer prevention in epithelial cells lining the colon (161). In a similar cell model, urolithins A and B induced the expression and activity of phase I and II enzymes, including cytochrome P450 mono-oxygenase 1A1 and UGT 1A10, and inhibited several SULTs (160). In a well-known rat model of inflammatory bowel disease, urolithins A and B reduced a number of inflammation markers (i.e., inducible NO synthase, COX-2, prostaglandin E [PGE] synthase, and PGE2 in colonic mucosa), favorably modulated the gut microbiota, preserved colonic architecture, but did not decrease oxidative stress in plasma and colon mucosa (271). Interestingly, in the same study, the formation of urolithins from punicalagin in rats fed a pomegranate extract was prevented by inflammation, suggesting that urolithin A, in particular, could be an active anti-inflammatory compound in healthy subjects, but that other molecules, arguably punicalagin, may be responsible for anti-inflammatory activity when a pathological condition appears. In a subsequent study with colon fibroblasts (163), urolithin A and B (10 μM) inhibited prostaglandin E2 production after IL-1β stimulation. Urolithin A, but not urolithin B, downregulated COX-2 and microsomal PGE synthase-1 (mPGES-1) mRNA expression and protein levels. Both urolithins inhibited NF-κB translocation to the nucleus. Slight, but significant, effects were found in the activation of MAPK pathways. Urolithin A lowered JNK phosphorylation state, and both urolithins inhibited p38 activation. Very interestingly, no urolithin-derived metabolites were found in the cell incubation medium, and only traces of urolithins were found inside the cells.
The current results, therefore, suggest that urolithins, the dihydroxy urolithin A in particular, have anti-inflammatory properties, and that the mode of action involves inhibition of NF-κB and MAPK activation, downregulation of COX-2 and mPGES-1 expression, and consequently, a reduction of PGE2 production. It must be pointed out that as urolithins were not able to efficiently enter the cells, interference for IL-1β membrane receptors cannot be excluded.
This anti-inflammatory ability of ellagitannin metabolites could be translated into a relevant cancer preventive effect, at least in the environment of the large intestine, where the concentration of these compounds is comparable to those applied in experimental in vitro models. To corroborate this hypothesis, in vitro urolithins were shown to inhibit 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1-mediated ethoxyresorufin-O-de-ethylase activity with IC50 values ranging from 57 μM for urolithin A to 75 μM for urolithin C, which may be reasonably achievable concentrations in physiological conditions in the colon. These compounds, applied at similar concentrations, exhibited dose- and time-dependent effects in decreasing cell proliferation and the clonogenic efficiency of HT-29 cells. Inhibition of cell proliferation was mediated through cell cycle arrest at the S phase, followed by the induction of apoptosis (241). These findings indicate that the urolithins released in the colon after consumption of ellagitannin-rich foods, such as walnuts, raspberries, or pomegranate juice (269), can potentially curtail the risk of colon cancer development by inhibiting cell proliferation and inducing apoptosis.
Another study investigated the effects of urolithins on Wnt signaling in a human 293T cell line using a luciferase reporter of canonical Wnt pathway-mediated transcriptional activation (414). Investigating this pathway is extremely relevant as Wnt proteins are a family of highly conserved, secreted signaling molecules playing a pivotal role in cellular development and carcinogenesis, with a large percentage (∼90%) of colon cancers arising from activating mutations in the Wnt pathway (250). Urolithin A was able to inhibit Wnt signaling at physiological intestinal concentrations, suggesting that ellagitannin-rich foods have the potential to prevent or counteract colon carcinogenesis through this novel mechanism.
Urolithin activity has also been investigated in the context of other forms of cancer. Seeram et al. (406) noted that urolithins accumulated in the prostate tissue of C57BL/6 wild-type male mice fed a pomegranate extract and demonstrated that urolithins were able to inhibit the growth of human prostate cancer cells in vitro. Subsequently, Kasimsetty et al. (241) reported that urolithin C, 8-O-methylurolithin A, and 8,9-di-O-methylurolithin C caused potent inhibition of CYP1-mediated ethoxyresorufin-O-de-ethylase activity also in 22Rv1 prostate carcinoma cells after a 24-h incubation at micromolar concentrations. Neutral red uptake assay results indicated that the same three metabolites induced profound cytotoxicity in the proximity of their CYP1 inhibitory IC50 values. Moreover, urolithins interfered with the expression of CYP1B1 protein, an established target in prostate cancer chemoprevention. Thus, urolithins were found to display a dual mode mechanism of cancer chemopreventive activity, by decreasing CYP1B1 activity and expression.
Urolithins A and B together with their acetylated, methylated, and sulfated analogs were examined for their ability to inhibit aromatase activity and testosterone-induced breast cancer cell proliferation at concentrations in the low micromolar range (1). Urolithin B was the most effective inhibitor of aromatase activity in a live cell assay. Kinetic analysis of urolithin B showed mixed inhibition, suggesting more than one inhibitory mechanism. Urolithin B was also the most effective in inhibiting testosterone-induced MCF-7aro cell proliferation, whereas the other urolithins were effective to a much lesser degree. These observations suggest that ellagitannin-derived urolithin B has potential for the prevention of estrogen-responsive breast cancers. In fact, both urolithins A and B had been previously shown to exert estrogenic activity in a dose-dependent manner, even at high concentrations, and without antiproliferative or toxic effects in estrogen-sensitive human breast cancer MCF-7 cells, whereas common phytoestrogens such as isoflavones are reported to inhibit cell proliferation only at high concentrations (270). Overall, urolithins exhibited weaker estrogenic activity than other well-known phytoestrogens, but displayed a slightly higher antiestrogenic activity.
In the previously cited antiglycative experimental model, urolithins A and B, together with pyrogallol (1,2,3-trihydroxybenzene), inhibited protein glycation by 37% and 44% at 1 and 2 μM, respectively, showing a potential effect in protecting circulating proteins during hyperglycemic conditions (476). Table 11 summarizes the mechanisms of action exerted by ellagitannin metabolites in models related to cancer and inflammation.
Table 11.
Mechanisms of Action Exerted by Ellagitannin Metabolites in Models Related to Cancer and Inflammation
| Metabolite | Mechanisms | Model/cell line | References |
|---|---|---|---|
| Urolithin A | PGE2 ↓, COX-2−, NF-κB ↓, JNK ↓ | Colon fibroblast, | 163 |
| Urolithin B | PGE2 ↓, COX-2 =, NF-κB ↓, JNK = | Colon fibroblast | 163 |
| Ellagic acid | PGE2 =, COX-2 =, NF-κB =, JNK = | Colon fibroblast | 163 |
| Urolithins A and B+ellagic acid | Cell cycle arrest, FGFR2-, DUSP6+, c-Myc+, | Caco-2, microarray/RT-PCR, WB | 160, 161 |
| Urolithin A | Normal cell cycle, FGFR2−, DUSP6+, c-Myc+, CYP1A1+, UGT1A10+ | Caco-2, microarray/RT-PCR; WB | 160, 161 |
| Urolithin B | Normal cell cycle, FGFR2−, DUSP6+, c-Myc+, CYP1A1+, UGT1A10+ | Caco-2, microarray/RT-PCR, WB | 160, 161 |
| Ellagic acid | Normal cell cycle, FGFR2−, DUSP6+, c-Myc+, CYP1A1+, UGT1A10+ | Caco-2, microarray/RT-PCR, WB | 160, 161 |
| Urolithin A | COX-2−, iNOS−, PTGES−, PGE2 ↓ | Rat inflammatory bowel disease, WB | 271 |
↓, signaling/effect inhibition; =, no significant change; +, expression induction (upregulation), −, expression inhibition (downregulation); c-Myc, v-myc myelocytomatosis viral oncogene homolog (avian); COX-2, cyclooxygenase-2; CYP1B1, cytochrome p450 1B1; DUSP-6, dual-specificity phosphatase 6; FGFR2, fibroblast growth factor receptor 2; iNOS, inducible nitric oxide synthases; JNK, c-Jun N-terminal kinase; PTGES, prostaglandin E synthase; Uro A/B, urolithin A/B; VEGF, vascular endothelial growth factor; WB, western blot.
The Eph–ephrin system coordinates the physiology and homeostasis of many adult organs, and therefore, an imbalance of Eph–ephrin function may contribute to a variety of diseases, including neurodegeneration, diabetes, CVDs, and cancer (347). It has recently been demonstrated that pyrogallol is able to functionally interfere with the EphA2–ephrinA1 system. The concentrations used were relatively high, but considering the very high levels that could be reached in the colon after consumption of a wide array of different (poly)phenol-containing foods, interactions with colonic Eph–ephrin protein in vivo are feasible (457) (Fig. 38).
FIG. 38.
The Eph–ephrin system regulates cell adhesion, shape and movements, cell proliferation, survival, differentiation, and secretion and plays a role in embryogenesis, neural development, plasticity and regeneration, angiogenesis, bone remodeling, insulin secretion, intestinal homeostasis, cancer suppression, and/or promotion (347). Pyrogallol (1,2,3-trihydroxybenzene), a common colonic metabolite of several (poly)phenolic compounds, has been reported as able to perturb the protein–protein interactions in a dose-dependent way (457). (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars.)
D. Flavan-3-ols
Once again, antioxidant activity of the main metabolites of flavan-3-ols has been tested in several studies. Starting with the phase II human metabolites, the 3′-O- and 4′-O-methyl derivatives of (+)-catechin and (−)-epicatechin, have been characterized, and their antioxidant activity was evaluated and compared to those of the corresponding quercetin metabolites. The antioxidant activity was assessed using the ferric-reducing antioxidant power (FRAP) assay and two methods based on the ability to scavenge the ABTS•+ radical cation at different pH values (121). The O-methylation at the B-ring level resulted in reduced antioxidant activity with respect to the parent compounds. However, the methylated metabolites still retained significant radical-scavenging activity at pH 7.4, suggesting that they could act as potential antioxidants under physiological conditions. The highest antioxidant activity was that of 3′-O-methyl-catechin at pH 7.4, comparable to those of its parent compound and the quercetin metabolites. In a more recent study, (−)-epigallocatechin-3-O-gallate exhibited antioxidant activity more than five times higher than that of Trolox, whereas that of 4′,4′′-di-O-methyl-epigallocatechin-3-O-gallate was similar to Trolox. This observation demonstrates that dimethylation of (−)-epigallocatechin-3-O-gallate results in a dramatic loss of its reducing activity (285). Applying the 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant assay, it has also been reported that epigallocatechin-3-O-gallate-3′-O-glucuronide and epigallocatechin-3-O-gallate-3-O-glucuronide have an antioxidant capacity similar to that of (−)-epigallocatechin-3-O-gallate. Epigallocatechin-3-O-gallate-4”-O-glucuronide, epigallocatechin-3-O-gallate-7-O-glucuronide, and epigallocatechin-7-O-glucuronide were less active than their parent compounds, and epigallocatechin-3′-O-glucuronide was much less active than (−)-epigallocatechin (286).
Regarding flavan-3-ol colon-derived catabolites, the antioxidant activity of 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (M6), 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone (M6′), 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4), and 5-(3′-hydroxyphenyl)-γ-valerolactone (M7) was calculated by Takagaki et al. (452). The order of their antioxidant activity in this study was M4>M6′>M6>M7.
As already discussed, the antioxidant activity of (poly)phenol metabolites, even when they retain the activity of their unmetabolized parent compounds, is not necessarily the most relevant feature of their putative biological activity. Koga and Meydani (251) obtained plasma extracts of flavonoid metabolites prepared after intragastric administration of pure compounds to rats. The extracts contained a mixture of sulfate, glucuronide, and methyl metabolites. The investigators measured the adhesion of U937 monocytic cells to human aortic endothelial cells and the production of ROS in these cells when they were pretreated with either pure compounds or plasma extracts from control or treated rats. The data obtained demonstrated that pretreatment of endothelial cells with methylated (+)-catechin metabolites inhibited U937 cell adhesion, whereas (+)-catechin had no effect. Generation of ROS in hydrogen peroxide-stimulated endothelium was inhibited by (+)-catechin, methyl-catechin, and,unexpectedly, the control plasma extract, whereas ROS generation in IL-1β-stimulated endothelial cells was inhibited only by methylcatechin. The merit of this 2001 report is that it was one of the first to point out that the metabolites of flavonoids, rather than their parent compounds, may contribute to the epidemiologically reported effects of flavonoids in terms of reducing the risk of CVD. However, the approach used and the tested metabolites are far from mimicking human physiology, as methylcatechin is not a common circulating metabolite in humans, and applying plasma extracts to cell cultures does not facilitate distinguishing between the molecules that exert a biological effect.
Methylated flavan-3-ols were reinvestigated in a more recent study that addressed the effects of (−)-epicatechin and some of its phase II human metabolites on NADPH oxidase activity in intact human umbilical vein endothelial cells (HUVECs) and in cell lysates (442). The parent flavan-3-ol proved to be an O2•+-scavenger, but did not inhibit NADPH oxidase activity, whereas the opposite was observed for 3′- and 4′-O-methyl-(−)-epicatechin. In contrast, the dimer procyanidin B2 and an epicatechin-O-glucuronide, in which the position of the glucuronide moiety was not stated, were both O2•+-scavengers and inhibited NADPH oxidase.
Lu et al. (286) reported that epicatechin metabolites isolated from human and rat urine inhibited peroxynitrite-mediated tyrosine nitration, with the following order of potency: (−)-epicatechin-3′-O-glucuronide >(−)-epicatechin >(−)-epicatechin-7-O-glucuronide=3′-O-methyl-(−)-epicatechin-7-O-glucuronide=4′-O-methyl-(−)-epicatechin-3′-O-glucuronide. These results demonstrate that the metabolites of (−)-epicatechin can prevent peroxynitrite-induced physiological damages, to some extent, as oxidation of tyrosine residues, which is associated with the nitration of proteins, is of particular interest in pathologies related to inflammation. The relevance of these metabolites in the context of inflammation has been confirmed in other studies. At concentrations of 2 and 10 μM, which can be considered physiologically relevant, several flavan-3-ol glucuronides inhibited the release of arachidonic acid and its metabolites from HT-29 cells (286). At both concentrations, epigallocatechin-3′-O-glucuronide was less effective than epigallocatechin-7-O-glucuronide, which had comparable activity to (−)-epigallocatechin. Ascorbic acid had no effect on arachidonic acid release at concentrations as high as 50 μM, suggesting a mechanism that does not involve antioxidant activity. In a more recent study (285), 4′,4′′-di-O-methyl-epigallocatechin-3-O-gallate selectively inhibited ICAM-1 expression with an IC50 value of 94 μM, whereas (−)-epigallocatechin-3-O-gallate and 4”-O-methyl-epigallocatechin-3-O-gallate had no effect. The inhibitory effect of 4′,4′′-di-O-methyl-epigallocatechin-3-O-gallate on adhesion molecule expression was not related to either its antioxidant-reducing capacity or an inhibition of NF-κB activation.
A few studies have investigated the biological activity of the microbial flavan-3-ol catabolites, the valerolactones. Two, M6 and its methylated analog 5-(3′-methoxy-4′-hydroxyphenyl)-γ-valerolactone, are catechin catabolites identified in human urine after the consumption of pine bark extract. These two molecules exhibited strong inhibitory activity toward metalloproteinases (MMP) 1, 2, and 9. Moreover, at the cellular level, 0.5 μM concentrations of both valerolactones resulted in a ∼50% inhibition of MMP-9 secretion. M6 was more effective in superoxide scavenging than (+)-catechin, ascorbic acid, and Trolox, whereas its 3′-methylated analog lacked scavenging activity (170). In another study, M4, M6, and their methoxy-derivatives were assessed for their ability to inhibit the growth of a panel of immortalized and malignant human cell lines using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. M4 was the most active catabolite tested on human esophageal squamous cell carcinoma cells (KYSE150), human colon adenocarcinoma cells (HT-29 and HCT-116), immortalized human intestinal epithelial cells (INT-407), and an immortalized rat intestinal epithelial cell line (IEC-6) (266). This valerolactone was also able to inhibit the production of NO by LPS-stimulated murine macrophages (RAW264.7). The growth inhibitory activity of M4 against immortalized and cancer cell lines allows us to hypothesize that this catabolite may contribute to the cancer preventive effect of green tea observed in epidemiological studies. The peculiar sensitivity of INT-407 cells relative to the other cell lines may indicate that the trihydroxyvalerolactone has some selectivity for premalignant cells, but this remains to be more thoroughly investigated. Moreover, the ability of M4 to inhibit the production of LPS-stimulated macrophages may indicate that it can contribute to the anti-inflammatory activities of green tea.
Although the valerolactone metabolites of flavan-3-ols have been found in human internal compartments at levels that vastly exceed those of many other flavonoid metabolites (277), there are relative few reports on their biological activities in in vitro test systems. This is because standards cannot be obtained from commercial sources. However, chemical procedures for their synthesis, and that of their sulfated and glucuronide metabolites, are available (25), so information on their mode of action is likely to be forthcoming in the near future.
E. Flavonols
Flavonols, quercetin in particular, are a notable exception to what has been written in this review, as studies dealing with the bioactivity of their human metabolites date back more than 10 years ago.
Quercetin metabolites retain a part of the antioxidant activity of their parent aglycone, and this has been demonstrated in several different experimental models. Indirectly, plasma obtained from rats fed quercetin was more resistant to copper sulfate-induced lipid peroxidation than the control plasma on the basis of the accumulation of hydroperoxides and the degradation of α-tocopherol. Based on the fact that quercetin is recovered in rat blood plasma principally as sulfate and glucuronide conjugates, these observations indicate that at least some metabolites of quercetin retain the ability to act as effective antioxidants (98). To clarify the antioxidant properties of one of the main phase II metabolites of quercetin in a cellular environment, the ability of quercetin-3-O-glucuronide (Fig. 15) to inhibit the production of H2O2-induced ROS in mouse 3T3-cultured fibroblasts was evaluated (418). When the cells were exposed to H2O2 in the presence of quercetin or its glucuronide, the metabolite was less active than the aglycone. In contrast, when fibroblasts were pretreated with the flavonols before exposure to H2O2, the glucuronide, but not the aglycone, inhibited the production of ROS. In a different model of oxidation, both 3′-methylation and 3-glucuronidation significantly reduced the ability of quercetin to protect against radical-induced [2,2′-azobis(2-methylpropionamidine)dihydrochloride] oxidation (283). In contrast, however, methylated quercetin metabolites (namely 3′-O- and 4′-O-methyl-quercetin) retained some antioxidant activity when tested in the FRAP and the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays (121). In another study, 3′-O-methylquercetin and quercetin-3′-O-sulfate were shown to have lower reducing activity than quercetin in the FRAP assay (285).
The inhibitory effect of quercetin-3-O-glucuronide on lipid peroxidation has been investigated using phosphatidylcholine large unilamellar vesicles as a plasma membrane model. The glucuronide inhibited lipid peroxidation generated via different initiators, but less effectively than quercetin. Ultrafiltration of phosphatidylcholine large unilamellar vesicles revealed that the metabolite exhibited a low, but significant, affinity for the membranes of phospholipid bilayers, and therefore may be able to act as an efficient antioxidant in cell membranes (417).
Quercetin colonic catabolites have also been evaluated for their antioxidant activity. When measured with the DPPH assay, 3′,4′-dihydroxyphenylacetic acid exerted a concentration-dependent scavenging effect in the range of 1–30 μM, whereas 4′-hydroxyphenylacetic acid was almost ineffective up to 500 μM (153). It has also been shown that 3′,4′-dihydroxyphenylacetic acid, 3,4-dihydroxybenzoic acid, and taxifolin exhibit antioxidant activity in the FRAP assay, but no reaction was observed with 3′-hydroxyphenylacetic acid, 4-hydroxybenzoic acid, and 3-(3′-hydroxyphenyl)propionic acid (221). In part, this contrasts with the findings of Pavlica and Gebhardt (349), who reported that the lipophilic metabolite 3,4-dihydroxytoluene exhibited strong antilipoperoxidation activity, whereas the hydrophilic metabolite 3′,4′-dihydroxyphenylacetic acid was less effective against oxidative stress induced in PC12 cells by peroxides and iron.
In a pioneering study, Day et al. (103) reported on the anti-inflammatory activity, measured as the ability to inhibit xanthine oxidase and lipoxygenase, of several quercetin glucuronides. The Ki for the inhibition of xanthine oxidase by the glucuronide metabolites was as follows: 4′>3′>7>3, with quercetin-4′-O-glucuronide being a particularly potent inhibitor (Ki=0.25 μM).
The anti-inflammatory activity of quercetin metabolites has subsequently been investigated by other groups with a particular attention being paid to the vascular system as a promising target of these molecules. It has, for instance, been shown that 3′-O-methyl-quercetin and 4′-O-methyl-quercetin inhibit the expression of ICAM-1 at physiological concentrations in human aortic endothelial cells. E-selectin expression was also suppressed by both metabolites to a somewhat lesser extent, whereas VCAM-1 was unaffected. In contrast, quercetin-3-O-glucuronide, quercetin-3′-O-sulfate, and phenolic acid catabolites of quercetin were unable to inhibit adhesion molecule expression (285). This is in partial agreement with a previous study in which quercetin-3′-O-sulfate, quercetin-3-O-glucuronide, and isorhamnetin 3-O-glucuronide either exhibited a reduced ability to inhibit the expression of these molecules compared with the parent aglycone or had no effect. However, all three metabolites inhibited VCAM-1 cell surface expression at a concentration of 2 μM (459). These results, taken together, indicate that quercetin metabolites, at physiological concentrations, can inhibit the expression of key molecules involved in monocyte recruitment during the early stages of atherosclerosis. However, in a study on human umbilical artery smooth muscle cells activated by TNF-α, these observations were not confirmed; quercetin-3′-O-sulfate, quercetin-3-O-glucuronide, and isorhamnetin-3-O-glucuronide had no effect on either TNFα-induced upregulation of adhesion molecules (VCAM-1 and ICAM-1) or chemokine expression (monocyte chemotactic protein-1 [MCP-1]) (496).
These studies suggest that the vascular anti-inflammatory effects of quercetin consumption are mediated through effects on endothelial cells, but not smooth muscle cells. Moreover, quercetin-3′-O-sulfate and quercetin-3-O-glucuronide exert anti-inflammatory effects on the vasculature, possibly through a mechanism involving inhibition of NFκB, by preventing LPS-induced changes in vascular responses in porcine coronary artery segments at physiological concentrations (5). It has also been reported that quercetin and isorhamnetin, but not quercetin-3-O-glucuronide, decrease mRNA and protein levels of TNF-α in murine RAW264.7 macrophages stimulated with LPS. Furthermore, no significant decreases in the mRNA levels of IL-1β, IL-6, macrophage inflammatory protein-1α, and inducible NO synthase or proinflammatory microRNA-155 were evident in response to quercetin glucuronide treatment (36). It can be concluded that the anti-inflammatory effectiveness of quercetin phase II metabolites is probably strongly linked to specific tissues and specific conditions, and that further research is needed to clarify these inconsistencies.
Modulation of endothelial function by quercetin metabolites, which has a strong connection with inflammation, has also been investigated. In a study involving thoracic aortic rings isolated from Wistar rats, quercetin-3-O-glucuronide, isorhamnetin-3-O-glucuronide, and quercetin-3′-O-sulfate had no direct vasorelaxant effects, nor did they modify endothelial function or the biological activity of NO. However, all metabolites, at marginally supraphysiological concentrations, partially prevented the impairment of the endothelial-derived NO response under conditions of high oxidative stress induced by diethyldithiocarbamic acid, a superoxide dismutase inhibitor, and protected the biological activity of exogenous NO. Quercetin-3′-O-sulfate and quercetin-3-O-glucuronide inhibited NADPH oxidase-derived O2 release, but this occurred only at very high concentrations. Quercetin-3-O-glucuronide was able to prevent the endothelial dysfunction induced by incubation with endothelin-1 (282). Subsequently, quercetin phase II conjugates were tested on vasoconstrictor and vasodilator responses in the porcine isolated coronary artery (451). Quercetin-3′-O-sulfate inhibited receptor-mediated contractions of the porcine isolated coronary artery by an endothelium-independent action and was able to counteract glyceryl trinitrate-induced tolerance in vitro, which may be beneficial for patients treated for angina pectoris.
Another study investigated the effect of quercetin sulfate and glucuronide metabolites on the prevention of high-glucose-induced apoptosis of HUVECs. HUVECs were exposed to high-glucose concentrations (33 mM) in the presence or absence of physiological concentrations of a mixture of sulfated and glucuronidated quercetin isolated from rat plasma. High-glucose-induced apoptosis was inhibited by both metabolites at nM concentrations in a dose-dependent manner, through a mechanism involving H2O2 quenching and the inhibition of JNK and caspase-3 activity (71).
Donnini et al. (117) observed opposing effects of quercetin metabolites on angiogenesis. While quercetin-3-O-glucuronide inhibited vascular endothelial growth factor (VEGF)-induced endothelial cell functions and angiogenesis, quercetin-3′-O-sulfate promoted endothelial cell proliferation and angiogenesis, and both were tested in the nM range. The inhibitory effect elicited by the glucuronide was associated with the inhibition of ERK1/2 phosphorylation elicited by VEGF. On the other hand, the activation of endothelial cells by the sulfate was associated with stimulation of VEGF receptor-2 and with downstream signaling activation (phosphatidylinositol-3 kinase/Akt and NO synthase pathways), ultimately responsible for ERK1/2 phosphorylation. This observation indicates how quercetin metabolism in vivo can drastically change its bioactivity, shifting it from one effect to the other.
The effects of quercetin phase II metabolites on lung cancer cells have also been investigated. Yang et al. (506) demonstrated that quercetin glucuronides (not fully identified, as they were prepared from the serum of quercetin-fed rabbits) were able to inhibit proliferation through G2/M arrest of the cell cycle and induce apoptosis via the caspase-3 cascade in the human lung cancer cell line NCI-H209. These results were partly confirmed by Yeh et al. (507), who investigated the effects of quercetin metabolite-enriched plasma (QMP, obtained from Mongolian gerbils 2 h after quercetin feeding) on the growth of A549 lung cancer cells and the possible mechanisms of these effects. QMP, but not control plasma, reduced cell growth and led to cell cycle arrest at the G2/M phase by downregulating the expression of cdk1 and cyclin B. QMP, but not control plasma or quercetin aglycone, significantly increased peroxisome proliferator-activated receptor gamma (PPAR-γ) expression, which was accompanied by an increase in phosphatase and tensin homolog deleted on chromosome 10 and a decrease in the phosphorylation of Akt. Moreover, quercetin-3-O-glucuronide and quercetin-3′-O-sulfate also significantly increased PPAR-γ expression in these cultured lung cancer cells. Finally, GW9662, a PPAR-γ antagonist, significantly suppressed the effects of QMP on cell proliferation and on the expression of cyclin B and cdk1. Taken together, these data suggest that the interaction with PPAR-γ could play a paramount role in the antiproliferative effects of quercetin metabolites.
The hypothesis of Terao and colleagues that glucuronide conjugates of quercetin are bioactive agents against ROS, but only as precursors of hydrophobic aglycones (216, 243), merits comment. Quercetin aglycone was predicted to be generated at the target site (i.e., the cardiovascular system or central nervous system) by the action of β-glucuronidase activity, under oxidative stress or inflammatory conditions, on quercetin-O-glucuronide metabolites. This hypothesis goes some way to restoring the reputation of at least some of the numerous studies performed with aglycones such as quercetin. However, as yet, the generation of quercetin has not been firmly established, and the presence of its glucuronide metabolites at the extracellular level would still require that all in vitro tests be performed with these molecules.
One of the main colonic catabolites of quercetin, 3′,4′-dihydroxyphenylacetic acid (Fig. 16), has been found to possess significant reducing power and free-radical scavenging activity (221). However, the same molecule has only limited activity in the prevention of cell death, glutathione depletion, lipid peroxidation, and production of ROS in differentiated PC12 cells, used as a model system of neuronal cells (349). This finding was confirmed by Verzelloni et al. (476), who observed a negligible effect of 3′,4′-dihydroxyphenylacetic acid, at physiological concentrations, on neuroblastoma cell protection against oxidative stress. This study also tested antiglycative activity and found that 3′,4′-dihydroxyphenylacetic acid was almost ineffective in preventing serum albumin glycation. However, this observation is in contrast to the results of Pashikanti et al. (346), who found that the phenolic acid catabolite was a powerful inhibitor of Nɛ-carboxymethyl lysine and Nɛ-carboxymethyl lysine–histone H1 adduct formation and ADP-ribose histone H1 glycation. Finally, Miene et al. (307) demonstrated that 3′,4′-dihydroxyphenylacetic acid was, like 3-(3′,4′-dihydroxyphenyl)propionic acid (dihydrocaffeic acid), able to act as an anti-inflammatory agent, possibly contributing to the chemopreventive potential of (poly)phenol catabolites in the gut.
F. Lignans and isoflavones
Phytoestrogens, plant-derived compounds structurally similar to estrogen, may have both agonistic and antagonistic actions toward estrogen receptors (460). There are two major classes of phytoestrogens: lignans, which occur in high amounts in flaxseeds (Fig. 5), and isoflavones, found in high concentrations in soy with the 7-O-glucoside and 7-O-(6′′-O-malonyl)glucoside of genistein and daidzein being the main components (Fig. 4) (see Section II.A.3).
After ingestion, plant lignans are converted by the intestinal microbiota in the upper part of the large bowel to enterolactone and enterodiol (Fig. 36), which are referred to as mammalian enterolignans (2). Chen et al. (73) reported that enterolactone, at μM concentrations, suppressed the growth of LNCaP prostate cancer cells by triggering apoptosis. The mechanism of action involved a dose-dependent loss of mitochondrial membrane potential, the release of cytochrome C, and cleavage of procaspase-3 and poly(ADP-ribose) polymerase. A series of other intracellular proteins (Akt, GSK-3B, MDM2, and p53) were shown to be involved in enterolactone-dependent apoptosis. To better understand how these estrogenic metabolites might influence breast cancer progression and to unravel their mechanisms of action, Carreau et al. (64) compared the ability of enterodiol and enterolactone to induce the transactivation of the two estrogen receptors (ERs), α and β, to modulate ERα target genes and MCF-7 breast cancer cell migration by acting on matrix MMPs at a concentration of 10 μM, which arguably is unlikely to be achieved via a lignan-rich diet. It was found that enterolignans have distinct abilities for the activation of ERα and ERβ and modulation of ERα mRNA and protein contents and cell proliferation. The immunomodulatory activity of enterolactone and enterodiol was also investigated by treating peripheral blood lymphocytes with increasing physiologically relevant concentrations of the two lignan metabolites after stimulus with LPS and anti-CD3 and anti-CD28 monoclonal antibodies (87). A dose-related inhibition of cell proliferation and cytokine production was observed, with enterolactone being the most effective metabolite. Additional experiments with THP-1 cells showed that both compounds prevented inhibitory-κB degradation and NF-κB activation, resulting in decreased TNF-α production.
After ingestion by humans, genistein and daidzein glycosides undergo a rapid degradation by the gut microbiota, giving rise to a wide range of metabolites, including dihydrodaidzein, dihydrogenistein, equol, and O-desmethylangolensin (15) (see Section II.D). Among these metabolites, equol has been investigated most extensively. It was reported to generate acute endothelium- and NO-dependent relaxation of aortic rings, a rapid, 2-min activation of endothelial nitric oxide synthase (eNOS) in human aortic and umbilical vein endothelial cells at physiological concentrations (1–100 nM) (232). The effects of equol on markers of immune function have been tested in human peripheral blood mononuclear cells. The evaluated variables included lytic activity of natural killer cells, proliferation, secretion of cytokines, and apoptosis/necrosis. In addition, the cells were treated with estrogenic antagonists to determine whether an estrogen receptor-mediated pathway was involved (169). It was shown that equol was a potent modulator of immune function, but the effect was evident only at high, nonphysiological concentrations (10–50 μM). Particularly noteworthy was the observation that the effects on cytokine secretion were not mediated by phytoestrogenic activity.
Li et al. (279) investigated the ability of equol to activate human and mouse pregnane X receptor (PXR), an inducer of drug metabolism. In human primary hepatocytes, equol was a potent activator of PXR, with consequent increase of CYP3A4 mRNA and immunoreactive protein expression, whereas genistein or daidzein was more effective on mouse PXR. Interestingly, these observations are consistent with species-specific differences in cytochrome P450-mediated drug and steroid metabolism.
Equol was also able to prevent oxidized LDL-stimulated apoptosis in HUVECs at a concentration of 1 μM, but was most effective at 10 μM. The mechanism of action was described as being related mainly to the control of superoxide and NO intracellular production (238). Endothelial function was also investigated by Rowlands et al. (394), who reported that equol-stimulated mitochondrial ROS modulate endothelial redox signaling and NO release, involving transactivation of epidermal growth factor receptor kinase and reorganization of the F-actin cytoskeleton, providing valuable insights for therapeutic strategies to restore endothelial function in CVD.
In another set of endothelial cells, human pulmonary artery endothelial cells, treatment with the HIV protease inhibitor ritonavir significantly reduced eNOS expression, and 1 μM equol effectively blocked this downregulation (74). In the same study, ritonavir significantly reduced endothelium-dependent relaxation in response to bradykinin in fresh porcine pulmonary artery rings, and equol was able to reverse this effect. The results of this study suggest that equol may have a clinical application in providing some prevention against HIV treatment-associated cardiovascular complications.
G. Flavanones
The interactions of a mixture of hesperetin 5-O- and 7-O-glucuronides with human fibroblasts and their implications for oxidative stress-induced cell death were examined by Proteggente et al. (362). The data obtained indicated that the hesperetin glucuronides, but not hesperetin, protect against UV-A-induced necrotic cell death. As it was recently shown that hesperetin may regulate primary rat osteoblast differentiation through bone morphogenetic protein signaling, the effect of hesperetin-7-O-glucuronide on primary rat osteoblast proliferation and differentiation was investigated by Trzeciakiewicz et al. (461). At a physiological concentration of 1 μM, the glucuronide did not affect proliferation, but enhanced differentiation by increasing alkaline phosphatase activity and mRNA expression. It also induced mRNA expression of Runx2 and Osterix, two transcription factors implicated in the regulation of osteoblast functions. Moreover, phosphorylation of Smad1/5/8 was enhanced by the glucuronide, whereas ERK 1/2 remained unchanged after 48 h, and receptor activator of nuclear factor κ-B ligand (RANKL) gene expression was significantly decreased. These results suggest that hesperetin-7-O-glucuronide may regulate osteoblast differentiation through specific transcription factor stimulation, and might in turn be implicated in the regulation of osteoblast/osteoclast communication.
Tables 12 and 13 represent the synthesis of the molecular actions exerted by (poly)phenolic metabolites in experimental models of CVDs and cancer, respectively. Figures 39 and 40 represent a graphical summary of the main mechanisms of action involved in the cardiovascular and cancer protective effects of some (poly)phenol metabolites, respectively.
Table 12.
Mechanisms of Action Exerted by (Poly)phenol Metabolites in Experimental Models Related to Cardiovascular Diseases
| Class | Metabolite | Mechanisms | Model | Reference |
|---|---|---|---|---|
| Anthocyanins | Protocatechuic acid | Monocyte adhesion ↓ | Mouse endothelial cells | 481 |
| VCAM1 ↓, ICAM1 ↓ | Apo-E mice | 481 | ||
| Chlorogenic acids | m-Coumaric acid, dihydroferulic acid | oxLDL = | Chemical LDL oxidation | 155 |
| Dihydroferulic acid, 3-(3′-hydroxyphenyl)propionic acid | Platelet reactivity ↓, P-selectin −, NOS ↑ | Platelets | 371 | |
| Flavan-3-ols | Mixture of plasma metabolites from rats | Monocyte adhesion ↓, ROS ↓ | Human aortic endothelial cells | 251 |
| 4′,4”-Di-O-methyl-EGCG | ICAM1 − | Human aortic endothelial cells | 285 | |
| Flavonols | 3′- and 4′-O-methyl-quercetin | ICAM1 −, E-selectin −, VCAM1 = | Human aortic endothelial cells | 285 |
| Quercetin-3′-O-sulfate, quercetin-3-O-glucuronide, isorhamnetin 3-O-glucuronide | ICAM1 =, VCAM 1 −, MCP1 = | HUVECs | 459 | |
| Quercetin-3′-O-sulfate, quercetin-3-O-glucuronide, isorhamnetin 3-O-glucuronide | VCAM1 =, ICAM1 =, MCP1 = | TNFα-activated human artery smooth muscle cells | 496 | |
| Quercetin-3′-O-sulfate, quercetin-3-O-glucuronide, isorhamnetin 3-O-glucuronide | Vasorelaxation =, NO =, NOS = | Rat thoracic aortic rings | 282 | |
| Lignans, isoflavones | Equol | Vasorelaxation ↑, NO ↑, eNOS ↑ | Human aortic rings, HUVECs | 232, 394 |
| Equol | PXR ↑, CYP3A4+ | Human primary hepatocytes | 279 | |
| Equol | eNOS+, relaxation | Ritonavir-induced eNOS downregulation and blockage of BK relaxation | 74 |
↑, signaling/effect activation; ↓, signaling/effect inhibition; =, no significant change; +, expression induction (upregulation); −, expression inhibition (downregulation); EGCG, (−)-epigallocatechin-3-O-gallate; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule 1; MCP-1, Monocyte chemotactic protein-1; NO, nitric oxide; NOS, nitric oxide synthase; oxLDL, oxidated low-density lipoprotein; PXR, pregnane X receptor; ROS, reactive oxygen species; TNF-α, tumour necrosis factor-α; VCAM-1, vascular cell adhesion molecule 1.
Table 13.
Mechanisms of Action Exerted by (Poly)phenol Metabolites in Experimental Models Related to Cancer
| Polyphenols | Metabolites | Mechanisms | Model/cell line | Reference |
|---|---|---|---|---|
| Anthocyanins | Protocatechuic acid | Apoptosis ↓, ROS ↓, caspases ↓ | Neural stem cells | 172 |
| Apoptosis ↑, JNK ↑, MAPK ↑ | Gastric adenocarcinoma | 280 | ||
| Proliferation ↓, JNK ↑, MAPK ↑ | HepG2 | 508 | ||
| ROS ↓, caspase3 ↓, Bcl2−, | PC12 | 173 | ||
| Various | Proliferation ↓ | Caco2 | 144 | |
| Ellagitannins | Urolithin A | Wnt ↓ | 293T | 414 |
| Urolithin A | Proliferation ↓ | LAPC-4 | 406 | |
| Various urolithins | EROD ↓, CYP1B1− | 22Rv1, prostate cancer | 241 | |
| Urolithin A and B | Aromatase ↓, proliferation ↓ | MCF7aro | 1 | |
| Pyrogallol | EphA2 ↓ | PC3 | 457 | |
| Flavan-3-ols | M4, M6 | Proliferation ↓ | Various cancer cells | 266 |
| Flavonols | Quercetin-3-O-glucuronide | VEGF ↓, ERK ↓ | Bovine coronary venular endothelial cells | 117 |
| Quercetin-3′-O-sulfate | VEGF ↑, ERK ↑ | Bovine coronary venular endothelial cells | 117 | |
| Quercetin-3′-O-sulfate Quercetin-3-O-glucuronide | Angiogenesis = | Rabbit cornea | 117 | |
| Lignans, isoflavones | Enterolactone | Proliferation ↓, apoptosis ↑ | LNCaP | 73 |
| Enterolactone | ERα +, proliferation = | MCF7 | 64 | |
| Enterodiol | ERα +, ERβ +, proliferation ↑ | MCF7 | 64 |
↑, signaling/effect activation; ↓, signaling/effect inhibition; =, no significant change; +expression induction (upregulation); −, expression inhibition (downregulation); Bbl2, B-cell lymphoma 2; ER, estrogen receptor; ERK, extracellular signal-regulated kinases; EROD, CYP1B1-mediated ethoxy resorufin-O-de-ethylase assay; M4, 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone; M6, 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone; MAPK, mitogen-activated protein kinase.
FIG. 39.
Graphical summary of some of the mechanisms involved in cardiovascular protection exerted by (poly)phenol metabolites. Protocatechuic acid (3,4-dihydroxybenzoic acid) impairs monocyte adhesion by inhibiting the nuclear content of p65, a subunit of NF-κB, and thus decreasing ICAM-1 and VCAM-1 expression. Moreover, equol induces eNOS expression by increasing NO production. eNOS, endothelial nitric oxide synthase; ICAM-1, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen-1; NF-κB, nuclear factor kappa-light-chain enhancer of activated B cells; NO, nitric oxide; PCA, protocatechuic acid; TNF-α, tumor necrosis factor-α; TNFR, TNF-α receptor. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars.)
FIG. 40.
Graphical summary of some of the mechanisms involved in pathways related to cancer. MAPK/ERK signaling is inhibited by quercetin-3-O-glucuronide and enhanced by PCA and quercetin-3′-O-sulfate. JNK activity is reduced by urolithin A and increased by protocatechuic acid. Pyrogallol (1,2,3-trihydroxybenzene) inhibits EphA2 kinase activation, interfering with ephrinA1 binding (see also Fig. 39). Arrows identify activation interactions, whereas truncated lines classify inhibition interactions. Dotted lines are used to simplify transduction pathways. ERK, extracellular signal-regulated kinases; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; RTK, receptor tyrosine kinase; VEGFR, vascular endothelial growth factor receptor. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars.)
VI. Feeding Studies and Evidence of Protective Effects
There is considerable epidemiological evidence indicating that the consumption of diets rich in fruit and vegetables is associated with a reduction in the risk of a number of chronic diseases, most notably CVD, specific cancers, and neurodegenerative diseases (24, 29, 54, 90, 99, 100, 137, 183, 231, 264, 276, 375). The beneficial effects of such diets have often been attributed to the (poly)phenols they contain. Indeed, associations have also been observed between the dietary intake of (poly)phenols and reductions in the risk of such chronic diseases (13, 83, 97, 193, 309). However, to confirm if such observations are genuine, and whether (poly)phenols are indeed responsible for them, well-powered and well-controlled human intervention trials are necessary.
In the last 5–10 years, numerous such trials have been conducted, although arguably, many are flawed, as they lack proper controls, do not provide a detailed compositional analysis of the foods that are being tested, and/or lack a relevant study population. For example, to make sound causal relationships between (poly)phenol intake and the clinical/physiological outcomes being assessed, a suitable control intervention is essential. A number of studies have strived to utilize such a control food/beverage, which is indistinguishable from the test food/beverage by means of taste and appearance, which is accurately matched for micro- and macronutrient composition, including other potential bioactive compounds.
In this section, we will present data relating principally to the role of (poly)phenols in human health, while considering the points raised above. In particular, we will focus on well-controlled, randomized human intervention studies examining the effects of (poly)phenol-rich foods/beverages against CVD, cancer, and neurodegenerative diseases, and/or clinically significant risk factors associated with such disorders.
A. Cardiovascular effects of dietary polyphenols
There have been a number of short-term, small-scale human intervention studies designed to test the effect of (poly)phenol-rich foods, and in particular those containing flavonoids, on well-characterized, medically significant CVD risk factors, including hypertension, endothelial dysfunction, lipid metabolism, and platelet activation. In this section, we report on human randomized controlled trials (RCTs), where at least one of these markers has been assessed (see Table 14). The following (poly)phenol-rich foods will be discussed: cocoa, red wine, berries, pomegranate, tea, coffee, citrus fruits, and nuts, although the amount and quality of the data vary.
Table 14.
Summary of Randomized Controlled Clinical Intervention Studies Investigating the Effect of Dietary Polyphenols on Human Vascular Function
| Food | (Poly)phenols per day | Study details | Effect | Reference |
|---|---|---|---|---|
| Cocoa | ||||
| Flavan-3-ol tablets | 234 mg CF | Parallel; 28 days; 32 healthy; HF vs. LF tablets | BP nc; p-selectin expression -; ADP, collagen-induced platelet aggregation- | 326 |
| Dark chocolate | 259 mg CF | Parallel; 28 days; 21 healthy; HF vs. LF dark chocolate | BP nc; FMD+1.3% 2 h after intake; Lipids nc | 131 |
| Dark chocolate | 168 mg CF | Crossover; 14 days; 56 healthy; dark vs. white chocolate | BP −5 mm Hg; −0.5 mM; LDL-C-0.5 Mm | 146 |
| Dark chocolate | ∼500 mg CP | Crossover; 15 days; 15 healthy; dark vs. white chocolate | SBP-6.4 mm Hg; HOMA-IR-0.78; QUICKI +0.042; lipids nc | 166 |
| Dark chocolate | 88 mg CF monomers | Crossover; 15 days; 20 hypertensive; dark vs. white chocolate | 24 h SBP-11.9 mm Hg; DBP-8.5 mm Hg; FMD+1.5%; TC-0.4 mM; LDL-C-0.4 mM; HOMA-IR-; QUICKI+, ISI+ | 168 |
| Dark chocolate | 1008 mg CP, 147 mg CF monomers | Crossover; 15 days; 19 IGT hypertensive; dark vs. white chocolate | 24 h SBP-4.52 mm Hg; DBP-4.17 mm Hg; FMD+∼1.3%; TC-6.5%; LDL-C−7.5%; HOMA-IR-; QUICKI+, SI+ | 165 |
| Dark chocolate | 30 mg CF | Crossover; 126 days; 44 hypertensive; dark vs. white chocolate | SBP-2.9 mm Hg; DBP-1.9 mm Hg; lipids nc | 453 |
| Dark chocolate+cocoa drink | 755 mg CF | Parallel; 42 days; 90 healthy; dark chocolate+cocoa drink vs. controls | BP nc; lipids nc | 89 |
| Cocoa drink | 900 mg CF | Crossover; 14 days; 40 hypertensive; high-flavan-3-ol vs. low-flavan-3-ol cocoa | BP nc; BAD+3.1%; lipids nc; QUICKI nc; SIclamp nc | 325 |
| Cocoa drink | 176 mg CF | Crossover; 1 day; 26 CVD or CAD; high-flavan-3-ol vs. low-flavan-3-ol cocoa | BP nc; FMD+2.9%, 2 h after intake | 187 |
| Cocoa drink | 176 mg CF | Crossover; 1 day; 11 smokers; high-flavan-3-ol vs. low-flavan-3-ol cocoa | BP nc; FMD+2.4%, 2 h after intake | 190 |
| Cocoa drink/epicatechin | 917 mg CF | Crossover; 1 day; 16 healthy; high-flavanol vs. low-flavan-3-ol cocoa or (−)-epicatechin vs. water | Cocoa: FMD+6% high flavan-3-ol 2 h after intake; (−)-epicatechin FMD+2%–3% 2 h after intake; PAT+ | 404 |
| 1–2 mg/kg Epi | ||||
| Cocoa drink | 3×306 mg CF;28–918 mg CF | Crossover; 7 days; 6 smokers; high-flavan-3-ol vs. low-flavan-3-ol cocoa crossover; 1 day; 11 smokers; 3 doses high-flavan-3-ol cocoa | BP nc; FMD+2.9% baseline day 1–8,+2.4% 2 h after intake; acute: FMD+4% 330 mg CF;+6% 918 mg CF 2 h after intake | 188 |
| Cocoa drink | 3×321 mg CF; 75–963 mg CF | Parallel; 30 days; 41 diabetics; high-flavan-3-ol vs. low-flavan-3-ol cocoa crossover; 1 day; 10 diabetics; 3 doses high-flavan-3-ol cocoa | BP nc; FMD+1% baseline day 1–30, +1.5% 2 h after intake; lipids nc acute: FMD+0.7% 371 mg CF; +1.7% 963 mg CF 2 h after intake | 22 |
| Cocoa drink | 750 mg CF | Crossover; 30 days; 16 CAD; high-flavan-3-ol vs. low-flavan-3-ol cocoa | SBP nc; DBP-4.2%; FMD+1.8% baseline day 1–30; Lipids nc | 189 |
| Dark chocolate | 46.8 mg CF monomers | Parallel; 15 days; 22 heart transplant recipients; dark chocolate vs. control | Coronary artery diameter+ | 143 |
| Cocoa drink | 446 mg CF | Parallel; 42 days; 32 hypercholesterol. postmenopausal; high-flavan-3-ol vs. low-flavan-3-ol cocoa | BP nc; FMD nc; blood flow+76%, HDL-C+6.6% | 486 |
| Cocoa drink | 2×402.5 mg CF | Crossover; 42 days; 37 overweight; sugar-sweetened vs. sugar-free cocoa vs. placebo | BP nc; lipids nc sugar:% FMD+1.5%; sugar-free% FMD+2.4% | 333 |
| Dark chocolate, sugar, and sugar-free cocoa drink | nd | Crossover; 1 day; 45 overweight; chocolate vs. placebo; sugar-free vs. sugar-sweetened cocoa vs. placebo | Chocolate: SBP-3.2 mm Hg, DBP-1.4 mm Hg, FMD+4.3%; sugar-free: SBP-2.1 mm Hg, DBP-1.2 mm Hg; FMD+5.7%; sugar cocoa: BP nc, FMD+2%, 2 h after intake | 136 |
| Dark chocolate, sugar, and sugar-free cocoa drink | nd | Crossover; 1 day; 45 overweight; chocolate vs. placebo; sugar-free vs. sugar-sweetened cocoa vs. placebo | Chocolate: SBP-3.2 mm Hg, DBP-1.4 mm Hg, FMD+4.3%; sugar-free: SBP-2.1 mm Hg, DBP-1.2 mm Hg; FMD+5.7%; sugar cocoa: BP nc, FMD+2%, 2 h after intake | 136 |
| Cocoa drink | 897 mg CF | Parallel; 1 day; 30 healthy; cocoa drink vs. caffeine control vs. water | Epinephrine–collagen hemostasis-, ADP-induced, and epinephrine-induced GPIIb/IIIa −, ADP-induced platelet P-selectin | 372 |
| Cocoa drink | 897 mg CF | Crossover; 1 day; 16 healthy; cocoa vs. aspirin vs. cocoa+aspirin | Epinephrine-stimulated platelet activation- | 350 |
| Red wine | ||||
| Red wine | 758.6 mg TP | Crossover; 30 days; 24 healthy; RW vs. DRW vs. beer vs. nothing (375 ml) | RW: ABP+2.9 mm Hg; HR+5 bpm; beer: ABP+1.9 mm Hg; HR+4.4 bpm | 512 |
| Red wine | nd | Crossover; 1 day; 11 healthy; RW vs. DRW vs. vodka vs. water (500 ml) | RW: HR+10–11 bpm 0.5–2 h, FMD+2% 2 h after intake; DRW: SBP+12 mm Hg 0.5 h, FMD+5%–2% 0.5–2 h; vodka: HR+11–12 bpm 0.5–2 h, FMD-2%–3% 0.5–2 h | 181 |
| Red wine | 27.3 mg TP. | Crossover; 1 day, 13 healthy; RW vs. ethanol vs. water (∼155–310 ml) | RW: HR+5.7 bpm, FMD-4.4% | 427 |
| Red and white wine | nd | Crossover; 1 day; 14 CAD; RW vs. WV (4 ml/kg BW) | RW: SBP-12 mm Hg, DBP-8 mm Hg, HR+4 bpm 1 h; FMD+1.6% 6 h WW: SBP-12 mm Hg, DBP-8 mm Hg, HR+4 bpm 1 h; FMD+3.1% 6 h after intake | 489 |
| Red wine | 560–840 mg TP, 58–87 mg ACN | Parallel; 30 days; 69 healthy; RW vs. red-grape tablet+water (2 doses) vs. placebo tablet+water (200–300 ml) | BP nc; RW: HDL-C+11%–16% | 178 |
| Red wine | 1000 mg TP | Parallel; 42 days; 45 hypercholesterol. postmenopausal; RW vs. DRW vs. water (400 ml) | BP nc; AIX, AP (Arterial stiffness) nc; RW: LDL-C-8%; HDL-C+17% | 329 |
| Red wine | 487–277 mg TP | Crossover; 1 day; 12 healthy; RW vs. DRW (250 ml) | DRW: FMD+1.7%; RW: HR+3 bpm (0.5–1 h after intake) | 3 |
| Red wine | 1645 μg TP | Crossover; 1 day; 15 CAD; RW vs. DRW (250 ml) | BP nc; RW:FMD-2.3% respect DRW 1 h after intake | 240 |
| Red wine | RW 2800 mg DRW 2790 mg TP | Crossover; 1 day; 9 healthy; RW vs. DRW vs. polyphenol-stripped RW vs. ethanol vs. water (3 ml/kg BW) | RW: FMD+1.7% 1 h after intake | 35 |
| Red wine | nd | Crossover; 1 day; 20 healthy; RW vs. low-polyphenol alcohol (3–6 glasses of 110 ml RW/240 ml LP) | BP nc; RW:FMD-6.8% 3 drinks, −7.42% 6 drinks; low-polyphenol drink: FMD-4.5% 3 drinks, −6.1% 6 drinks | 196 |
| Red wine | nd | Parallel; 60 days; 20 Acute coronary syndrome; RW vs. alcohol abstinence (250 ml/day) | RW and control: FMD+0.03–0.05 (ratio EDD/EID) | 174 |
| Red wine | nd | Parallel; 14 days; 17 type 2 diabetics; RW vs. RW abstinence (360 ml/day) | Plethysmography (endothelial function) nc; lipids nc; insulin sensitivity+43% (hyperinsulinemic clamp) | 330 |
| Red wine | nd | Parallel; 90 days; 44 healthy; RW vs. RW abstinence (150–300 ml/day) | LDL-C–0.3 mM | 245 |
| Red wine | 1081 mg TP | Crossover; 28 days; 40 healthy; RW vs. gin (320 ml RW, 30 g ethanol/day) | BP nc; RW and gin: HDL-C+0.1 mM | 135 |
| Red wine | nd | Crossover; 28 days; 11 healthy; RW vs. DRW vs. alcohol+fruit juice (320 ml, 30 g ethanol/day) | RW and alcohol: collagen-induced platelet aggregation and fibrinogen levels- | 352 |
| Red wine and white wine | nd | Crossover; 1 day; 24 healthy; RW vs. WW (300 ml) | ADP and collagen-induced platelet aggregation nc | 248 |
| Red wine | nd | Crossover; 1 day; 12 healthy; RW vs. water (4.36 ml/kg BW) | RW: Platelet aggregation+ | 138 |
| Red wine, white wine, grape juice | nd | Crossover; 28 days; 24 healthy; RW vs. WW vs. grape juice vs. resveratrol-enriched grape juice (375 mL wine, 500 mL juice/day) | BP nc; WW: ADP- and thrombin-induced platelet aggregation-; RW: thrombin-induced platelet aggregation- | 339 |
| Red wine and white wine | nd | Crossover; 28 days; 13 healthy; RW vs. WW (23–32 g alcohol/day) | RW and WW: collagen- and thrombin-induced platelet aggregation- | 292 |
| Resveratrol capsules | 30–270 mg Resveratrol | Crossover; 1 day; 19 overweight obese prehypertensive; 30 vs. 90 vs. 270 mg resveratrol vs. placebo | FMD+2.5–3.6% (30–270 mg dose), FMD dose-dependent correlated plasma resveratrol | 498 |
| Tea | ||||
| Black tea | nd | Crossover; 28 days; 65 healthy; BT vs. placebo (6 cups/day) | BP nc; lipids nc | 32 |
| Black and green tea | nd | Crossover; 7 days/1 day; 13 hypertensive/20 healthy; BT vs. GT vs. water vs. caffeine+water (5/4 cups) | Chronic 24 h ABP nc; acute 1 h after intake BP nc; acute 30 min after intake: BT: SBP+10.7 mm Hg, DBP+5.1 mm Hg; GT: SBP+5.5 mm Hg, DBP+3.1 mm Hg | 203 |
| Black tea | 1350 mg TP | Crossover; 28 days/1 day; 66 CAD; BT vs. water (900 ml BT per day/450 ml BT) | SBP+5 mm Hg 2 h after intake; FMD+3.5% baseline day 1–28, +3.4% 2 h after intake day 1; +4.8% 2 h after intake day 28 | 124 |
| Black tea | nd | Parallel; 28 days; 21 healthy mild high TC/TAG; BT vs. water (5 cups/day) | BP nc; FMD+2.3%; lipids nc | 205 |
| Black tea | nd | Crossover; 1 day; 20 healthy; BT+dairy fat vs. water+dairy fat (1 cup) | Collagen and ADP-induced platelet aggregation nc; lipids nc | 204 |
| Decaffeinated green tea | 928 mg TF (GT), 870 mg TF(extract) | Parallel; 56 days; 35 obese or MS; GT vs. GT extract+water vs. water (4 cups/day) | BP nc; lipids nc; BMI and weight- | 28 |
| Decaffeinated green tea | 800 mg TF | Crossover; 42 days; 67 obese or overweight; DGT vs. placebo capsules | 24 h ABP nc; lipids nc; body weight- | 53 |
| Green tea | 400 mg TF vs. 100 mg TF | Parallel; 63 days; 51 healthy; high- vs. low-catechin GT (500 ml/day) | BP nc; lipids nc | 426 |
| Black tea | nd | Crossover; 1 day; 20 CAD; BT vs. water vs. BT+high-fat meal vs. water+high-fat meal (3 cups) | BT: SBP+9.3 mm Hg (3.5 h after intake), FMD nc; BT+high-fat meal: BP nc, FMD+1.7% (4 h after intake) | 200 |
| Black tea | nd | Crossover; 1 day; 17 renal transplant recipients; BT vs. water (500 ml) | FMD+6% (2 h after intake) | 11 |
| Black tea | 560 mg TF | Crossover; 1 day; 16 healthy postmenopausal; BT vs. BT+milk vs. water | BT: FMD+4% 2 h after intake BT+milk: FMD nc 2 h after intake | 284 |
| Green tea | nd | Crossover, 1 day; 14 healthy; GT vs. caffeine vs. water (6 g of GT, 125 mg of caffeine) | GT: SBP+8.2 mm Hg max 90 min after intake; FMD+3.7% max 30 min after intake; caffeine: SBP+7.6 mm Hg, FMD nc | 7 |
| Green and Black tea | 506,280 μmol TF (GT,BT) | Crossover; 1 day; 21 healthy women; GT vs. BT vs. water (5 g, 500 ml) | GT: FMD+4.8% 2 h after intake BT: FMD+4.1% 2 h after intake | 226 |
| Black tea | nd | Crossover; 1 day; 22 healthy; BT vs. water (2 g, 250 ml) | Collagen- and ADP-induced platelet aggregation nc; p-selectin- | 206 |
| Black tea | 100, 200, 400, 800 mg TF | Crossover, 1 week; 19 healthy; 4 BT doses vs. control | SBP-2.6%; DBP-2.2%; FMD+1.2% (100 mg), +1.3% (200 mg), +1.8% (400 mg), +2.5% (800 mg) | 167 |
| Green Tea | 460 mg TF | Parallel; 30 days; 40 chronic renal failure; green tea vs. water (5 g, 500 ml/day) | BP nc; FMD+2.98%; HOMA-IR nc; QUICKI nc | 344 |
| EGCG capsule | 2×150 mg EGCG | Crossover; 14 days; 42 CAD; EGCG vs. placebo | BP nc; FMD+1.5% 2 h after intake; lipids nc | 490 |
| Green and black tea | GT: 642 mg TF BT:215 mg TF | Crossover; 28 days; BT vs. GT vs. water (900 ml; 6 cups, 3 g tea/day) | Lipids nc | 471 |
| Black tea and coffee | 127 mg TF | Crossover; 28 days, 10 healthy; BT vs. coffee (600 ml) | Lipids nc | 296 |
| Green and black tea | GT:852 mg TF BT:240 mg TF GTC:2488 mg | Parallel, 28 days, 59 smokers; BT vs. GT vs. GT capsules vs. water (3 g tea or 3.9 g GT extract, 6×150 ml) | Lipids nc | 360 |
| Black tea | 25.2 mg TF 74.4 mg TP | Parallel; 84 days; 77 healthy; BT vs. water (9 g BT/day, 3×200 ml) | TAG-0.72 mM, ratio LDL/HDL-C-0.4, Glucose-1.5 mM | 21 |
| Berries | ||||
| Concord grape juice | nd | Parallel; 60 days; 40 hypertensive; grape juice vs. placebo (5.5 ml/kg BW) | SBP-7.2 mm Hg, DBP-6.2 mm Hg | 345 |
| Chokeberry extract | 127.5 mg Pro, 63.7 mg ACN | Parallel; 45 days; 44 MI on statins; chokeberry vs. placebo (3×85 mg) | SBP-11 mm Hg, DBP-7.2 mm Hg | 331 |
| Mixed berries | 837 mg TP 515 mg ACN 240 mg Pro | Parallel; 56 days; 72 middle age risk CVD; mix of frozen berries vs. porridge, sugar water, and marmalade sweets | SBP-1.5 mm Hg; HDL-C+0.08 mM; collagen- and ADP-induced platelet aggregation-11% | 132 |
| Blueberry | nd | Parallel; 56 days; 48 obese MS; freeze-dried blueberry+water vs. water (50 g=350 g fresh blueberry) | SBP-7.8 mm Hg, DBP-2.5 mm Hg; lipids nc | 27 |
| Hibiscus sabdariffa tea | 21.1 mg ACN 65.5 mg TP | Parallel, 42 days, 65 pre- or hypertensive; tea vs. placebo (3×240 ml) | SBP-7.2 mm Hg; DBP-3.1 mm Hg | 300 |
| Blueberry | nd | Parallel; 21 days; 20 healthy smokers; blueberry vs. placebo (250 g fresh blueberry) | BP nc | 297 |
| Concord grape juice | 245.6 mg TP/75 kg BW | Crossover; 60 days; 65 pre- or hypertensive; grape juice vs. placebo (525 ml/75 kg BW) | 24 h ABP nc; PWV nc; PAT nc | 114 |
| Blueberry | 1462 mg TP | Parallel; 32 obese; 45 days; freeze-dried blueberry smoothie vs. placebo (45 g=280 g fresh blueberry) | BP nc; insulin sensitivity+1.7 (mg·kg)/(FFM·min) | 448 |
| Cranberry juice | 835 mg TP 94 mg ACN | Crossover; 28 days; 44 CHD; cranberry juice vs. placebo (480 ml) | BP nc; FMD nc; PWV-8.3 m/s; lipids nc | 115 |
| Anthocyanin extract | 640 mg ACN | Crossover; 28 days; 31 hypertensive; anthocyanin vs. placebo (8 capsule/day) | 24 h ABP nc; BP nc | 182 |
| Grape | 384 mg TP | Crossover; 28 days; 44 healthy pre- and postmenopausal; freeze-dried grape vs. placebo (36 g=200 g fresh grapes) | BP nc; premenop: TAG-0.2 mM, LDL-C-0.3 mM; postmenop: TAG-0.1 mM, LDL-C-0.1 mM | 509 |
| Grape seed Extract | 186.9 mg TP | Crossover; 28 days; 50 CVD or at risk; muscadine grape seed vs. placebo (2 capsule/day) | BP nc; FMD nc; lipids nc | 302 |
| Grape and grape seed extract | 800 mg TP | Crossover; 14 days; 35 healthy; wine grape vs. grape seed vs. placebo (6 capsule/day) | BP nc; FMD nc; lipids nc | 472 |
| Grape seed extract | 200 mg Pro | Crossover, 56 days, 28 smokers; grape seed extract vs. placebo (2 capsule/day) | BP nc; FMD nc; lipids nc; collagen- and ADP-induced platelet aggregation nc | 488 |
| Anthocyanin extract | 320 mg ACN | Parallel/crossover; 90/1 days; 140/12 hypercholesterolemic; anthocyanin vs. placebo capsules (4 capsule/day) | BP nc; FMD+2.9%; HDL-C+0.15 mM; LDL-C-0.35 mM acute: FMD+2.7% (1 h),+1.8% (2 h) | 511 |
| Red grape extract | 600 mg TP | Parallel; 1 day; 30 CHD; red-grape extract+water vs. water (20 ml) | FMD+1.92% after 1 h intake | 275 |
| Purple grape juice | nd | Parallel; 14 days; 20 coronary disease; purple-grape juice vs. placebo (525 ml/75 kg BW) | HDL-C+0.13 mM; ADP/TRAP/PMA-induced platelet aggregation nc; p-selectin nc | 6 |
| Pomegranate | ||||
| Pomegranate juice | nd | Parallel; 90 days; 45 CHD and MI; pomegranate juice vs. placebo (240 ml/day) | BP nc; stress-induced ischemia- | 450 |
| Pomegranate juice | nd | Parallel; 540 days; 289 moderate-risk CHD pomegranate juice vs. placebo (240 ml/day) | BP nc; CIMT nc | 101 |
| Citrus fruit | ||||
| Orange juice Hesperidin | 240 mg hesperidin 47.5 mg narirutin | Crossover; 28 days; 24 healthy overweight; orange juice vs. control drink+hesperidin vs. control drink+placebo (500 ml) | Orange juice: DBP-5.5 mm Hg chronic, Ach-vasorelaxation:+105% 6 h after intake; hesperidin: DBP-3.2 mm Hg chronic, Ach-vasorelaxation+68.5% (6 h after intake); Lipids nc | 315 |
| Hesperidin | 500 mg | Crossover; 21 days; 24 metabolic syndrome; hesperidin vs. placebo | FMD+2.48% | 382 |
| Coffee | ||||
| Coffee | nd | Crossover; 1 day; 17 healthy; caffeinated vs. decaffeinated coffee (1 cup) (80 mg vs.<2 mg caffeine) | Coffee: DBP+4 mm Hg, HR-8 bpm, FMD-3.2 to −5.6% (0.5–2 h after intake); decaffeinated coffee: BP nc; HR-7 bpm, FMD nc | 343 |
| Coffee | nd | Crossover; 1 day; 20 healthy; caffeinated vs. decaffeinated espresso coffee (1 cup, 130 mg vs. 5 mg caffeine) | Coffee: SBP+3 mm Hg, DBP+4 mm Hg, FMD-1.4 to −1.7% (0.5–1 h after intake); decaff: BP nc; HR-7 bpm, FMD nc; HOMA-IR nc | 58 |
| Coffee | nd | Crossover; 1 day; 10 healthy; coffee vs. caffeine plus water (200 ml, 180 mg caffeine vs. 180 mg caffeine) | Coffee: collagen- and arachidonic-induced platelet aggregation- | 332 |
| Nuts | ||||
| Walnuts | nd | Crossover; 28 days; 18 hyperchol; 40–65 g walnuts/day vs. similar-energy and fat-content diet | BP nc; FMD+2.3%; TC-0.21 mM; LDL-C-0.11 mM | 388 |
| Walnuts | nd | Crossover; 56 days; 24 type 2 diabetes; walnut diet vs. walnut abstinence; 56 g walnut/day | BP nc; FMD+2.2%; Lipids nc; HOMA-IR nc | 288 |
| Hazelnut | nd | Crossover; 56 days; 15 hypercholesterolemic; low-fat diet vs. low-fat hazelnut diet (40g/day) | TAG-0.73 mM; HDL-C+0.14 mM; ratio TC/HDL-C-0.02; ratio LDL-C/HDL-C−0.01 | 304 |
ABP, ambulatory blood pressure; ACN, anthocyanins; AIx, augmentation index; AP, augmentation pressure; BAD, brachial artery diameter; BMI, body–mass index; BP, Blood pressure; BT, black tea; BW, body weight; CAD, coronary artery disease; CF, cocoa flavan-3-ols; CHD, coronary heart disease; CIMT, carotid intima-media thickness; CVD, cardiovascular disease; DBP, diastolic blood pressure; DGT, decaffeinated green tea; DRW, dealcoholized red wine; EDD, Endothelial-dependent dilation; EID, endothelial-independent dilation; FMD, flow-mediated dilation; GT, green tea; GTC, green tea capsules; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment–insulin resistance; HR, heart rate; ISI, insulin sensitivity index; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; MS, metabolic syndrome; nc, no change; nd, not detected; PAT, peripheral artery tonometry; Pro, procyanidins; PWV, pulse wave velocity; QUICKI, quantitative insulin sensitivity check index; RW, red wine; SBP, systolic blood pressure; SI clamp, glucose clamp index of insulin sensitivity; TAG, triacylglycerols; TC, total cholesterol; TF, tea flavan-3-ols; TP, total polyphenols; WW, white wine.
1. Cocoa
There is good evidence to suggest that cocoa-derived (poly)phenols may have beneficial effects on blood pressure (BP) in healthy subjects and, in particular, hypertensive individuals (89, 112, 146, 165, 166, 168, 211, 325, 326, 453, 454). A meta-analysis of 10 RCTs comprising healthy, normotensive, pre-, and hypertensive individuals reported a decrease of 4.5 mm Hg in systolic BP and 2.5 mm Hg in diastolic BP after intake of flavan-3-ol-rich chocolate or flavan-3-ol-rich cocoa for 2–18 weeks (112). However, caution should be expressed over the fact that seven of the studies used in the meta-analysis were noted to have used white chocolate, which is very different in appearance, taste, and nutrient content from dark chocolate, as a control for the high-flavan-3-ol test intervention. A more recent meta-analysis of 20 randomized controlled studies concluded that systolic BP decreased by 1.63 mm Hg, whereas diastolic BP and heart rate were unaffected after the consumption of flavan-3-ol-rich cocoa (421). Looking closely at these meta-analyses, it is evident that there are large differences in the quality of the selected studies.
With regard to endothelial function, a well-known surrogate marker for vascular health, flavan-3-ol-rich cocoa (176–963 mg of flavan-3-ol monomers and oligomers), has been shown to acutely improve endothelium-dependent vasodilation assessed by flow-mediated dilation (FMD) via increasing plasma NO bioavailability in healthy individuals and in patients with coronary artery disease (CAD), hypertension, or diabetes, compared to a well-matched control containing 0–75 mg flavan-3-ols (22, 102, 136, 187, 188, 190, 404). These vascular improvements correlated in time with changes in plasma flavan-3-ol metabolites, and were repeatable with pure (−)-epicatechin intake, suggesting a cause-and-effect relationship between flavan-3-ols and vascular improvements (404). Flavan-3-ol-rich cocoa has also been shown to reverse endothelial dysfunction in smokers (190) and to improve endothelium-dependent coronary vasomotion in heart transplant recipients (143).
Short-term flavan-3-ol-rich cocoa intake has also been shown to increase the FMD response and hyperemic brachial artery blood flow after 2–12-week consumption in obese, hypercholesterolemic, hypertensive, and healthy subjects (102, 131, 165, 168, 333, 486). A sustained increase in baseline FMD levels and an additional acute-on chronic increase in the FMD response were also reported in, respectively, healthy smokers and diabetics, after 7 and 30 days of flavan-3-ol-rich cocoa consumption (22, 188). Nine studies on cocoa and endothelial function were included in the meta-analysis of Shrime et al. (421), which concluded that flavan-3-ol-rich cocoa increased FMD in average by 1.53%. A dose-dependent effect was also observed with FMD increasing up to a 500 mg intake of flavan-3-ols and decreasing at higher doses. In the same meta-analysis, consumption of flavonoid-rich cocoa was also found to increase high-density lipoprotein (HDL) cholesterol by 0.046 mM and decrease LDL cholesterol (LDL-C) by 0.07 mM. An indication of dose dependency between the flavan-3-ol and HDL cholesterol (HDL-C) was also observed. Furthermore, subgroup analysis revealed that HDL-C tended to increase in longer studies with low-fat consumption, and LDL and total cholesterol tended to decrease in shorter studies, in studies with younger populations, and in trials with high-fat consumption (421).
The effects of cocoa flavan-3-ol intervention on a number of other CVD risk factors have also been investigated. For example, there is good evidence to suggest that they may improve insulin resistance (102, 165, 166, 168). Notably, after 2–12 weeks of cocoa flavan-3-ol intake of 500–1000 mg/day, a decrease of 0.94 points in the homeostasis model assessment of insulin resistance and a decrease of 4.95 points in the insulin sensitivity index were observed. However, there were no changes in the quantitative insulin sensitivity check index and fasting glucose levels. Together, these results suggest that cocoa intake may improve insulin sensitivity in healthy, hypertensive, and obese individuals (421). There are also extensive data regarding the effects of cocoa intake on platelet function (209, 326, 350, 373). Inhibition of platelet activation has been observed at 2 and 6 h post-flavan-3-ol-rich cocoa/chocolate consumption in healthy individuals (209, 373). Such acute observations are supported by chronic intervention studies where cocoa flavan-3-ols improve platelet function after 28 days of supplementation (326). Although less effective, an aspirin-like effect for flavan-3-ols has been suggested, in inhibiting platelet activation (350).
2. Red wine
It is well accepted that excessive alcohol consumption leads to increased BP in normotensive and hypertensive subjects (364, 365), whereas low/moderate consumption reduces specific cardiovascular risk factors (51, 384). Reports regarding the effects of red wine and red wine (poly)phenols on BP are in most case inconsistent. While there is extensive evidence to support the influence of wine intake on cardiovascular health (86, 116, 281), controversy remains whether red wine in particular exerts beneficial effects compared with other alcoholic beverages (47, 469) or simply alleviates the detrimental influence of alcohol on BP (55, 512). In acute studies with healthy volunteers, no changes in BP were observed, but an increase in the heart rate was reported after red wine consumption (181, 427), whereas in CAD patients, a decrease in systolic and diastolic BP was noted together with an increase in heart rate, 1 h postwine (red and white) intake (489). Other studies have reported no changes in hemodynamics or BP after medium-term daily intake of either red wine or its dealcoholized equivalent (178, 329). However, in the majority of these studies, no proper control was used.
Several studies report the effect of red wine and/or its dealcoholized equivalent on endothelial function, although again, there is inconsistency in the findings. For example, some studies indicate that dealcoholized wine, but not red wine, induces an increase in FMD (3, 240); others suggest the opposite (35); and another suggests that both red wine and dealcoholized red wine are equally effective (181). Other studies have shown no effect, or even a decrease, in FMD after red wine or alcohol consumption (196, 427). The reason for these inconsistencies may be the increase in the baseline brachial artery diameter due to alcohol reported in all the studies, which will affect FMD responses, as pointed out by Spaak et al. (427). With regard to longer-term (2 weeks to 2 months) red wine consumption, the majority of studies suggest that there is no significant effect on FMD or arterial stiffness (174, 329, 330, 512).
In addition to BP and endothelial function, several studies have also reported that regular medium-term intake of red wine (2–12 weeks) may increase HDL-C and lower LDL-C (178, 245, 328, 378). As there were no controls used in these studies with alcohol, it remains unclear as to whether the alcohol itself, rather than (poly)phenols, was responsible for the favorable effects of red wine. Indeed, HDL-C has been found to increase after a 28-day intake of either red wine or gin (135). Acute studies have failed to show a positive effect on platelet function after red wine consumption (138, 248). However, red wine, dealcoholized red wine, white wine, and alcohol all appear to have the same beneficial effects on platelet function after 4 weeks of intake (292, 339, 352), which suggests that the positive effects of moderate consumption of wines on platelet function seem to be due to their alcohol content.
Resveratrol (Fig. 13), a stilbene found in grape seed, has been postulated to be partly responsible for the beneficial vascular effects of red wine. Indeed, 30, 90, or 290 mg of trans-resveratrol has been shown to improve endothelial function in overweight subjects in a dose-dependent manner (498), and animal studies support this, as resveratrol reportedly improves survival rates, endothelium-dependent smooth muscle relaxation, cardiac contractile, and mitochondrial function in a hypertensive model of heart failure (379). However, while the conjecture that resveratrol may underpin the vasoactive effects of wine seems logical, the majority of red wines actually contain very little of the stilbenes, and thus it is unlikely that it is responsible for the beneficial effects of red wine in vivo (see Section III.J)
3. Tea
Tea is also a rich source of flavan-3-ols; although unlike those found in cocoa and red wine, they are predominantly gallated (Fig. 9). As with wine, there is some controversy regarding the effects of tea on BP, with the presence of caffeine likely to confuse the issue if studies are not properly controlled (211, 301). Meta-analysis (211) has indicated that acute, but not chronic, black tea intake affects systolic and diastolic BP in healthy subjects. In agreement with this, regular consumption of both green and black tea (1–9 weeks) have been shown not to significantly influence BP (28, 32, 123, 203, 204, 426, 454)
With regard to endothelial function, the meta-analysis by Hooper et al. (211) indicates that black tea consumption significantly increases the FMD response after acute (+1.7%) and long-term consumption (+3.4%), whereas that of Ras et al. (369), reporting on nine RCTs, concluded that moderate consumption of black (seven studies) and green (three studies) tea (2–3×500 ml cups/day) improved FMD by 2.6%. Subgroup analysis indicated that FMD increases occurred in healthy, diseased, young, and old subjects, and in acute (7, 11, 123, 200, 226, 284) and short-term consumption (1–4 weeks) (123, 167, 204, 344) studies in response to both green and black tea. As with in the case of cocoa flavan-3-ols, long-term tea consumption has been shown to produce a sustained increase in baseline FMD levels and an additional acute-on chronic increase in the FMD response (123). Acute intervention with the tea flavan-3-ol, (−)-epigallocatechin-3-O-gallate, also led to improvements in endothelial function, suggesting that the gallate may be partly responsible for the vascular effects of tea (490).
With regard to other risk factors for CVD, many human intervention studies have indicated that neither green nor black tea intake has an effect on blood lipids (32, 296, 360, 426, 471). However, a recent study with healthy individuals has reported that black tea decreases triglyceride levels by 35.8% and the LDL/HDL plasma cholesterol ratio by 20.3% after 12 weeks of consumption (21). Human intervention studies in both healthy and CAD subjects indicate no effect on platelet aggregation (124, 204, 206). In contrast, a decrease in platelet activation has been reported in healthy men after a 6-week consumption of black tea (443), whereas 4 weeks of consumption brought about a decrease in p-selectin, a marker of platelet activation (206).
4. Berries
A decrease in BP has been reported after chronic intakes (6–8 weeks) of mixed berries, anthocyanin-rich tea, and chokeberry and blueberry extracts by hypertensive individuals, myocardial infarction survivors, and those with markers of the metabolic syndrome (27, 132, 300, 331, 345). In contrast, a similar number of studies have reported a lack of efficacy of chronic intake of anthocyanin-rich foods, such as blueberry and cranberry juice in healthy individuals, chronic smokers, people with dyslipidemia, obese subjects, stage 1 hypertensive individuals, and CAD patients (114, 115, 182, 297, 366, 448). Similarly, grapes and grape seed extract have also been shown not to influence BP in healthy or diseased individuals (302, 472, 488, 509). Animal studies have been more positive, showing BP-lowering effects of berry extracts after long-term consumption, but only in models of hypertension (130, 415, 497). There is no evident reason on why some studies show a positive effect on BP and others not, although different types of berries may contain very different profiles of (poly)phenols (41).
Improvements in vascular function (increased FMD) have been reported acutely with pure anthocyanins (320 mg) (511), after consumption of a grape extract (72, 275) administered with or without a high-fat meal (72), and after consumption of cranberry juice (4 weeks; decrease in carotid femoral pulse wave velocity) (115). Despite these promising results, other studies have failed to observe a positive effect on endothelial function after consumption of grapes (302, 472, 488). Berry and grape consumption has also been shown to lead to increases in HDL-C (6, 132, 366, 511) and decreases in LDL-C (509) in healthy, at risk, and diseased individuals. Others have reported no changes in blood lipids despite observing changes in other CVD markers (72, 114, 115, 127, 302, 472). The evidence for berries, including Concord grape juice, inhibiting platelet aggregation is positive (6, 132, 357), and recent evidence suggests that berry consumption may improve insulin sensitivity (448).
5. Pomegranate
Pomegranate has been suggested to have antiatherosclerotic effects in human studies and animal models of disease (17, 19, 101, 239, 389, 450). The consumption of pomegranate juice (3 months, 240 ml of pomegranate juice/day) has been reported to lead to significant decreases in stress-induced ischemia, assessed by myocardial perfusion imaging at rest and during stress (treadmill test exercise) with which may decrease myocardial ischemia and improve myocardial perfusion in patients with coronary heart disease (CHD) (450). Furthermore, daily intake of 240 ml of pomegranate juice for 18 months improved carotid intima-media thickness (CIMT) of at risk individuals relative to a control (similar characteristics, color, and energy), although only in those with the most adverse tertiles for blood lipids (101). Although an uncontrolled intervention study (i.e., the control was no consumption), a daily 50-ml consumption of pomegranate juice for 12 months has also been shown to induce a 12% decrease in systolic BP and a 30% decrease in CIMT in patients with carotid artery stenosis (18).
Many animal investigations have supported these these reported cardiovascular effects in humans (17, 19, 239, 389). In an atherosclerotic mouse model, a 3-month supplementation with pomegranate juice, pomegranate fruit liquid extract, pomegranate (poly)phenol powder extract, and, in particular, a pomegranate ground flower extract led to significant decreases in the atherosclerotic lesion area relative to water-fed controls along with decreases in serum glucose and cholesterol levels (19). Furthermore, intervention with pomegranate juice extract for 4 weeks reduced the mean arterial BP, decreased the vascular reactivity in response to catecholamines, and reversed biochemical changes induced by angiotensin II in diabetic rats (311). Further, better-controlled human and animal studies are required to conclude on the likely efficacy of pomegranate against CVD.
6. Citrus fruit
Medium-term (4–5 week), daily consumption of flavanone-rich grapefruit (374) and orange (315) juice has been shown to induce decreases in diastolic BP. In support of these findings, consumption of pure hesperidin, 500 mg/day for 3 weeks, resulted in significant increases in the FMD response compared with a placebo (382). Also, both orange juice and hesperidin significantly improve acetylcholine-mediated vasodilation 6 h after ingestion (315). Unlike endothelial function, no changes in plasma lipids were observed after intakes of either orange juice or pure hesperidin (315), although one study suggests that consumption of 750 ml, but not 250 or 500 ml of orange juice, may significantly increase HDL-C (21%) and triacylglycerol (30%) and decrease the LDL/HDL cholesterol ratio by 16% in hypercholesterolemia patients (263). Although the evidence is limited, the fact that two studies have utilized purified flavonoids provides additional weight to previous investigations (404, 490), which suggest that flavonoids themselves are the causal agents in mediating vascular benefits.
7. Coffee
A meta-analysis of 11 intervention studies regarding coffee consumption reports that coffee intake increases systolic BP by 2.4 mm Hg and diastolic BP by 1.2 mm Hg (224). A correlation between the number of cups of coffee consumed and the increase in BP was observed, and the effect of coffee on BP was reported to be greater in studies with younger populations. These findings are supported by another meta-analysis of 16 RCTs examining coffee and caffeine intake for at least 7 days (334). Here, an increase of 1.22 mm Hg in systolic BP and 0.49 mm Hg in diastolic BP was observed in response to coffee consumption. However, in studies where caffeine on its own was supplemented, increases in BP were higher (4.16 mm Hg for systolic and 2.41 mg Hg for diastolic BP), suggesting that coffee (poly)phenols, most notably chlorogenic acids (see Section II.B), may limit the negative effects of caffeine on BP. A more recent meta-analysis, looking at hypertensive subjects, reports an acute increase in BP 3 h after ingestion of 200–300 mg of caffeine, but no significant effect for longer-term consumption (305). Indeed, analysis of cohort studies provides no evidence of an association between habitual coffee consumption and a higher risk of CVD in hypertensive individuals (305). Indeed, long-term, moderate coffee consumption has been associated with a lower risk of CHD in women (503).
In healthy subjects, acute coffee intake (one cup) led to a decrease in the FMD response, with no effect on endothelial function reported for decaffeinated coffee (58, 343). However, if the dose of decaffeinated coffee is increased, an improved FMD response may be measured (59), suggesting that coffee (poly)phenols are able to improve acute endothelial function. Caffeinated coffee intake appears to elevate serum lipids and cholesterol in a dose-dependent manner in patients with hyperlipidemia (223), whereas coffee consumption, but not caffeine, has been found to decrease platelet aggregation acutely (332).
8. Nuts
Whole, unprocessed nuts are rich in flavan-3-ols, flavonols, anthocyanins, and, most notably, procyanidins and phenolic acids (37). A recent review of 19 RCTs concluded that consumption of nuts has no effect on BP (66). However, the majority of these studies were uncontrolled, and BP was often a secondary outcome measure. Improvements in FMD have been observed after 4 weeks of walnut or pistachio consumption in healthy, hypercholesterolemia, or type 2 diabetes patients compared to the same diet without nuts (288, 388, 401). In contrast, hazelnuts (40 g/day; 4 weeks) failed to elicit an FMD effect (304). Studies with nuts, perhaps expectedly, have given more emphasis to plasma lipid changes. A meta-analysis considering 13 studies concluded that consumption of walnuts for periods of 4–24 weeks is capable of decreasing total and LDL-C, whereas HDL-C and triglycerides were unaffected (23), while another systematic review reported a decrease in total and LDL-C after short-term consumption of almonds, peanuts, pecan nuts, and walnuts, with human studies of 2–3-week duration (316). Whether or not these changes are due to the (poly)phenols found in nuts is presently unclear, although it seems likely that unsaturated fats, mainly oleic and linoleic acids, may contribute to lipid-lowering effects in vivo.
B. (Poly)phenols and neurodegenerative diseases
A combination of preclinical and epidemiological studies suggests that (poly)phenols may be effective in reversing neurodegenerative pathology and age-related declines in neurocognitive performance, although at present, a direct association between (poly)phenol consumption and improvement in neurological health has not been made. The potential of (poly)phenols to improve neurological health appears to be related to a number of mechanisms, including their ability to interact with intracellular neuronal and glial signaling, to influence the peripheral and cerebrovascular blood flow, and to reduce neuronal damage and losses induced by neurotoxins and neuroinflammation (148, 327, 424, 428, 430–432, 492). This section will examine the beneficial effects of flavonoids on memory, learning, and neurocognitive performance.
There are a number of studies that have indicated that the consumption of flavonoid-rich plant foods and extracts may also be capable of inducing improvements in cognitive performance (83, 276, 429, 431). Human observational and interventional studies have hinted at the potential benefits of such foods and their regular intake [reviewed in (289)]. The efficacy of isoflavones on cognition and aspects of memory are well reported (67, 122, 141, 145, 198, 214, 255, 259), and may relate to their potential to mimic the actions of estrogens in the brain (33, 191), or to influence the synthesis of acetylcholine and neurotrophic factors (341, 342). With regard to other flavonoids, human data are somewhat scarcer. Observational data have suggested that the regular and moderate intake of flavonoid-rich foods, such as wine, berries, and cocoa, may result in cognitive benefits (83, 276, 335). In addition, two intervention studies have examined the effects of flavonoid-rich extracts of Ginkgo biloba in older adults with (274) and without (310) Alzheimer's disease (AD); two detailed the effects of procyanidin-rich pine bark extracts on healthy older adults (355, 397); and one examined the effects of flavan-3-ol-rich cocoa on cognitive function in young healthy female adults (149).
In terms of more commonly consumed flavonoid-rich foods, intervention trials have shown that grape juice, blueberry, and cocoa intake results in positive effects on cognitive outcome measures (257, 258, 403), although there are studies that suggest no changes in neurophysiological effects (89). With respect to berries, there is a large body of animal behavioral evidence that supports the efficacy of flavonoids on memory and learning. Grape, pomegranate, strawberry, and blueberry (1%–2% [w/w] freeze-dried fruit/fruit juice), as well as pure (−)-epicatechin and quercetin, have been shown capable of affecting several aspects of memory and learning, notably rapid (484) and slow (180, 229, 230, 416, 495) memory acquisition, short-term working memory (207, 363, 368, 484, 491), long-term reference memory (65, 179), reversal learning (207, 484), and memory retention/retrieval (473). For example, there is extensive animal evidence suggesting that blueberries are effective at reversing age-related deficits in spatial working memory (9, 26, 65, 368, 383, 423, 491, 494). In addition, (−)-epicatechin at a dose of 500 μg/g enhances the retention of rat spatial memory, especially when combined with exercise (473). These observations suggest that flavonoids may be causal agents in inducing the behavioral effects. In addition, two intervention studies have examined the effects of flavonoid-rich extracts of G. biloba in older adults with (274) and without (310) AD; two detailed the effects of procyanidin-rich pine bark extracts on healthy older adults (355, 397); and one examined the effects of flavan-3-ol-rich cocoa on cognitive function in young healthy female adults (149). While these data suggest an effect of flavonoids in preventing cognitive impairment, at this time, it is not possible to conclude on cause-and-effect relationships between these components and biological effects.
Blueberry-derived flavonoids may also act to enhance the efficiency of spatial memory indirectly by acting on the dentate gyrus (DG), the hippocampal subregion most sensitive to the effects of aging (56, 425). Blueberry supplementation has been shown to significantly increase the proliferation of precursor cells in the DG of aged rats (65). This link between DG neurogenesis, cognitive performance, and aging is well documented (120, 246, 260, 420, 441), and may represent another mechanism by which fruits rich in (poly)phenols may improve memory by acting on the hippocampus. In addition to those with berries, animal studies with tea (70) and pomegranate juice (180) or pure flavonols such as quercetin, rutin (363), or fisetin (290) have provided further evidence that dietary flavonoids are beneficial in reversing the course of neuronal and behavioral aging. There are also lines of evidence suggesting that these effects may not be restricted to memory and learning, but may also exert positive changes in psychomotor activity in older animals (229, 422).
C. (Poly)phenols and cancer
A number of epidemiological studies have investigated the relationship between the intake of fruits and vegetables and cancer incidence, with many indicating a positive correlation (29, 137, 140, 157, 510) and others suggesting no association (44, 272, 273). While a number of in vitro experiments have suggested that (poly)phenols may influence carcinogenesis and tumor development (61, 126, 306, 356, 470), very few clinical trials have been conducted regarding the role of (poly)phenols in cancer prevention, incidence, or mortality. Two trials have investigated the effects of green tea extracts on oral cancer (278, 463). The first suggested that they may reduce the oral mucosa leukoplakia size, a precursor lesion for oral cancer, following a 6 months of intake of 3 g/day of a mixed tea extract in capsules (278), whereas the more recent study failed to observe similar reductions in oral premalignant lesions or histology after a 12-week green tea extract supplementation, or any changes in cancer incidence 27 months after trial completion (463). Furthemore, tea (poly)phenol intake (100–600 mg/day) did not decrease serum pepsinogen levels, an indicator of increased risk of stomach cancer, after a year of intake (177), or alter abnormal cell proliferation or lesion formation in patients with esophageal precancerous lesions (5 mg of decaffeinated green tea extract/day for 1 year) (483). Data have been more promising in men with high-grade prostatic intraepithelial neoplasia, a precursor of prostate cancer, where daily consumption of green tea catechin capsules (200 mg of flavan-3-ols/day) for 1 year resulted in only one tumor being diagnosed among the 30 subjects in the green tea group versus 9 cancers diagnosed in the similarly sized control group (30).
It has been suggested that soy isoflavones may have protective effects against breast cancer (460, 501). However, a meta-analysis bringing together eight RCTs investigating the effect of isoflavone consumption (40–120 mg/day, 0.5–3 years) on the breast density (a biomarker of breast cancer risk) reported no significant effects, although it appeared that there might be a small increase in the breast density of premenopausal women (212). The clinical relevance of these findings was questioned by the authors and needs to be further investigated.
Other clinical studies have focused on how (poly)phenol interventions influence DNA oxidation/damage, which has been correlated with cancer risk (34). For example, a variety of (poly)phenol-rich foods have been found to protect against lymphocyte DNA damage, including anthocyanin-rich juice (700 ml of a red mixed berry juice/day; 4 weeks) (487), blood-orange juice (300 ml/day, 3 weeks) (381), purple potato (150 g/day, 6 weeks) (242), and kiwi fruit (500 ml juice, 1 day) (82). Other trials have reported a decrease in urinary levels of 8-hydroxydeoxyguanosine, which is a biomarker of oxidative DNA damage, following the regular consumption of green tea (poly)phenols [4 cups/day, 146 mg total (poly)phenols/cup, 4 months; 500/1000 mg (poly)phenols/day, 3 months] (176, 287). Interventions aimed at increasing overall fruit and vegetable intake have also been suggested to induce significant reductions in oxidative DNA damage (230, 231, 446, 455), although such findings are disputed (52, 313). Indeed, no influence on DNA oxidation is reported for a dealcoholized, procyanidin-rich wine (500 ml/day, 7 mg procyanidins/kg body weight, 4 weeks) (152), cranberry juice (750 ml/day, 2 weeks) (127), a mixture of grapes and berries (200 g/day, 2 weeks) (63), or after blackcurrant juice consumption (666 ml/day, 397 mg anthocyanins/day, 3 weeks) (312). The variability observed here is likely to be influenced by the intervention, its duration, the health status of the study population, and differences in methodology.
A summary of the potential effects of dietary (poly)phenols in humans is illustrated in Figure 41.
FIG. 41.
A summary of the potential health benefits of dietary (poly)phenols. (To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars.)
VII. Conclusions
A number of small-scale, human intervention studies have shed light on the beneficial effects of various (poly)phenol-rich foods on CVD risk factors. However, many of these studies are limited in that they lack proper control or do not fully characterize their intervention, in particular the (poly)phenol content. For example, if a study uses nothing or water as a control, such a control may well lack the various macro- and micronutrients that are part of the (poly)phenol-rich test intervention, and which may themselves exert efficacy (e.g., vitamin C/E, folate, and Se). Others do not report the (poly)phenol content and the compositional analysis of the test food/drink, creating significant problems when comparing different studies with the same food/beverage. Smaller issues, such as a failure to blind the study and a failure to record compliance and absorption/metabolism data, also limit the interpretation of the existing data. At present, uncertainty remains regarding which specific (poly)phenols are directly responsible for beneficial actions in vivo, as many of these foods/beverages contain a diversity of (poly)phenols classes (see Section II).
At present, the strongest evidence for the efficacy of (poly)phenols against chronic disease exists for flavan-3-ol-rich foods, in particular cocoa in respect to CVD risk. Many flavan-3-ol studies have used adequate controls in double-blind randomized studies, with well-characterized intervention foods/drinks. Additionally, they have strived to assess flavan-3-ol absorption and metabolism, a factor critical in building potential cause-and-effect relationships. Flavan-3-ol studies have been further strengthened by interventions, indicating that pure flavan-3-ols mimic the vascular effects exerted by flavan-3-ol-rich foods. As such, there is good evidence to suggest that the acute and/or regular consumption of flavan-3-ol rich cocoa or tea can have a positive effect on the vascular system, mainly through their effects on endothelial function, NO bioavailability, and BP. However, the long-term effects of flavan-3-ol interventions are presently unclear, and there are issues to resolve with respect to the efficacy of tea and caffeine.
Presently, the evidence for anthocyanins, flavonols and flavanones, stilbenes, hydroxycinnamates, and phenolic acids, based on studies with berries, pomegranate, red wine or citrus fruits, and coffee, is inconclusive, mainly due to the insufficiencies in the study design mentioned above. The diversity of (poly)phenols in berries and wine also makes it more difficult to assess putative health effects of individual (poly)phenols. Future studies will need to utilize other pure (poly)phenols in well-designed RCTs, before conclusions can be drawn regarding their specific biological function. Recent studies with flavanone-rich orange juice in parallel with pure flavanones are encouraging, in particular the positive effects exerted by pure flavanones on endothelial function and BP. Data with nuts are undoubtedly complicated by the presence of unsaturated fats, which are also known to be protective against various vascular risk factors. Future work with nuts will need to isolate specific (poly)phenol fractions for intervention to shed further light on the efficacy of nut (poly)phenols.
While there appears to be vast literature regarding the potential of (poly)phenols to improve cardiovascular health, at present, there is insufficient evidence to correlate individual (poly)phenols, with the exception of flavan-3-ol monomers and flavanones, with benefits against CVD risk, particularly with regard to long-term ingestion and risk. As such, additional long-term RCTs are required that utilize better-defined interventions, which are properly controlled and blinded, and that assess physiological endpoints linked to disease. Such studies should include detailed compositional analysis of the (poly)phenol-rich foods and control food/beverage carried out using validated analytical methods. If possible, future studies should include a pure (poly)phenol arm and controls designed accordingly to investigate the efficacy of specific (poly)phenols on functional outcomes. If such control arms are not possible (i.e., lack of a food grade quality, pure (poly)phenol in sufficient quantity), it is crucial that studies attempt to assess the absorption and metabolism of the (poly)phenols from the food/beverage that functional outcomes can be directly correlated to the presence of (poly)phenols/metabolites in the circulation. Ideally, what now required are long-term, large population RCT studies with healthy or at-risk individuals and with clinically relevant endpoints. Such studies are likely to be expensive, will require collaboration between academia and industry, and will run over a sufficiently long time frame (e.g., 3–12 months) to meet health claim criteria. Ultimately, (poly)phenols also need to be tested in the context of other unavoidable CVD risk factors, such as age, sex, and/or ethnicity. Inclusions of these are likely to increase the study population size considerably, and thus such studies will require cooperation between multiple centers, each running a cohort with the same dietary intervention/control. The outcomes of such future studies, if positive, may ultimately be used to make specific dietary recommendations regarding the efficacy of (poly)phenols in preventing the CVD risk.
Currently, there are also not enough data to clearly associate (poly)phenol consumption to improvements in neurological health. While there is extensive animal evidence for the actions of flavan-3-ols (from cocoa, wine, grape, etc.) and anthocyanins (from blueberry, grape, raspberry, etc.) on memory and learning, this still needs to be translated into humans before firm efficacy conclusions can be made. In such studies, intervention periods may need to be very long (12–24 months) before significant benefits emerge. There are limited data in support of a causal relationship between the consumption of flavonoids and behavioral outcomes. To make such relationships, future intervention studies will be required to utilize better-characterized intervention materials, more appropriate controls, and more rigorous clinical outcomes, such as well-designed human cognitive testing along with hemodynamic and structural changes measurable using MRI and fMRI techniques. Such hemodynamic changes may be further compared to changes in the gray matter density and to biomarkers of neural stem and progenitor cells using proton nuclear magnetic resonance spectroscopy. Such an approach will be essential to provide links between flavonoid intake and brain function in a mechanistic, dynamic, and quantitative way.
Another mechanism by which (poly)phenols may influence brain disease is through the modification of a number of risk factors common to AD, vascular dementia, hypertension, and CVD (26, 48). Modulation of these classical cardiovascular risk factors by flavonoids, as detailed in the previous section, may result in reductions in both typical and atypical cognitive function (43, 50). The ability of flavonoids to exert peripheral vascular changes (30, 66, 78, 83, 86, 97, 98, 147, 226) may facilitate more efficient cerebral blood flow (CBF), something that is known to deteriorate with age, to be vital for optimal brain function, and to be decreased in patients with dementia (163, 201, 395, 485). In this respect, flavan-3-ol-rich cocoa has also been shown to exert a positive effect on CBF through the middle cerebral artery in humans (55, 74, 80, 113). In support of these findings, arterial spin-labeling sequence magnetic resonance imaging also indicates that flavan-3-ols increase CBF up to a maximum of 2 h after ingestion (142).
In relation to dementia, there is a lack of human clinical evidence to date to make solid conclusions regarding function in patient populations, although the consumption of (poly)phenol-rich vegetables, fruit juices, and red wine has been reported to delay the onset of the disease (13, 50). However, the vast majority of data in support of their effects against dementia derive from animal studies that have been focused to a greater degree on the mechanism of action rather than functional outcomes. Further studies are required before conclusions may be drawn. As intervention times will have to be long, careful consideration needs will have to be afforded to whether supplementation with individual flavonoids, flavonoid combinations, or simply clearer dietary recommendations on flavonoid intake (including a potentially recommended daily intake level) represents the best initial approach for the rapid translation of these findings into effective interventions for individuals affected by dementia.
Evidence relating to the anticancer effects of (poly)phenols is limited. The majority of available clinical evidence has been with green tea/(poly)phenols in populations at a high risk of cancer development, with results proving inconclusive. In particular, there are some promising data indicating that regular consumption of green tea may retard oral and prostate cancer development. With regard to oral cancer, the efficacy may be apparent due to the direct interaction between (poly)phenols and cancer cells in the mouth, and suggest that similar effects against other GIT cancers (esophagus, stomach, small, and large intestine) may be possible. Future studies need to be designed in collaboration with cancer biologists and clinicians to test this hypothesis.
While a few of studies suggest that (poly)phenol-rich foods prevent lymphocyte DNA damage, no direct link with a decrease in cancer risk can be established from those studies. Undoubtedly, the oxidation of DNA may contribute to mutation rates and cancer risk; however, it is unlikely that such a reduction in lymphocyte DNA damage would impact on cancer development in sites outside the GIT, for example, breast, colon, and lung. As discussed earlier, more well-controlled, long-term intervention studies are needed, in particular those that measure tumor size, cancer incidence, and even cancer mortality as endpoints. Such endpoints are needed in the absence of suitable biomarkers of cancer risk, and should be measured routinely in healthy individuals or cancer patients. Until such studies have been conducted, it will be difficult to fully assess the impact of (poly)phenol-rich foods on cancer development and long-term risk.
Finally, a number of studies have been recently appearing in the literature overcoming previous limitations in the approaches used to unravel the putative mechanisms of action behind the protective effects of (poly)phenols. Identifying metabolites and catabolites and testing them in cell-based experimental models at physiological concentrations are the only ways to fully understand if the in vivo observations actually have molecular basis. While most of the published articles on (poly)phenol bioactivity still describe the effects of molecules that are only present in planta, detailed and elegant studies applying human and microbial metabolites have established reasonable mechanisms through which some candidates in this immense class of plant secondary metabolites may exert their beneficial actions. In this context, it is apparent that they do not act simply as antioxidants in vivo, and their diverse effects are, in most instances, based on more complex and specific modes of action, as discussed in Section V. Along with medium long-term RCTs, new and more refined molecular approaches will be welcome to finally and fully understand how these molecules, which have been relegated to the promising category for far too long, interact with human physiological and pathological processes. As yet, it is too premature to consider the potential use of (poly)phenolic compounds as therapeutic agents.
Abbreviations Used
- ABC
adenosine triphosphate-binding cassette
- ABP
ambulatory blood pressure
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ACN
anthocyanins
- AD
Alzheimer's disease
- AGEs
advanced glycation end products
- AIx
augmentation index
- AP
augmentation pressure
- ApoE
apolipoprotein E
- BAD
brachial artery diameter
- BMI
body–mass index
- BMP
bone morphogenetic protein
- BP
blood pressure
- BT
black tea
- BW
body weight
- CAD
coronary artery disease
- CBF
cerebral blood flow
- CBG
cytosolic β-glucosidase
- CF
cocoa flavanols
- CHD
coronary heart disease
- CIMT
carotid intima-media thickness
- Cmax
peak plasma concentration
- CML
carboxymethyl lysine
- Co-A
co-enzyme A
- COMT
catechol-O-methyltransferase
- COX-2
cyclooxygenase-2
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DG
dentate gyrus
- DGT
decaffeinated green tea
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- DRW
dealcoholized red wine
- EDD
endothelial-dependent dilation
- EGCG
(−)-epigallocatechin-3-O-gallate
- EID
endothelial-independent dilation
- eNOS
endothelial nitric oxide synthase
- ER
estrogen receptors
- ERK
extracellular-signal-regulated kinases
- EST
esterase
- FMD
flow-mediated dilation
- FRAP
ferric-reducing antioxidant power
- GIT
gastrointestinal tract
- GLT
glucose transporter
- GSTT2
glutathione S-transferase T2
- GT
green tea
- GTC
green tea capsules
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment–insulin resistance
- HPAEC
human pulmonary artery endothelial cells
- HPLC
high-performance liquid chromatography
- HPLC-MS
HPLC–mass spectrometry
- HR
heart rate
- HUVECs
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- IL-1β
interleukin-1 beta
- ISI
insulin sensitivity index
- JNK
c-Jun N-terminal kinase
- LDL
low-density lipoprotein
- LDL-C
low-density lipoprotein cholesterol
- LPH
lactase phloridzin hydrolase
- LPS
lipopolysaccharide
- M4
5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone
- M6′
5-(3′,5′-dihydroxyphenyl)-γ-valerolactone
- M6
5-(3′,4′-dihydroxyphenyl)-γ-valerolactone
- M7
5-(3′-hydroxyphenyl)-γ-valerolactone
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- MI
myocardial infarction
- MMP
metalloproteinases
- mPGES-1
microsomal PGE synthase-1
- MPP+
1-methyl-4-phenylpyridinium ion
- MRP
multidrug resistance protein
- MS
metabolic syndrome
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- nc
no change
- nd
not detected
- NFκB
nuclear factor κB
- NO
nitric oxide
- ORAC
oxygen-radical absorbance capacity
- PARP
poly(ADP-ribose) polymerase
- PAT
peripheral artery tonometry
- PGE
prostaglandin E
- PP
(poly)phenol aglycone
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- PP-met
polyphenol sulfate/glucuronide/methyl metabolites
- Pro
procyanidins
- PWV
pulse wave velocity
- PXR
pregnane X receptor
- QMP
quercetin metabolite-enriched plasma
- QUICKI
quantitative insulin sensitivity check index
- RA
reductase
- RANKL
receptor activator of nuclear factor κ-B ligand
- RCTs
randomized controlled trials
- ROS
reactive oxygen species
- RTV
ritonavir
- RW
red wine
- SBP
systolic blood pressure
- SGLT1
sodium-dependent glucose transporter 1
- SI clamp
glucose clamp index of insulin sensitivity
- SULT
sulfotransferase
- T½
elimination half-life
- TAG
triacylglycerols
- TC
total cholesterol
- TF
tea flavan-3-ols
- Tmax
time of peak plasma concentration
- TNF-α
tumour necrosis factor-α
- TP
total polyphenols
- UGT
uridine-5′-diphosphate glucuronosyltransferase
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- WW
white wine
Footnotes
Flavonoids and related compounds are also referred to as polyphenols. However, a number of compounds, including hydroxycinnamates and phenolic acids, have only one phenolic ring. In this review, therefore, the term (poly)phenolic compounds will be used.
This is because, as will be discussed later in the text, at a similar level of intake, the peak plasma concentration for quercetin metabolites can be ∼500 mM and that of (−)-epicatechin metabolites ∼200 nM. In contrast, urinary excretion of the flavonol metabolites corresponds to ∼4% of intake, while that of the flavan-3-ol is ∼30% of the ingested dose.
In a subsequent orange juice feeding study, urinary hesperetin metabolites that were identified were hesperetin-7-O-glucuronide, hesperetin-5-O-glucuronide, hesperetin-3′-O-glucuronide, hesperetin-5,7-O-diglucuronide, hesperetin-3′,7-O-diglucuronide, and hesperetin-3′-O-sulfate along with a hesperetin-O-glucuronide-O-sulfate (Fig. 17) (46).
Acknowledgment
Supported by “Progetto AGER,” grant n. 2011-0283.
References
- 1.Adams LS. Zhang Y. Seeram NP. Heber D. Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res. 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 3.Agewall S. Wright S. Doughty RN. Whalley GA. Duxbury M. Sharpe N. Does a glass of red wine improve endothelial function? Eur Heart J. 2000;21:74–78. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB. Sung B. The relationship between inflammation and cancer is analogous to that between fuel and fire. Oncology. 2011;25:414–418. [PubMed] [Google Scholar]
- 5.Al-Shalmani S. Suri S. Hughes DA. Kroon PA. Needs PW. Taylor MA. Tribolo S. Wilson VG. Quercetin and its principal metabolites, but not myricetin, oppose lipopolysaccharide-induced hyporesponsiveness of the porcine isolated coronary artery. Br J Pharmacol. 2011;162:1485–1497. doi: 10.1111/j.1476-5381.2010.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers AR. Varghese S. Vitseva O. Vita JA. Freedman JE. The antiinflammatory effects of purple grape juice consumption in subjects with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2004;24:e179–e180. doi: 10.1161/01.ATV.0000143479.97844.af. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos N. Vlachopoulos C. Aznaouridis K. Baou K. Vasiliadou C. Pietri P. Xaplanteris P. Stefanadi E. Stefanadis C. The acute effect of green tea consumption on endothelial function in healthy individuals. Eur J Cardiovasc Prev Rehabil. 2008;15:300–305. doi: 10.1097/HJR.0b013e3282f4832f. [DOI] [PubMed] [Google Scholar]
- 8.Almeida L. Vaz-da-Silva M. Falcão A. Soares E. Costa R. Loureiro AI. Fernandes-Lopes C. Rocha JF. Nunes T. Wright L. Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 9.Andres-Lacueva C. Shukitt-Hale B. Galli RL. Jauregui O. Lamuela-Raventos RM. Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 10.Appeldoom MM. Vincken J-P. Aura A-M. Hollman PC. Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J Agric Food Chem. 2009;57:1084–1092. doi: 10.1021/jf803059z. [DOI] [PubMed] [Google Scholar]
- 11.Ardalan MR. Tarzamni MK. Shoja MM. Tubbs RS. Rahimi-Ardabili B. Ghabili K. Khosroshahi HT. Black tea improves endothelial function in renal transplant recipients. Transplant Proc. 2007;39:1139–1142. doi: 10.1016/j.transproceed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Aron PM. Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res. 2008;52:79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 13.Arts IC. Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 14.Auger C. Hara Y. Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr. 2008;138:1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- 15.Aura A-M. Colon-derived microbial metabolites of dietary phenolic compounds. In: Packer L, editor; Cadenas H, editor. Oxidative Stress and Disease. Vol. 30. Boca Raton, FL: CRC Press; 2012. pp. 201–231. Flavonoids and Related Compounds: Bioavailability and Function, edited by Spencer JPE and Crozier A. [Google Scholar]
- 16.Aura A-M. Oikarinen S. Mutanen M. Heinonen SM. Adlercreutz HC. Virtanen H. Poutanen KS. Suitability of a batch in vitro fermentation model using human faecal microbiota for prediction of conversion of flaxseed lignans to enterolactone with reference to an in vivo rat model. Eur J Nutr. 2006;45:45–51. doi: 10.1007/s00394-005-0561-z. [DOI] [PubMed] [Google Scholar]
- 17.Aviram M. Dornfeld L. Kaplan M. Coleman R. Gaitini D. Nitecki S. Hofman A. Rosenblat M. Volkova N. Presser D. Attias J. Hayek T. Fuhrman B. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans. Drugs Exp Clin Res. 2002;28:49–62. [PubMed] [Google Scholar]
- 18.Aviram M. Rosenblat M. Gaitini D. Nitecki S. Hoffman A. Dornfeld L. Volkova N. Presser D. Attias J. Liker H. Hayek T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23:423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Aviram M. Volkova N. Coleman R. Dreher M. Reddy MK. Ferreira D. Rosenblat M. Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: studies in vivo in atherosclerotic apolipoprotein e-deficient (E 0) mice and in vitro in cultured macrophages and lipoproteins. J Agric Food Chem. 2008;56:1148–1157. doi: 10.1021/jf071811q. [DOI] [PubMed] [Google Scholar]
- 20.Baba S. Osakabe N. Yasuda A. Natsume M. Takizawa T. Nakamura T. Terao J. Bioavailability of (−)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic Res. 2000;33:635–641. doi: 10.1080/10715760000301151. [DOI] [PubMed] [Google Scholar]
- 21.Bahorun T. Luximon-Ramma A. Neergheen-Bhujun VS. Gunness TK. Googoolye K. Auger C. Crozier A. Aruoma OI. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev Med. 2012;54:S98–S102. doi: 10.1016/j.ypmed.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Balzer J. Rassaf T. Heiss C. Kleinbongard P. Lauer T. Merx M. Heussen N. Gross HB. Keen CL. Schroeter H. Kelm M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 23.Banel DK. Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barberger-Gateau P. Raffaitin C. Letenneur L. Berr C. Tzourio C. Dartigues JF. Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 25.Barron D. Smarrito-Menozzi C. Fumeaux R. Viton F. Synthesis of dietary phenolic metabolites and isotopically-labeled dietary phenolics. In: Spencer JPE, editor; Crozier A, editor; Packer L, editor; Cadenas H, editor. Bioavailability and Function of Flavonoids. Oxidative Stress and Disease. Vol. 30. Boca Raton, FL: CRC Press; 2012. pp. 233–280. [Google Scholar]
- 26.Barros D. Amaral OB. Izquierdo I. Geracitano L. do Carmo Bassols Raseira M. Henriques AT. Ramirez MR. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol Biochem Behav. 2006;84:229–234. doi: 10.1016/j.pbb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Basu A. Du M. Leyva MJ. Sanchez K. Betts NM. Wu M. Aston CE. Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140:1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu A. Sanchez K. Leyva MJ. Wu M. Betts NM. Aston CE. Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29:31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 29.Benetou V. Orfanos P. Lagiou P. Trichopoulos D. Boffetta P. Trichopoulou A. Vegetables and fruits in relation to cancer risk: evidence from the Greek EPIC cohort study. Cancer Epidemiol Biomarkers Prev. 2008;17:387–392. doi: 10.1158/1055-9965.EPI-07-2665. [DOI] [PubMed] [Google Scholar]
- 30.Bettuzzi S. Brausi M. Rizzi F. Castagnetti G. Peracchia G. Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 31.Bialonska D. Kasimsetty SG. Khan SI. Ferreira D. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J Agric Food Chem. 2009;57:10181–10186. doi: 10.1021/jf9025794. [DOI] [PubMed] [Google Scholar]
- 32.Bingham SA. Vorster H. Jerling JC. Magee E. Mulligan A. Runswick SA. Cummings JH. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br J Nutr. 1997;78:41–55. doi: 10.1079/bjn19970117. [DOI] [PubMed] [Google Scholar]
- 33.Birge SJ. Is there a role for estrogen replacement therapy in the prevention and treatment of dementia? J Am Geriatr Soc. 1996;44:865–870. doi: 10.1111/j.1532-5415.1996.tb03749.x. [DOI] [PubMed] [Google Scholar]
- 34.Bjelland S. Seeberg E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Boban M. Modun D. Music I. Vukovic J. Brizic I. Salamunic I. Obad A. Palada I. Dujic Z. Red wine induced modulation of vascular function: separating the role of polyphenols, ethanol, and urates. J Cardiovasc Pharm. 2006;47:695–701. doi: 10.1097/01.fjc.0000211762.06271.ce. [DOI] [PubMed] [Google Scholar]
- 36.Boesch-Saadatmandi C. Loboda A. Wagner AE. Stachurska A. Jozkowicz A. Dulak J. Döring F. Wolffram S. Rimbach G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. J Nutr Biochem. 2011;22:293–299. doi: 10.1016/j.jnutbio.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Bolling BW. Chen CY. McKay DL. Blumberg JB. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24:1–32. doi: 10.1017/S095442241100014X. [DOI] [PubMed] [Google Scholar]
- 38.Bonetti A. Marotti I. Dinelli G. Urinary excretion of kaempferol from common beans (Phaseolus vulgaris L.) in humans. Int J Food Sci. 2007;58:261–269. doi: 10.1080/09637480601154228. [DOI] [PubMed] [Google Scholar]
- 39.Boocock DJ. Faust GE. Patel KR. Schinas AM. Brown VA. Ducharme MP. Booth TD. Crowell JA. Perloff M. Gescher AJ. Steward WP. Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 40.Boocock DJ. Patel KR. Faust GE. Normolle DP. Marczylo TH. Crowell JA. Brenner DE. Booth TD. Gescher A. Steward WP. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. Chromatogr B. 2007;848:182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borges G. Degeneve A. Mullen W. Crozier A. Identification of flavonoid and phenolic antioxidants in blackcurrants, blueberries, raspberries, redcurrants and cranberries. J Agric Food Chem. 2010;58:3901–3909. doi: 10.1021/jf902263n. [DOI] [PubMed] [Google Scholar]
- 42.Borges G. Mullen W. Mullan A. Lean MEJ. Roberts SA. Crozier A. Bioavailability of multiple components following acute ingestion of a polyphenol-rich drink. Mol Nutr Food Res. 2010;5:S268–S277. doi: 10.1002/mnfr.200900611. [DOI] [PubMed] [Google Scholar]
- 43.Borges G. Roowi S. Rouanet J-M. Duthie GG. Lean MEJ. Crozier A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol Nutr Food Res. 2007;51:714–725. doi: 10.1002/mnfr.200700024. [DOI] [PubMed] [Google Scholar]
- 44.Botterweck AA. van den Brandt PA. Goldbohm RA. A prospective cohort study on vegetable and fruit consumption and stomach cancer risk in The Netherlands. Am J Epidemiol. 1998;148:842–853. doi: 10.1093/oxfordjournals.aje.a009709. [DOI] [PubMed] [Google Scholar]
- 45.Braune A. Engst W. Blaut M. Degradation of neohesperidin dihydrochalcone by human intestinal bacteria. J Agric Food Chem. 2005;53:1782–1790. doi: 10.1021/jf0484982. [DOI] [PubMed] [Google Scholar]
- 46.Bredsdorff L. Nielson ILF. Rasmussen SE. Cornett C. Barron D. Bouisset F. Offord E. Williamson G. Absorption, conjugation and excretion of the flavanones, naringenin and hesperetin from α-rhamnosidase−treated orange juice in human subjects. Br J Nutr. 2010;103:1602–1609. doi: 10.1017/S0007114509993679. [DOI] [PubMed] [Google Scholar]
- 47.Brenn T. The Tromso heart study: alcoholic beverages and coronary risk factors. J Epidemiol Community Health. 1986;40:249–256. doi: 10.1136/jech.40.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breteler MM. Vascular risk factors for Alzheimer's disease: an epidemiologic perspective. Neurobiol Aging. 2000;21:153–160. doi: 10.1016/s0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 49.Brett GM. Hollands W. Needs PW. Teucher B. Dainty JR. Davis BD. Brodbelt JS. Kroon PA. Absorption, metabolism and excretion of flavanones from singles portion of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanones excretion. Br J Nutr. 2008;101:1–12. doi: 10.1017/S000711450803081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bridges JW. French MR. Smith RL. Williams RT. The fate of benzoic acid in various species. Biochem J. 1970;118:47–51. doi: 10.1042/bj1180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brien SE. Ronksley PE. Turner BJ. Mukamal KJ. Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Briviba K. Bub A. Moseneder J. Schwerdtle T. Hartwig A. Kulling S. Watzl B. No differences in DNA damage and antioxidant capacity between intervention groups of healthy, nonsmoking men receiving 2, 5, or 8 servings/day of vegetables and fruit. Nutr Cancer. 2008;60:164–170. doi: 10.1080/01635580701621346. [DOI] [PubMed] [Google Scholar]
- 53.Brown AL. Lane J. Holyoak C. Nicol B. Mayes AE. Dadd T. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr. 2011;106:1880–1889. doi: 10.1017/S0007114511002376. [DOI] [PubMed] [Google Scholar]
- 54.Buijsse B. Weikert C. Drogan D. Bergmann M. Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010;31:1616–1623. doi: 10.1093/eurheartj/ehq068. [DOI] [PubMed] [Google Scholar]
- 55.Bulpitt CJ. Shipley MJ. Semmence A. The contribution of a moderate intake of alcohol to the presence of hypertension. J Hypertens. 1987;5:85–91. doi: 10.1097/00004872-198702000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Burke SN. Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 57.Burkon A. Somoza V. Quantification of free and protein bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides–two novel resveratrol metabolites in human plasma. Mol Nutr Food Res. 2008;52:549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 58.Buscemi S. Verga S. Batsis JA. Donatelli M. Tranchina MR. Belmonte S. Mattina A. Re A. Cerasola G. Acute effects of coffee on endothelial function in healthy subjects. Eur J Clin Nutr. 2010;64:483–489. doi: 10.1038/ejcn.2010.9. [DOI] [PubMed] [Google Scholar]
- 59.Buscemi S. Verga S. Batsis JA. Tranchina MR. Belmonte S. Mattina A. Re A. Rizzo R. Cerasola G. Dose-dependent effects of decaffeinated coffee on endothelial function in healthy subjects. Eur J Clin Nutr. 2009;63:1200–1205. doi: 10.1038/ejcn.2009.51. [DOI] [PubMed] [Google Scholar]
- 60.Calani L. Del Rio D. Luisa Callegari M. Morelli L. Brighenti F. Updated bioavailability and 48 h excretion profile of flavan-3-ols from green tea in humans. Int J Food Sci Nutr. 2012;63:513–521. doi: 10.3109/09637486.2011.640311. [DOI] [PubMed] [Google Scholar]
- 61.Calomme M. Pieters L. Vlietinck A. Vanden Berghe D. Inhibition of bacterial mutagenesis by citrus flavonoids. Planta Med. 1996;62:222–226. doi: 10.1055/s-2006-957864. [DOI] [PubMed] [Google Scholar]
- 62.Carkeet C. Clevidence BA. Novotny JA. Anthocyanin excretion by humans increases linearly with increasing strawberry dose. J Nutr. 2008;138:897–902. doi: 10.1093/jn/138.5.897. [DOI] [PubMed] [Google Scholar]
- 63.Carmen Ramirez-Tortosa M. Garcia-Alonso J. Luisa Vidal-Guevara M. Quiles JL. Jesus Periago M. Linde J. Dolores Mesa M. Ros G. Abellan P. Gil A. Oxidative stress status in an institutionalised elderly group after the intake of a phenolic-rich dessert. Br J Nutr. 2004;91:943–950. doi: 10.1079/bjn20041146. [DOI] [PubMed] [Google Scholar]
- 64.Carreau C. Flouriot G. Bennetau-Pelissero C. Potier M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERα transcriptional activation in human breast cancer cells. J Steroid Biochem Mol Biol. 2008;110:176–185. doi: 10.1016/j.jsbmb.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 65.Casadesus G. Shukitt-Hale B. Stellwagen HM. Zhu X. Lee HG. Smith MA. Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 66.Casas-Agustench P. Lopez-Uriarte P. Ros E. Bullo M. Salas-Salvado J. Nuts, hypertension and endothelial function. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 1):S21–S33. doi: 10.1016/j.numecd.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Casini ML. Marelli G. Papaleo E. Ferrari A. D'Ambrosio F. Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006;85:972–978. doi: 10.1016/j.fertnstert.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 68.Cerdá B. Espín JC. Parra S. Martínez P. Tomás-Barberán FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43:205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 69.Cerdá B. Tomás-Barberán FA. Espín JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem. 2005;53:227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 70.Chan YC. Hosoda K. Tsai CJ. Yamamoto S. Wang MF. Favorable effects of tea on reducing the cognitive deficits and brain morphological changes in senescence-accelerated mice. J Nutr Sci Vitaminol. 2006;52:266–273. doi: 10.3177/jnsv.52.266. [DOI] [PubMed] [Google Scholar]
- 71.Chao CL. Hou YC. Chao PD. Weng CS. Ho FM. The antioxidant effects of quercetin metabolites on the prevention of high glucose-induced apoptosis of human umbilical vein endothelial cells. Br J Nutr. 2009;101:1165–1170. doi: 10.1017/S0007114508073637. [DOI] [PubMed] [Google Scholar]
- 72.Chaves AA. Joshi MS. Coyle CM. Brady JE. Dech SJ. Schanbacher BL. Baliga R. Basuray A. Bauer JA. Vasoprotective endothelial effects of a standardized grape product in humans. Vascul Pharmacol. 2009;50:20–26. doi: 10.1016/j.vph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Chen LH. Fang J. Li H. Demark-Wahnefried W. Lin X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol Cancer Ther. 2007;6:2581–2590. doi: 10.1158/1535-7163.MCT-07-0220. [DOI] [PubMed] [Google Scholar]
- 74.Cheng C. Wang X. Weakley SM. Kougias P. Lin PH. Yao Q. Chen C. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Nutr. 2010;140:12–17. doi: 10.3945/jn.109.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chow HH. Cai Y. Alberts DS. Hakim I. Dorr R. Shahi F. Crowell JA. Yang CS. Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and Polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–58. [PubMed] [Google Scholar]
- 76.Chu LM. Lassaletta AD. Robich MP. Sellke FW. Resveratrol in the prevention and treatment of coronary artery disease. Curr Atheroscl Rep. 2011;13:439–446. doi: 10.1007/s11883-011-0202-3. [DOI] [PubMed] [Google Scholar]
- 77.Clavel T. Henderson G. Alpert C-A. Philippe C. Rigottier-Gois L. Doré J. Blaut M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol. 2005;71:6077–6085. doi: 10.1128/AEM.71.10.6077-6085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clifford MN. Anthocyanins—nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1063–1072. [Google Scholar]
- 79.Clifford MN. Copeland EL. Bloxsidge JP. Mitchell LA. Hippuric acid as a major excretion product associated with black tea consumption. Xenobiotica. 2000;30:317–326. doi: 10.1080/004982500237703. [DOI] [PubMed] [Google Scholar]
- 80.Clifford MN. Crozier A. Phytochemicals in teas and tisanes and their bioavailability. In: Crozier A, editor; Ashihara H, editor; Tomás-Barbéran F, editor. Teas, Cocoa and Coffee: Plant Secondary Metabolites and Health. Oxford: Blackwell Publishing; 2011. pp. 45–98. [Google Scholar]
- 81.Coldham NG. Darby C. Hows M. King LJ. Zhang AQ. Saurer MJ. Comparative metabolism of genistein in human and rat gut microflora: detection and identification of the end-products of metabolism. Xenobiotica. 2002;32:45–62. doi: 10.1080/00498250110085809. [DOI] [PubMed] [Google Scholar]
- 82.Collins BH. Horska A. Hotten PM. Riddoch C. Collins AR. Kiwifruit protects against oxidative DNA damage in human cells and in vitro. Nutr Cancer. 2001;39:148–153. doi: 10.1207/S15327914nc391_20. [DOI] [PubMed] [Google Scholar]
- 83.Commenges D. Scotet V. Renaud S. Jacqmin-Gadda H. Barberger-Gateau P. Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–363. doi: 10.1023/a:1007614613771. [DOI] [PubMed] [Google Scholar]
- 84.Corder R. Red wine, chocolate and vascular health: developing the evidence base. Heart. 2008;94:821–823. doi: 10.1136/hrt.2008.143909. [DOI] [PubMed] [Google Scholar]
- 85.Corder R. Crozier A. Kroon PA. Drinking your health? It's too early to say. Nature. 2003;426:119. doi: 10.1038/426119d. [DOI] [PubMed] [Google Scholar]
- 86.Cordova AC. Jackson LS. Berke-Schlessel DW. Sumpio BE. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–439. doi: 10.1016/j.jamcollsurg.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 87.Corsini E. Dell'Agli M. Facchi A. De Fabiani E. Lucchi L. Boraso MS. Marinovich M. Galli CL. Enterodiol and enterolactone modulate the immune response by acting on nuclear factor-κB (NF-κB) signaling. J Agric Food Chem. 2010;58:6678–6684. doi: 10.1021/jf100471n. [DOI] [PubMed] [Google Scholar]
- 88.Coward L. Smith M. Kirk M. Barnes S. Chemical modification of isoflavones in soyfoods during cooking and processing. Am J Clin Nutr. 1998;68:1486S–1491S. doi: 10.1093/ajcn/68.6.1486S. [DOI] [PubMed] [Google Scholar]
- 89.Crews WD., Jr. Harrison DW. Wright JW. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. Am J Clin Nutr. 2008;87:872–880. doi: 10.1093/ajcn/87.4.872. [DOI] [PubMed] [Google Scholar]
- 90.Crowe FL. Roddam AW. Key TJ. Appleby PN. Overvad K. Jakobsen MU. Tjonneland A. Hansen L. Boeing H. Weikert C. Linseisen J. Kaaks R. Trichopoulou A. Misirli G. Lagiou P. Sacerdote C. Pala V. Palli D. Tumino R. Panico S. Bueno-de-Mesquita HB. Boer J. van Gils CH. Beulens JW. Barricarte A. Rodriguez L. Larranaga N. Sanchez MJ. Tormo MJ. Buckland G. Lund E. Hedblad B. Melander O. Jansson JH. Wennberg P. Wareham NJ. Slimani N. Romieu I. Jenab M. Danesh J. Gallo V. Norat T. Riboli E. Fruit and vegetable intake and mortality from ischaemic heart disease: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. Eur Heart J. 2011;32:1235–1243. doi: 10.1093/eurheartj/ehq465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crozier A, editor; Ashihara H, editor; Tomás-Barbéran F, editor. Teas, Cocoa and Coffee: Plant Secondary Metabolites and Health. Oxford: Blackwell Publishing; 2011. [Google Scholar]
- 92.Crozier A. Borges G. Ryan D. The glass that cheers: phenolic and polyphenolic constituents and the beneficial effects of moderate red wine consumption. Biochemist. 2010;32:4–9. [Google Scholar]
- 93.Crozier A. Jaganath IB. Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 94.Crozier A. McDonald MS. Lean MEJ. Black C. Quantitative analysis of the flavonoid content of tomatoes, onions, lettuce and celery. J Agric Food Chem. 1997;45:590–595. [Google Scholar]
- 95.Crozier A. Yokota T. Jaganath IB. Marks S. Saltmarsh M. Clifford MN. Secondary metabolites as dietary components in plant-based foods and beverages. In: Crozier A, editor; Clifford MN, editor; Ashihara H, editor. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Oxford: Blackwell Publishing; 2006. pp. 208–302. [Google Scholar]
- 96.Crozier TWM. Stalmach A. Lean MEJ. Crozier A. Espresso coffees, caffeine and chlorogenic acid intake: potential health implications. Food Funct. 2012;3:30–33. doi: 10.1039/c1fo10240k. [DOI] [PubMed] [Google Scholar]
- 97.Cutler GJ. Nettleton JA. Ross JA. Harnack LJ. Jacobs DR., Jr. Scrafford CG. Barraj LM. Mink PJ. Robien K. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women's Health Study. Int J Cancer. 2008;123:664–671. doi: 10.1002/ijc.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.da Silva EL. Piskula MK. Yamamoto N. Moon JH. Terao J. Quercetin metabolites inhibit copper ion-induced lipid peroxidation in rat plasma. FEBS Lett. 1998;430:405–408. doi: 10.1016/s0014-5793(98)00709-1. [DOI] [PubMed] [Google Scholar]
- 99.Dai Q. Borenstein AR. Wu Y. Jackson JC. Larson EB. Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am J Med. 2006;119:751–759. doi: 10.1016/j.amjmed.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dauchet L. Amouyel P. Hercberg S. Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136:2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 101.Davidson MH. Maki KC. Dicklin MR. Feinstein SB. Witchger M. Bell M. McGuire DK. Provost JC. Liker H. Aviram M. Effects of consumption of pomegranate juice on carotid intima-media thickness in men and women at moderate risk for coronary heart disease. Am J Cardiol. 2009;104:936–942. doi: 10.1016/j.amjcard.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 102.Davison K. Coates AM. Buckley JD. Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes. 2008;32:1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 103.Day AJ. Bao Y. Morgan MR. Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med. 2000;29:1234–1243. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 104.Day AJ. Canada FJ. Diaz JC. Kroon PA. Mclauchlan R. Faulds CB. Plumb GW. Morgan MR. Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phloridzin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 105.Day AJ. Mellon F. Barron D. Sarrazin G. Morgan MRA. Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35:941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 106.Day AJ. Morgan MRA. Methods of polyphenol extraction from biological fluids and tissues. In: Santos-Buelga C, editor; Williamson G, editor. Methods in Polyphenol Analysis. Cambridge: Royal Society of Chemistry; 2003. pp. 17–47. [Google Scholar]
- 107.Daykin CA. van Duynhoven JP. Groenewegen A. Dachtler M. van Amelsvoort JM. Mulder TP. Nuclear magnetic resonance spectroscopic based studies of the metabolism of black tea polyphenols in humans. J Agric Food Chem. 2005;53:1428–1434. doi: 10.1021/jf048439o. [DOI] [PubMed] [Google Scholar]
- 108.Del Rio D. Calani L. Cordero C. Salvatore S. Pellegrini N. Brighenti F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition. 2010;26:1110–1116. doi: 10.1016/j.nut.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 109.Del Rio D. Costa LG. Lean ME. Crozier A. Polyphenols and health: what compounds are involved? Nutr Metab Cardiovasc Dis. 2010;20:1–6. doi: 10.1016/j.numecd.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 110.Del Rio D. Stewart AJ. Mullen W. Burns J. Lean MEJ. Brighenti F. Crozier A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J Agric Food Chem. 2004;52:2807–2815. doi: 10.1021/jf0354848. [DOI] [PubMed] [Google Scholar]
- 111.Déprez S. Brezillon C. Rabot S. Philippe C. Mila I. Lapierre C. Scalbert A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr. 2000;130:2733–2738. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- 112.Desch S. Schmidt J. Kobler D. Sonnabend M. Eitel I. Sareban M. Rahimi K. Schuler G. Thiele H. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 113.Dinges DF. Cocoa flavanols, cerebral blood flow, cognition, and health: going forward. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S221–S223. doi: 10.1097/00005344-200606001-00019. [DOI] [PubMed] [Google Scholar]
- 114.Dohadwala MM. Hamburg NM. Holbrook M. Kim BH. Duess MA. Levit A. Titas M. Chung WB. Vincent FB. Caiano TL. Frame AA. Keaney JF., Jr. Vita JA. Effects of Concord grape juice on ambulatory blood pressure in prehypertension and stage 1 hypertension. Am J Clin Nutr. 2010;92:1052–1059. doi: 10.3945/ajcn.2010.29905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dohadwala MM. Holbrook M. Hamburg NM. Shenouda SM. Chung WB. Titas M. Kluge MA. Wang N. Palmisano J. Milbury PE. Blumberg JB. Vita JA. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93:934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dohadwala MM. Vita JA. Grapes and cardiovascular disease. J Nutr. 2009;139:1788S–1793S. doi: 10.3945/jn.109.107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Donnini S. Finetti F. Lusini L. Morbidelli L. Cheynier V. Barron D. Williamson G. Waltenberger J. Ziche M. Divergent effects of quercetin conjugates on angiogenesis. Br J Nutr. 2006;95:1016–1023. doi: 10.1079/bjn20061753. [DOI] [PubMed] [Google Scholar]
- 118.Donovan JL. Manach C. Faulks RM. Kroon PA. Absorption and metabolism of dietary secondary metabolites. In: Crozier A, editor; Clifford MN, editor; Ashihara H, editor. Plant Secondary Metabolites. Occurrence, Structure and Role in the Human Diet. Oxford: Blackwell Publishing; 2006. pp. 303–351. [Google Scholar]
- 119.Donovan JL. Manach C. Rios L. Morand C. Scalbert A. Rémésy C. Procyanidins are not bioavailable in rats fed a single meal containing a grape seed extract or the procyanidin dimer B3. Br J Nutr. 2002;87:299–306. doi: 10.1079/bjnbjn2001517. [DOI] [PubMed] [Google Scholar]
- 120.Drapeau E. Mayo W. Aurousseau C. Le MM. Piazza PV. Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dueñas M. González-Manzano S. González-Paramás A. Santos-Buelga C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J Pharm Biomed Anal. 2010;51:443–449. doi: 10.1016/j.jpba.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Duffy R. Wiseman H. File SE. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol Biochem Behav. 2003;75:721–729. doi: 10.1016/s0091-3057(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 123.Duffy SJ. Keaney JF., Jr. Holbrook M. Gokce N. Swerdloff PL. Frei B. Vita JA. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 124.Duffy SJ. Vita JA. Holbrook M. Swerdloff PL. Keaney JF., Jr Effect of acute and chronic tea consumption on platelet aggregation in patients with coronary artery disease. Arterioscler Thromb Vasc. 2001;21:1084–1089. doi: 10.1161/01.atv.21.6.1084. [DOI] [PubMed] [Google Scholar]
- 125.DuPont MS. Day AJ. Bennet RN. Mellon FA. Kroon PA. Absorption of kaempferol from endive, source of kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 2004;58:947–954. doi: 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- 126.Duthie SJ. Dobson VL. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur J Nutr. 1999;38:28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- 127.Duthie SJ. Jenkinson AM. Crozier A. Mullen W. Pirie L. Kyle J. Yap LS. Christen P. Duthie GG. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. 2006;45:113–122. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 128.Eeckhaut E. Struijs K. Possemiers S. Vincken JP. Keukeleire DD. Verstraete W. Metabolism of lignan macromolecule into eneterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J Agric Food Chem. 2008;56:4806–4812. doi: 10.1021/jf800101s. [DOI] [PubMed] [Google Scholar]
- 129.El Mohsen MA. Marks J. Kuhnle G. Moore K. Debnam E. Kaila Srai S. Rice-Evans C. Spencer JP. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br J Nutr. 2006;95:51–58. doi: 10.1079/bjn20051596. [DOI] [PubMed] [Google Scholar]
- 130.Elks CM. Reed SD. Mariappan N. Shukitt-Hale B. Joseph JA. Ingram DK. Francis J. A blueberry-enriched diet attenuates nephropathy in a rat model of hypertension via reduction in oxidative stress. PLoS One. 2011;6:e24028. doi: 10.1371/journal.pone.0024028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Engler MB. Engler MM. Chen CY. Malloy MJ. Browne A. Chiu EY. Kwak HK. Milbury P. Paul SM. Blumberg J. Mietus-Snyder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 132.Erlund I. Koli R. Alfthan G. Marniemi J. Puukka P. Mustonen P. Mattila P. Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87:323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 133.Espín JC. García-Conesa MT. Tomás-Barberán FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 134.Espín JC. González-Barrio R. Cerdá B. López-Bote C. Rey AI. Tomás-Barberán FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem. 2007;55:10476–10485. doi: 10.1021/jf0723864. [DOI] [PubMed] [Google Scholar]
- 135.Estruch R. Sacanella E. Mota F. Chiva-Blanch G. Antunez E. Casals E. Deulofeu R. Rotilio D. Andres-Lacueva C. Lamuela-Raventos RM. de Gaetano G. Urbano-Marquez A. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: a randomised cross-over trial. Nutr Metab Cardiovasc Dis. 2011;21:46–53. doi: 10.1016/j.numecd.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 136.Faridi Z. Njike VY. Dutta S. Ali A. Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr. 2008;88:58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 137.Favero A. Parpinel M. Franceschi S. Diet and risk of breast cancer: major findings from an Italian case-control study. Biomed Pharmacother. 1998;52:109–115. doi: 10.1016/S0753-3322(98)80088-7. [DOI] [PubMed] [Google Scholar]
- 138.Fehr M. Galliard-Grigioni KS. Reinhart WH. Influence of acute alcohol exposure on hemorheological parameters and platelet function in vivo and in vitro. Clin Hemorheol Microcirc. 2008;39:351–358. [PubMed] [Google Scholar]
- 139.Felgines C. Talavéra S. Gonthier M-P. Texier O. Scalbert A. Lamaison JL. Rémésy C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J Nutr. 2003;133:1269–1301. doi: 10.1093/jn/133.5.1296. [DOI] [PubMed] [Google Scholar]
- 140.Feskanich D. Ziegler RG. Michaud DS. Giovannucci EL. Speizer FE. Willett WC. Colditz GA. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst. 2000;92:1812–1823. doi: 10.1093/jnci/92.22.1812. [DOI] [PubMed] [Google Scholar]
- 141.File SE. Jarrett N. Fluck E. Duffy R. Casey K. Wiseman H. Eating soya improves human memory. Psychopharmacology. 2001;157:430–436. doi: 10.1007/s002130100845. [DOI] [PubMed] [Google Scholar]
- 142.Fisher ND. Sorond FA. Hollenberg NK. Cocoa flavanols and brain perfusion. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S210–S214. doi: 10.1097/00005344-200606001-00017. [DOI] [PubMed] [Google Scholar]
- 143.Flammer AJ. Hermann F. Sudano I. Spieker L. Hermann M. Cooper KA. Serafini M. Luscher TF. Ruschitzka F. Noll G. Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- 144.Forester SC. Waterhouse AL. Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells. J Agric Food Chem. 2010;58:5320–5327. doi: 10.1021/jf9040172. [DOI] [PubMed] [Google Scholar]
- 145.Fournier LR. Ryan Borchers TA. Robison LM. Wiediger M. Park JS. Chew BP. McGuire MK. Sclar DA. Skaer TL. Beerman KA. The effects of soy milk and isoflavone supplements on cognitive performance in healthy, postmenopausal women. J Nutr Health Aging. 2007;11:155–164. [PubMed] [Google Scholar]
- 146.Fraga CG. Actis-Goretta L. Ottaviani JI. Carrasquedo F. Lotito SB. Lazarus S. Schmitz HH. Keen CL. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin Dev Immunol. 2005;12:11–17. doi: 10.1080/10446670410001722159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fraga CG. Litterio MC. Prince PD. Calabro V. Piotrkowski B. Galleano M. Cocoa flavanols: effects on vascular nitric oxide and blood pressure. J Clin Biochem Nutr. 2011;48:63–67. doi: 10.3164/jcbn.11-010FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fraga CG. Oteiza PI. Dietary flavonoids: role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic Biol Med. 2011;51:813–823. doi: 10.1016/j.freeradbiomed.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 149.Francis ST. Head K. Morris PG. Macdonald IA. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S215–S220. doi: 10.1097/00005344-200606001-00018. [DOI] [PubMed] [Google Scholar]
- 150.Gao HM. Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gee JM. DuPont SM. Day AJ. Plumb GW. Williamson G. Johnson IT. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J Nutr. 2000;130:2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- 152.Giovannelli L. Pitozzi V. Luceri C. Giannini L. Toti S. Salvini S. Sera F. Souquet JM. Cheynier V. Sofi F. Mannini L. Gori AM. Abbate R. Palli D. Dolara P. Effects of de-alcoholised wines with different polyphenol content on DNA oxidative damage, gene expression of peripheral lymphocytes, and haemorheology: an intervention study in post-menopausal women. Eur J Nutr. 2011;50:19–29. doi: 10.1007/s00394-010-0111-1. [DOI] [PubMed] [Google Scholar]
- 153.Glässer G. Graefe EU. Struck F. Veit M. Gebhardt R. Comparison of antioxidative capacities and inhibitory effects on cholesterol biosynthesis of quercetin and potential metabolites. Phytomedicine. 2002;9:33–40. doi: 10.1078/0944-7113-00080. [DOI] [PubMed] [Google Scholar]
- 154.Goldberg DM. Yan J. Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 155.Gómez-Ruiz JA. Leake DS. Ames JM. In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem. 2007;55:6962–6969. doi: 10.1021/jf0710985. [DOI] [PubMed] [Google Scholar]
- 156.Gonthier MP. Donovan JL. Texier O. Felgines C. Remesy C. Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35:837–844. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 157.González CA. Pera G. Agudo A. Bueno-de-Mesquita HB. Ceroti M. Boeing H. Schulz M. Del Giudice G. Plebani M. Carneiro F. Berrino F. Sacerdote C. Tumino R. Panico S. Berglund G. Siman H. Hallmans G. Stenling R. Martinez C. Dorronsoro M. Barricarte A. Navarro C. Quiros JR. Allen N. Key TJ. Bingham S. Day NE. Linseisen J. Nagel G. Overvad K. Jensen MK. Olsen A. Tjonneland A. Buchner FL. Peeters PH. Numans ME. Clavel-Chapelon F. Boutron-Ruault MC. Roukos D. Trichopoulou A. Psaltopoulou T. Lund E. Casagrande C. Slimani N. Jenab M. Riboli E. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Cancer. 2006;118:2559–2566. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 158.González-Barrio R. Borges G. Mullen W. Crozier A. Bioavailability of raspberry anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J Agric Food Chem. 2010;58:3933–3939. doi: 10.1021/jf100315d. [DOI] [PubMed] [Google Scholar]
- 159.González-Barrio R. Edwards CA. Crozier A. Colonic catabolism of ellagitannins, ellagic acid and raspberry anthocyanins: in vivo and in vitro studies. Drug Met Dispos. 2011;39:1680–1688. doi: 10.1124/dmd.111.039651. [DOI] [PubMed] [Google Scholar]
- 160.González-Sarrías A. Azorín-Ortuño M. Yáñez-Gascón MJ. Tomás-Barberán FA. García-Conesa MT. Espín JC. Dissimilar in vitro and in vivo effects of ellagic acid and its microbiota-derived metabolites, urolithins, on the cytochrome P450 1A1. J Agric Food Chem. 2009;57:5623–5632. doi: 10.1021/jf900725e. [DOI] [PubMed] [Google Scholar]
- 161.González-Sarrías A. Espín JC. Tomás-Barberán FA. García-Conesa MT. Gene expression, cell cycle arrest and MAPK signaling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins. Mol Nutr Food Res. 2009;53:686–698. doi: 10.1002/mnfr.200800150. [DOI] [PubMed] [Google Scholar]
- 162.González-Sarrías A. Giménez-Bastida JA. García-Conesa MT. Gómez-Sánchez MB. García-Talavera NV. Gil-Izquierdo A. Sánchez-Alvarez C. Fontana-Compiano LO. Morga-Egea JP. Pastor-Quirante FA. Martínez-Díaz F. Tomás-Barberán FA. Espín JC. Occurrence of urothins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland after consumption of walnuts and pomegranate juice. Mol Nutr Food Res. 2010;54:311–322. doi: 10.1002/mnfr.200900152. [DOI] [PubMed] [Google Scholar]
- 163.González-Sarrías A. Larrosa M. Tomás-Barberán FA. Dolara P. Espín JC. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr. 2010;104:503–512. doi: 10.1017/S0007114510000826. [DOI] [PubMed] [Google Scholar]
- 164.Gosnay SL. Bishop JA. New SA. Catterick J. Clifford MN. Estimation of the mean intakes of fourteen classes of dietary phenolics in a population of young British women aged 20–30 years. Proc Nutr Soc. 2002;61:125A. [Google Scholar]
- 165.Grassi D. Desideri G. Necozione S. Lippi C. Casale R. Properzi G. Blumberg JB. Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 166.Grassi D. Lippi C. Necozione S. Desideri G. Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 167.Grassi D. Mulder TP. Draijer R. Desideri G. Molhuizen HO. Ferri C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J Hypertens. 2009;27:774–781. doi: 10.1097/HJH.0b013e328326066c. [DOI] [PubMed] [Google Scholar]
- 168.Grassi D. Necozione S. Lippi C. Croce G. Valeri L. Pasqualetti P. Desideri G. Blumberg JB. Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 169.Gredel S. Grad C. Rechkemmer G. Watzl B. Phytoestrogens and phytoestrogen metabolites differentially modulate immune parameters in human leukocytes. Food Chem Toxicol. 2008;46:3691–3696. doi: 10.1016/j.fct.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 170.Grimm T. Schäfer A. Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol) Free Radic Biol Med. 2004;36:811–822. doi: 10.1016/j.freeradbiomed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 171.Grumer HD. Formation of hippuric acid from phenylalanine labelled with carbon-14 in phenylketonuric subjects. Nature. 1961;189:63–64. doi: 10.1038/189063a0. [DOI] [PubMed] [Google Scholar]
- 172.Guan S. Ge D. Liu TQ. Ma XH. Cui ZF. Protocatechuic acid promotes cell proliferation and reduces basal apoptosis in cultured neural stem cells. Toxicol In Vitro. 2009;23:201–208. doi: 10.1016/j.tiv.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 173.Guan S. Jiang B. Bao YM. An LJ. Protocatechuic acid suppresses MPP+-induced mitochondrial dysfunction and apoptotic cell death in PC12 cells. Food Chem Toxicol. 2006;44:1659–1666. doi: 10.1016/j.fct.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 174.Guarda E. Godoy I. Foncea R. Perez DD. Romero C. Venegas R. Leighton F. Red wine reduces oxidative stress in patients with acute coronary syndrome. Int J Cardiol. 2005;104:35–38. doi: 10.1016/j.ijcard.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 175.Hagl S. Deusser H. Soyalan B. Janzowski C. Will F. Dietrich H. Albert FW. Rohner S. Richling E. Colonic availability of polyphenols and D-(−)-quinic acid after apple smoothie consumption. Mol Nutr Food Res. 2011;55:368–377. doi: 10.1002/mnfr.201000252. [DOI] [PubMed] [Google Scholar]
- 176.Hakim IA. Harris RB. Brown S. Chow HH. Wiseman S. Agarwal S. Talbot W. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J Nutr. 2003;133:3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- 177.Hamajima N. Tajima K. Tominaga S. Matsuura A. Kuwabara M. Okuma K. Tea polyphenol intake and changes in serum pepsinogen levels. Jpn J Cancer Res. 1999;90:136–143. doi: 10.1111/j.1349-7006.1999.tb00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hansen AS. Marckmann P. Dragsted LO. Finne Nielsen IL. Nielsen SE. Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur J Clin Nutr. 2005;59:449–455. doi: 10.1038/sj.ejcn.1602107. [DOI] [PubMed] [Google Scholar]
- 179.Haque AM. Hashimoto M. Katakura M. Tanabe Y. Hara Y. Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. 2006;136:1043–1047. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 180.Hartman RE. Shah A. Fagan AM. Schwetye KE. Parsadanian M. Schulman RN. Finn MB. Holtzman DM. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 181.Hashimoto M. Kim S. Eto M. Iijima K. Ako J. Yoshizumi M. Akishita M. Kondo K. Itakura H. Hosoda K. Toba K. Ouchi Y. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am J Cardiol. 2001;88:1457–1560. doi: 10.1016/s0002-9149(01)02137-3. A9. [DOI] [PubMed] [Google Scholar]
- 182.Hassellund SS. Flaa A. Sandvik L. Kjeldsen SE. Rostrup M. Effects of anthocyanins on blood pressure and stress reactivity: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens. 2012;26:396–404. doi: 10.1038/jhh.2011.41. [DOI] [PubMed] [Google Scholar]
- 183.He FJ. Nowson CA. Lucas M. MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 184.Heinonen S-M. Nurmi T. Liukkonen K. Poutanen K. Wähälä K. Deyama T. Nishibe S. Adlercreutz H. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem. 2001;49:3178–3186. doi: 10.1021/jf010038a. [DOI] [PubMed] [Google Scholar]
- 185.Heinonen S-M. Wähälä K. Adlercreutz H. Identification of urinary metabolites of the red clover isoflavones formononetin and biochanin A in human subjects. J Agric Food Chem. 2004;52:6802–6809. doi: 10.1021/jf0492767. [DOI] [PubMed] [Google Scholar]
- 186.Heinonen S-M. Wähälä K. Liukkonen K-H. Aura AM. Poutanen K. Adlercreutz H. Studies of the in vitro intestinal metabolism of isoflavones aid in the identification of their urinary metabolites. J Agric Food Chem. 2004;52:2640–2646. doi: 10.1021/jf030681s. [DOI] [PubMed] [Google Scholar]
- 187.Heiss C. Dejam A. Kleinbongard P. Schewe T. Sies H. Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 188.Heiss C. Finis D. Kleinbongard P. Hoffmann A. Rassaf T. Kelm M. Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol. 2007;49:74–80. doi: 10.1097/FJC.0b013e31802d0001. [DOI] [PubMed] [Google Scholar]
- 189.Heiss C. Jahn S. Taylor M. Real WM. Angeli FS. Wong ML. Amabile N. Prasad M. Rassaf T. Ottaviani JI. Mihardja S. Keen CL. Springer ML. Boyle A. Grossman W. Glantz SA. Schroeter H. Yeghiazarians Y. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:218–224. doi: 10.1016/j.jacc.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 190.Heiss C. Kleinbongard P. Dejam A. Perre S. Schroeter H. Sies H. Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 191.Henderson VW. Estrogen-containing hormone therapy and Alzheimer's disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 192.Henning SM. Niu Y. Lee NH. hames GD. Minutti RR. Wang H. Go VL. Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 193.Hertog MG. Sweetnam PM. Fehily AM. Elwood PC. Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 194.Hertog MGL. Hollman PCH. Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in The Netherlands. J Agric Food Chem. 1993;41:1242–1246. [Google Scholar]
- 195.Hertog MGL. Hollman PCH. van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines and fruit juices in The Netherlands. J Agric Food Chem. 1992;40:2379–2383. [Google Scholar]
- 196.Hijmering ML. de Lange DW. Lorsheyd A. Kraaijenhagen RJ. van de Wiel A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: a small trial in healthy volunteers. Neth J Med. 2007;65:29–35. [PubMed] [Google Scholar]
- 197.Hirsh J. Hyperactive platelets and complications of coronary artery disease. N Engl J Med. 1987;316:1543–1544. doi: 10.1056/NEJM198706113162410. [DOI] [PubMed] [Google Scholar]
- 198.Ho SC. Chan AS. Ho YP. So EK. Sham A. Zee B. Woo JL. Effects of soy isoflavone supplementation on cognitive function in Chinese postmenopausal women: a double-blind, randomized, controlled trial. Menopause. 2007;14:489–499. doi: 10.1097/GME.0b013e31802c4f4f. [DOI] [PubMed] [Google Scholar]
- 199.Ho Y. Lee Y-L. Hsu K-Y. Determination of (+)-catechin in plasma by high-performance liquid chromatography using fluorescence detection. J Chromatogr B. 1995;665:383–389. doi: 10.1016/0378-4347(94)00535-d. [DOI] [PubMed] [Google Scholar]
- 200.Hodgson JM. Burke V. Puddey IB. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J Hypertens. 2005;23:47–54. doi: 10.1097/00004872-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 201.Hodgson JM. Chan SY. Puddey IB. Devine A. Wattanapenpaiboon N. Wahlqvist ML. Lukito W. Burke V. Ward NC. Prince RL. Croft KD. Phenolic acid metabolites as biomarkers for tea- and coffee-derived polyphenol exposure in human subjects. Br J Nutr. 2004;91:301–306. doi: 10.1079/BJN20031046. [DOI] [PubMed] [Google Scholar]
- 202.Hodgson JM. Morton LW. Puddey IB. Beilin LJ. Croft KD. Gallic acid metabolites are markers of black tea intake in humans. J Agric Food Chem. 2000;48:2276–2280. doi: 10.1021/jf000089s. [DOI] [PubMed] [Google Scholar]
- 203.Hodgson JM. Puddey IB. Burke V. Beilin LJ. Jordan N. Effects on blood pressure of drinking green and black tea. J Hypertens. 1999;17:457–463. doi: 10.1097/00004872-199917040-00002. [DOI] [PubMed] [Google Scholar]
- 204.Hodgson JM. Puddey IB. Burke V. Beilin LJ. Mori TA. Chan SY. Acute effects of ingestion of black tea on postprandial platelet aggregation in human subjects. Br J Nutr. 2002;87:141–145. doi: 10.1079/BJN2001499. [DOI] [PubMed] [Google Scholar]
- 205.Hodgson JM. Puddey IB. Burke V. Watts GF. Beilin LJ. Regular ingestion of black tea improves brachial artery vasodilator function. Clin Sci. 2002;102:195–201. [PubMed] [Google Scholar]
- 206.Hodgson JM. Puddey IB. Mori TA. Burke V. Baker RI. Beilin LJ. Effects of regular ingestion of black tea on haemostasis and cell adhesion molecules in humans. Eur J Clin Nutr. 2001;55:881–886. doi: 10.1038/sj.ejcn.1601231. [DOI] [PubMed] [Google Scholar]
- 207.Hoffman JR. Donato A. Robbins SJ. Ginkgo biloba promotes short-term retention of spatial memory in rats. Pharmacol Biochem Behav. 2004;77:533–539. doi: 10.1016/j.pbb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 208.Hoikkala A. Mustonen E. Saastamoinen I. Jokela T, Taponen J, Saloniemi H, and Wähälä K. High levels of equol in organic skimmed Finnish cow milk. Mol Nutr Food Res. 2007;51:782–786. doi: 10.1002/mnfr.200600222. [DOI] [PubMed] [Google Scholar]
- 209.Holt RR. Actis-Goretta L. Momma TY. Keen CL. Dietary flavanols and platelet reactivity. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S187–S196. doi: 10.1097/00005344-200606001-00014. discussion S206–S209. [DOI] [PubMed] [Google Scholar]
- 210.Holt RR. Lazarus SA. Sullards MC. Zhu QY. Schramm DD. Hammerstone JF. Fraga CG. Schmitz HH. Keen CL. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 211.Hooper L. Kroon PA. Rimm EB. Cohn JS. Harvey I. Le Cornu KA. Ryder JJ. Hall WL. Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 212.Hooper L. Madhavan G. Tice JA. Leinster SJ. Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16:745–760. doi: 10.1093/humupd/dmq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hosoda K. Furuta T. Ishii K. Metabolism and disposition of iosflavone conjugated metabolites in humans after ingestion of Kinako. Drug Metab Disp. 2011;39:1762–1767. doi: 10.1124/dmd.111.038281. [DOI] [PubMed] [Google Scholar]
- 214.Howes JB. Bray K. Lorenz L. Smerdely P. Howes LG. The effects of dietary supplementation with isoflavones from red clover on cognitive function in postmenopausal women. Climacteric. 2004;7:70–77. doi: 10.1080/13697130310001651490. [DOI] [PubMed] [Google Scholar]
- 215.Huang J. de Paulis T. May JM. Antioxidant effects of dihydrocaffeic acid in human EA.hy926 endothelial cells. J Nutr Biochem. 2004;15:722–729. doi: 10.1016/j.jnutbio.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 216.Ishisaka A. Ichikawa S. Sakakibara H. Piskula MK. Nakamura T. Kato Y. Ito M. Miyamoto K. Tsuji A. Kawai Y. Terao J. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects on rats. Free Radic Biol Med. 2011;51:1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 217.Ito H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011;77:1110–1115. doi: 10.1055/s-0030-1270749. [DOI] [PubMed] [Google Scholar]
- 218.Jaganath IB. Crozier A. Overview of health promoting compounds in fruits and vegetables. In: Fraga C, editor. Phenolic Compounds of Plant Origin and Health: The Biochemistry Behind Their Nutritional and Pharmacological Value. Chichester, United Kingdom: Wiley; 2009. pp. 1–48. [Google Scholar]
- 219.Jaganath IB. Crozier A. Flavonoid metabolism. In: Ashihara H, editor; Crozier A, editor; Komamine A, editor. Plant Metabolism and Biotechnology. Chichester, United Kingdom: Wiley; 2011. pp. 285–312. [Google Scholar]
- 220.Jaganath IB. Mullen W. Edwards CA. Crozier A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic Res. 2006;40:1035–1046. doi: 10.1080/10715760600771400. [DOI] [PubMed] [Google Scholar]
- 221.Jaganath IB. Mullen W. Lean ME. Edwards CA. Crozier A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic Biol Med. 2009;47:1180–1189. doi: 10.1016/j.freeradbiomed.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 222.Janle EM. Lila MA. Grannan M. Wood W. Higgins A. Yousef GG. Rogers R. Kim H. Jackson GS. Ho L. Weaver CM. Pharmacokinetics and tissue distribution of 14C-labeled grape polyphenols in the periphery and central nervous system following oral administration. J Med Food. 2010;13:926–933. doi: 10.1089/jmf.2009.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jee SH. He J. Appel LJ. Whelton PK. Suh I. Klag MJ. Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2001;153:353–362. doi: 10.1093/aje/153.4.353. [DOI] [PubMed] [Google Scholar]
- 224.Jee SH. He J. Whelton PK. Suh I. Klag MJ. The effect of chronic coffee drinking on blood pressure: a meta-analysis of controlled clinical trials. Hypertension. 1999;33:647–652. doi: 10.1161/01.hyp.33.2.647. [DOI] [PubMed] [Google Scholar]
- 225.Joannou GE. Kelly G. Reeder AY. Waring M. Nelson C. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J Steroid Biochem Mol Biol. 1995;54:167–184. doi: 10.1016/0960-0760(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 226.Jochmann N. Lorenz M. Krosigk A. Martus P. Bohm V. Baumann G. Stangl K. Stangl V. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br J Nutr. 2008;99:863–868. doi: 10.1017/S0007114507838992. [DOI] [PubMed] [Google Scholar]
- 227.Johnsen NF. Hausner H. Olsen A. Tetens I. Christensen J. Knudsen KE. Overvad K. Tjønneland A. Intake of whole grains and vegetables determines the plasma enterolactone concentration of Danish women. J Nutr. 2004;134:2691–2697. doi: 10.1093/jn/134.10.2691. [DOI] [PubMed] [Google Scholar]
- 228.Johnson K. Sharp P. Clifford MN. Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005;579:1653–1657. doi: 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 229.Joseph JA. Shukitt-Hale B. Denisova NA. Bielinski D. Martin A. McEwen JJ. Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Joseph JA. Shukitt-Hale B. Denisova NA. Prior RL. Cao G. Martin A. Taglialatela G. Bickford PC. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Joshipura KJ. Hu FB. Manson JE. Stampfer MJ. Rimm EB. Speizer FE. Colditz G. Ascherio A. Rosner B. Spiegelman D. Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 232.Joy S. Siow RC. Rowlands DJ. Becker M. Wyatt AW. Aaronson PI. Coen CW. Kallo I. Jacob R. Mann GE. The isoflavone equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J Biol Chem. 2006;281:27335–27345. doi: 10.1074/jbc.M602803200. [DOI] [PubMed] [Google Scholar]
- 233.Kahle K. Huemmer W. Kempf M. Scheppach W. Erk T. Richling E. Polyphenols are extensively metabolized in the human gastrointestinal tract after apple juice consumption. J Agric Food Chem. 2007;55:10695–10614. doi: 10.1021/jf071942r. [DOI] [PubMed] [Google Scholar]
- 234.Kahle K. Kempf M. Schreier P. Scheppach W. Schrenk D. Kautenburger T. Hecker D. Huemmer W. Ackermann M. Richling E. Intestinal transit and systemic metabolism of apple polyphenols. Eur J Nutr. 2011;50:507–522. doi: 10.1007/s00394-010-0157-0. [DOI] [PubMed] [Google Scholar]
- 235.Kahle K. Kraus M. Scheppach W. Ackermann M. Ridder F. Richling E. Studies on apple and blueberry fruit constituents: do the polyphenols reach the colon after ingestion? Mol Nutr Food Res. 2006;50:418–423. doi: 10.1002/mnfr.200500211. [DOI] [PubMed] [Google Scholar]
- 236.Kahle K. Kraus M. Scheppach W. Richling E. Colonic availability of apple polyphenols—a study in ileostomy subjects. Mol Nutr Food Res. 2005;49:1143–1150. doi: 10.1002/mnfr.200500132. [DOI] [PubMed] [Google Scholar]
- 237.Kalt W. Blumberg JB. McDonald JE. Vinqvist-Tymchuk MR. Fillmore SA. Graf BA. O'Leary JM. Milbury PE. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 238.Kamiyama M. Kishimoto Y. Tani M. Utsunomiya K. Kondo K. Effects of equol on oxidized low-density lipoprotein-induced apoptosis in endothelial cells. J Atheroscler Thromb. 2009;16:239–249. doi: 10.5551/jat.1057. [DOI] [PubMed] [Google Scholar]
- 239.Kaplan M. Hayek T. Raz A. Coleman R. Dornfeld L. Vaya J. Aviram M. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J Nutr. 2001;131:2082–2089. doi: 10.1093/jn/131.8.2082. [DOI] [PubMed] [Google Scholar]
- 240.Karatzi K. Papamichael C. Aznaouridis K. Karatzis E. Lekakis J. Matsouka C. Boskou G. Chiou A. Sitara M. Feliou G. Kontoyiannis D. Zampelas A. Mavrikakis M. Constituents of red wine other than alcohol improve endothelial function in patients with coronary artery disease. Coron Artery Dis. 2004;15:485–490. doi: 10.1097/00019501-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 241.Kasimsetty SG. Bialonska D. Reddy MK. Thornton C. Willett KL. Ferreira D. Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. J Agric Food Chem. 2009;57:10636–10644. doi: 10.1021/jf902716r. [DOI] [PubMed] [Google Scholar]
- 242.Kaspar KL. Park JS. Brown CR. Mathison BD. Navarre DA. Chew BP. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J Nutr. 2011;141:108–111. doi: 10.3945/jn.110.128074. [DOI] [PubMed] [Google Scholar]
- 243.Kawai Y. Nishikawa T. Shiba Y. Saito S. Murota K. Shibata N. Kobayashi M. Kanayama M. Uchida K. Terao J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries. Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 244.Kawai Y. Tanaka H. Murota K. Naito M. Terao J. (−)-Epicatechin gallate accumulates in foamy macrophages in human atherosclertic aorta: implication in the anti-atherosclerotic actions of tea catechins. Biochem Biophys Res Comm. 2008;374:527–532. doi: 10.1016/j.bbrc.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 245.Kechagias S. Zanjani S. Gjellan S. Leinhard OD. Kihlberg J. Smedby O. Johansson L. Kullberg J. Ahlstrom H. Lindstrom T. Nystrom FH. Effects of moderate red wine consumption on liver fat and blood lipids: a prospective randomized study. Ann Med. 2011;43:545–554. doi: 10.3109/07853890.2011.588246. [DOI] [PubMed] [Google Scholar]
- 246.Kempermann G. Kuhn HG. Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kida T. Suzuki M. Matsumoto N. Nanjo F. Hara Y. Identification of biliary metabolites of (−)-epigallocatechin gallate in rats. J Agric Food Chem. 2000;48:4151–4155. doi: 10.1021/jf000386x. [DOI] [PubMed] [Google Scholar]
- 248.Kikura M. Levy JH. Safon RA. Lee MK. Szlam F. The influence of red wine or white wine intake on platelet function and viscoelastic property of blood in volunteers. Platelets. 2004;15:37–41. doi: 10.1080/0953710032000158772. [DOI] [PubMed] [Google Scholar]
- 249.Kilkkinen A. Stumpf K. Pietinen P. Valsta LM. Tapanainen H. Adlercreutz H. Determinants of serum enterolactone concentration. Am J Clin Nutr. 2001;73:1094–1100. doi: 10.1093/ajcn/73.6.1094. [DOI] [PubMed] [Google Scholar]
- 250.Klaus A. Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 251.Koga T. Meydani M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am J Clin Nutr. 2001;73:941–948. doi: 10.1093/ajcn/73.5.941. [DOI] [PubMed] [Google Scholar]
- 252.Kohri T. Nanjo F. Suziki M. Seto R. Matsumoto N. Yamakawa M. Hojo H. Hara Y. Desai D. Amin S. Conaway CC. Chung FL. Synthesis of (−)-[4-3H]epigallocatechin gallate and its metabolic fate in rats after intravenous administration. J Agric Food Chem. 2001;49:1042–1048. doi: 10.1021/jf0011236. [DOI] [PubMed] [Google Scholar]
- 253.Kottra G. Daniel H. Flavonoid glycosides are not transported by the human Na+/glucose transporter when expressed in Xenopus laevis oocytes, but effectively inhibit electrogenic glucose uptake. J Pharmacol Exp Ther. 2007;322:829–835. doi: 10.1124/jpet.107.124040. [DOI] [PubMed] [Google Scholar]
- 254.Krafczyk N. Glomb MA. Characterization of phenolic compounds in rooibos tea. J Agric Food Chem. 2008;56:3368–3376. doi: 10.1021/jf703701n. [DOI] [PubMed] [Google Scholar]
- 255.Kreijkamp-Kaspers S. Kok L. Grobbee DE. de Haan EH. Aleman A. Lampe JW. van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 256.Kreuz A. Joubert E. Waldmann KH. Ternes W. Aspalathin, a flavonoid in Aspalathus linearis (rooibos), is absorbed by pig intestine as a C-glycoside. Nutr Res. 2008;28:690–701. doi: 10.1016/j.nutres.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 257.Krikorian R. Nash TA. Shidler MD. Shukitt-Hale B. Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr. 2010;103:730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- 258.Krikorian R. Shidler MD. Nash TA. Kalt W. Vinqvist-Tymchuk MR. Shukitt-Hale B. Joseph JA. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58:3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kritz-Silverstein D. Von MD. Barrett-Connor E. Bressel MA. Isoflavones and cognitive function in older women: the Soy and Postmenopausal Health In Aging (SOPHIA) Study. Menopause. 2003;10:196–202. doi: 10.1097/00042192-200310030-00004. [DOI] [PubMed] [Google Scholar]
- 260.Kuhn HG. Dickinson-Anson H. Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kuhnert N. Clifford MN. Muller A. Oxidative cascade reactions yielding polyhydroxy-theaflavins and theacitrins in the formation of black tea thearubigins: evidence by tandem LC−MS. Food Funct. 2010;1:180–199. doi: 10.1039/c0fo00066c. [DOI] [PubMed] [Google Scholar]
- 262.Kuhnert N. Dryman JW. Obuchowicz J. Clifford MN. Witt M. Mass spectrometric characterization of black tea thearubigins leading to an oxidative cascade hypothesis for thearubigin formation. Rapid Comm Mass Spectrom. 2010;24:3387–3404. doi: 10.1002/rcm.4778. [DOI] [PubMed] [Google Scholar]
- 263.Kurowska EM. Spence JD. Jordan J. Wetmore S. Freeman DJ. Piche LA. Serratore P. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr. 2000;72:1095–1100. doi: 10.1093/ajcn/72.5.1095. [DOI] [PubMed] [Google Scholar]
- 264.La Vecchia C. Chatenoud L. Franceschi S. Soler M. Parazzini F. Negri E. Vegetables and fruit and human cancer: update of an Italian study. Int J Cancer. 1999;82:151–152. doi: 10.1002/(sici)1097-0215(19990702)82:1<151::aid-ijc25>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 265.Lai M-Y. Hou Y-C. Hsiu S-L. Chen C-C. Chao P-D-L. Relative flavone bioavailability of Scutellariae Radix between traditional decoction and commercial power preparation in humans. J Food Drug Anal. 2002;10:75–80. [Google Scholar]
- 266.Lambert JD. Rice JE. Hong J. Hou Z. Yang CS. Synthesis and biological activity of the tea catechin metabolites, M4 and M6 and their methoxy-derivatives. Bioorg Med Chem Lett. 2005;15:873–876. doi: 10.1016/j.bmcl.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 267.Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int. 2011;44:1150–1160. [Google Scholar]
- 268.Langcake P. Pryce RJ. A new class of phytoalexins from grapevines. Experientia. 1977;33:151–152. doi: 10.1007/BF02124034. [DOI] [PubMed] [Google Scholar]
- 269.Larrosa M. García-Conesa MT. Espin JC. Tomás-Barberán FA. Bioavailability and metabolism of ellagic acid and ellagitannins. In: Spencer JPE, editor; Crozier A, editor; Packer L, editor; Cadenas H, editor. Flavonoids and Related Compounds: Bioavailability and Function. Oxidative Stress and Disease. Vol. 30. Boca Raton, FL: CRC Press; 2012. pp. 183–199. [Google Scholar]
- 270.Larrosa M. González-Sarrías A. García-Conesa MT. Tomás-Barberán FA. Espín JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611–1620. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 271.Larrosa M. González-Sarrías A. Yáñez-Gascón MJ. Selma MV. Azorín-Ortuño M. Toti S. Tomás-Barberán F. Dolara P. Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 272.Larsson SC. Andersson SO. Johansson JE. Wolk A. Fruit and vegetable consumption and risk of bladder cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2008;17:2519–2522. doi: 10.1158/1055-9965.EPI-08-0407. [DOI] [PubMed] [Google Scholar]
- 273.Larsson SC. Hakansson N. Naslund I. Bergkvist L. Wolk A. Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:301–305. doi: 10.1158/1055-9965.EPI-05-0696. [DOI] [PubMed] [Google Scholar]
- 274.Le Bars PL. Velasco FM. Ferguson JM. Dessain EC. Kieser M. Hoerr R. Influence of the severity of cognitive impairment on the effect of the Ginkgo biloba extract EGb 761 in Alzheimer's disease. Neuropsychobiology. 2002;45:19–26. doi: 10.1159/000048668. [DOI] [PubMed] [Google Scholar]
- 275.Lekakis J. Rallidis LS. Andreadou I. Vamvakou G. Kazantzoglou G. Magiatis P. Skaltsounis AL. Kremastinos DT. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:596–600. doi: 10.1097/00149831-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 276.Letenneur L. Proust-Lima C. Le GA. Dartigues JF. Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- 277.Li C. Lee MJ. Sheng S. Meng X. Prabhu S. Winnik B. Huang B. Chung JY. Yan S. Ho CT. Yang CS. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol. 2000;13:177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 278.Li N. Sun Z. Han C. Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc Soc Exp Biol Med. 1999;220:218–224. doi: 10.1046/j.1525-1373.1999.d01-37.x. [DOI] [PubMed] [Google Scholar]
- 279.Li Y. Ross-Viola JS. Shay NF. Moore DD. Ricketts ML. Human CYP3A4 and murine Cyp3A11 are regulated by equol and genistein via the pregnane X receptor in a species-specific manner. J Nutr. 2009;139:898–904. doi: 10.3945/jn.108.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lin HH. Chen JH. Huang CC. Wang CJ. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signalling activation. Int J Cancer. 2007;120:2306–2316. doi: 10.1002/ijc.22571. [DOI] [PubMed] [Google Scholar]
- 281.Lippi G. Franchini M. Favaloro EJ. Targher G. Moderate red wine consumption and cardiovascular disease risk: beyond the “French paradox”. Semin Thromb Hemost. 2010;36:59–70. doi: 10.1055/s-0030-1248725. [DOI] [PubMed] [Google Scholar]
- 282.Lodi F. Jimenez R. Moreno L. Kroon PA. Needs PW. Hughes DA. Santos-Buelga C. González-Paramas A. Cogolludo A. Lopez-Sepulveda R. Duarte J. Perez-Vizcaino F. Glucuronidated and sulfated metabolites of the flavonoid quercetin prevent endothelial dysfunction but lack direct vasorelaxant effects in rat aorta. Atherosclerosis. 2009;204:34–39. doi: 10.1016/j.atherosclerosis.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 283.Loke WM. Proudfoot JM. McKinley AJ. Needs PW. Kroon PA. Hodgson JM. Croft KD. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J Agric Food Chem. 2008;56:3609–3615. doi: 10.1021/jf8003042. [DOI] [PubMed] [Google Scholar]
- 284.Lorenz M. Jochmann N. von Krosigk A. Martus P. Baumann G. Stangl K. Stangl V. Addition of milk prevents vascular protective effects of tea. Eur Heart J. 2007;28:219–223. doi: 10.1093/eurheartj/ehl442. [DOI] [PubMed] [Google Scholar]
- 285.Lotito SB. Zhang WJ. Yang CS. Crozier A. Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51:454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lu H. Meng X. Li C. Sang S. Patten C. Sheng S. Hong J. Bai N. Winnik B. Ho CT. Yang CS. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003;31:452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- 287.Luo H. Tang L. Tang M. Billam M. Huang T. Yu J. Wei Z. Liang Y. Wang K. Zhang ZQ. Zhang L. Wang JS. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 288.Ma Y. Njike VY. Millet J. Dutta S. Doughty K. Treu JA. Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33:227–232. doi: 10.2337/dc09-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Macready AL. Kennedy OB. Ellis JA. Williams CM. Spencer JP. Butler LT. Flavonoids and cognitive function: a review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009;4:227–242. doi: 10.1007/s12263-009-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Maher P. Akaishi T. Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci U S A. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Manach C. Williamson G. Morand C. Scalbert A. Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:2230S–2242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 292.Mansvelt EP. van Velden DP. Fourie E. Rossouw M. van Rensburg SJ. Smuts CM. The in vivo antithrombotic effect of wine consumption on human blood platelets and hemostatic factors. Ann N Y Acad Sci. 2002;957:329–332. doi: 10.1111/j.1749-6632.2002.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 293.Manzano S. Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res. 2010;54:1773–1780. doi: 10.1002/mnfr.201000019. [DOI] [PubMed] [Google Scholar]
- 294.Marks SC. Mullen W. Borges G. Crozier A. Absorption, metabolism, and excretion of cider dihydrochalcones in healthy humans and subjects with an ileostomy. J Agric Food Chem. 2009;57:2009–2015. doi: 10.1021/jf802757x. [DOI] [PubMed] [Google Scholar]
- 295.Matsumoto H. Nakamura Y. Iida H. Ito K. Ohguro H. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp Eye Res. 2006;83:348–356. doi: 10.1016/j.exer.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 296.McAnlis GT. McEneny J. Pearce J. Young IS. Black tea consumption does not protect low density lipoprotein from oxidative modification. Eur J Clin Nutr. 1998;52:202–206. doi: 10.1038/sj.ejcn.1600540. [DOI] [PubMed] [Google Scholar]
- 297.McAnulty SR. McAnulty LS. Morrow JD. Khardouni D. Shooter L. Monk J. Gross S. Brown V. Effect of daily fruit ingestion on angiotensin converting enzyme activity, blood pressure, and oxidative stress in chronic smokers. Free Radic Res. 2005;39:1241–1248. doi: 10.1080/10715760500306836. [DOI] [PubMed] [Google Scholar]
- 298.McDonald MS. Hughes M. Lean MEJ. Burns J. Matthews D. Crozier A. Survey of the free and conjugated flavonol content of sixty five red wines of different geographical origins. J Agric Food Chem. 1998;46:368–375. doi: 10.1021/jf970677e. [DOI] [PubMed] [Google Scholar]
- 299.McGhie TK. Aingie GD. Barnet LE. Cooney JM. Jensen DJ. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolised by both human and rats. J Agric Food Chem. 2003;51:4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- 300.McKay DL. Chen CY. Saltzman E. Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr. 2010;140:298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 301.McMullen MK. Whitehouse JM. Shine G. Towell A. Habitual coffee and tea drinkers experienced increases in blood pressure after consuming low to moderate doses of caffeine; these increases were larger upright than in the supine posture. Food Funct. 2011;2:197–203. doi: 10.1039/c0fo00166j. [DOI] [PubMed] [Google Scholar]
- 302.Mellen PB. Daniel KR. Brosnihan KB. Hansen KJ. Herrington DM. Effect of muscadine grape seed supplementation on vascular function in subjects with or at risk for cardiovascular disease: a randomized crossover trial. J Am Coll Nutr. 2010;29:469–475. doi: 10.1080/07315724.2010.10719883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Meng X. Sang S. Zhu N. Lu H. Sheng S. Lee MJ. Ho CT. Yang CS. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem Res Toxicol. 2002;15:1042–1050. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- 304.Mercanligil SM. Arslan P. Alasalvar C. Okut E. Akgul E. Pinar A. Geyik PO. Tokgozoglu L. Shahidi F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur J Clin Nutr. 2007;61:212–220. doi: 10.1038/sj.ejcn.1602518. [DOI] [PubMed] [Google Scholar]
- 305.Mesas AE. Leon-Munoz LM. Rodriguez-Artalejo F. Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr. 2011;94:1113–1126. doi: 10.3945/ajcn.111.016667. [DOI] [PubMed] [Google Scholar]
- 306.Middleton E., Jr. Kandaswami C. Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 307.Miene C. Weise A. Glei M. Impact of polyphenol metabolites produced by colonic microbiota on expression of COX-2 and GSTT2 in human colon cells (LT97) Nutr Cancer. 2011;63:653–662. doi: 10.1080/01635581.2011.552157. [DOI] [PubMed] [Google Scholar]
- 308.Milder IEJ. Kuijsteen A. Arts IC. Feskens EJ. Kampman E. Hollman PC. Van ’t Veer P. Relation between plasma enterodiol and enterolactone and dietary intake of lignans in a Dutch endoscopy-based population. J Nutr. 2007;137:1266–1271. doi: 10.1093/jn/137.5.1266. [DOI] [PubMed] [Google Scholar]
- 309.Mink PJ. Scrafford CG. Barraj LM. Harnack L. Hong CP. Nettleton JA. Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 310.Mix JA. Crews WD., Jr An examination of the efficacy of Ginkgo biloba extract EGb761 on the neuropsychologic functioning of cognitively intact older adults. J Altern Complement Med. 2000;6:219–229. doi: 10.1089/acm.2000.6.219. [DOI] [PubMed] [Google Scholar]
- 311.Mohan M. Waghulde H. Kasture S. Effect of pomegranate juice on Angiotensin II-induced hypertension in diabetic Wistar rats. Phytother Res. 2010;24(Suppl 2):S196–S203. doi: 10.1002/ptr.3090. [DOI] [PubMed] [Google Scholar]
- 312.Moller P. Loft S. Alfthan G. Freese R. Oxidative DNA damage in circulating mononuclear blood cells after ingestion of blackcurrant juice or anthocyanin-rich drink. Mutat Res. 2004;551:119–126. doi: 10.1016/j.mrfmmm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 313.Moller P. Vogel U. Pedersen A. Dragsted LO. Sandstrom B. Loft S. No effect of 600 grams fruit and vegetables per day on oxidative DNA damage and repair in healthy nonsmokers. Cancer Epidemiol. 2003;12:1016–1022. [PubMed] [Google Scholar]
- 314.Monge P. Solheim E. Scheline RR. Dihydrochalcone metabolism in the rat: phloretin. Xenobiotica. 1984;14:917–924. doi: 10.3109/00498258409151490. [DOI] [PubMed] [Google Scholar]
- 315.Morand C. Dubray C. Milenkovic D. Lioger D. Martin JF. Scalbert A. Mazur A. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. 2011;93:73–80. doi: 10.3945/ajcn.110.004945. [DOI] [PubMed] [Google Scholar]
- 316.Mukuddem-Petersen J. Oosthuizen W. Jerling JC. A systematic review of the effects of nuts on blood lipid profiles in humans. J Nutr. 2005;135:2082–2089. doi: 10.1093/jn/135.9.2082. [DOI] [PubMed] [Google Scholar]
- 317.Mulder T. van Platerink CJ. Wijnand Schuyl PJ. van Amelsvoort JM. Analysis of theaflavins in biological fluids using liquid chromatography–electrospray mass spectrometry. J Chromatogr B. 2001;760:271–279. doi: 10.1016/s0378-4347(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 318.Mulder TP. Rietveld AG. van Amelsvoort JM. Consumption of both black tea and green tea results in an increase in the excretion of hippuric acid into urine. Am J Clin Nutr. 2005;81:256S–260S. doi: 10.1093/ajcn/81.1.256S. [DOI] [PubMed] [Google Scholar]
- 319.Mullen W. Archeveque M-A. Edwards CA. Crozier A. Bioavailability and metabolism of orange juice flavanones in humans: impact of a full fat yogurt. J Agric Food Chem. 2008;56:11157–11164. doi: 10.1021/jf801974v. [DOI] [PubMed] [Google Scholar]
- 320.Mullen W. Borges G. Donovan JL. Edwards CA. Serafini M. Lean MEJ. Crozier A. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan-3-ol metabolites in humans. Am J Clin Nut. 2009;89:1784–1791. doi: 10.3945/ajcn.2008.27339. [DOI] [PubMed] [Google Scholar]
- 321.Mullen W. Edwards CA. Crozier A. Absorption, excretion and metabolic profiling of methyl-, glucuronyl-, glucosyl and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br J Nutr. 2006;96:107–116. doi: 10.1079/bjn20061809. [DOI] [PubMed] [Google Scholar]
- 322.Mullen W. Edwards CA. Serafini M. Crozier A. Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J Agric Food Chem. 2008;56:713–719. doi: 10.1021/jf072000p. [DOI] [PubMed] [Google Scholar]
- 323.Mullen W. Rouanet J-M. Auger C. Teissedre P-L. Caldwell ST. Hartley RC. Lean MEJ. Edwards CA. Crozier A. Bioavailability of [2-14C]quercetin-4′-glucoside in rats. J Agric Food Chem. 2008;56:12127–12137. doi: 10.1021/jf802754s. [DOI] [PubMed] [Google Scholar]
- 324.Mullen W. McGinn J. Lean MEJ. MacLean MR. Gardner P. Duthie GG. Crozier A. (2002). Ellagitannins, flavonoids and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J Agric Food Chem. 2002;50:5191–5196. doi: 10.1021/jf020140n. [DOI] [PubMed] [Google Scholar]
- 325.Muniyappa R. Hall G. Kolodziej TL. Karne RJ. Crandon SK. Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr. 2008;88:1685–1696. doi: 10.3945/ajcn.2008.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Murphy KJ. Chronopoulos AK. Singh I. Francis MA. Moriarty H. Pike MJ. Turner AH. Mann NJ. Sinclair AJ. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- 327.Nagahama Y. Nabatame H. Okina T. Yamauchi H. Narita M. Fujimoto N. Murakami M. Fukuyama H. Matsuda M. Cerebral correlates of the progression rate of the cognitive decline in probable Alzheimer's disease. Eur Neurol. 2003;50:1–9. doi: 10.1159/000070851. [DOI] [PubMed] [Google Scholar]
- 328.Naissides M. Mamo JC. James AP. Pal S. The effect of chronic consumption of red wine on cardiovascular disease risk factors in postmenopausal women. Atherosclerosis. 2006;185:438–445. doi: 10.1016/j.atherosclerosis.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 329.Naissides M. Pal S. Mamo JC. James AP. Dhaliwal S. The effect of chronic consumption of red wine polyphenols on vascular function in postmenopausal women. Eur J Clin Nutr. 2006;60:740–745. doi: 10.1038/sj.ejcn.1602377. [DOI] [PubMed] [Google Scholar]
- 330.Napoli R. Cozzolino D. Guardasole V. Angelini V. Zarra E. Matarazzo M. Cittadini A. Sacca L. Torella R. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metab Clin Exper. 2005;54:306–313. doi: 10.1016/j.metabol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 331.Naruszewicz M. Laniewska I. Millo B. Dluzniewski M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI) Atherosclerosis. 2007;194:e179–e184. doi: 10.1016/j.atherosclerosis.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 332.Natella F. Nardini M. Belelli F. Pignatelli P. Di Santo S. Ghiselli A. Violi F. Scaccini C. Effect of coffee drinking on platelets: inhibition of aggregation and phenols incorporation. Br J Nutr. 2008;100:1276–1282. doi: 10.1017/S0007114508981459. [DOI] [PubMed] [Google Scholar]
- 333.Njike VY. Faridi Z. Shuval K. Dutta S. Kay CD. West SG. Kris-Etherton PM. Katz DL. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol. 2011;149:83–88. doi: 10.1016/j.ijcard.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 334.Noordzij M. Uiterwaal CS. Arends LR. Kok FJ. Grobbee DE. Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23:921–928. doi: 10.1097/01.hjh.0000166828.94699.1d. [DOI] [PubMed] [Google Scholar]
- 335.Nurk E. Refsum H. Drevon CA. Tell GS. Nygaard HA. Engedal K. Smith AD. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr. 2009;139:120–127. doi: 10.3945/jn.108.095182. [DOI] [PubMed] [Google Scholar]
- 336.Ottaviani JL. Momma TY. Heiss C. Kwik-Uribe C. Schroeter H. Keen CL. The stereochemical configuration of flavonols influences the level and metabolism of flavonols in humans and their biological activity in vivo. Free Radic Biol Med. 2011;50:237–244. doi: 10.1016/j.freeradbiomed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 337.Ottaviani JI. Momma TY. Kuhnle GK. Keen CL. Schroeter H. Structurally related (−)-epicatechin metabolites in humans: assessment using de novo chemically synthesized authentic standards. Free Radic Biol Med. 2012;52:1403–1412. doi: 10.1016/j.freeradbiomed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 338.Ozeki Y. Matsuba Y. Abe Y. Umemoto N. Sasaki N. Pigment biosynthesis I. Anthocyanins. In: Ashihara H, editor; Crozier A, editor; Komamine A, editor. Plant Metabolism and Biotechnology. Chichester, United Kingdom: Wiley; 2011. pp. 155–181. [Google Scholar]
- 339.Pace-Asciak CR. Rounova O. Hahn SE. Diamandis EP. Goldberg DM. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin Chim Acta. 1996;246:163–182. doi: 10.1016/0009-8981(96)06236-5. [DOI] [PubMed] [Google Scholar]
- 340.Paixão J. Dinis TC. Almeida LM. Dietary anthocyanins protect endothelial cells against peroxynitrite-induced mitochondrial apoptosis pathway and Bax nuclear translocation: an in vitro approach. Apoptosis. 2011;16:976–989. doi: 10.1007/s10495-011-0632-y. [DOI] [PubMed] [Google Scholar]
- 341.Pan Y. Anthony M. Clarkson TB. Effect of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proc Soc Exp Biol Med. 1999;221:118–125. doi: 10.1046/j.1525-1373.1999.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 342.Pan Y. Anthony M. Clarkson TB. Evidence for up-regulation of brain-derived neurotrophic factor mRNA by soy phytoestrogens in the frontal cortex of retired breeder female rats. Neurosci Lett. 1999;261:17–20. doi: 10.1016/s0304-3940(98)00994-x. [DOI] [PubMed] [Google Scholar]
- 343.Papamichael CM. Aznaouridis KA. Karatzis EN. Karatzi KN. Stamatelopoulos KS. Vamvakou G. Lekakis JP. Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci. 2005;109:55–60. doi: 10.1042/CS20040358. [DOI] [PubMed] [Google Scholar]
- 344.Park CS. Kim W. Woo JS. Ha SJ. Kang WY. Hwang SH. Park YW. Kim YS. Ahn YK. Jeong MH. Green tea consumption improves endothelial function but not circulating endothelial progenitor cells in patients with chronic renal failure. Int J Cardiol. 2010;145:261–262. doi: 10.1016/j.ijcard.2009.09.471. [DOI] [PubMed] [Google Scholar]
- 345.Park YK. Kim JS. Kang MH. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: double-blind, placebo controlled intervention trial. BioFactors. 2004;22:145–147. doi: 10.1002/biof.5520220128. [DOI] [PubMed] [Google Scholar]
- 346.Pashikanti S. de Alba DR. Boissonneault GA. Cervantes-Laurean D. Rutin metabolites: novel inhibitors of nonoxidative advanced glycation end products. Free Radic Biol Med. 2010;48:656–663. doi: 10.1016/j.freeradbiomed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 347.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 348.Patel KR. Brown VA. Jones DJ. Britton RG. Hemingway D. Miller AS. West KP. Booth TD. Perloff M. Crowell JA. Brenner DE. Steward WP. Gescher AJ. Brown K. Clinical pharmacology of resveratrol and Its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Pavlica S. Gebhardt R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010;86:79–86. doi: 10.1016/j.lfs.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 350.Pearson DA. Paglieroni TG. Rein D. Wun T. Schramm DD. Wang JF. Holt RR. Gosselin R. Schmitz HH. Keen CL. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002;106:191–197. doi: 10.1016/s0049-3848(02)00128-7. [DOI] [PubMed] [Google Scholar]
- 351.Pedersen A. Johansen C. Grønbaek M. Relations between amount and type of alcohol and colon and rectal cancer in a Danish population based cohort study. Gut. 2003;52:861–867. doi: 10.1136/gut.52.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Pellegrini N. Pareti FI. Stabile F. Brusamolino A. Simonetti P. Effects of moderate consumption of red wine on platelet aggregation and haemostatic variables in healthy volunteers. Eur J Clin Nutr. 1996;50:209–213. [PubMed] [Google Scholar]
- 353.Peñalvo JL. Haajanen KM. Botting N. Adlercreutz H. Quantification of lignans in food using isotope dilution gas chromatography/mass spectrometry. J Agric Food Chem. 2005;53:9342–9347. doi: 10.1021/jf051488w. [DOI] [PubMed] [Google Scholar]
- 354.Peñalvo JL. Heinonen S-M. Aura A-M. Adlercreutz H. Dietary sesamin is converted to enterolactone in humans. J Nutr. 2005;135:1056–1062. doi: 10.1093/jn/135.5.1056. [DOI] [PubMed] [Google Scholar]
- 355.Pipingas A. Silberstein RB. Vitetta L. Rooy CV. Harris EV. Young JM. Frampton CM. Sali A. Nastasi J. Improved cognitive performance after dietary supplementation with a Pinus radiata bark extract formulation. Phytother Res. 2008;22:1168–1174. doi: 10.1002/ptr.2388. [DOI] [PubMed] [Google Scholar]
- 356.Plaumann B. Fritsche M. Rimpler H. Brandner G. Hess RD. Flavonoids activate wild-type p53. Oncogene. 1996;13:1605–1614. [PubMed] [Google Scholar]
- 357.Polagruto JA. Gross HB. Kamangar F. Kosuna K. Sun B. Fujii H. Keen CL. Hackman RM. Platelet reactivity in male smokers following the acute consumption of a flavanol-rich grape seed extract. J Med Food. 2007;10:725–730. doi: 10.1089/jmf.2007.402. [DOI] [PubMed] [Google Scholar]
- 358.Poquet L. Clifford MN. Williamson G. Effect of dihydrocaffeic acid on UV irradiation of human keratinocyte HaCaT cells. Arch Biochem Biophys. 2008;476:196–204. doi: 10.1016/j.abb.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 359.Poulose SM. Shukitt-Hale B. Effects of flavonoids on cognitive performance. In: Spencer JPE, editor; Crozier A, editor; Packer L, editor; Cadenas H, editor. Flavonoids and Related Compounds: Bioavailability and Function. Oxidative Stress and Disease. Vol. 30. Boca Raton, FL: CRC Press; 2012. pp. 393–411. [Google Scholar]
- 360.Princen HM. van Duyvenvoorde W. Buytenhek R. Blonk C. Tijburg LB. Langius JA. Meinders AE. Pijl H. No effect of consumption of green and black tea on plasma lipid and antioxidant levels and on LDL oxidation in smokers. Arterioscler Thromb Vasc Biol. 1998;18:833–841. doi: 10.1161/01.atv.18.5.833. [DOI] [PubMed] [Google Scholar]
- 361.Prior RL. Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006;40:1014–1028. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]
- 362.Proteggente AR. Basu-Modak S. Kuhnle G. Gordon MJ. Youdim K. Tyrrell R. Rice-Evans CA. Hesperetin glucuronide, a photoprotective agent arising from flavonoid metabolism in human skin fibroblasts. Photochem Photobiol. 2003;78:256–261. doi: 10.1562/0031-8655(2003)078<0256:hgapaa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 363.Pu F. Mishima K. Irie K. Motohashi K. Tanaka Y. Orito K. Egawa T. Kitamura Y. Egashira N. Iwasaki K. Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007;104:329–334. doi: 10.1254/jphs.fp0070247. [DOI] [PubMed] [Google Scholar]
- 364.Puddey IB. Beilin LJ. Vandongen R. Regular alcohol use raises blood pressure in treated hypertensive subjects. A randomised controlled trial. Lancet. 1987;1:647–651. doi: 10.1016/s0140-6736(87)90413-2. [DOI] [PubMed] [Google Scholar]
- 365.Puddey IB. Beilin LJ. Vandongen R. Rouse IL. Rogers P. Evidence for a direct effect of alcohol consumption on blood pressure in normotensive men. A randomized controlled trial. Hypertension. 1985;7:707–713. doi: 10.1161/01.hyp.7.5.707. [DOI] [PubMed] [Google Scholar]
- 366.Qin Y. Xia M. Ma J. Hao Y. Liu J. Mou H. Cao L. Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–492. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- 367.Radnai B. Tucsek Z. Bognar Z. Antus C. Mark L. Berente Z. Gallyas F., Jr. Sumegi B. Veres B. Ferulaldehyde, a water-soluble degradation product of polyphenols, inhibits the lipopolysaccharide-induced inflammatory response in mice. J Nutr. 2009;139:291–297. doi: 10.3945/jn.108.097386. [DOI] [PubMed] [Google Scholar]
- 368.Ramirez MR. Izquierdo I. do Carmo Bassols Raseira M. Zuanazzi JA. Barros D. Henriques AT. Effect of lyophilised Vaccinium berries on memory, anxiety and locomotion in adult rats. Pharmacol Res. 2005;52:457–462. doi: 10.1016/j.phrs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 369.Ras RT. Zock PL. Draijer R. Tea consumption enhances endothelial-dependent vasodilation; a meta-analysis. PLoS One. 2011;6:e16974. doi: 10.1371/journal.pone.0016974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Rechner AR. Kroner C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb Res. 2005;116:327–334. doi: 10.1016/j.thromres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 371.Rechner AR. Smith MA. Kuhnle G. Gibson GR. Debnam ES. Srai SK. Moore KP. Rice-Evans CA. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med. 2004;36:212–225. doi: 10.1016/j.freeradbiomed.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 372.Rein D. Lotito S. Holt RR. Keen CL. Schmitz HH. Fraga CG. Epicatechin in plasma: in vivo determination and effect of chocolate consumption on plasma oxidative status. J Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- 373.Rein D. Paglieroni TG. Wun T. Pearson DA. Schmitz HH. Gosselin R. Keen CL. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 374.Reshef N. Hayari Y. Goren C. Boaz M. Madar Z. Knobler H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am J Hypertens. 2005;18:1360–1363. doi: 10.1016/j.amjhyper.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 375.Riboli E. Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 376.Richelle M. Tavazzi I. Enslen M. Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur J Clin Nutr. 1999;53:22–26. doi: 10.1038/sj.ejcn.1600673. [DOI] [PubMed] [Google Scholar]
- 377.Richling E. Bioavailability of dihydrochalcones. In: Spencer JPE, editor; Crozier A, editor; Packer L, editor; Cadenas H, editor. Flavonoids and Related Compounds: Bioavailability and Function. Oxidative Stress and Disease. Vol. 30. Boca Raton, FL: CRC Press; 2012. pp. 157–165. [Google Scholar]
- 378.Rifler JP. Lorcerie F. Durand P. Delmas D. Ragot K. Limagne E. Mazue F. Riedinger JM. d'Athis P. Hudelot B. Prost M. Lizard G. Latruffe N. A moderate red wine intake improves blood lipid parameters and erythrocytes membrane fluidity in post myocardial infarct patients. Mol Nutr Food Res. 2012;56:345–351. doi: 10.1002/mnfr.760. [DOI] [PubMed] [Google Scholar]
- 379.Rimbaud S. Ruiz M. Piquereau J. Mateo P. Fortin D. Veksler V. Garnier A. Ventura-Clapier R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6:e26391. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Rios LY. Bennett RN. Lazarus SA. Rémésy C. Scalbert A. Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002;76:1106–1110. doi: 10.1093/ajcn/76.5.1106. [DOI] [PubMed] [Google Scholar]
- 381.Riso P. Visioli F. Gardana C. Grande S. Brusamolino A. Galvano F. Galvano G. Porrini M. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. J Agric Food Chem. 2005;53:941–947. doi: 10.1021/jf0485234. [DOI] [PubMed] [Google Scholar]
- 382.Rizza S. Muniyappa R. Iantorno M. Kim JA. Chen H. Pullikotil P. Senese N. Tesauro M. Lauro D. Cardillo C. Quon MJ. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E782–E792. doi: 10.1210/jc.2010-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Rolls ET. Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 384.Ronksley PE. Brien SE. Turner BJ. Mukamal KJ. Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Roomi MW. Ivanov V. Kalinovsky T. Niedzwiecki A. Rath M. Anticancer effect of lysine, proline, arginine, ascorbic acid and green tea extract on human renal adenocarcinoma line 786-0. Oncol Rep. 2006;16:943–947. [PubMed] [Google Scholar]
- 386.Roowi S. Mullen W. Edwards CA. Crozier A. Yoghurt impacts on the excretion of phenolic acids derived from colonic breakdown of orange juice flavanones in humans. Mol Nutr Food Res. 2009;53:S68–S75. doi: 10.1002/mnfr.200800287. [DOI] [PubMed] [Google Scholar]
- 387.Roowi S. Stalmach A. Mullen W. Lean MEJ. Edwards CA. Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem. 2010;58:1296–1304. doi: 10.1021/jf9032975. [DOI] [PubMed] [Google Scholar]
- 388.Ros E. Nunez I. Perez-Heras A. Serra M. Gilabert R. Casals E. Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 389.Rosenblat M. Volkova N. Coleman R. Aviram M. Pomegranate byproduct administration to apolipoprotein e-deficient mice attenuates atherosclerosis development as a result of decreased macrophage oxidative stress and reduced cellular uptake of oxidized low-density lipoprotein. J Agric Food Chem. 2006;54:1928–1935. doi: 10.1021/jf0528207. [DOI] [PubMed] [Google Scholar]
- 390.Roura E. Almajano MP. Mata-Bilbao ML. Andrés-Lacueva C. Estruch R. Lamuela-Raventós RM. Human urine: epicatechin metabolites and antioxidant activity after cocoa intake. Free Radic Res. 2007;41:943–949. doi: 10.1080/10715760701435236. [DOI] [PubMed] [Google Scholar]
- 391.Roura E. Andés-Lacueva C. Estruch R. Mata-Bilbao ML. Izquierdo-Pulido M. Waterhouse AL. Lamuela-Raventós RM. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann Nutr Metab. 2007;51:493–498. doi: 10.1159/000111473. [DOI] [PubMed] [Google Scholar]
- 392.Roura E. Andrés-Lacueva C. Estruch R. Lourdes Mata Bilbao M. Izquierdo-Pulido M. Lamuela-Raventós RM. The effects of milk as a food matrix for polyphenols on the excretion profile of cocoa (−)-epicatechin metabolites in healthy human subjects. Br J Nutr. 2008;100:846–851. doi: 10.1017/S0007114508922534. [DOI] [PubMed] [Google Scholar]
- 393.Roura E. Andrés-Lacueva C. Jáuregui O. Badia E. Estruch R. Izquierdo-Pulido M. Lamuela-Raventós RM. Rapid liquid chromatography tandem mass spectrometer assay to quantify plasma (−)-epicatechin metabolites after ingestion of a standard portion of cocoa beverage in humans. J Agric Food Chem. 2005;53:6190–6194. doi: 10.1021/jf050377u. [DOI] [PubMed] [Google Scholar]
- 394.Rowlands DJ. Chapple S. Siow RC. Mann GE. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: roles for F-actin and GPR30. Hypertension. 2011;57:833–840. doi: 10.1161/HYPERTENSIONAHA.110.162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Ruitenberg A. den Heijer T. Bakker SL. van Swieten JC. Koudstaal PJ. Hofman A. Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 396.Russell WR. Labat A. Scobbie L. Duncan SH. Availability of blueberry phenolics for microbial metabolism in the colon and the potential inflammatory implications. Mol Nutr Food Res. 2007;51:726–731. doi: 10.1002/mnfr.200700022. [DOI] [PubMed] [Google Scholar]
- 397.Ryan J. Croft K. Mori T. Wesnes K. Spong J. Downey L. Kure C. Lloyd J. Stough C. An examination of the effects of the antioxidant Pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. 2008;22:553–562. doi: 10.1177/0269881108091584. [DOI] [PubMed] [Google Scholar]
- 398.Sakakibara H. Ogawa T. Koyanagi A. Kobayashi S. Goda T. Kumazawa S. Kobayashi H. Shimoi K. Distribution and excretion of bilberry anthocyanins in mice. J Agric Food Chem. 2009;57:7681–7686. doi: 10.1021/jf901341b. [DOI] [PubMed] [Google Scholar]
- 399.Sakata Y. Zhuang H. Kwansa H. Koehler RC. Doré S. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol. 2010;224:325–329. doi: 10.1016/j.expneurol.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Sang S. Lee MJ. Yang I. Buckley B. Yang CS. Human urinary metabolite profile of tea polyphenols analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry with data-dependent acquisition. Rapid Comm Mass Spectrom. 2008;22:1567–1578. doi: 10.1002/rcm.3546. [DOI] [PubMed] [Google Scholar]
- 401.Sari I. Baltaci Y. Bagci C. Davutoglu V. Erel O. Celik H. Ozer O. Aksoy N. Aksoy M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study. Nutrition. 2010;26:399–404. doi: 10.1016/j.nut.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 402.Sarwar N. Thompson AJ. Di Angelantonio E. Markers of inflammation and risk of coronary heart disease. Dis Markers. 2009;26:217–225. doi: 10.3233/DMA-2009-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Scholey AB. French SJ. Morris PJ. Kennedy DO. Milne AL. Haskell CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. 2010;24:1505–1514. doi: 10.1177/0269881109106923. [DOI] [PubMed] [Google Scholar]
- 404.Schroeter H. Heiss C. Balzer J. Kleinbongard P. Keen CL. Hollenberg NK. Sies H. Kwik-Uribe C. Schmitz HH. Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Science Daily. Red wine compound resveratrol demonstrates significant health effects. www.sciencedaily.com/releases/2009/06/090611174052.htm. 2009. www.sciencedaily.com/releases/2009/06/090611174052.htm
- 406.Seeram NP. Aronson WJ. Zhang Y. Henning SM. Moro A. Lee RP. Sartippour M. Harris DM. Rettig M. Suchard MA. Pantuck AJ. Belldegrun A. Heber D. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 407.Seeram NP. Henning SM. Zhang Y. Suchard M. Li Z. Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 408.Self HL. Brown RR. Price JM. Quantitative studies on the metabolites of tryptophan in the urine of swine. J Nutr. 1960;70:21–25. doi: 10.1093/jn/70.1.21. [DOI] [PubMed] [Google Scholar]
- 409.Setchell KD. Brown NM. Lydeking-Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 410.Setchell KD. Brown NM. Zimmer-Nechemias L. Brashear WT, Wolfe BE, Kirschner AS, and Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 411.Setchell KD. Faughnan MS. Zimmer-Nechemias L. Brown NM. Wolfe BE. Brashear WT. Desai D. Oldfield MF. Botting NP. Cassidy A. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labelled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–419. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 412.Shahrzad S. Bitsch I. Determination of gallic acid and its metabolites in human plasma and urine by high-performance liquid chromatography. J Chromatogr B. 1998;705:87–95. doi: 10.1016/s0378-4347(97)00487-8. [DOI] [PubMed] [Google Scholar]
- 413.Shahrzad S. Aoyagi K. Winter A. Koyama A. Bitsch I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr. 2001;131:1207–1210. doi: 10.1093/jn/131.4.1207. [DOI] [PubMed] [Google Scholar]
- 414.Sharma M. Li L. Celver J. Killian C. Kovoor A. Seeram NP. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J Agric Food Chem. 2010;58:3965–3969. doi: 10.1021/jf902857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Shaughnessy KS. Boswall IA. Scanlan AP. Gottschall-Pass KT. Sweeney MI. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr Res. 2009;29:130–138. doi: 10.1016/j.nutres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 416.Shif O. Gillette K. Damkaoutis CM. Carrano C. Robbins SJ. Hoffman JR. Effects of Ginkgo biloba administered after spatial learning on water maze and radial arm maze performance in young adult rats. Pharmacol Biochem Behav. 2006;84:17–25. doi: 10.1016/j.pbb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 417.Shirai M. Moon JH. Tsushida T. Terao J. Inhibitory effect of a quercetin metabolite, quercetin 3-O-β-D-glucuronide, on lipid peroxidation in liposomal membranes. J Agric Food Chem. 2001;49:5602–5608. doi: 10.1021/jf010713g. [DOI] [PubMed] [Google Scholar]
- 418.Shirai M. Yamanishi R. Moon JH. Murota K. Terao J. Effect of quercetin and its conjugated metabolite on the hydrogen peroxide-induced intracellular production of reactive oxygen species in mouse fibroblasts. Biosci Biotechnol Biochem. 2002;66:1015–1021. doi: 10.1271/bbb.66.1015. [DOI] [PubMed] [Google Scholar]
- 419.Shoji T. Masumoto S. Moriichi N. Akiyama H. Kanda T. Ohtake Y. Goda Y. Apple procyanidin oligomers absorption in rats after oral administration: analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J Agric Food Chem. 2006;54:884–892. doi: 10.1021/jf052260b. [DOI] [PubMed] [Google Scholar]
- 420.Shors TJ. Townsend DA. Zhao M. Kozorovitskiy Y. Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Shrime MG. Bauer SR. McDonald AC. Chowdhury NH. Coltart CE. Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. 2011;141:1982–1988. doi: 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 422.Shukitt-Hale B. Carey A. Simon L. Mark DA. Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 423.Shukitt-Hale B. Cheng V. Joseph JA. Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci. 2009;12:135–140. doi: 10.1179/147683009X423292. [DOI] [PubMed] [Google Scholar]
- 424.Small SA. Chawla MK. Buonocore M. Rapp PR. Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Small SA. Tsai WY. DeLaPaz R. Mayeux R. Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 426.Sone T. Kuriyama S. Nakaya N. Hozawa A. Shimazu T. Nomura K. Rikimaru S. Tsuji I. Randomized controlled trial for an effect of catechin-enriched green tea consumption on adiponectin and cardiovascular disease risk factors. Food Nutr Res. 2011;55 doi: 10.3402/fnr.v55i0.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Spaak J. Merlocco AC. Soleas GJ. Tomlinson G. Morris BL. Picton P. Notarius CF. Chan CT. Floras JS. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol. 2008;294:H605–H612. doi: 10.1152/ajpheart.01162.2007. [DOI] [PubMed] [Google Scholar]
- 428.Spencer JP. Beyond antioxidants: the cellular and molecular interactions of flavonoids and how these underpin their actions on the brain. Proc Nutr Soc. 2010;69:244–260. doi: 10.1017/S0029665110000054. [DOI] [PubMed] [Google Scholar]
- 429.Spencer JP. Flavonoids: modulators of brain function? Br J Nutr. 2008;99(E Suppl 1):ES60–ES77. doi: 10.1017/S0007114508965776. [DOI] [PubMed] [Google Scholar]
- 430.Spencer JP. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–1161. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- 431.Spencer JP. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010;104(Suppl 3):S40–S47. doi: 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- 432.Spencer JP. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2:257–273. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Spencer JPE, editor; Crozier A, editor; Packer L, editor; Cadenas H, editor. Flavonoids, Related Compounds: Bioavailability, Function. Oxidative Stress and Disease. Vol. 30. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 434.Stalmach A. Edwards CA. Wightman J. Crozier A. Identification of (poly)phenolic compounds in Concord grape juice and their metabolites in human plasma and urine after juice consumption. J Agric Food Chem. 2011;59:9512–9522. doi: 10.1021/jf2015039. [DOI] [PubMed] [Google Scholar]
- 435.Stalmach A. Edwards CA, Wightman J, and Crozier A. Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of Concord grape juice by humans. Mol Nutr Food Res. 2012;56:497–509. doi: 10.1002/mnfr.201100566. [DOI] [PubMed] [Google Scholar]
- 436.Stalmach A. Mullen W. Barron D. Uchida K. Yokota T. Cavin C. Steiling H. Williamson G. Crozier A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine following the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Met Disp. 2009;37:1759–1768. doi: 10.1124/dmd.109.028019. [DOI] [PubMed] [Google Scholar]
- 437.Stalmach A. Mullen W. Pecorari M. Serafini M. Crozier A. Bioavailability of C-linked dihydrochalcone and flavanone glucosides in humans following ingestion of unfermented and fermented rooibos teas. J Agric Food Chem. 2009;57:7104–7111. doi: 10.1021/jf9011642. [DOI] [PubMed] [Google Scholar]
- 438.Stalmach A. Mullen W. Steiling H. Williamson G. Lean MEJ. Crozier A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol Nutr Food Res. 2010;54:323–334. doi: 10.1002/mnfr.200900194. [DOI] [PubMed] [Google Scholar]
- 439.Stalmach A. Steiling H. Williamson G. Crozier A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch Biochem Biophys. 2010;501:98–105. doi: 10.1016/j.abb.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 440.Stalmach A. Troufflard S. Serafini M. Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res. 2009;53:S44–S53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 441.Stangl D. Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Steffen Y. Gruber C. Schewe T. Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 443.Steptoe A. Gibson EL. Vuononvirta R. Hamer M. Wardle J. Rycroft JA. Martin JF. Erusalimsky JD. The effects of chronic tea intake on platelet activation and inflammation: a double-blind placebo controlled trial. Atherosclerosis. 2007;193:277–282. doi: 10.1016/j.atherosclerosis.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 444.Stoupi S. Williamson G. Drynan JW. Barron D. Clifford MN. A comparison of the in vitro biotransformation of (−)-epicatechin and procyanidin B2 by human faecal microbiota. Mol Nutr Food Res. 2010;54:747–759. doi: 10.1002/mnfr.200900123. [DOI] [PubMed] [Google Scholar]
- 445.Stoupi S. Williamson G. Drynan JW. Barron D. Clifford MN. Procyanidin B2 catabolism by human fecal microflora: partial characterization of ‘dimeric’ intermediates. Arch Biochem Biophys. 2010;501:73–78. doi: 10.1016/j.abb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 446.Stoupi S. Williamson G. Viton F. Barron D. King LJ. Brown JE. Clifford MN. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C]procyanidin B2 in male rats. Drug Met Disp. 2010;38:287–291. doi: 10.1124/dmd.109.030304. [DOI] [PubMed] [Google Scholar]
- 447.Strack D. Wray V. Anthocyanins. In: Harborne JB, editor. The Flavonoids: Advances in Research Since 1986. London: Chapman and Hall; 1992. pp. 1–22. [Google Scholar]
- 448.Stull AJ. Cash KC. Johnson WD. Champagne CM. Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140:1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Suganuma M. Okabe S. Oniyama M. Tada Y. Ito H. Fujiki H. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 450.Sumner MD. Elliott-Eller M. Weidner G. Daubenmier JJ. Chew MH. Marlin R. Raisin CJ. Ornish D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol. 2005;96:810–814. doi: 10.1016/j.amjcard.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 451.Suri S. Liu XH. Rayment S. Hughes DA. Kroon PA. Needs PW. Taylor MA. Tribolo S. Wilson VG. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br J Pharmacol. 2010;159:566–575. doi: 10.1111/j.1476-5381.2009.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Takagaki A. Otani S. Nanjo F. Antioxidative activity of microbial metabolites of (−)-epigallocatechin gallate produced in rat intestines. Biosci Biotechnol Biochem. 2011;75:582–585. doi: 10.1271/bbb.100683. [DOI] [PubMed] [Google Scholar]
- 453.Taubert D. Roesen R. Lehmann C. Jung N. Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 454.Taubert D. Roesen R. Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167:626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 455.Thompson HJ. Heimendinger J. Gillette C. Sedlacek SM. Haegele A. O'Neill C. Wolfe P. In vivo investigation of changes in biomarkers of oxidative stress induced by plant food rich diets. J Agric Food Chem. 2005;53:6126–6132. doi: 10.1021/jf050493x. [DOI] [PubMed] [Google Scholar]
- 456.Thompson HJ. Heimendinger J. Haegele A. Sedlacek SM. Gillette C. O'Neill C. Wolfe P. Conry C. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis. 1999;20:2261–2266. doi: 10.1093/carcin/20.12.2261. [DOI] [PubMed] [Google Scholar]
- 457.Tognolini M. Giorgio C. Mohamed IH. Barocelli E. Calani L. Reynaud E. Dangles O. Borges G. Crozier A. Brighenti F. Del Rio D. Perturbation of the EphA2-EphrinA1 system by colonic (poly)phenol catabolites in human prostate cancer cells. J Agric Food Chem. 2012 doi: 10.1021/jf205305m. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 458.Tomás-Barberán FA. Cienfuegos-Jovellanos E. Marín A. Muguerza B. Gil-Izquierdo A. Cerda B. Zafrilla P. Morillas J. Mulero J. Ibarra A. Pasamar MA. Ramón D. Espín JC. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J Agric Food Chem. 2007;55:3926–3935. doi: 10.1021/jf070121j. [DOI] [PubMed] [Google Scholar]
- 459.Tribolo S. Lodi F. Connor C. Suri S. Wilson VG. Taylor MA. Needs PW. Kroon PA. Hughes DA. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–56. doi: 10.1016/j.atherosclerosis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 460.Trock BJ. Hilakivi-Clarke L. Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 461.Trzeciakiewicz A. Habauzit V. Mercier S. Barron D. Urpi-Sarda M. Manach C. Offord E. Horcajada MN. Molecular mechanism of hesperetin-7-O-glucuronide, the main circulating metabolite of hesperidin, involved in osteoblast differentiation. J Agric Food Chem. 2010;58:668–675. doi: 10.1021/jf902680n. [DOI] [PubMed] [Google Scholar]
- 462.Tsang C. Auger C. Mullen W. Bornet A. Rouanet JM. Crozier A. Teissedre PL. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr. 2005;94:170–181. doi: 10.1079/bjn20051480. [DOI] [PubMed] [Google Scholar]
- 463.Tsao AS. Liu D. Martin J. Tang XM. Lee JJ. El-Naggar AK. Wistuba I. Culotta KS. Mao L. Gillenwater A. Sagesaka YM. Hong WK. Papadimitrakopoulou V. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res. 2009;2:931–941. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Unno T. Kondo K, Itakura H, and Takeo T. Analysis of (−)-epigallocatechin gallate in human serum obtained after ingesting green tea. Biosci Biotechnol Biochem. 1996;60:2066–2068. doi: 10.1271/bbb.60.2066. [DOI] [PubMed] [Google Scholar]
- 465.Urpi-Sarda M. Monagas M. Khan N. Lamuela-Raventos RM. Santos-Buelga C. Sacanella E. Castell M. Permanyer J. Andres-Lacueva C. Epicatechin, procyanidins and phenolic microbial metabolites after cocoa intake in humans and rats. Anal Bioanal Chem. 2009;394:1545–1556. doi: 10.1007/s00216-009-2676-1. [DOI] [PubMed] [Google Scholar]
- 466.Urpi-Sarda M. Ramiro-Puig E. Romos-Romero S. Llorach R. Castell M. González-Manzano S. Santos-Buelga C. Andres-Lacueva C. Distribution of epicatechin metabolites in lymphoid tissues and testes of young rats with a cocoa-enriched diet. Br J Nutr. 2010;103:1393–1397. doi: 10.1017/S0007114509993473. [DOI] [PubMed] [Google Scholar]
- 467.Urpi-Sarda M. Zamora-Ros R. Lamuela-Raventos R. Cherubini A. Jauregui O. de la Torre R. Covas MI. Estruch R. Jaeger W. Andres-Lacueva C. HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clin Chem. 2007;53:292–299. doi: 10.1373/clinchem.2006.071936. [DOI] [PubMed] [Google Scholar]
- 468.van de Wetering K. Burkon A. Feddema W. Bot A. de Jonge H. Somoza V. Borst P. Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol Pharmacol. 2009;7:876–885. doi: 10.1124/mol.108.052019. [DOI] [PubMed] [Google Scholar]
- 469.van de Wiel A. de Lange DW. Cardiovascular risk is more related to drinking pattern than to the type of alcoholic drinks. Neth J Med. 2008;66:467–473. [PubMed] [Google Scholar]
- 470.van Erk MJ. Roepman P. van der Lende TR. Stierum RH. Aarts JM. van Bladeren PJ. van Ommen B. Integrated assessment by multiple gene expression analysis of quercetin bioactivity on anticancer-related mechanisms in colon cancer cells in vitro. Eur J Nutr. 2005;44:143–156. doi: 10.1007/s00394-004-0503-1. [DOI] [PubMed] [Google Scholar]
- 471.van het Hof KH. de Boer HS. Wiseman SA. Lien N. Westrate JA. Tijburg LB. Consumption of green or black tea does not increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 1997;66:1125–1132. doi: 10.1093/ajcn/66.5.1125. [DOI] [PubMed] [Google Scholar]
- 472.van Mierlo LA. Zock PL. van der Knaap HC. Draijer R. Grape polyphenols do not affect vascular function in healthy men. J Nutr. 2010;140:1769–1773. doi: 10.3945/jn.110.125518. [DOI] [PubMed] [Google Scholar]
- 473.van Praag H. Lucero MJ. Yeo GW. Stecker K. Heivand N. Zhao C. Yip E. Afanador M. Schroeter H. Hammerstone J. Gage FH. Plant-derived flavanol (−)-epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Vastrano BC. Chen Y. Zhu N. Ho CT. Zhou Z. Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000;48:253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 475.Vaz-da-Silva M. Loureiro AI. Falcao A. Nunes T. Rocha JF. Fernandes-Lopes C. Soares E. Wright L. Almeida L. Soares-da-Silva P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int J Clin Pharmacol Ther. 2008;46:564–570. doi: 10.5414/cpp46564. [DOI] [PubMed] [Google Scholar]
- 476.Verzelloni E. Pellacani C. Tagliazucchi D. Tagliaferri S. Calani L. Costa LG. Brighenti F. Borges G. Crozier A. Conte A. Del Rio D. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol Nutr Food Res. 2011;55(Suppl 1):S35–S43. doi: 10.1002/mnfr.201000525. [DOI] [PubMed] [Google Scholar]
- 477.Verzelloni E. Tagliazucchi D. Del Rio D. Calani L. Conte A. Antiglycative and antioxidative properties of coffee fractions. Food Chem. 2011;124:1430–1435. [Google Scholar]
- 478.Vitaglione P. Donnarumma G. Napolitano A. Galvano F. Gallo A. Scalfi L. Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 479.Vitaglione P. Sforza S. Galaverna G. Ghidini C. Caporaso N. Vescovi PP. Fogliano V. Marchelli R. Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 480.Walle T. Hsieh F. DeLegge MH. Oatis JE., Jr. Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Met Disp. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 481.Wang D. Wei X. Yan X. Jin T. Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. 2010;58:12722–12728. doi: 10.1021/jf103427j. [DOI] [PubMed] [Google Scholar]
- 482.Wang JF. Schramm DD. Holt RR. Ensunsa JL. Fraga CG. Schmitz HH. Keen CL. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J Nutr. 2000;130:2115S–2119S. doi: 10.1093/jn/130.8.2115S. [DOI] [PubMed] [Google Scholar]
- 483.Wang LD. Zhou Q. Feng CW. Liu B. Qi YJ. Zhang YR. Gao SS. Fan ZM. Zhou Y. Yang CS. Wei JP. Zheng S. Intervention and follow-up on human esophageal precancerous lesions in Henan, northern China, a high-incidence area for esophageal cancer. Cancer Chemother. 2002;29(Suppl 1):159–172. [PubMed] [Google Scholar]
- 484.Wang Y. Wang L. Wu J. Cai J. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br J Pharmacol. 2006;148:147–153. doi: 10.1038/sj.bjp.0706720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Wang Z. Fernandez-Seara M. Alsop DC. Liu WC. Flax JF. Benasich AA. Detre JA. Assessment of functional development in normal infant brain using arterial spin labeled perfusion MRI. Neuroimage. 2008;39:973–978. doi: 10.1016/j.neuroimage.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Wang-Polagruto JF. Villablanca AC. Polagruto JA. Lee L. Holt RR. Schrader HR. Ensunsa JL. Steinberg FM. Schmitz HH. Keen CL. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S177–S186. doi: 10.1097/00005344-200606001-00013. discussion S206–S209. [DOI] [PubMed] [Google Scholar]
- 487.Weisel T. Baum M. Eisenbrand G. Dietrich H. Will F. Stockis JP. Kulling S. Rufer C. Johannes C. Janzowski C. An anthocyanin/polyphenolic-rich fruit juice reduces oxidative DNA damage and increases glutathione level in healthy probands. Biotechnol J. 2006;1:388–397. doi: 10.1002/biot.200600004. [DOI] [PubMed] [Google Scholar]
- 488.Weseler AR. Ruijters EJ. Drittij-Reijnders MJ. Reesink KD. Haenen GR. Bast A. Pleiotropic benefit of monomeric and oligomeric flavanols on vascular health—a randomized controlled clinical pilot study. PLoS One. 2011;6:e28460. doi: 10.1371/journal.pone.0028460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Whelan AP. Sutherland WH. McCormick MP. Yeoman DJ. de Jong SA. Williams MJ. Effects of white and red wine on endothelial function in subjects with coronary artery disease. Int Med J. 2004;34:224–228. doi: 10.1111/j.1444-0903.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 490.Widlansky ME. Hamburg NM. Anter E. Holbrook M. Kahn DF. Elliott JG. Keaney JF., Jr. Vita JA. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr. 2007;26:95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Williams CM. El Mohsen MA. Vauzour D. Rendeiro C. Butler LT. Ellis JA. Whiteman M. Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 492.Williams RJ. Spencer JP. Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 493.Williamson G. Clifford MN. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr. 2010;104(Suppl 3):S48–S66. doi: 10.1017/S0007114510003946. [DOI] [PubMed] [Google Scholar]
- 494.Willis LM. Shukitt-Hale B. Joseph JA. Recent advances in berry supplementation and age-related cognitive decline. Curr Opin Clin Nutr Metab Care. 2009;12:91–94. doi: 10.1097/MCO.0b013e32831b9c6e. [DOI] [PubMed] [Google Scholar]
- 495.Winter JC. The effects of an extract of Ginkgo biloba, EGb 761, on cognitive behavior and longevity in the rat. Physiol Behav. 1998;63:425–433. doi: 10.1016/s0031-9384(97)00464-2. [DOI] [PubMed] [Google Scholar]
- 496.Winterbone MS. Tribolo S. Needs PW. Kroon PA. Hughes DA. Physiologically relevant metabolites of quercetin have no effect on adhesion molecule or chemokine expression in human vascular smooth muscle cells. Atherosclerosis. 2009;202:431–438. doi: 10.1016/j.atherosclerosis.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 497.Wiseman W. Egan JM. Slemmer JE. Shaughnessy KS. Ballem K. Gottschall-Pass KT. Sweeney MI. Feeding blueberry diets inhibits angiotensin II-converting enzyme (ACE) activity in spontaneously hypertensive stroke-prone rats. Can J Physiol Pharmacol. 2011;89:67–71. doi: 10.1139/y10-101. [DOI] [PubMed] [Google Scholar]
- 498.Wong RH. Howe PR. Buckley JD. Coates AM. Kunz I. Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 499.Woods E. Clifford MN. Gibbs M. Hampton S. Arendt J. Morgan L. Estimation of mean intakes of 14 classes of dietary phenols in a population of male shift workers. Proc Nutr Soc. 2003;62:60A. [Google Scholar]
- 500.Woodward G. Kroon P. Cassidy A. Kay C. Anthocyanin stability and recovery: implications for the analysis of clinical and experimental samples. J Agric Food Chem. 2009;57:5271–5278. doi: 10.1021/jf900602b. [DOI] [PubMed] [Google Scholar]
- 501.Wu AH. Yu MC. Tseng CC. Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Wu CH. Lin MC. Wang HC. Yang MY. Jou MJ. Wang CJ. Rutin inhibits oleic acid induced lipid accumulation via reducing lipogenesis and oxidative stress in hepatocarcinoma cells. J Food Sci. 2011;76:T65–T72. doi: 10.1111/j.1750-3841.2010.02033.x. [DOI] [PubMed] [Google Scholar]
- 503.Wu JN. Ho SC. Zhou C. Ling WH. Chen WQ. Wang CL. Chen YM. Coffee consumption and risk of coronary heart diseases: a meta-analysis of 21 prospective cohort studies. Int J Cardiol. 2009;137:216–225. doi: 10.1016/j.ijcard.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 504.Wu X. Cao G. Prior RL. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J Nutr. 2002;132:1865–1871. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]
- 505.Wu X. Pittman HE. McKay S. Prior RL. Aglycones and sugar moieties alter anthocyanin absorption and metabolism after berry consumption in weanling pigs. J Nutr. 2005;135:2417–2424. doi: 10.1093/jn/135.10.2417. [DOI] [PubMed] [Google Scholar]
- 506.Yang JH. Hsia TC. Kuo HM. Chao PD. Chou CC. Wei YH. Chung JG. Inhibition of lung cancer cell growth by quercetin glucuronides via G2/M arrest and induction of apoptosis. Drug Metab Dispos. 2006;34:296–304. doi: 10.1124/dmd.105.005280. [DOI] [PubMed] [Google Scholar]
- 507.Yeh SL. Yeh CL. Chan ST. Chuang CH. Plasma rich in quercetin metabolites induces G2/M arrest by upregulating PPAR-γ expression in human A549 lung cancer cells. Planta Med. 2011;77:992–998. doi: 10.1055/s-0030-1250735. [DOI] [PubMed] [Google Scholar]
- 508.Yip EC. Chan AS. Pang H. Tam YK. Wong YH. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol Toxicol. 2006;22:293–302. doi: 10.1007/s10565-006-0082-4. [DOI] [PubMed] [Google Scholar]
- 509.Zern TL. Wood RJ. Greene C. West KL. Liu Y. Aggarwal D. Shachter NS. Fernandez ML. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 510.Zhang SM. Hunter DJ. Rosner BA. Giovannucci EL. Colditz GA. Speizer FE. Willett WC. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin's lymphoma among women. Cancer Epidemiol Biomarkers Prev. 2000;9:477–485. [PubMed] [Google Scholar]
- 511.Zhu Y. Xia M. Yang Y. Liu F. Li Z. Hao Y. Mi M. Jin T. Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem. 2011;57:1524–1533. doi: 10.1373/clinchem.2011.167361. [DOI] [PubMed] [Google Scholar]
- 512.Zilkens RR. Burke V. Hodgson JM. Barden A. Beilin LJ. Puddey IB. Red wine and beer elevate blood pressure in normotensive men. Hypertension. 2005;45:874–879. doi: 10.1161/01.HYP.0000164639.83623.76. [DOI] [PubMed] [Google Scholar]
- 513.Zini A. Del Rio D. Stewart AJ. Mandrioli J. Merelli E. Sola P. Nichelli P. Serafini M. Brighenti F. Edwards CA. Crozier A. Do flavan-3-ols from green tea reach the human brain. Nutr Neurosci. 2006;9:57–61. doi: 10.1080/10284150600637739. [DOI] [PubMed] [Google Scholar]