Abstract
Primate societies are based on face recognition. Face recognition mechanisms have been studied most extensively in humans and macaque monkeys. In both species, multiple brain areas specialized for face processing have been found, and their functional properties are characterized with increasing detail, so we can now begin to address questions about similarities and differences of face-recognition systems across species with 25 million years of separate evolution. Both systems are organized into multiple face-selective cortical areas in spatial arrangements and with functional specializations, implying both hierarchical and parallel modes of information processing. Yet open questions about homologies remain. To address these, future studies employing similar techniques and experimental designs across multiple species are needed to identify a putative core primate face processing system and to understand its differentiations into the multiple branches of the primate order.
Introduction
Faces are special: we encounter them more frequently than almost any other type of object, and they provide a rich source of diverse social information [1]. This is the case for virtually all primate species. If this is so, then even the first living primate's brain should have contained machinery for face recognition. We should therefore expect to find remnants of this system in the evolved face processing systems of living primates today. But what is the evidence for this? Specialization of brain areas for face processing have, to date, only been studied extensively in two primate species, humans and macaque monkeys, which belong to the catarrhine group of primates. This group is thought to have split some 25 million years ago into the old world monkeys, which macaques belong to, and hominoids, which humans belong to [2,3]. What properties do the macaque and human face-processing systems share? And can we infer homology from these commonalities? Recent investigations into the human and macaque face-processing systems allow us to begin answering these questions. They further offer insights into mechanisms of object recognition, principles of brain organization, and the evolution of social brains. The better we understand homologies between the systems, the better we can use data available from only one species to understand the other. Research into face-recognition systems will deepen our understanding of the evolution of social perceptual abilities. Here, we review selected findings on human and macaque face processing systems in order to highlight similarities and differences between the two species and delineate directions for future research.
Face-selective brain areas in humans and macaque monkeys
Faces activate special areas within those parts of the visual system that are dedicated to object recognition [4-9]. In humans, numerous functional magnetic resonance imaging (fMRI) studies have reported three occipito-temporal areas that respond significantly more strongly to faces than any other non-face objects. These face-selected areas were found in the lateral occipital cortex, the occipital face area (OFA) of the mid fusiform gyrus [10], the fusiform face area (FFA) [7], and one within the posterior part of the superior temporal sulcus (STS), the STS-FA [11] (Fig. 1A, right). As imaging technology has improved and more diverse stimulus sets have been used, further areas and subdivisions have been found: the FFA can be subdivided into an anterior and a posterior part [12,13]; it is accompanied by one or two more anterior face areas [13-15]; similarly, the STS-FA appears to be only one of multiple face areas inside the STS [16,17]; and a face-selective prefrontal region was found in or close to the inferior frontal gyrus (IFG) [14,17,18]. Thus, it appears that face processing in humans is supported by an array of specialized face areas (see Table 1). In macaque monkeys, fMRI and electrophysiological recording studies have revealed five face areas inside the STS (Fig. 1A, left), one in anterior inferotemporal cortex on the ventral surface of the temporal lobe [14] (Table 1B), and three in the frontal lobe [19]. A further face-selective region might exist on or outside the dorsal lip of the STS [13], and a recent study reported three further areas on the ventral surface of the temporal lobe [20] (see Table 1B). Thus, in both human and macaque monkey, using the same technique, multiple face-selective areas are found to exist; they are found primarily in the temporal lobe, with lower frequency in the frontal lobe; they reside within object-selective cortex; and, while there are still some uncertainties, the numbers of face areas might match exactly in both species. This match is not trivial given the huge difference in absolute size of macaque and human brain and the variation in the number of cortical fields across species [21]. If there is a one-to-one mapping between human and macaque face areas, then how could it have been established?
Figure 1. Functional correspondence between human and monkey face areas.
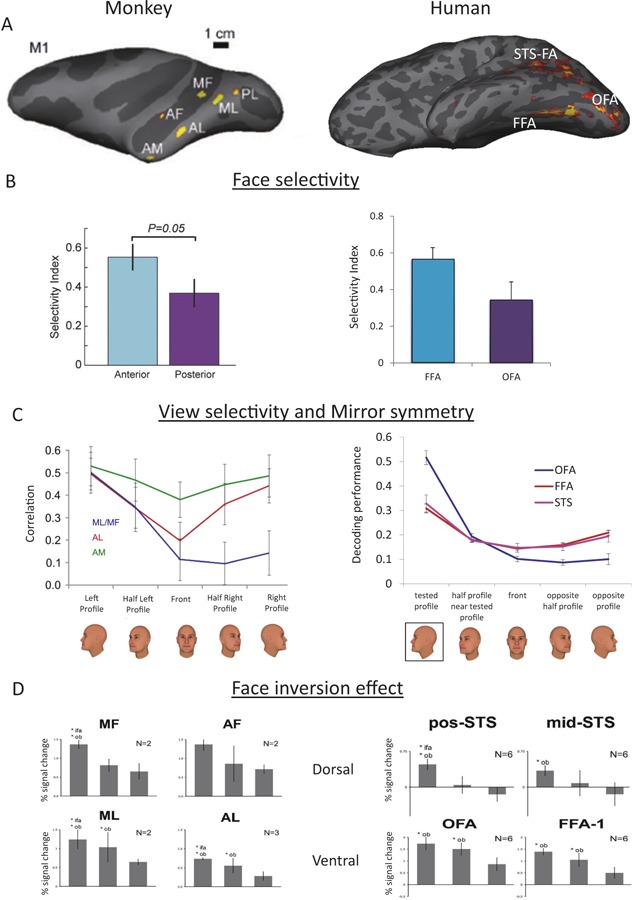
(A) Left: In the macaque temporal lobe, six face patches have been consistently found by fMRI. They have been referred to, from posterior to anterior, as the posterior lateral (PL), medial lateral (ML, medial fundus (MF), anterior lateral (AL), anterior fundus (AF), and anterior medial (AM) face patch. Right: In the human temporal lobe, three face-selective brain regions have been frequently described: the fusiform face area (FFA), the occipital face area (OFA), and the STS face area (STS-FA). (B) Left: Anterior face areas in monkeys show higher face selectivity than posterior areas (modified from ref [47]). Right: The human FFA shows higher face selectivity than the OFA (based on data collected by Erez & Yovel, submitted). (C) Left: Tuning to head orientation in ML/MF, AL, and AM. Correlation coefficients between population activity vectors to twenty five faces at five different head orientations were computed based on data from ref [49]. Only cross-individual correlations were computed. Error bars indicate one standard deviation. Head orientation coding is strongly mirror-symmetrical in AL, weakly in AM, and not mirror-symmetrical in MF/ML. Right: Evidence for mirror-symmetric coding of head orientation in human FFA and STS-FA, but not OFA was found using multivoxel-pattern analysis [51]. (D) Inversion effect is larger in the dorsal (monkey MF/AF and human STS) than the ventral face areas (monkey ML/AL and human OFA/FFA). Inverted faces show similar response to upright objects in the dorsal areas but higher response than upright objects in the ventral areas (reconstructed from Pinsk [13]).
Table 1A. Approximate correspondence of human face-selective areas (defined by contrast faces>objects) across studies.
| Study | Face-selective areas | |||||||
|---|---|---|---|---|---|---|---|---|
| Haxby et al [60] | Inferior OccipitalGyrus | Lateral Fusiform Gyrus | STS | |||||
| Kanwisher & Yovel [59] | OFA | FFA | fSTS | |||||
| Tsao et al [14] | OFA | FFA | AFP1 | AFP2 | STS-FA | |||
| Pinsk et al [13] | OFA | FFA1 | FFA2 | AT | postSTS/midSTS | antSTS | ||
| Rajimehr et al [15] | OFA/IOG | FFA | ATFP | STS | ||||
| Weiner and Grill-Spector [31] | IOG-face | posFus-face | midFus-face | pSTS1 | ||||
| Pitcher et al [17] | OFA | FFA | pSTS2 | aSTS3 | ||||
1the STS face areas reported in the other studies are anterior to this one
2also reported pcSTS, which may correspond to postSTS in Pinsk et al., [13] and pSTS in Weiner and Grill-Spector, [31]
3was found only with dynamic stimuli
OFA – Occipital Face Area, FFA – Fusiform Face Area, AFP – Anterior Face Patch, STS-FA – superior temporal sulcus- face area, fSTS – face superior temporal sulcus, pSTS – posterior STS, aSTS – anterior STS
Table 1B. Approximate correspondence of macaque face-selective areas (defined by contrast faces>objects) across studies.
| Study | Face-selective areas | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tsao et al [14] | PL | ML | MF | AL | AF | AM | |||
| Pinsk et al [13] | ML | MF | AL | AF | AD | ||||
| Rajimehr et al [15] | Posterior temporal face patch (PTFP) | Anterior temporal face patch (AFTP) | |||||||
| Bell et al [47] | Posterior face-selective region | Anterior face-selective region | |||||||
| Ku et al 1 [20] | pSTS | mSTS | aSTS/TEad | AMTS | |||||
| Issa et al [64] | PL | ML | AL | AM | |||||
1Ku et al. further report on vV4, TEpd, TF and EC in the temporal lobe (see Table 2)
IOG – Inferior Occipital Gyrus, ATFP – Anterior Temporal Face patch
posFus – posterior Fusiform; midFus – middle Fusiform; TEad – anterior dorsal portion of temporal area “E” of von Economo and Koskinas. AMTS – anterior medial temporal sulcus
Similarities and differences between human and macaque face-processing systems
Homology of brain areas is classically established with a set of criteria including (i) relative location, (ii) cytoarchitecture/immunohistochemistry/gene expression profiles, (iii) connectivity, and (iv) functional similarity [22-25]. Below, we review existing evidence for each of these four criteria and discuss the mappings between human and macaque face areas they suggest.
Relative location
The location of face areas, relative to other cortical areas and relative to each other, provides a first constraint on homologies. Temporal lobe face areas in both species are organized along an occipitotemporal axis [14], embedded in the larger organization of the entire visual system that progresses from early visual areas occipitally to more high-level, object-selective areas temporally [26-28]. It is, thus, most parsimonious to assume that homologies between face areas conform to this pattern. Similarly, in both species, face areas are found at more ventral and at more dorsal locations, and it appears most plausible to assume that ordering of areas along this dorso-ventral axis would be preserved across species. These considerations lead to a first scenario (Table 2, Scenario 1).
Table 2. Correspondence between human and monkey face areas based on relative and absolute anatomical location and connectivity.
| Scenario 1 | ||||||
|---|---|---|---|---|---|---|
| Posterior-anterior axis | Species | |||||
| Dorso-Ventral Axis | dorsal | MF | AF | Macaque | ||
| pSTS | aSTS | Human | ||||
| ventral | PL | ML | AL | AM | Macaque | |
| OFA | pFFA | mFFA | AFP | Human | ||
Three scenarios indicating putative correspondences between macaque and human face areas along the posterior-anterior and dorso-ventral anatomical axis
However, human and macaque face-processing systems also exhibit a striking difference in spatial location [14]: while most of the macaque face areas are located inside or close to the STS, the majority of human areas are located at more ventral locations. A recent report on additional, more ventrally located macaque face areas [20], thus, raises the possibility, in agreement with the above interpretative scheme, that human and macaque STS face areas might correspond, as do the more ventral areas of the temporal lobe (Table 2, Scenario 2). However, in this scenario, a stunning mismatch between the numbers of face areas across species inside/around the STS and along the ventral part of inferotemporal cortex remains unexplained.
Understanding the organization of face-processing systems requires not only understanding of the relative position of face areas towards each other but also their embedding into the rest of the brain. Systematic mapping of macaque and human visual cortices onto each other reveals an overall shift of areas ventrally from the STS in humans compared to macaque monkeys [29], corresponding to overall areal expansion in this region [30]. Thus, the fact that human face areas are found on the ventral surface of the temporal lobe, while macaque face areas are mostly found on the lateral surface is consistent with this overall pattern. In agreement with this interpretation, landmark-based cross-species warping of the macaque onto the human brain, estimates that macaque middle face patches in the STS correspond to human FFA [8,15], and macaque anterior area AM map onto human AFP [15] (Table 2, Scenario 1).
Our understanding of the organization of the human face areas has been greatly advanced recently by investigations of their relative location to body- and motion-selective areas [12,31,32]. In humans, a systematic pattern of alternating face- and body-selective areas, some surrounding motion-selective area MT in an alternating pattern was found. Similarly, the macaque face- and limb-selective areas have been found to be located in the same neighborhood as each other [8,13,33] and as motion-selective areas in the STS [34]. However, in contrast to the human brain, no face area has been found dorsal to area MT in the macaque brain, no circular arrangements of face- and body-areas have been demonstrated, and fewer body areas (two) have been found [33]. Further comparative studies are necessary to gain a complete understanding of how the spatial maps of functional specializations [35] that embed face area maps between human and macaque.
Differences in cytoarchitecture, immunohistochemistry and gene expression
While cytoarchitectonic, immunohistochemical, and gene expression differences across brain areas provide, arguably, the strongest clues for homology, these important pieces of information are only beginning to be used to identify putative anatomical specializations of face areas in humans and macaque monkeys. Cytoarchitectonic differences across the cortex [36] have, together with connectivity data, been used to parcel the STS of macaque monkeys [37] into subdivisions, which correlate with functional specializations [38]. Thus, it is important to know where exactly inside the STS macaque face patches are located (and especially where they are located relative to cytoarchitectonically defined STS subregions) in order to establish each area's identity and allow for thorough comparisons with human areas. Human STS is cytoarchitecturally subdivided as well [39], as are the ventral portions of the temporal lobe in which the fusiform face areas reside [40]. Correlating functional specializations with cytoarchitecture should be a major future research focus, as difficult as it is, with cytoarchitecture typically performed post-mortem. But the development of neuroimaging correlates of the cyto- and myeloarchitecture [41,42] might provide new tools to allow for these structure/function correlations.
Connectivity
Connectivity among brain areas constitutes an important third anatomical criterion for establishing homology across species. In macaque monkeys, using electrical stimulation with fMRI, strong connections have been found between the majority of face areas [43], notably also along the dorsoventral axis. In humans, diffusion tensor imaging (DTI) studies suggest strong connections between the OFA and FFA, but weak connections between these areas and the STS [44]. Studies of functional connectivity also report strong linkage of OFA and FFA [45,46]. These findings suggest that, in humans, two face-processing streams might exist, a dorsal and a ventral one, with only weak connections between them. In this scenario (Table 2, Scenario 3), the ventral human face-processing system would find a homolog in the entire macaque face-processing system, both richly interconnected; yet the STS face area(s) would constitute an evolutionarily new development of humans or hominoids, without correspondence in the macaque brain. This scenario is independently supported by the finding of a face area directly dorsal to the MT/MST complex [12], which does not appear to have a counterpart in the macaque.
Functional specializations
Functional specializations of face areas, currently, provide the richest source of information for a cross-species comparison of face-processing systems (Table 3). Of particular relevance are experiments demonstrating clear functional dissociations between face areas.
Table 3. Organization principles for establishing homologies between the human and monkey face processing systems.
| Posterior-Anterior Organization | |
| Relative Location of Areas | |
| Hierarchy Receptive Field Size49-50 | |
| Magnitude of Face Selectivity17,47 | |
| Mirror Symmetry49,51-52 | |
| Individual Selectivity49,53-54 | |
| Dorso-Ventral Organization | |
| Relative Location of Areas | |
| Face Inversion Effect13 | |
| Motion Sensitivity17-18,34 | |
Correspondence between human and monkey face areas based on functional similarities suggests two organization principles along the posterior-anterior and dorso-ventral anatomical axis.
Face selectivity
The most basic feature of a face-selective area is how much more strongly it responds to faces than to non-face stimuli. Bell et al. [47] reported greater face-selectivity in the anterior than posterior face areas in the monkey (Fig. 2B, left). Similarly, human fMRI studies reveal greater selectivity in the more anterior area FFA than in the posterior area OFA (e.g. [17]) (Fig. 2B, right).
Hierarchical organization
Hierarchical object recognition systems are characterized by increasing object selectivity from one processing level to the next and concomitantly increasing abstraction from (invariance to) accidental image features like position, size or object orientation [48]. In macaque monkeys, receptive field sizes increase from posterior to anterior face areas, and face-selective responses grow more and more position-invariant [49]. Similarly, in humans, the OFA shows stronger responses to contralateral than ipsilateral faces, whereas the FFA shows similar responses to faces in both visual fields [50].
As position invariance increases along the face-processing hierarchy, so does invariance to head orientation. Cells in the middle face areas (MF and ML) are tuned to the orientation of face stimuli, firing maximally for one particular head orientation; cells in more anterior area AL showed a mirror-symmetric tuning to head orientation; and cells in the most anterior area AM were highly invariant to head orientation [49]. In humans, fMRI data indicate that the representation of view in the OFA was similar to early visual areas and consistent with view-selectivity and a mirror symmetric representation in the FFA [51,52] (Fig. 1C).
With increasing invariance to position and head orientation, a higher specificity for facial identity is built up from the middle face areas, via AL to AM in the macaque brain [49]. In humans, facial identity selectivity (but not invariance to head orientation) was reported anterior to the FFA in one study [53] and inside and anterior to the FFA (invariant to facial expressions) in another [54]. Future studies establishing the relationship of this selectivity to the anterior face areas will be necessary to confirm the now suggestive functional similarity to macaque area AM.
A hierarchical organization of face areas predicts increasing response latencies from posterior to anterior areas. In the macaque, response latencies, as gathered from single neuron firing rates (and the local field potential's first face-selective component), increase from 88 ms (126 ms) in the middle face areas to 104 ms (133 ms) in AL and to 124 ms (145 ms) in AM [49]. In humans, a transcranial magnetic stimulation study suggests that the OFA is maximally engaged in face processing at 100-110 ms after stimulus onset [55,56], consistent with the results of a simultaneous electroencephalogram-fMRI study that found that the OFA face-selectivity is correlated with face-selectivity at 110 ms. The FFA in this latter EEG-fMRI study was correlated with the face-selective N170 event-related potential component [57], indicating later processing time of about 60 ms.
Taken together, both human and macaque face-processing systems are organized along the occipito-temporal axis (OFA-FFA-aFA and PL-ML/MF-AL/AF-AM) [14] in a processing hierarchy. Mirror-symmetry of head orientation tuning in monkey area AL and human FFA and STS suggest homology, but correspondences between the other face areas remain unclear (Table 1, Scenario 1).
Face inversion
Recognition of upside down relative to upright stimuli drops more severely for faces than non-face objects [58]. A larger face inversion effect was found in anterior than in posterior face areas in macaques [47], and in FFA and STS-FA compared with the OFA in humans [59]. Pinsk et al. [13] reported a smaller inversion effect in ventral (probably ML and AL) than in dorsal (probably MF and AF) macaque face areas. Furthermore, the response to inverted faces did not differ from upright objects in the monkey dorsal face areas, similar to the human STS-FA, whereas the response to inverted faces was higher than the response to upright objects in the monkey ventral face areas, similar to the human OFA and FFA (Figure 1D). These findings suggest a correspondence between the monkey dorsal face areas (MF and AF) and the human STS-FA and the ventral monkey face (ML and AL) areas and the human OFA and FFA (Table 2, Scenario 2).
Selectivity for facial motion
A similar division of labor between dorsal and ventral face-processing areas, proposed early on [60,61], is suggested by studies of facial motion. In macaque monkeys, selectivity for facial motion is found more dorsally inside the STS or even dorsally to it [34]. In humans, the STS-FA is exquisitely tuned to facial motion, while the OFA and the FFA respond similarly to static and moving faces [17,18]. Thus, in addition to an occpitotemporal axis of hierarchical organization, both human and macaque face-processing systems are also organized along a dorso-ventral axis characterized by functional differentiation (face inversion and facial motion) and indicating parallel organization of face-processing systems (Table 2, Scenario 2)
Conclusions and outlook
Comparing face areas across two primate species has provided us with important insights into the neural mechanisms of face recognition. Yet, in trying to establish homologies between the systems, we still need to consider several very different models seriously. The model most consistent with current data is one of two face-processing streams, a dorsal and a ventral one, each organized in a hierarchical fashion (Table 2, Scenario 1, Table 3). Yet, more needs to be learned about functional specializations within and outside face areas, their connections and anatomical underpinnings to reach certainty about the evolution of face-recognition systems in primates. This will also require insights into the brains of additional primate species, in particular the great apes [62], new-world monkeys, and non-primate species (e.g. sheep [63]) for in- and out-group comparisons. Future research into functional specializations of face areas should utilize, as much as possible, common stimulus designs, behavioral paradigms, and experimental techniques.
Acknowledgments
We thank Vadim Axelrod for critically reading the manuscript and providing Figure 1A (human), Shih-pi Ku for discussion of Scenario 2, David Pitcher for discussion of Table 1, Gerhard Roth for advice on evolutionary neuroanatomy, Tim Kietzmann for discussion of mirror symmetry in the human brain and four reviewers for their very insightful comments.
Abbreviations
- AF
anterior fundus
- AFP
anterior face patch
- AL
anterior lateral
- AM
anterior medial
- DTI
diffusion tensor imaging
- FFA
fusiform face area
- fMRI
functional magnetic resonance imaging
- IFG
inferior frontal gyrus
- MF
medial fundus
- ML
medial lateral
- MST
medial superior temporal
- MT
middle temporal
- OFA
occipital face area
- PL
posterior lateral
- STS
superior temporal sulcus
Disclosure
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/5/10
Contributor Information
Galit Yovel, Email: gality@post.tau.ac.il.
Winrich A. Freiwald, Email: wfreiwald@rockefeller.edu.
References
- 1.Allison, Puce, McCarthy Social perception from visual cues: role of the STS region. Trends Cogn Sci (Regul Ed) 2000;4:267–78. doi: 10.1016/S1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 2.Stewart CB, Disotell TR. Primate evolution - in and out of Africa. Curr Biol. 1998;8:R582–8. doi: 10.1016/S0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- 3.Hedges SB, Dudley J, Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- 4.Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46:369–84. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- 5.Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philos Trans R Soc Lond B Biol Sci. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- 6.Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb Cortex. 1994;4:544–54. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- 7.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989828
- 8.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RBH. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–95. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eifuku S, Souza WC de, Tamura R, Nishijo H, Ono T. Neuronal correlates of face identification in the monkey anterior temporal cortical areas. J Neurophysiol. 2004;91:358–71. doi: 10.1152/jn.00198.2003. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989829
- 11.Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3:80–4. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989830
- 12.Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. Neuroimage. 2010;52:1559–73. doi: 10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989831
- 13.Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J Neurophysiol. 2009;101:2581–600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/1165814
- 14.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci USA. 2008;105:19514–9. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajimehr R, Young JC, Tootell RBH. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc Natl Acad Sci USA. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989832
- 16.Carlin JD, Calder AJ, Kriegeskorte N, Nili H, Rowe JB. A head view-invariant representation of gaze direction in anterior superior temporal sulcus. Curr Biol. 2011;21:1817–21. doi: 10.1016/j.cub.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitcher D, Dilks DD, Saxe RR, Triantafyllou C, Kanwisher N. Differential selectivity for dynamic versus static information in face-selective cortical regions. Neuroimage. 2011;56:2356–63. doi: 10.1016/j.neuroimage.2011.03.067. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/14276379
- 18.Fox CJ, Iaria G, Barton JJS. Defining the face processing network: optimization of the functional localizer in fMRI. Hum Brain Mapp. 2009;30:1637–51. doi: 10.1002/hbm.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989838
- 19.Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008;11:877–9. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/1119021
- 20.Ku S, Tolias AS, Logothetis NK, Goense J. fMRI of the face-processing network in the ventral temporal lobe of awake and anesthetized macaques. Neuron. 2011;70:352–62. doi: 10.1016/j.neuron.2011.02.048. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989840
- 21.Krubitzer L. In search of a unifying theory of complex brain evolution. Ann N Y Acad Sci. 2009;1156:44–67. doi: 10.1111/j.1749-6632.2009.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Striedter GF, Northcutt RG. Biological hierarchies and the concept of homology. Brain Behav Evol. 1991;38:177–89. doi: 10.1159/000114387. [DOI] [PubMed] [Google Scholar]
- 23.Payne BR. Evidence for visual cortical area homologs in cat and macaque monkey. Cereb Cortex. 1993;3:1–25. doi: 10.1093/cercor/3.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Pritz MB. Comparisons and homology in adult and developing vertebrate central nervous systems. Brain Behav Evol. 2005;66:222–33. doi: 10.1159/000088127. [DOI] [PubMed] [Google Scholar]
- 25.Krubitzer LA, Seelke AMH. Cortical evolution in mammals: the bane and beauty of phenotypic variability. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10647–54. doi: 10.1073/pnas.1201891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J Neurophysiol. 1994;71:856–67. doi: 10.1152/jn.1994.71.3.856. [DOI] [PubMed] [Google Scholar]
- 27.Logothetis NK, Sheinberg DL. Visual object recognition. Annu Rev Neurosci. 1996;19:577–621. doi: 10.1146/annurev.ne.19.030196.003045. [DOI] [PubMed] [Google Scholar]
- 28.Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci (Regul Ed) 2013;17:26–49. doi: 10.1016/j.tics.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orban GA, van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci (Regul Ed) 2004;8:315–24. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989842
- 30.van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–25. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Weiner KS, Grill-Spector K. Neural representations of faces and limbs neighbor in human high-level visual cortex: evidence for a new organization principle. Psychol Res. 2013;77:74–97. doi: 10.1007/s00426-011-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989843
- 32.Weiner KS, Grill-Spector K. Not one extrastriate body area: using anatomical landmarks, hMT+, and visual field maps to parcellate limb-selective activations in human lateral occipitotemporal cortex. Neuroimage. 2011;56:2183–99. doi: 10.1016/j.neuroimage.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989845
- 33.Popivanov ID, Jastorff J, Vanduffel W, Vogels R. Stimulus representations in body-selective regions of the macaque cortex assessed with event-related fMRI. Neuroimage. 2012;63:723–41. doi: 10.1016/j.neuroimage.2012.07.013. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989847
- 34.Furl N, Hadj-Bouziane F, Liu N, Averbeck BB, Ungerleider LG. Dynamic and static facial expressions decoded from motion-sensitive areas in the macaque monkey. J Neurosci. 2012;32:15952–62. doi: 10.1523/JNEUROSCI.1992-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717963080
- 35.Haxby JV, Guntupalli JS, Connolly AC, Halchenko YO, Conroy BR, Gobbini MI, Hanke M, Ramadge PJ. A common, high-dimensional model of the representational space in human ventral temporal cortex. Neuron. 2011;72:404–16. doi: 10.1016/j.neuron.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13370091
- 36.Brodmann K. Comparative localization studies in the brain cortex, its fundamentals represented on the basis of its cellular architecture. Leipzig: Barth; 1909. [Google Scholar]
- 37.Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-X. [DOI] [PubMed] [Google Scholar]
- 38.Baylis GC, Rolls ET, Leonard CM. Functional subdivisions of the temporal lobe neocortex. J Neurosci. 1987;7:330–42. doi: 10.1523/JNEUROSCI.07-02-00330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morosan P, Schleicher A, Amunts K, Zilles K. Multimodal architectonic mapping of human superior temporal gyrus. Anat Embryol. 2005;210:401–6. doi: 10.1007/s00429-005-0029-1. [DOI] [PubMed] [Google Scholar]
- 40.Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlberg H, Amunts K. Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989849
- 41.Barazany D, Assaf Y. Visualization of cortical lamination patterns with magnetic resonance imaging. Cereb Cortex. 2012;22:2016–23. doi: 10.1093/cercor/bhr277. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989851
- 42.Eickhoff SB, Paus T, Caspers S, Grosbras M, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989852
- 43.Moeller S, Freiwald WA, Tsao DY. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320:1355–9. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gschwind M, Pourtois G, Schwartz S, van de Ville D, Vuilleumier P. White-matter connectivity between face-responsive regions in the human brain. Cereb Cortex. 2012;22:1564–76. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989855
- 45.Zhu Q, Zhang J, Luo YLL, Dilks DD, Liu J. Resting-state neural activity across face-selective cortical regions is behaviorally relevant. J Neurosci. 2011;31:10323–30. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/13135958
- 46.Davies-Thompson J, Andrews TJ. Intra- and interhemispheric connectivity between face-selective regions in the human brain. J Neurophysiol. 2012;108:3087–95. doi: 10.1152/jn.01171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989857
- 47.Bell AH, Hadj-Bouziane F, Frihauf JB, Tootell RBH, Ungerleidery LG. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. J Neurophysiol. 2009;101:688–700. doi: 10.1152/jn.90657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/1135903
- 48.Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat Neurosci. 1999;2:1019–25. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- 49.Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010;330:845–51. doi: 10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/14055956
- 50.Hemond CC, Kanwisher NG, Beeck HP op de. A preference for contralateral stimuli in human object- and face-selective cortex. PLoS ONE. 2007;2:e574. doi: 10.1371/journal.pone.0000574. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/717989860
- 51.Axelrod V, Yovel G. Hierarchical processing of face viewpoint in human visual cortex. J Neurosci. 2012;32:2442–52. doi: 10.1523/JNEUROSCI.4770-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.f1000.com/prime/14021978
- 52.Kietzmann TC, Swisher JD, König P, Tong F. Prevalence of selectivity for mirror-symmetric views of faces in the ventral and dorsal visual pathways. J Neurosci. 2012;32:11763–72. doi: 10.1523/JNEUROSCI.0126-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600–5. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nestor A, Vettel JM, Tarr MJ. Task-specific codes for face recognition: how they shape the neural representation of features for detection and individuation. PLoS ONE. 2008;3:e3978. doi: 10.1371/journal.pone.0003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitcher D, Goldhaber T, Duchaine B, Walsh V, Kanwisher N. Two critical and functionally distinct stages of face and body perception. J Neurosci. 2012;32:15877–85. doi: 10.1523/JNEUROSCI.2624-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitcher D, Walsh V, Yovel G, Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Curr Biol. 2007;17:1568–73. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 57.Sadeh B, Podlipsky I, Zhdanov A, Yovel G. Event-related potential and functional MRI measures of face-selectivity are highly correlated: a simultaneous ERP-fMRI investigation. Hum Brain Mapp. 2010;31:1490–501. doi: 10.1002/hbm.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin RK. Looking at upside-down faces. J exp psychol. 1969;81:141–5. doi: 10.1037/h0027474. [DOI] [Google Scholar]; http://www.f1000.com/prime/717989861
- 59.Yovel G, Kanwisher N. The neural basis of the behavioral face-inversion effect. Curr Biol. 2005;15:2256–62. doi: 10.1016/j.cub.2005.10.072. [DOI] [PubMed] [Google Scholar]
- 60.Haxby JV, Hoffman E A, Gobbini MI. The distributed humans neural system for face perception. Trends Cogn Sci. 2000;4:223–33. doi: 10.1016/S1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 61.Calder AJ, Young AW. Understanding the recognition of facial identity and facial expression. Nat Rev Neurosci. 2005;6:641–51. doi: 10.1038/nrn1724. [DOI] [PubMed] [Google Scholar]
- 62.Parr LA, Hecht E, Barks SK, Preuss TM, Votaw JR. Face processing in the chimpanzee brain. Curr Biol. 2009;19:50–3. doi: 10.1016/j.cub.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kendrick KM, Baldwin BA. Cells in temporal cortex of conscious sheep can respond preferentially to the sight of faces. Science. 1987;236:448–50. doi: 10.1126/science.3563521. [DOI] [PubMed] [Google Scholar]
- 64.Issa EB, DiCarlo JJ. Precedence of the eye region in neural processing of faces. J Neurosci. 2012;32:16666–82. doi: 10.1523/JNEUROSCI.2391-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


