Abstract
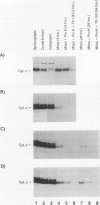
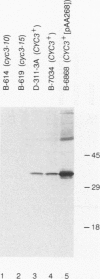
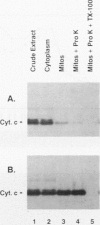
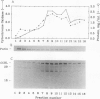
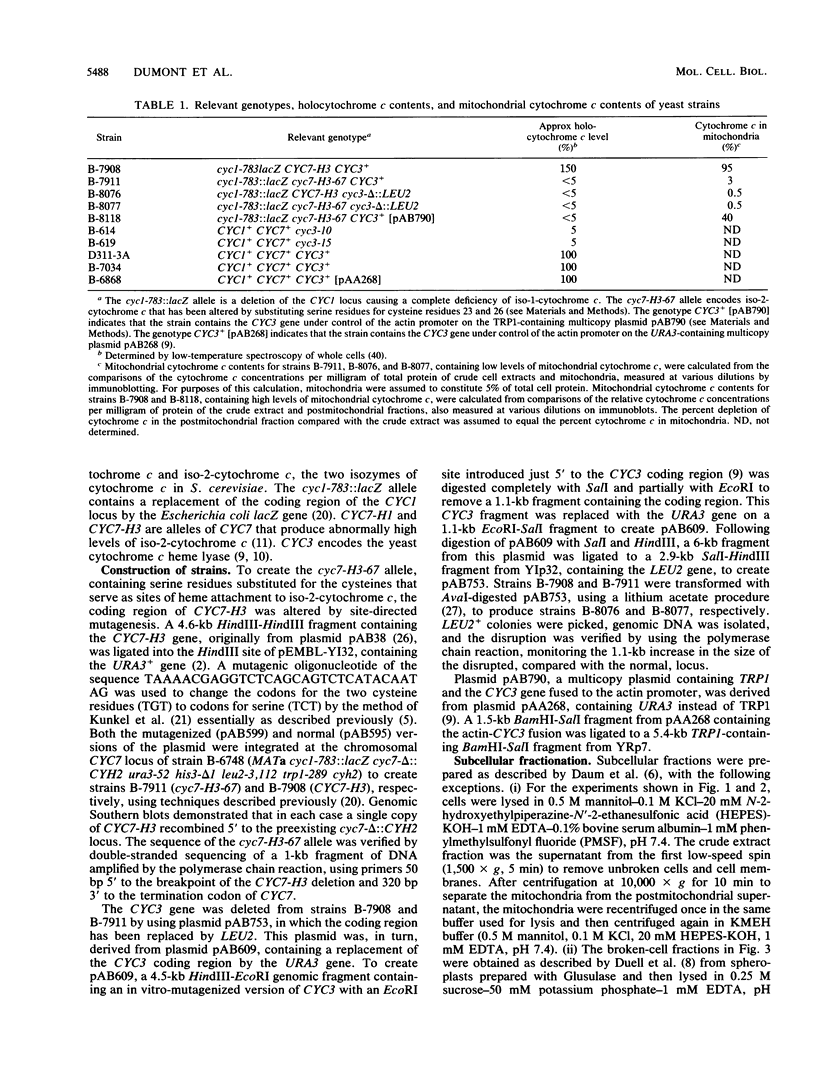
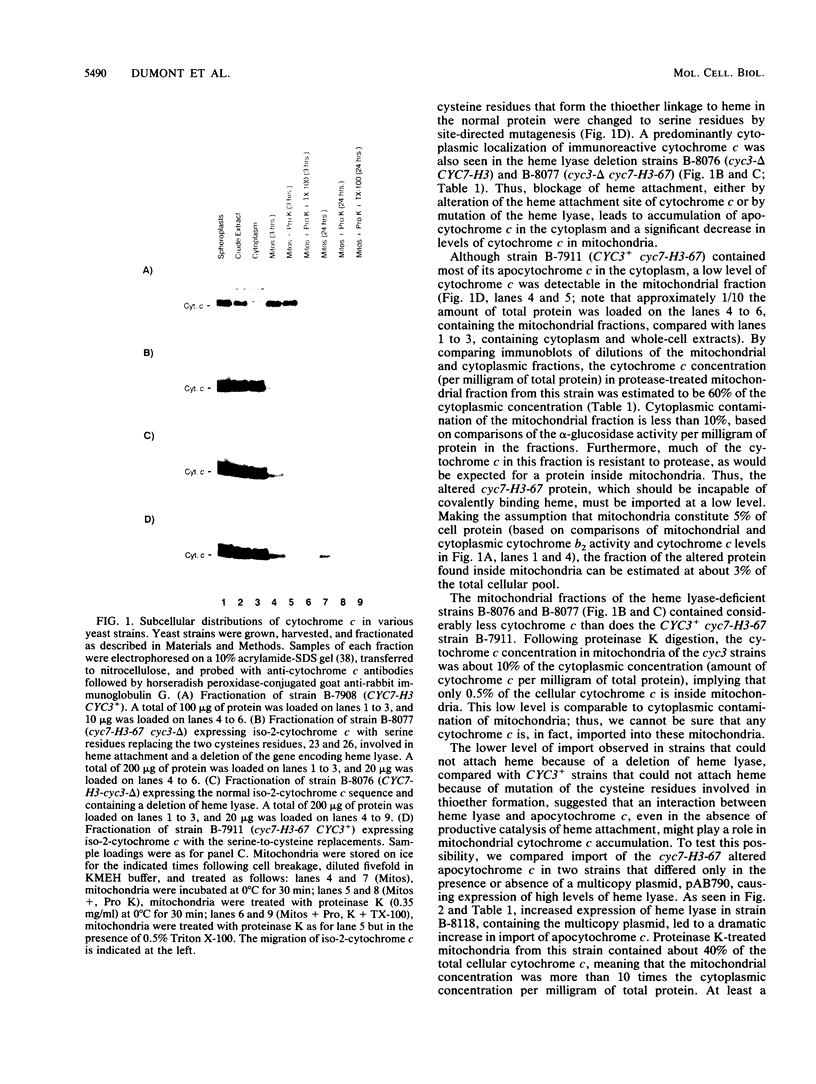
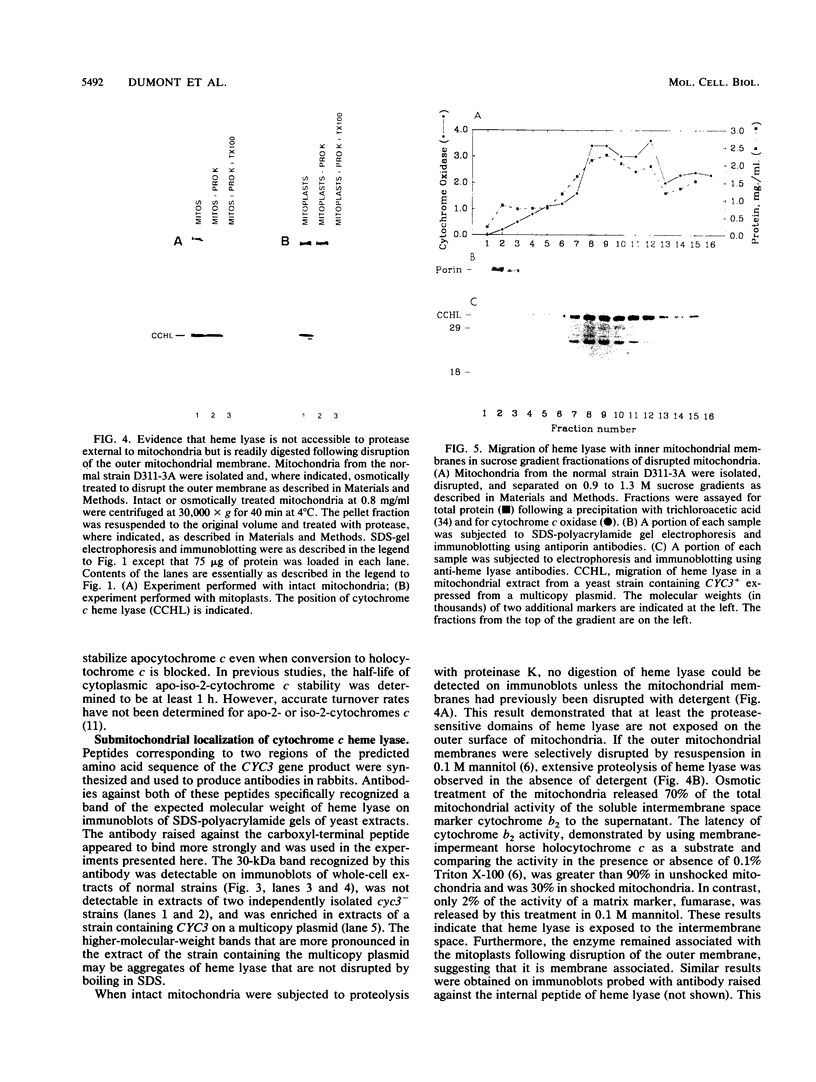
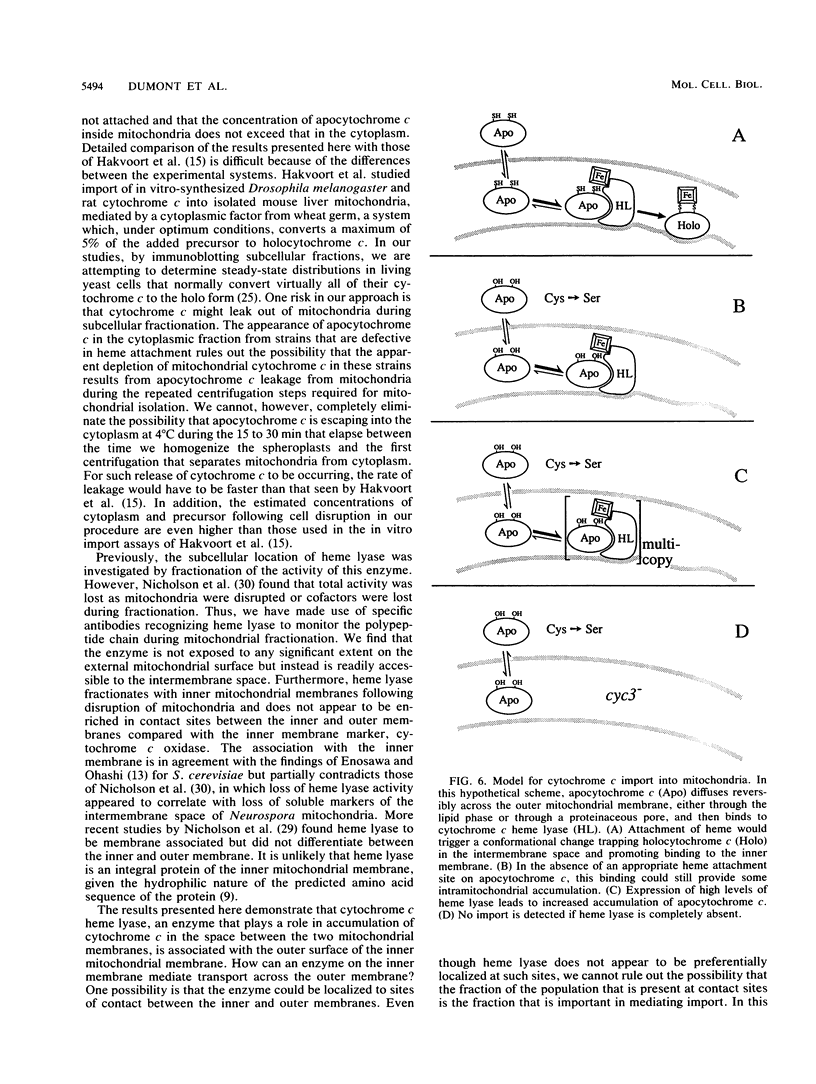
Heme is covalently attached to cytochrome c by the enzyme cytochrome c heme lyase. To test whether heme attachment is required for import of cytochrome c into mitochondria in vivo, antibodies to cytochrome c have been used to assay the distributions of apo- and holocytochromes c in the cytoplasm and mitochondria from various strains of the yeast Saccharomyces cerevisiae. Strains lacking heme lyase accumulate apocytochrome c in the cytoplasm. Similar cytoplasmic accumulation is observed for an altered apocytochrome c in which serine residues were substituted for the two cysteine residues that normally serve as sites of heme attachment, even in the presence of normal levels of heme lyase. However, detectable amounts of this altered apocytochrome c are also found inside mitochondria. The level of internalized altered apocytochrome c is decreased in a strain that completely lacks heme lyase and is greatly increased in a strain that overexpresses heme lyase. Antibodies recognizing heme lyase were used to demonstrate that the enzyme is found on the outer surface of the inner mitochondrial membrane and is not enriched at sites of contact between the inner and outer mitochondrial membranes. These results suggest that apocytochrome c is transported across the outer mitochondrial membrane by a freely reversible process, binds to heme lyase in the intermembrane space, and is then trapped inside mitochondria by an irreversible conversion to holocytochrome c accompanied by folding to the native conformation. Altered apocytochrome c lacking the ability to have heme covalently attached accumulates in mitochondria only to the extent that it remains bound to heme lyase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Lactic dehydrogenase and cytochrome b2 of baker's yeast; purification and crystallization. Biochem J. 1959 Mar;71(3):492–499. doi: 10.1042/bj0710492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldari C., Cesareni G. Plasmids pEMBLY: new single-stranded shuttle vectors for the recovery and analysis of yeast DNA sequences. Gene. 1985;35(1-2):27–32. doi: 10.1016/0378-1119(85)90154-4. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982 Feb;28(2):395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Basile G., Di Bello C., Taniuchi H. Formation of an iso-1-cytochrome c-like species containing a covalently bonded heme group from the apoprotein by a yeast cell-free system in the presence of hemin. J Biol Chem. 1980 Aug 10;255(15):7181–7191. [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G., Hickey D. R., McLendon D., McLendon G., Sherman F. Dramatic thermostabilization of yeast iso-1-cytochrome c by an asparagine----isoleucine replacement at position 57. Proc Natl Acad Sci U S A. 1989 Jan;86(2):496–499. doi: 10.1073/pnas.86.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Drygas M. E., Lambowitz A. M., Nargang F. E. Cloning and analysis of the Neurospora crassa gene for cytochrome c heme lyase. J Biol Chem. 1989 Oct 25;264(30):17897–17906. [PubMed] [Google Scholar]
- Dumont M. D., Mathews A. J., Nall B. T., Baim S. B., Eustice D. C., Sherman F. Differential stability of two apo-isocytochromes c in the yeast Saccharomyces cerevisiae. J Biol Chem. 1990 Feb 15;265(5):2733–2739. [PubMed] [Google Scholar]
- Dumont M. E., Ernst J. F., Hampsey D. M., Sherman F. Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. EMBO J. 1987 Jan;6(1):235–241. doi: 10.1002/j.1460-2075.1987.tb04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M. E., Ernst J. F., Sherman F. Coupling of heme attachment to import of cytochrome c into yeast mitochondria. Studies with heme lyase-deficient mitochondria and altered apocytochromes c. J Biol Chem. 1988 Nov 5;263(31):15928–15937. [PubMed] [Google Scholar]
- Dumont M. E., Richards F. M. Insertion of apocytochrome c into lipid vesicles. J Biol Chem. 1984 Apr 10;259(7):4147–4156. [PubMed] [Google Scholar]
- Enosawa S., Ohashi A. Localization of enzyme for heme attachment to apocytochrome c in yeast mitochondria. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1145–1150. doi: 10.1016/s0006-291x(86)80163-2. [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Taniuchi H., Anfinsen C. B. On the role of heme in the formation of the structure of cytochrome c. J Biol Chem. 1973 May 10;248(9):3188–3195. [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- Hakvoort T. B., Sprinkle J. R., Margoliash E. Reversible import of apocytochrome c into mitochondria. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4996–5000. doi: 10.1073/pnas.87.13.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey D. M., Das G., Sherman F. Yeast iso-1-cytochrome c: genetic analysis of structural requirements. FEBS Lett. 1988 Apr 25;231(2):275–283. doi: 10.1016/0014-5793(88)80834-2. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Hennig B., Neupert W. Assembly of cytochrome c. Apocytochrome c is bound to specific sites on mitochondria before its conversion to holocytochrome c. Eur J Biochem. 1981 Dec;121(1):203–212. doi: 10.1111/j.1432-1033.1981.tb06450.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laz T. M., Pietras D. F., Sherman F. Differential regulation of the duplicated isocytochrome c genes in yeast. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4475–4479. doi: 10.1073/pnas.81.14.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- Matner R. R., Sherman F. Differential accumulation of two apo-iso-cytochromes c in processing mutants of yeast. J Biol Chem. 1982 Aug 25;257(16):9811–9821. [PubMed] [Google Scholar]
- McKnight G. L., Cardillo T. S., Sherman F. An extensive deletion causing overproduction of yeast iso-2-cytochrome c. Cell. 1981 Aug;25(2):409–419. doi: 10.1016/0092-8674(81)90059-3. [DOI] [PubMed] [Google Scholar]
- Moerschell R. P., Das G., Sherman F. Transformation of yeast directly with synthetic oligonucleotides. Methods Enzymol. 1991;194:362–369. doi: 10.1016/0076-6879(91)94027-a. [DOI] [PubMed] [Google Scholar]
- Nargang F. E., Drygas M. E., Kwong P. L., Nicholson D. W., Neupert W. A mutant of Neurospora crassa deficient in cytochrome c heme lyase activity cannot import cytochrome c into mitochondria. J Biol Chem. 1988 Jul 5;263(19):9388–9394. [PubMed] [Google Scholar]
- Nicholson D. W., Hergersberg C., Neupert W. Role of cytochrome c heme lyase in the import of cytochrome c into mitochondria. J Biol Chem. 1988 Dec 15;263(35):19034–19042. [PubMed] [Google Scholar]
- Nicholson D. W., Köhler H., Neupert W. Import of cytochrome c into mitochondria. Cytochrome c heme lyase. Eur J Biochem. 1987 Apr 1;164(1):147–157. doi: 10.1111/j.1432-1033.1987.tb11006.x. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Neupert W. Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye S. H., Scarpulla R. C. In vivo expression and mitochondrial targeting of yeast apoiso-1-cytochrome c fusion proteins. Mol Cell Biol. 1990 Nov;10(11):5753–5762. doi: 10.1128/mcb.10.11.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent S. A., Fenimore C. M., Bostian K. A. Vector systems for the expression, analysis and cloning of DNA sequences in S. cerevisiae. Yeast. 1985 Dec;1(2):83–138. doi: 10.1002/yea.320010202. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pon L., Moll T., Vestweber D., Marshallsay B., Schatz G. Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J Cell Biol. 1989 Dec;109(6 Pt 1):2603–2616. doi: 10.1083/jcb.109.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Rietveld A., de Kruijff B. Is the mitochondrial precursor protein apocytochrome c able to pass a lipid barrier? J Biol Chem. 1984 Jun 10;259(11):6704–6707. [PubMed] [Google Scholar]
- SHERMAN F., SLONIMSKI P. P. RESPIRATION-DEFICIENT MUTANTS OF YEAST. II. BIOCHEMISTRY. Biochim Biophys Acta. 1964 Jul 15;90:1–15. doi: 10.1016/0304-4165(64)90113-8. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sprinkle J. R., Hakvoort T. B., Koshy T. I., Miller D. D., Margoliash E. Amino acid sequence requirements for the association of apocytochrome c with mitochondria. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5729–5733. doi: 10.1073/pnas.87.15.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen E., Rysavy R., Babul G. The conformation of horse heart apocytochrome c. J Biol Chem. 1972 Dec 25;247(24):8074–8077. [PubMed] [Google Scholar]