Abstract
Aims: There are no effective treatments for chronic pulmonary hypertension in infants with cardiopulmonary disorders associated with hypoxia, such as those with chronic lung disease. These patients often have poor or inconsistent pulmonary dilator responses to inhaled nitric oxide (iNO) therapy for unknown reasons. One possible explanation for poor responsiveness to iNO is reduced NO bioavailability caused by interactions between reactive oxygen species (ROS) and NO. Our major aim was to determine if strategies to reduce ROS improve dilator responses to the NO donor, S-nitroso-N-acetyl-penicillamine (SNAP), in resistance pulmonary arteries (PRAs) from a newborn piglet model of chronic pulmonary hypertension. Results: The dilation to SNAP was significantly impaired in PRAs from piglets with chronic hypoxia-induced pulmonary hypertension. ROS scavengers, including cell-permeable and impermeable agents to degrade hydrogen peroxide (H2O2), improved dilation to SNAP in PRAs from chronically hypoxic piglets. Treatment with agents to inhibit nitric oxide synthase and NADPH oxidase, potential enzymatic sources of ROS, also improved dilation to SNAP in PRAs from hypoxic piglets. Innovation: Our studies are the first to utilize a newborn model of chronic pulmonary hypertension to evaluate the impact of a number of potential therapeutic strategies for ROS removal on responses to exogenous NO in the vessels most relevant to the regulation of pulmonary vascular resistance (PRA). Conclusions: Strategies aimed at reducing ROS merit further evaluation and consideration as therapeutic approaches to improve responses to iNO in infants with chronic pulmonary hypertension. Antioxid. Redox Signal. 18, 1727–1738.
Introduction
It has been known for decades that some infants suffering from chronic cardiopulmonary disorders associated with persistent or episodic hypoxia, such as congenital diaphragmatic hernia or bronchopulmonary dysplasia, develop pulmonary hypertension (1, 2, 20, 28, 46). Once diagnosed with pulmonary hypertension, these infants have only a 40%–50% survival rate (29). Infants with chronic progressive pulmonary hypertension have poor and erratic pulmonary dilator responses to inhaled nitric oxide (iNO) therapy (4, 27, 36, 46). Unfortunately, little progress has been made in developing effective alternative therapies for these infants (1, 2, 20, 46). One potential explanation for poor iNO responsiveness is that the reactive oxygen species (ROS), superoxide, interacts with and thereby reduces NO bioavailability.
Innovation.
Human infants with chronic pulmonary hypertension have no known effective treatments. Utilizing a newborn piglet model of chronic pulmonary hypertension, we performed studies with the vessels most relevant to the regulation of pulmonary vascular resistance, resistance-level pulmonary arteries (PRAs). Our findings are the first to show strategies for the removal of reactive oxygen species that improve PRA responses to exogenous nitric oxide (NO) in a model of chronic neonatal pulmonary hypertension. These findings serve as the underpinnings for future studies that could lead to clinical trials to improve responses to inhaled NO and provide a desperately needed therapy for human infants suffering from chronic pulmonary hypertension.
We previously provided evidence that ROS are involved with the pathogenesis of chronic progressive pulmonary hypertension in newborn piglets exposed to prolonged in vivo hypoxia (9). The major purpose of this study was to evaluate whether ROS-reducing strategies enhance pulmonary vascular responses to exogenous NO in resistance pulmonary arteries (PRAs) from newborn piglets with chronic hypoxia-induced pulmonary hypertension.
Results
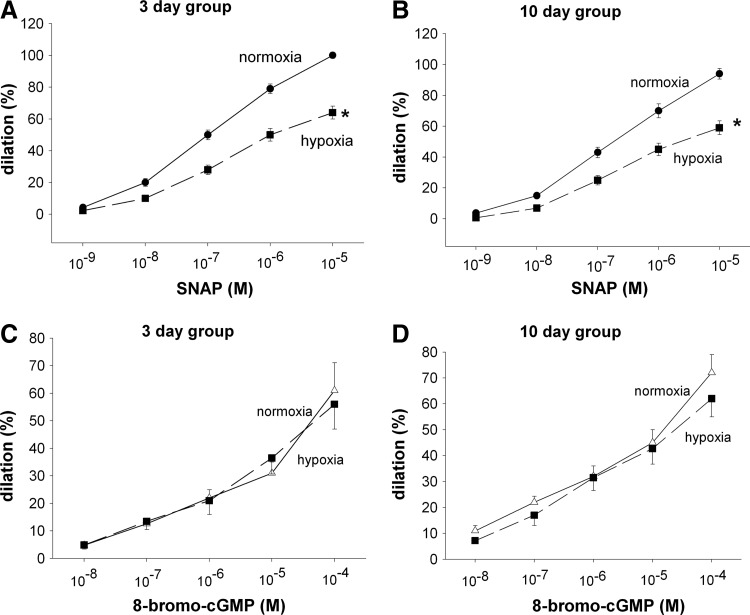
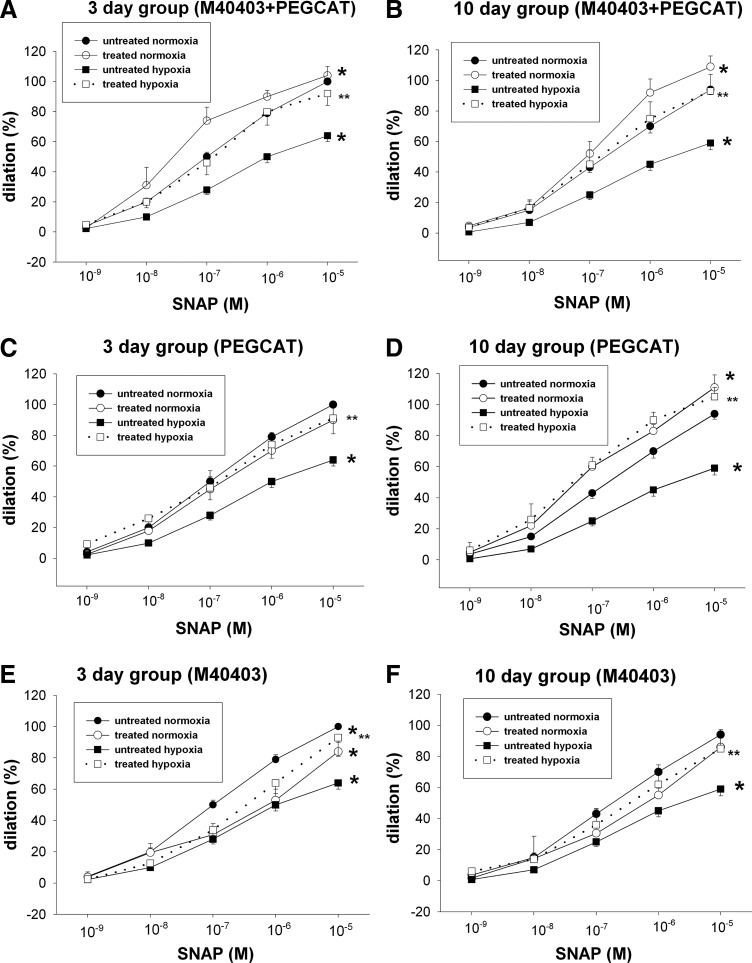
Responses to the NO donor, S-nitroso-N-acetyl-penicillamine (SNAP), were diminished in PRAs from hypoxic piglets in both the 3- (Fig. 1A) and 10- (Fig. 1B) day exposure groups. These diminished responses to SNAP were not due to inability to dilate to the second messenger, cyclic GMP (Fig. 1C, D). Combined treatment with cell-permeable agents to remove both superoxide (M40403) and hydrogen peroxide (H2O2) (polyethylene glycol [PEG]-catalase [CAT]) completely restored responses to SNAP in arteries from hypoxic piglets to the same level observed in arteries from comparable age normoxic piglets (Fig. 2A, B). Moreover, the sole use of either PEG-CAT or M40403also improved dilation to SNAP in PRAs from both groups of hypoxic piglets. Indeed, with exception of the effect of M40403 in the 3-day hypoxic piglets (Fig. 2E), the sole treatment with either M40403 or PEG-CAT increased dilation to SNAP in hypoxic PRAs to levels similar to those found in PRAs from normoxic piglets (Fig. 2C, D, F).
FIG. 1.
Vasodilation to the nitric oxide (NO) donor S-nitroso-N-acetyl-penicillamine (SNAP) (A, B) and to 8-bromo-cyclic GMP (C and D) in resistance pulmonary arteries (PRAs) from piglets raised in normoxia or hypoxia for 3 days. (A: 3-day normoxic, n=39; 3-day hypoxic, n=41; C: 3-day normoxia, n=9; 3-day hypoxia, n=13) or 10 days (B: 10-day normoxic, n=35; 10-day hypoxic, n=39; D: 10-day normoxia, n=13; 10-day hypoxia, n=11). Values are mean±SEM. *different from the normoxic dose–response curve; p<0.05.
FIG. 2.
Effect of antioxidant treatment by M40403+polyethylene glycol-catalase (PEG-CAT), (A, B) PEG-CAT alone (C, D), or M40403 alone (E, F) on responses to SNAP in PRAs from piglets raised in normoxia or hypoxia for 3 days. (A: untreated 3-day normoxia, n=39; M40403+PEG-CAT-treated 3-day normoxia, n=7; untreated 3-day hypoxia, n=41; M40403+PEG-CAT-treated 3-day hypoxia, n=10; C: untreated 3-day normoxia, n=39; PEG-CAT-treated 3-day normoxia, n=8; untreated 3-day hypoxia, n=41; PEG-CAT-treated 3-day hypoxia, n=10; E: untreated 3-day normoxia, n=39; M40403-treated 3-day normoxia, n=10, untreated 3-day hypoxia, n=41; M40403-treated 3-day hypoxia, n=11) or 10 days (B: untreated 10-day normoxia, n=35; M40403+PEG-CAT-treated 10-day normoxia, n=9; untreated 10-day hypoxia, n=39; M40403+PEG-CAT-treated 10-day hypoxia, n=11; D: untreated 10-day normoxia, n=35; PEG-CAT treated 10-day normoxia, n=7; untreated 10-day hypoxia, n=39; PEG-CAT treated 10-day hypoxia, n=7; F: untreated 10-day normoxia, n=35; M40403-treated 10-day normoxia, n=12; untreated 10-day hypoxia, n=39; M40403 treated 10-day hypoxia, n=11). Values are mean±SEM. *different from the untreated normoxia dose–response curve; **different from the untreated hypoxia dose–response curve; p<0.05.
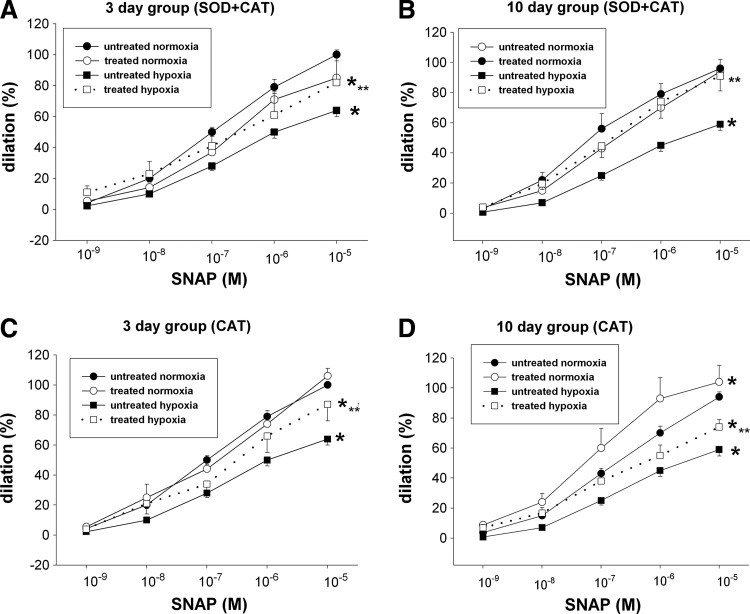
In PRAs from normoxic piglets, combined treatments with cell-permeable agents, M40403 plus PEG-CAT, further enhanced dilation to SNAP (Fig. 2A, B). Sole treatment with M40403 or PEG-CAT augmented (Fig. 2D), diminished (Fig. 2E, F), or had no impact (Fig. 2C) on dilation responses to SNAP in normoxic PRAs. Similarly, normoxic PRAs treated with cell-impermeable agents, CAT or CAT+superoxide dismutase (SOD), exhibited responses to SNAP that were nearly as robust (Fig. 3A–C) or more robust (Fig. 3D) than responses to SNAP in untreated vessels.
FIG. 3.
Effect of superoxide dismutase (SOD)+CAT (A, B) and CAT alone (C, D) on responses to SNAP in PRAs from piglets raised in normoxia or hypoxia for 3 days. (A: untreated 3-day normoxia, n=39; SOD+CAT-treated 3-day normoxia, n=8; untreated 3-day hypoxia, n=41; SOD+CAT-treated 3-day hypoxia, n=10; C: untreated 3-day normoxia, n=39; CAT-treated 3-day normoxia, n=9; untreated 3-day hypoxia, n=41; CAT-treated 3-day hypoxia, n=10) or 10 days (B: untreated 10-day normoxia, n=35; SOD+CAT-treated 10-day normoxia, n=7; untreated 10-day hypoxia, n=39; SOD+CAT-treated 10-day hypoxia, n=8; D: untreated 10-day normoxia, n=35; CAT-treated 10-day normoxia, n=8; untreated 10-day hypoxia, n=39; CAT-treated 10-day hypoxia, n=12). Values are mean±SEM. *different from the untreated normoxia dose–response curve; **different from the untreated hypoxia dose–response curve; p<0.05.
Responses to SNAP in hypoxic PRAs were improved by combined treatment with cell-impermeable agents, SOD+CAT (Fig. 3A, B). Moreover, treatment with CAT improved responses to SNAP in both groups of hypoxic PRAs even when used without a superoxide-removing agent (Fig. 3C, D). When CAT was inactivated by incubation with 3-amino-1,2,4-triazole, hypoxic PRAs no longer exhibited enhanced responses to SNAP: dilation to 10−5 M SNAP was 51%+7% for vehicle-treated hypoxic PRAs, n=4; versus 45%+4% for CAT incubated with 3-amino-1,2,4-triazole-treated PRAs, n=4; versus 94%±12% for CAT-treated PRAs, n=4. Of note, with exception of the effect of SOD+CAT in the 10-day hypoxic piglets (Fig. 3B), combined or sole treatment with cell-impermeable agents (SOD and/or CAT) did not fully restore responses in hypoxic arteries to those in normoxic arteries (Fig. 3A, C, D).
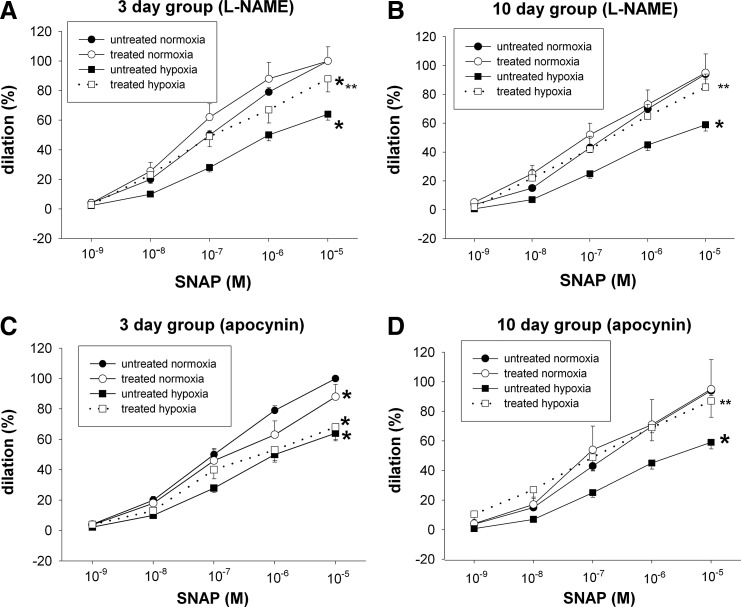
We next explored the effect of agents that target potential enzymatic sources of ROS. The nitric oxide synthase (NOS) inhibitor, L-NAME, improved dilator responses to SNAP in PRAs from both the 3- and 10-day hypoxic groups (Fig. 4A, B). By comparison, the NADPH oxidase (NOX) inhibitor, apocynin, increased dilation to SNAP in PRAs from piglets exposed to 10 days of hypoxia (Fig. 4D), but had no significant effect on dilation to SNAP in PRAs from piglets exposed to 3 days of hypoxia (Fig. 4C).
FIG. 4.
Effect of the endothelial nitric oxide synthase (eNOS) inhibitor L-NAME (A, B) and NADPH oxidase inhibitor apocynin (C, D) on responses to SNAP in PRAs from piglets raised in normoxia or hypoxia for 3 days. (A: untreated 3-day normoxia, n=39; L-NAME-treated 3-day normoxia, n=12; untreated 3-day hypoxia, n=41; L-NAME treated 3-day hypoxia, n=14; C: untreated 3-day normoxia, n=39; apocynin-treated 3-day normoxia, n=23; untreated 3-day hypoxia, n=41; apocynin-treated 3-day hypoxia, n=17) or 10 days (B: untreated 10-day normoxia, n=35; L-NAME-treated 10-day normoxia, n=7; untreated 10-day hypoxia, n=39; L-NAME-treated 10-day hypoxia, n=11; D: untreated 10-day normoxia, n=35; apocynin-treated 10-day normoxia, n=6; untreated 10-day hypoxia, n=39; apocynin-treated 10-day hypoxia, n=9). Values are mean±SEM. *different from the untreated normoxia dose–response curve; **different from the untreated hypoxia dose–response curve; p<0.05.
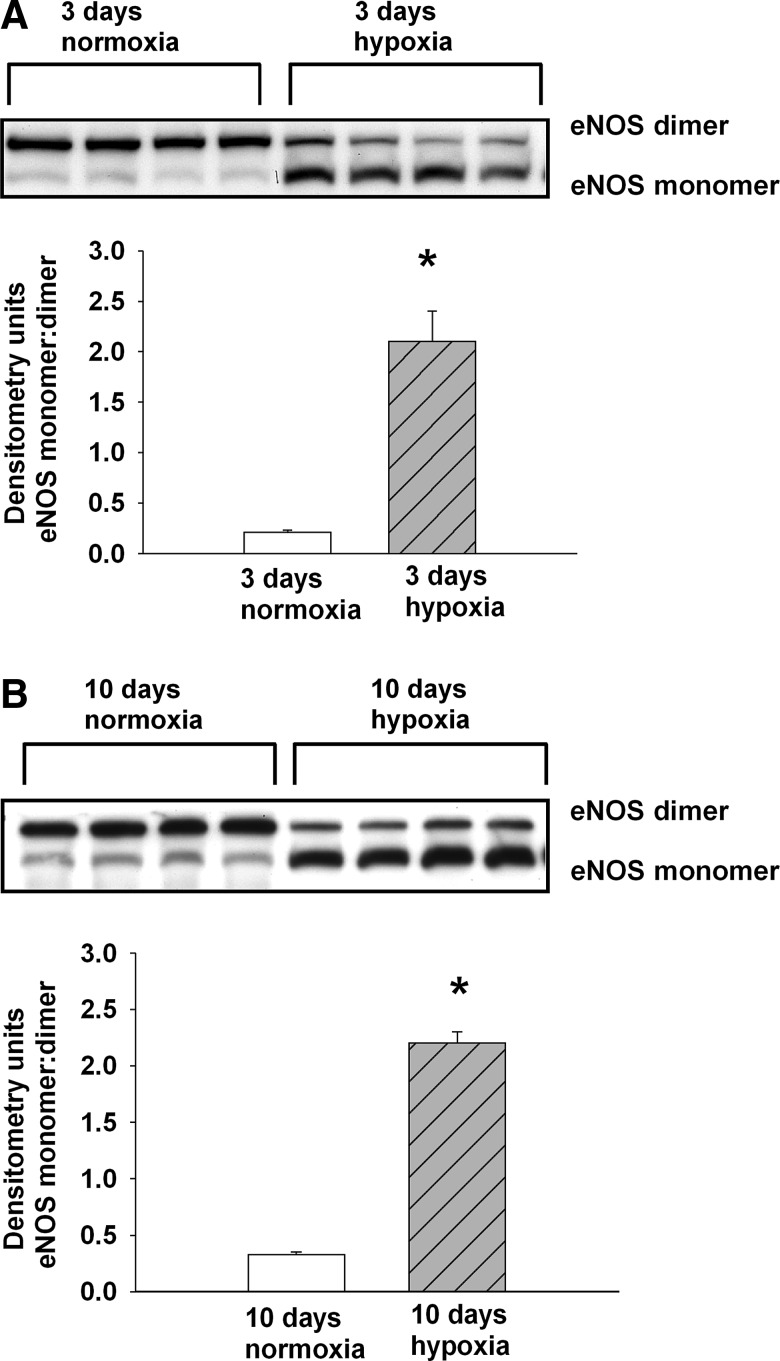
To further evaluate endothelial NOS (eNOS) as an enzymatic source of ROS in hypoxic PRAs, we performed studies to assess eNOS uncoupling. Consistent with eNOS uncoupling, the monomer-to-dimer ratio of eNOS is increased in PRA homogenates from both groups of hypoxic piglets (Fig. 5A, B).
FIG. 5.
Immunoblot results and corresponding densitometry for the eNOS monomer/dimer ratio for PRA homogenates from piglets raised in normoxia or hypoxia for (A) 3 days: 3-day normoxia, n=4; 3-day hypoxia; n=4 or (B) 10 days: 10-day normoxia, n=4; 10-day hypoxia, n=4. Values are mean±SEM. *different from normoxia; p<0.05.
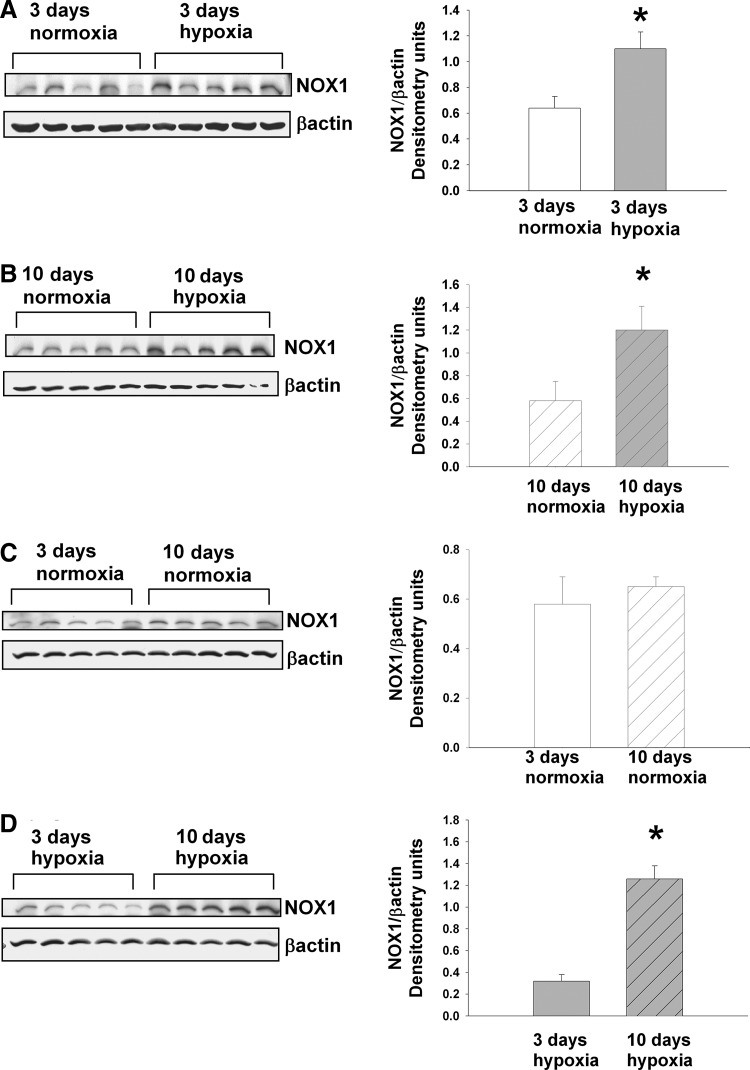
We also evaluated the possibility that hypoxia alters NOX1 protein expression and that the contribution from NOX1 differs between piglets exposed to 3- and 10-day hypoxia. NOX1 expression was greater in PRAs from both groups of hypoxic piglets than in PRAs from comparable age normoxic piglets (Fig. 6A, B). There was no difference in NOX1 expression in normoxic piglets in the 3- and 10-day groups (Fig. 6C). However, we found greater NOX1 expression in PRA homogenates from piglets exposed to hypoxia for 10 days than for 3 days (Fig. 6D).
FIG. 6.
Immunoblot results and corresponding densitometry for NOX1. (A) NOX1 and β-actin protein abundances in PRA homogenates from piglets raised in normoxia (n=5) or hypoxia (n=5) for 3 days. (B) NOX1 and β-actin protein abundances in PRA homogenates from piglets raised in normoxia (n=5) or hypoxia (n=5) for 10 days. (C) NOX1 and β-actin protein abundances in PRA homogenates from piglets raised in normoxia for 3 days (n=5) or 10 days (n=5). (D) NOX1 and β-actin protein abundances in PRA homogenates from piglets raised in hypoxia for 3 days (n=5) or 10 days (n=5). Values are mean±SEM. *different from normoxia; p<0.05.
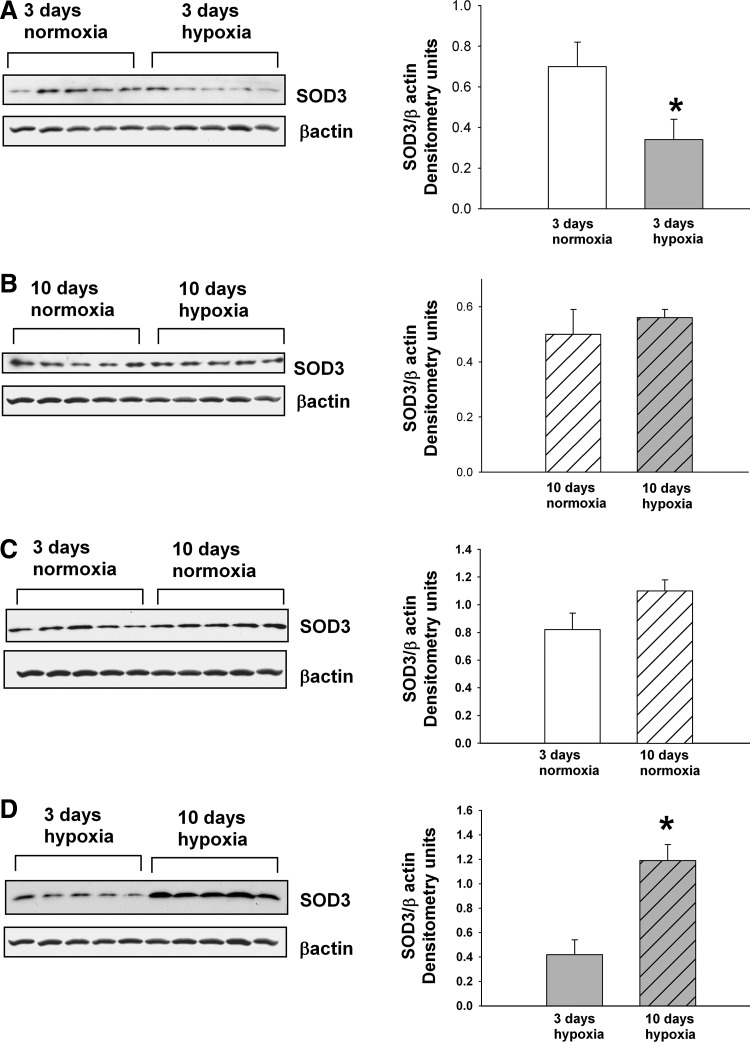
Extracellular SOD (SOD3) is an antioxidant enzyme that scavenges ROS specifically in the extracellular compartment (42). Immunoblot analyses demonstrate that SOD3 expression was decreased in PRA homogenates from piglets exposed to 3 (Fig. 7A), but not to 10, days (Fig. 7B) of hypoxia. There was no developmental difference in SOD3 expression with advancing postnatal age (Fig. 7C). SOD3 expression increased with more prolonged hypoxic exposure (Fig. 7D).
FIG. 7.
Immunoblot results and corresponding densitometry for SOD3. (A) SOD3 and β-actin protein abundances in PRA homogenates from piglets raised in normoxia (n=5) or hypoxia (n=5) for 3 days. (B) SOD3 β-actin protein abundances in PRA homogenates from piglets raised in normoxia (n=5) or hypoxia (n=5) for 10 days. (C). SOD3 and β-actin protein abundances in PRA homogenates from piglets raised in normoxia for 3 days (n=5) or 10 days (n=5). (D) SOD3 and β-actin protein abundances in PRA homogenates from piglets raised in hypoxia for 3 days (n=5) or 10 days (n=5). Values are mean±SEM. *different from normoxia; p<0.05.
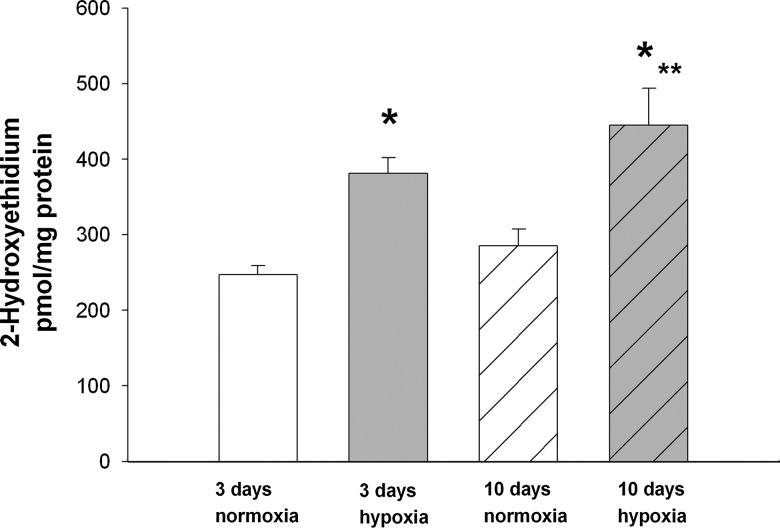
As assessed by high-performance liquid chromatography (HPLC), greater amounts of intracellular superoxide as measured by 2-hydroxyethidium were found in PRAs from piglets raised in hypoxia for 3 or 10 days than in PRAs from age-matched piglets raised in normoxia (Fig. 8). In addition, the amount of superoxide increased when hypoxic exposure was extended from 3 to 10 days.
FIG. 8.
Superoxide production as assessed by high-performance liquid chromatography determination of 2-hydroxyethidium in PRAs from piglets raised in normoxia or hypoxia for 3 days (3-day normoxia, n=5; 3-day hypoxia, n=5) or 10 days (10-day normoxia, n=5; 10-day hypoxia, n=5). Values are mean±SEM. *different from a comparable age group of normoxic piglets; **different from piglets raised in 3-day hypoxia; p<0.05.
Discussion
Consistent with our previous findings (9), this study provides additional evidence that ROS are involved with the pathogenesis of pulmonary hypertension in newborn piglets exposed to either 3 or 10 days of in vivo hypoxia (9). This study confirms that expression of NOX1, an enzymatic source of ROS, was greater in PRAs from piglets exposed to either 3 or 10 days of in vivo hypoxia than in PRAs from comparable age groups of normoxic piglets. We add to our previous findings and now show that NOX1 expression increases when in vivo hypoxia is extended from 3 to 10 days. Another new finding in this study is that intracellular superoxide progressively increases with a greater duration of in vivo hypoxic exposure.
Moreover, we found evidence of impaired antioxidant capacity in PRAs from piglets exposed to chronic in vivo hypoxia (9). We have previously reported that SOD1 was diminished in PRAs from piglets exposed to either 3 or 10 days of hypoxia (9). In this study, we show that another antioxidant enzyme, SOD3, is reduced in PRAs from piglets exposed to 3 days of hypoxia, but then increases when hypoxic exposure is extended to 10 days. Taken all together, our findings suggest that an imbalance in ROS generating and antioxidant defense systems occurs in PRAs during in vivo exposure to chronic hypoxia, and that the specific contribution from each system might change with progressive exposure to hypoxia. It follows that targeting ROS could be a useful therapeutic maneuver, but that the impact from manipulating a particular target might differ depending on the duration of chronic hypoxia-induced pulmonary hypertension.
Along these lines, a major new finding in this study is that strategies to remove ROS or to limit their production improve PRA responses to exogenous NO in piglets exposed to either 3- or 10-day in vivo hypoxia. Other investigators have provided evidence that pulmonary vascular responses to exogenous NO are improved by ROS-removing agents in both adult and newborn animals with pulmonary hypertension (6, 26, 31, 40, 45, 49). Similar to our findings with M40403, in these previous studies, the strategies for ROS removal included use of PEG-SOD, a cell-permeable agent that dismutates superoxide to H2O2. It is well known that H2O2 has vasoactive effects, causing either dilation or constriction depending on the species, vascular bed, and presence or absence of vascular disease (3, 37). Indeed, we previously found that H2O2 causes dilation in PRAs from both normoxic and chronically hypoxic piglets, although the magnitude of response was much less in the hypoxic than in normoxic animals (13). In this study, we wanted to eliminate vasodilation from H2O2 as a mechanism by which the ROS-removing agents improve functional responses to exogenous NO. Therefore, in addition to evaluating the sole influence of superoxide removal with an SOD mimetic, M40403, in some studies, we also used an agent to degrade H2O2. It is well known that superoxide can directly interact with, and thereby reduce, NO (23). Thus, the mechanism for enhanced NO responsiveness from the ROS-removing treatments in our study is likely to be due, at least in part, to an attenuation of NO inactivation and an increase in the amount of NO available to activate the downstream signaling target, soluble guanylate cyclase (sGC).
Unlike superoxide, there is no described interaction between NO and H2O2. Therefore, our findings that the sole use of H2O2-degrading agents improved functional responses to exogenous NO were initially surprising. However, H2O2 has been shown to contribute to superoxide production via a number of mechanisms (3, 5). For example, it is known that H2O2 can activate NOXs to produce superoxide (35, 44, 50). H2O2 has also been shown to cause eNOS uncoupling by NOX-dependent mechanisms (5), leading to an increase of superoxide and decrease of NO production. Moreover, by decreasing cellular levels of the eNOS cofactor, BH4, (5), H2O2 can disrupt NOS dimer formation and further increase eNOS uncoupling (43). Furthermore, H2O2 is known to inhibit both SOD1 and SOD3 activities in a dose-dependent fashion (21, 25, 48). Thus, by influencing a number of mechanisms that are modulated by H2O2, CAT and PEG-CAT could reduce superoxide.
In a fetal lamb ductal-ligation model of persistent pulmonary hypertension of the newborn (PPHN), PEG-CAT was recently shown to both enhance SOD3 activity and reduce superoxide levels (48). PEG-CAT also improved responses to an NO donor in isolated pulmonary arteries from the fetal lamb PPHN model (49). The use of the cell-impermeable agent, CAT, was not evaluated in the lamb model. Thus, we extend the prior observations made in the fetal lamb PPHN model and show for the first time that without the use of a superoxide reducing agent, both CAT and PEG-CAT improve pulmonary vascular responses to exogenous NO in a newborn piglet model of chronic progressive pulmonary hypertension. H2O2, an important signaling molecule in normal vessels (11, 44), may not need to be pathologically elevated for CAT and PEG-CAT to impact vascular responses. It is probable that any reduction in H2O2, including reductions below basal, physiologic levels, could increase SOD1 and SOD3 activity (21, 25, 48), thereby reduce superoxide, and explain the improved responses to NO donors in the hypoxic arteries. In line with this, we previously reported that H2O2 levels were either unchanged or decreased in PRAs from piglets with chronic hypoxia-induced pulmonary hypertension (9).
Unlike PRAs from hypoxic piglets, ROS-removing agents did not consistently increase NO responses in PRAs from normoxic, control piglets. In light of the multiple roles that ROS play in normal cell signaling, this is not surprising; ROS removal may adversely alter some normal vascular responses (11, 47).
The effectiveness of the cell-impermeable and permeable forms of ROS-removing agents is of interest. NO will be inactivated at any site in the vascular wall, where superoxide is elevated (21). SOD3 removes extracellular superoxide and prevents extracellular destruction of NO (41, 42). SOD1, which is localized in the cytosol, preserves NO within the cell (21). Thus, both SOD3 and SOD1 help preserve NO as it traverses cells and extracellular spaces to activate its major molecular target, sGC, in smooth muscle. Due in part to our aforementioned findings of alterations in SOD1 and SOD3 during exposure to in vivo hypoxia, one might predict greater effectiveness from cell-permeable than cell-impermeable agents. Indeed, our findings are consistent with this prediction. In most study groups, only the cell-permeable ROS-removing treatments restored responses to exogenous NO in hypoxic PRAs to levels similar to those found in normoxic PRAs. Nonetheless, our findings also indicate that extracellular ROS play an important role in reducing the effectiveness of exogenous NO therapy in our model of chronic pulmonary hypertension.
It may be desirable to target the sources of elevated ROS. Our findings suggest that eNOS is a source of the ROS that decreases the effectiveness of exogenous NO. In situations where eNOS is uncoupled (as demonstrated by reduced dimer formation in hypoxic PRAs), electrons flow to molecular oxygen, leading to production of superoxide rather than NO (22). The superoxide produced from uncoupled eNOS would be released intracellularly and undergo dismutation to H2O2, which, in turn, can traverse the plasma membrane. Thus, uncoupled eNOS could contribute to both intracellular and extracellular ROS. This explains the marked ability of NOS inhibition to improve PRA responses to exogenous NO in hypoxic PRAs in our study.
NOX family enzymes are also a likely source of ROS that reduces the effectiveness of exogenous NO. Apocynin inhibits only those NOX enzymes such as NOX1 that require assembly with p47 phox, p67 phox, or their homologs for activation and generation of ROS (7, 8). NOX1 has been shown to be located in caveolae on the cell surface and release superoxide extracellularly (24, 33). NOX1 can also be located within cells and release superoxide inside vesicles (32, 38). In the latter case, superoxide may affect cytosolic signaling after crossing the membranes via anion channels (32, 38). In both the cytosolic and extracellular locations, superoxide may directly interact with NO or undergo dismutation to H2O2. Hence, NOX1 could contribute to both extracellular and intracellular ROS, explaining the ability of apocynin treatment to restore the diminished responses to exogenous NO in PRAs from piglets exposed to 10-day hypoxia.
Given our findings of hypoxia-induced elevations in NOX1, the lack of effectiveness of apocynin therapy in PRAs from piglets exposed to 3-day hypoxia was surprising to us. Taken together, our findings with apocynin suggest that NOX1-dependent ROS are sufficient to impair responses to exogenous NO after 10, but not after only 3, days of hypoxia. Therefore, our findings indicate that the functional impact of apocynin treatment differs depending on the duration of exposure to in vivo hypoxia.
In summary, our findings suggest that elevations in ROS, which can be attributed to uncoupled eNOS and increased NOX1 expression, contribute to the poor functional responses to exogenous NO found in PRAs of piglets with chronic progressive hypoxia-induced pulmonary hypertension. Our findings also point out the potential efficacy of agents that solely target H2O2 degradation. Of note, studies with the fetal ductal-ligation lamb model have provided evidence to support ROS reduction as a therapeutic strategy for infants suffering from PPHN at birth (19). Our findings are the first to suggest that a similar strategy might provide a desperately needed therapy for infants with chronic pulmonary hypertension. We acknowledge that caution is needed when extrapolating findings in experimental animals to humans. Nonetheless, our findings provide important new data supporting the contention that ROS-reducing agents merit further evaluation and consideration as a novel therapy to improve responses to exogenous NO in infants suffering from postnatal forms of chronic pulmonary hypertension due to cardiopulmonary conditions associated with chronic hypoxia.
Materials and Methods
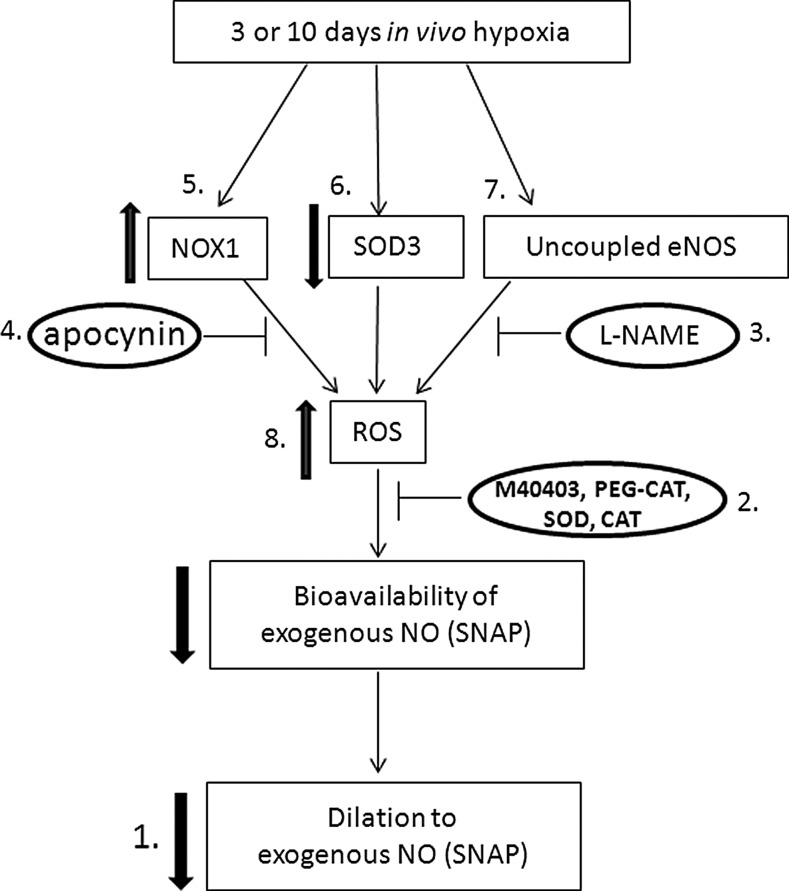
A schematic of our overall study design is presented in Figure 9.
FIG. 9.
Schematic of experimental design. Please see the Methods section for details on each point from 1 to 8.
Animals: Chronic hypoxia model of pulmonary hypertension
Newborn pigs (2 days old) were placed in a hypoxic normobaric environment for either 3 or 10 days. Oxygen content was regulated at 10%–12% O2 (PiO2 64–78 Torr). CO2 was absorbed with soda lime, and PiCO2 was maintained at 3–6 Torr. For normoxic (control) animals, piglets were studied on the day of arrival from the vendor, at postnatal ages of either 5–6 or 12–14 days, that is, comparable postnatal ages to the hypoxic piglets on the day of study. We have previously found no differences in vascular responses between piglets raised in a room-air environment and piglets raised by the vendor (15, 16). At the time of study, all piglets were preanesthetized with ketamine (30 mg/kg im) and acepromazine (2 mg/kg im) and then anesthetized with pentobarbital (10 mg/kg iv). Then, heparin was administered (1,000 IU/kg iv), and all animals were exsanguinated. Next, the thorax was opened, and the lungs were removed and placed in cold (4°C) Krebs until use. All experimental protocols were followed in adherence to the National Institutes of Health guidelines for the use of experimental animals and approved by the Animal Care and Use Committee of the Vanderbilt University Medical Center. This animal resource facility is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Use.
Cannulated artery preparation
Piglet resistance-level pulmonary arteries (PRAs, 80–200-μm diameter) were isolated, cannulated, and pressurized for a continuous measurement of diameter using our previously published methods (12, 17).
Cannulated artery protocols
Each artery was equilibrated for 30–60 min to establish a basal tone. Normoxic (control) and hypoxic arteries were equilibrated at transmural pressures that represent in vivo pressures (15, 16): the normoxic arteries at a transmural pressure of 15-cm H2O and the hypoxic arteries at a transmural pressure of 25-cm H2O. We have found no effect between these transmural pressures on pulmonary arterial responses (18). After establishment of a basal tone, all arteries were tested for viability by contraction to the thromboxane mimetic, U46619 (10−8M). To check for a functional endothelium in normoxic arteries, responses to acetylcholine, ACh (10−6 M), were evaluated. We previously found that hypoxic arteries constricted to ACh, but dilated to the calcium ionophore, A23187 (18). Therefore, responses to A23187 were used to check for a functional endothelium in hypoxic arteries.
We performed studies to determine whether responses to the NO donor, SNAP, were altered by chronic in vivo hypoxia (number 1 in Fig. 9). We previously found minimal-to-no responses to SNAP in vessels from normoxic and hypoxic piglets studied at a basal tone (14). Therefore, for these studies, the vessel tone was elevated by addition of either endothelin (10−10 to 10−9 M) or U46619 (10−9 to 10−8 M) to the reservoir to achieve a 40%–50% decrease in the vessel diameter. Cumulative doses of SNAP (10−9–10−5 M) were then added to the reservoir while the vessel diameter was continuously monitored. Other vessels were studied to determine whether impairments in smooth-muscle-cell relaxation to the NO second messenger, cyclic GMP, were involved in altered responses to SNAP. For these studies, after elevating the tone by 40%–50%, changes in the vessel diameter to cumulative doses of 8-bromo-cyclic GMP (10−8 to 10−4 M) were measured.
We then performed studies to evaluate the influence of ROS on vessel responses to SNAP (number 2 in Fig. 9). To evaluate the contribution of endogenous superoxide and H2O2, an SOD mimetic, which dismutates superoxide to H2O2, and/or an H2O2-decomposing enzyme, which converts H2O2 to H2O, was added to the reservoir of some vessels 20 min before measuring changes in the diameter to SNAP (10−9–10−5 M). We used either the cell-permeable M40403 (3 μg/ml) or the cell-impermeable SOD (250 U/ml) as the SOD mimetic. Either the cell-permeable PEG-CAT (250 U/ml) or cell-impermeable CAT (250 U/ml) was used as the H2O2-decomposing agent. To evaluate the effect of enzymatically inactive CAT, in some studies, we used CAT that was incubated with 3-amino-1,2,4-triazole [5×10−2 M;(34, 39)], a CAT inhibitor.
We also performed studies to evaluate the influence on SNAP responses from potential enzymatic sources of ROS, NOS, and NOX (numbers 3 and 4 in Fig. 9). To evaluate the influence of NOS, responses to cumulative doses of SNAP were measured 20 min after adding the NOS antagonist, L-NAME (10−3 M), to the reservoir. To evaluate the influence of NOX, similar studies were performed after adding the NOX inhibitor, apocynin, 10−6 M.
In some studies, responses to the vehicle used for solubilization of the ROS-removing agent, L-NAME, or apocynin were evaluated.
Immunoblot analysis of SOD3, NOX1, and eNOS dimers/monomers
Following our previously published methods (12, 17), frozen samples of small pulmonary arteries (≤300-μm diameter) were used for immunoblot analysis of SOD3 (1:500; antibody from ABCAM, Cambridge, MA) and NOX1 (1:500; antibody from Santa Cruz Biotechnology, Santa Cruz, CA) (numbers 5 and 6 in Fig. 9). Using nonboiled lysates and low-temperature sodium dodecyl sulfate–polyacrylamide gel electrophoresis, eNOS dimers/monomers were immunoblotted (1:2000; eNOS antibody from BD-Transduction Laboratory, San Diego, CA) as described elsewhere (30) (number 7 in Fig. 9). The membranes were developed using enhanced chemiluminescence reagents and captured on an X-ray film. Similar procedures were followed to reprobe the membranes for β-actin (Sigma-Aldrich, St. Louis, MO). The bands for each protein were quantified using densitometry.
Superoxide assessment with HPLC
We evaluated superoxide production in samples of frozen small pulmonary arteries (≤300-μm diameter) by measuring the formation of 2-hydroxyethidium from dihydroethidium using HPLC analysis (10) (number 8 in Fig. 9). Hydroxyethidium was expressed per milligram protein. In some samples, PEG-SOD (100 U/ml) was added 1 h before addition of dihydroethidium. PEG-SOD inhibited the dihydroethidium signal by 60%.
Drugs
SNAP and U46619 were from Biomol (Farmingdale, NY). SNAP was solubilized in DMSO. L-NAME and SOD were from Cayman (Ann Arbor, MI) and were solubilized in saline. Apocynin and endothelin were from EMD Biosciences (La Jolla, CA) and were solubilized, respectively, in DMSO or ethanol. PEG-CAT, CAT, 8-bromo-cyclic GMP, and 3-amino-1,2,4, triazole were from Sigma-Aldrich and were solubilized, respectively, in distilled H2O, KH2PO4 buffer, or saline. M40403 was a generous gift from Activbiotics (Lexington, MA) and was solubilized in 26 mM NaHCO3. Concentrations for each drug listed in cannulated artery protocols are final concentrations in the vessel bath.
Statistics
Data are presented as means±SEM. For cannulated artery studies, the %change from the baseline diameter in response to each dose of SNAP or 8-bromo-cyclic GMP was compared between the control and hypoxic groups or between the untreated and treated vessels of the control and hypoxic groups using a linear mixed-effect model. There were no differences in the responses to SNAP between any vehicle-treated and untreated vessels in both the control and hypoxic groups of animals. Therefore, for statistical analysis, the untreated vessel group includes a combination of the vehicle-treated and untreated vessels. Findings with arteries studied at an elevated tone were similar regardless of the agent used for preconstriction, that is, endothelin or U46619, so that results with both agents were combined for statistical analysis. Each artery was exposed to multiple concentrations of SNAP or 8-bromo-cyclic GMP. Therefore, we included a random intercept to account for the correlation arising from taking repeated observations on the same vessel. For Western blot analysis and superoxide determination by HPLC, an unpaired t-test or ANOVA with Fisher's protected least significant difference post hoc test was used to compare values between groups of piglets. The level of statistical significance was p<0.05.
Abbreviations Used
- CAT
catalase
- eNOS
endothelial nitric oxide synthase
- H2O2
hydrogen peroxide
- HPLC
high-performance liquid chromatography
- iNO
inhaled nitric oxide
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- PEG
polyethylene glycol
- PPHN
persistent pulmonary hypertension of the newborn
- PRAs
resistance pulmonary arteries
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- SNAP
S-nitroso-N-acetyl-penicillamine
- SOD
superoxide dismutase
Acknowledgments
This work was supported by RO1 HL68572 (CDF) and by RO1 HL97566 (CDF).
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child. 2002;87:F15–F18. doi: 10.1136/fn.87.1.F15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JC. Zwerdling R. Ehrenkranz R. Gaultier C. Geggel R. Greenough A. Kleinman R. Klijanowicz A. Martinez F. Ozdemir A. Panitch HB. Phelps D. Nickerson BG. Stein MT. Tomezsko J. Anker JV. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168:356–396. doi: 10.1164/rccm.168.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Ardanaz N. Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med. 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 4.Banks BA. Seri I. Ischiropoulos H. Merrill J. Rychik J. Ballard RA. Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics. 1999;103:610–618. doi: 10.1542/peds.103.3.610. [DOI] [PubMed] [Google Scholar]
- 5.Boulden BM. Widder JD. Allen JC. Smith DA. Al-Baldawi RN. Harrison DG. Dikalov SI. Jo H. Dudley SJ. Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med. 2006;41:810–817. doi: 10.1016/j.freeradbiomed.2006.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan LA. Steinhorn RH. Wedgwood S. Mata-Greenwood E. Roark EA. Russell JA. Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 7.Brown DI. Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifuentes ME. Pagano PJ. Targeting reactive oxygen species in hypertension. Curr Opin Nephrol Hypertens. 2006;15:179–186. doi: 10.1097/01.mnh.0000214776.19233.68. [DOI] [PubMed] [Google Scholar]
- 9.Dennis KE. Aschner JL. Milatovic D. Schmidt JW. Aschner M. Kaplowitz MR. Zhang Y. Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L596–L607. doi: 10.1152/ajplung.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikalova AE. Bikineyeva AT. Budzyn K. Nazarewicz RR. McCann L. Lewis W. Harrison DG. Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng J. Damrauer SM. Lee M. Sellke FW. Ferran C. Abid R. Endothelium-dependent coronary vasodilatation requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol. 2010;30:1703–1710. doi: 10.1161/ATVBAHA.110.209726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fike C. Slaughter JC. Kaplowitz MR. Zhang Y. Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L881–L888. doi: 10.1152/ajplung.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fike CD. Aschner JL. Slaughter JC. Kaplowitz MR. Zhang Y. Pfister SL. Pulmonary arterial responses to reactive oxygen species are altered in newborn piglets with chronic hypoxia-induced pulmonary hypertension. Pediatr Res. 2011;70:136–141. doi: 10.1203/PDR.0b013e3182207ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fike CD. Aschner JL. Zhang Y. Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn pigs. Am J Physiol Lung Cell Mol Physiol. 2004;286:1244–1254. doi: 10.1152/ajplung.00345.2003. [DOI] [PubMed] [Google Scholar]
- 15.Fike CD. Kaplowitz MR. Chronic hypoxia alters nitric oxide-dependent pulmonary vascular responses in lungs of newborn pigs. J Appl Physiol. 1996;81:2078–2087. doi: 10.1152/jappl.1996.81.5.2078. [DOI] [PubMed] [Google Scholar]
- 16.Fike CD. Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol. 1994;77:2853–2862. doi: 10.1152/jappl.1994.77.6.2853. [DOI] [PubMed] [Google Scholar]
- 17.Fike CD. Kaplowitz MR. Zhang Y. Pfister SL. Cyclooxygenase-2 and an early stage of chronic hypoxia-induced pulmonary hypertension in newborn pigs. J Appl Physiol. 2004;98:1111–1118. doi: 10.1152/japplphysiol.00810.2004. [DOI] [PubMed] [Google Scholar]
- 18.Fike CD. Pfister SL. Kaplowitz MR. Madden JA. Cyclooxygenase contracting factors and altered pulmonary vascular responses in chronically hypoxic newborn piglets. J Appl Physiol. 2002;92:67–74. doi: 10.1152/jappl.2002.92.1.67. [DOI] [PubMed] [Google Scholar]
- 19.Firth AL. Yuan JX-J. Bringing down the ROS: a new therapeutic approach for PPHN. Am J Physiol Lung Cell Mol Physiol. 2008;295:L976–L978. doi: 10.1152/ajplung.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald D. Evans N. Asperen PV. Henderson-Smart D. Subclinical persisting pulmonary hypertension in chronic neonatal lung disease. Arch Dis Child. 1994;70:F118–F122. doi: 10.1136/fn.70.2.f118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukai T. Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gielis JF. Lin JY. Wingler K. Schil PEYV. Schmidt HH. Moens AL. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic Biol Med. 2011;50:765–776. doi: 10.1016/j.freeradbiomed.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Gryglewski RJ. Palmer RMJ. Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 24.Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 25.Hink HU. Santanam N. Dikalov S. McCann L. Nguyen AD. Parthasarathy S. Harrison DG. Fukai T. Peroxidase properties of extracellular superoxide dismutase, role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. 2002;22:1402–1408. doi: 10.1161/01.atv.0000027524.86752.02. [DOI] [PubMed] [Google Scholar]
- 26.Jernigan NL. Resta TC. Walker BR. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L947–L955. doi: 10.1152/ajplung.00215.2003. [DOI] [PubMed] [Google Scholar]
- 27.Karamanoukian HL. Glick PL. Zayek M. Steinhorn RH. Zwass MS. Fineman JR. Morin FC. Inhaled nitric oxide in congenital hypoplasia of the lungs due to diaphragmatic hernia or oligohydramnios. Pediatrics. 1994;94:715–718. [PubMed] [Google Scholar]
- 28.Keller RL. Moore P. Teitel D. Hawgood S. McQuitty J. Fineman JR. Abnormal vascular tone in infants and children with lung hypoplasia: findings from cardiac catheterization and the response to chronic therapy. Pediatr Crit Care Med. 2006;7:589–594. doi: 10.1097/01.PCC.0000244401.53189.CB. [DOI] [PubMed] [Google Scholar]
- 29.Khemani E. McElhinney DB. Rhein L. Andrade O. Lacro RV. Thomas KC. Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 30.Klatt P. Schmidt K. Lehner D. Glatter O. Bachinger HP. Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. EMBO J. 1995;14:3687–3695. doi: 10.1002/j.1460-2075.1995.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konduri GG. Bakhutashvili I. Eis A. Pritchard K. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1812–H1820. doi: 10.1152/ajpheart.00425.2006. [DOI] [PubMed] [Google Scholar]
- 32.Lassegue B. How does the chloride/proton antiporter ClC-3 control NADPH oxidase. Circ Res. 2007;101:648–650. doi: 10.1161/CIRCRESAHA.107.161869. [DOI] [PubMed] [Google Scholar]
- 33.Lassegue B. Griendling KK. NADPH Oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SY. Suh JK. Choi JH. Jeon WJ. Cheong MA. Effect of ketorolac and diclofenac on the impairment of endothelium-dependent relaxation by reactive oxygen species in rabbit abdominal aorta. Korean J Anesthesiol. 2010;59:196–202. doi: 10.4097/kjae.2010.59.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W-G. Miller FJ. Zhang HJ. Spitz DR. Oberly LW. Weintraub NL. H2O2-induced O2− production by a non-phagocytic NADPH oxidase causes oxidant injury. J Biol Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonnqvist PA. Jonsson B. Winberg P. Frostell CG. Inhaled nitric oxide in infants with develooping or established chronic lung disease. Acta Paediatr. 1995;84:1188–1192. doi: 10.1111/j.1651-2227.1995.tb13522.x. [DOI] [PubMed] [Google Scholar]
- 37.Lucchesi PA. Belmadani S. Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 38.Miller FJ. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kB in vascular smooth muscle cells requires signalling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 39.Milligan SA. Hoeffel JM. Goldstein IM. Flick MR. Effect of catalase on endotoxin-induced acute lung injury in unanesthetized sheep. Am Rev Respir Dis. 1988;137:420–428. doi: 10.1164/ajrccm/137.2.420. [DOI] [PubMed] [Google Scholar]
- 40.Norton CE. Jernigan NL. Kanagy NL. Walker BR. Resta TC. Intermittent hypoxia augments pulmonary vascular smooth muscle reactivity to NO: regulation by reactive oxygen species. J Appl Physiol. 2011;111:980–988. doi: 10.1152/japplphysiol.01286.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nozik-Grayck E. Sullivan HB. Piantadosi CA. Extracellular superoxide dismutase. Int J Biochem Cell Biol. 2005;37:2466–24671. doi: 10.1016/j.biocel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Oury TD. Day BJ. Crapo JD. Extracellular suerpoxide dismutase: a regulator of nitric oxide bioavailability. Lab Invest. 1996;75:617–636. [PubMed] [Google Scholar]
- 43.Panda K. Rosenfeld RJ. Ghosh S. Meade AL. Getzoff ED. Stuehr DJ. Distinct dimer interaction and regulation in nitric-oxide synthase types 1, 2, and 3. J Biol Chem. 2002;277:31020–31030. doi: 10.1074/jbc.M203749200. [DOI] [PubMed] [Google Scholar]
- 44.Seshiah PN. Weber DS. Rocic P. Valppu L. Taniyama Y. Griendling KK. Angiotensin II stimulation of NADPH oxidase activity upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 45.Steinhorn RH. Albert G. Swartz DD. Russell JA. Levine CR. Davis JM. Recombinant human superoxide dismutase enhances the effect ofinhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:834–839. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 46.Subhedar N. Recent advances in diagnosis and management of pulmonary hypertension in chronic lung disease. Acta Paediatr. 2004;444(Suppl):29–32. doi: 10.1111/j.1651-2227.2004.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki YJ. Forman HJ. Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 48.Wedgwood S. Lakshminrusimha S. Fukai T. Russell JA. Schumacker PT. Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal. 2011;15:1497–1506. doi: 10.1089/ars.2010.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wedgwood S. Steinhorn RH. Bunderson M. Wilham J. Lakshminrusimha S. Brennan LA. Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2005;289:L660–L666. doi: 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zinkevich NS. Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647–H653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]