Abstract
Alzheimer’s disease (AD) involves progressive accumulation of amyloid β-peptide (Aβ) and neurofibrillary pathologies, and glucose hypometabolism in brain regions critical for memory. The 3xTgAD mouse model was used to test the hypothesis that a ketone ester–based diet can ameliorate AD pathogenesis. Beginning at a presymptomatic age, 2 groups of male 3xTgAD mice were fed a diet containing a physiological enantiomeric precursor of ketone bodies (KET) or an isocaloric carbohydrate diet. The results of behavioral tests performed at 4 and 7 months after diet initiation revealed that KET-fed mice exhibited significantly less anxiety in 2 different tests. 3xTgAD mice on the KET diet also exhibited significant, albeit relatively subtle, improvements in performance on learning and memory tests. Immunohistochemical analyses revealed that KET-fed mice exhibited decreased Aβ deposition in the subiculum, CA1 and CA3 regions of the hippocampus, and the amygdala. KET-fed mice exhibited reduced levels of hyperphosphorylated tau deposition in the same regions of the hippocampus, amygdala, and cortex. Thus, a novel ketone ester can ameliorate proteopathic and behavioral deficits in a mouse AD model.
Keywords: Cognitive deficits, Hippocampus, Amygdala, 3xTgAD, Frontotemporal lobe dementia, Energy Metabolism, Anxiety
1. Introduction
By 2050, the number of patients with Alzheimer’s disease (AD) in the United States is expected to approach 13 million (Thies and Bleiler, 2011). Although there have been advances in the diagnosis of probable AD (McKhann et al., 2011), all clinical trials of interventions aimed at slowing disease progression in patients with mild cognitive impairment (MCI) and AD have failed (Feldman et al., 2007; Petersen et al., 2005; Winblad et al., 2008). The self-aggregation and accumulation of extracellular amyloid β-peptide (Aβ) and intracellular hyperphosphorylated tau (pTau) with cognitive impairment are defining features of AD (Selkoe, 1997). Although mutations in the β-amyloid precursor protein (APP) and the APP-cleaving enzyme presenilin-1 cause rare cases of early-onset familial AD (Bertram et al., 2010), most cases of AD occur after the age of 65 years and have no known cause.
Increasing evidence suggests a role for a chronic positive energy balance resulting from excessive caloric intake and a sedentary lifestyle (and associated insulin resistance) during midlife as AD risk factors (Kapogiannis and Mattson, 2011; Xu et al., 2011). Aβ pathology and cognitive deficits are exacerbated by a high-fat diet (Julien et al., 2010) and diabetes (Takeda et al., 2010), and are ameliorated by dietary energy restriction (Halagappa et al., 2007; Patel et al., 2005; Wang et al., 2005) in mouse models of AD. Moreover, pilot clinical studies have reported improvement in cognitive function and reduced progression of hypometabolism assessed by fluorodeoxyglucose (FDG) positron emission tomography (PET) in patients with AD after intranasal administration of insulin (Craft et al., 2012).
Ketone bodies are an alternative fuel for brain cells when glucose availability is insufficient. The neuroprotective potential of ketones is supported by the well-known efficacy of fasting and ketogenic diets in the treatment of epilepsy (Conklin, 1922; Wilder, 1921). The metabolism of ketone bodies mimics some actions of insulin (Sato et al., 1995) and can overcome insulin resistance (Kashiwaya et al., 1997), suggesting a potential therapeutic benefit of ketone bodies in AD (Kashiwaya et al., 2000). Consistent with the latter possibility, intermittent energy restriction increases levels of circulating β-hydroxybutyrate (Johnson et al., 2007) and ameliorates cognitive impairment in a mouse model of AD (Halagappa et al., 2007).
A ketogenic diet decreased Aβ levels in the brain of AD mice (Van Der Auwera et al., 2005), suggesting that ketone bodies might suppress the pathogenic processes associated with cognitive impairment in AD. However, the latter study did not evaluate the therapeutic potential of ketone bodies per se, and it is not known if ketone bodies can ameliorate behavioral deficits in AD models. There have been no published reports of ketone bodies improving behavioral function and decreasing progression of Aβ and pTau pathologies in AD models. In this study, a novel ketone ester comprised of D-β-hydroxybutyrate and (R)-1,3-butanediol, a precursor of the physiological forms of ketone bodies, was used. The impact of the ketone ester–based diet on the progression of AD-like Aβ and pTau pathologies and behavioral abnormalities in the triple transgenic mouse model of AD, 3xTgAD mice, was examined (Oddo et al., 2003).
2. Materials and methods
2.1. Animals, diet, and study overview
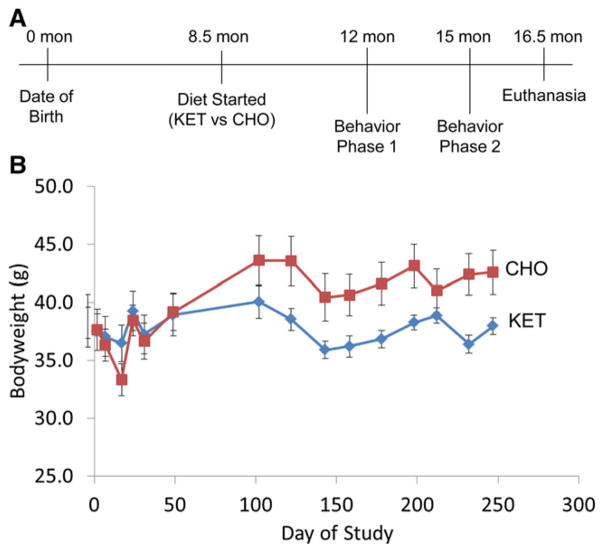
The initial generation and characterization of 3xTgAD mice have been reported previously (Oddo et al., 2003). The mice used in the present study were from a colony that had been back-crossed onto a C57BL/6 genetic background for 8 generations and characterized in our previous studies (Liu et al., 2010; Romberg et al., 2011; Rothman et al., 2012). Mice were housed at the National Institute on Aging Biomedical Research Center in Baltimore, MD. Thirty male 3xTgAD mice were housed in groups of 2 to 3 mice per cage under a standard 12-hour light and dark circadian cycle (lights off at 18:00 hours). When the mice were 8.5 months old, they were randomly assigned to 2 dietary groups of 15 mice per group (Fig. 1): (1) a diet containing a ketone ester (comprised of D-β-hydroxybutyrate and (R)-1,3-butanediol) (KET); or (2) a carbohydrate-enriched diet (CHO) based on the American Institute of Nutrition 1993 (AIN-93) dietary recommendation for laboratory rodents on a maintenance diet (Table 1) (Reeves et al., 1993). This purified diet with defined nutrients allows modification of 1 component while keeping other essential nutrients constant. The mice were fed a 4- to 5-g pellet (10.8–13.5 kcal) at approximately 06:00 hours each day. Body weight was measured once a week during the first 6 weeks and then once a month for the remainder of the study. To maintain body weight, supplemental NIH-31 pellets were provided to mice that exhibited a weight loss of more than 20% for a limited period during the first 6 weeks. Behavioral tests (phase 1 and phase 2) were performed when the mice were 12 months and 15 months old (Fig. 1).
Fig. 1.
Experimental design and body weight of mice during the course of the dietary intervention. (A) To characterize the behavioral effects of the ketone ester on 3xTgAD mice, we developed a strategy to analyze the cognitive performance of the mice at 12 months (phase 1) and at 15 months (phase 2). The dietary intervention was started at 8.5 months with initiation of either a carbohydrate-enriched (CHO) or a ketone ester (KET) diet. The mice were euthanized at the age of 16.5 months after completion of all behavioral testing for phase 2. (B) Body weight of 3xTgAD mice in the CHO and KET diet groups during the course of the study. Values are the mean and SEM (n = 11–15 mice per group).
Table 1.
Diet composition
| Ingredient | Product information | CHO | KET |
|---|---|---|---|
| Diet recipe (g/1000 g diet) | |||
| Casein | Bio-Serv, product no. 1100 | 120 | 120 |
| Cellulose (fiber) | Bio-Serv, product no. 3425 | 50 | 50 |
| Corn starch | Giant brand | 137 | 85 |
| Sucrose | Giant brand, pure cane sugar | 257 | 160 |
| Soybean oil | Pure Wesson soybean oil | 25 | 25 |
| Salt mix, AIN-93, GMX | Bio-Serv, product no. F8538 | 35 | 35 |
| Vitamin mix, AIN-93 | Bio-Serv, product no. F8001 | 10 | 10 |
| Choline chloride, USP | Bio-Serv, product no. 6105 | 2 | 2 |
| L-Methionine | Bio-Serv, product no. 1350 | 1.5 | 1.5 |
| L-Cystine | Bio-Serv, product no. 1160 | 1.5 | 1.5 |
| Acesulfame K | Sigma-Aldrich, catalog no. 04054 | 10 | 10 |
| tert-Butylhydroquinone | Sigma-Aldrich, catalog no. 112941 | 0.14 | 0.14 |
| Ketone ester | Produced in-house (R. Veech Laboratory) | 0 | 125 |
| Sugar-free Jell-O | Kraft brand, raspberry flavor | 100 | 100 |
| Water | Distilled | 251 | 275 |
| Diet composition (% kcal) | |||
| Carbohydrate | 64.9 | 43.5 | |
| Protein | 23.9 | 23.9 | |
| Fat | 8.2 | 8.2 | |
| Ketone ester | 0 | 21.5 | |
| Energy content (kcal/g) | 2.7 | 2.7 | |
The custom carbohydrate-enriched (CHO) and ketone ester (KET) diets were produced in-house in accordance with nutritional guidelines set forth by AIN in 1993 for rodent maintenance diets. The resulting diets both contained 2.7 kcal/g. The only difference in the 2 diets came from the addition of 21.5% by calories of ketone ester in the KET group. This difference was coupled with a 21.5% increase in carbohydrate content in the CHO group.
2.2. Euthanasia procedure
When the mice were 16.5 months old, they were euthanized and their brains collected at 10:00 hours according to previous protocols (Halagappa et al., 2007). Briefly, 1 brain hemisphere was immersed in 4% paraformaldehyde/phosphate-buffered saline (PBS) and kept at 4 °C until analysis. The hippocampus, cortex, striatum, and cerebellum were removed from the other hemisphere, immediately frozen, and subsequently stored at −80 °C. All procedures were approved by the Animal Care and Use Committee of the National Institute on Aging.
2.3. Open field testing
Spontaneous activity of mice in an open field test was quantified using the MEDOFA-MS system (Med Associates, St. Albans, VT, USA). Motion of the mouse was traced with infrared light– sensitive photocells with the apparatus placed in a 120-lx ventilated box. The dimensions of the arena were 40.6 cm × 40.6 cm; the inner 20.32 cm was defined as the center zone and outside was defined as the peripheral zone. Mice were placed in the center of the open field and assessed by measuring ambulatory counts, ambulatory time, and total distance covered over a period of 10 minutes.
2.4. Elevated plus maze testing
The apparatus consisted of a plus sign–shaped maze, elevated 60 cm from the floor with each arm measuring 25 cm × 5 cm; 2 arms lacked side walls (open arms) and 2 arms were enclosed in 30-cm high walls (closed arms). Each mouse was placed in the middle of the maze facing an open arm. After 5 minutes of testing, mice were returned to their home cages. Arm preference was automatically analyzed using the ANY-maze video tracking software (Stoelting, Wood Dale, IL, USA), and time spent in each arm was recorded. Elevated plus maze experiments were performed under a light intensity of 1300 lx.
2.5. Fear conditioning testing
Fear conditioning tests were performed in chambers with internal dimensions of 20 cm × 16 cm × 20.5 cm (MEDVFC-NR-M, Med Associates, St. Albans, VT, USA). A house light (CM1820 bulb) mounted directly above the chamber provided illumination. In the training session, mice were placed in a contextual conditioning chamber and allowed to explore the chamber for 2 minutes. At the end of 2 minutes, the audio tone (conditional stimulus: 5 kHz, 70 dB) was administered for 28 seconds followed by the foot shock (unconditional stimulus: 0.5 mA) from the metal grid on the floor for 2 seconds. Foot shock intensity was determined in a preliminary test on a separate cohort of mice to be the minimal applicable intensity that elicited a response. Each session lasted for 30 seconds and total experimental time was 5 minutes. The movement of mice was recorded. Tests that recorded no movement for more than 1 second were counted as freezing. On day 2, both contextual and cued conditioning tests were performed. In the contextual fear session, mice were returned to the conditioning chamber for 5 minutes without any shock or tone. The time of freezing was recorded and used as an index of contextual memory. After 3 hours of rest, the tone-associated, cued conditioning tests were performed. Mice were returned to the chamber, but in a different context. The floor was covered by a white plastic board and a black A-frame contextual plastic insert was placed inside the room. Mice were allowed to explore the chamber for 5 minutes without any audio tone followed by 5 30-second audio tones 30 seconds apart. Time freezing during and between the audio tones was recorded and used as an index of cued memory.
2.6. Morris water maze testing
A circular tank (diameter 160 cm) was filled with water (28 ± 1 °C) rendered opaque by the addition of nontoxic water paint (Palmer Paints Products Inc, Troy, MI, USA). Before all learning and memory tests, visible platform training was performed to facilitate motivation and habituation and to exclude any impairment of visual abilities or physical performance. Mice that demonstrated a difference in latency to reach the platform or swim speed of more than 3 standard deviations from the mean during the visible swim trials were excluded. For the visible platform training (4 trials per day for 3 days in phase 1, and for 4 days in phase 2), a square platform (14 cm × 14 cm) was placed above the water level with a black metal object attached. For the hidden platform maze test, the black metal on the platform was removed and the platform was submerged 0.5 to 0.8 mm below the surface of the water in the geometric center of 1 quadrant of the tank. Memory acquisition trials (the hidden test) consisted of 4 trials per day for 5 days. For each trial, the mouse was released facing the wall in a quadrant other than the quadrant with the platform. Termination of the trial was determined by either the mouse being able to find the platform or when 90 seconds had elapsed. If the mouse did not find the platform within 90 seconds, it was guided to the platform. Mice were placed on the platform for 30 seconds at the end of each trial. After the completion of the fourth trial on each day, the mouse was dried and returned to its home cage. Twenty-four hours after the final trial, the platform was removed and probe trials (60 seconds, 1 time) were performed.
Phase 1 acquisition and probe trials were performed in a dark room with 4 white illuminated cues (Artograph light box, 30.5 × 25.4 cm) placed around the tank. At completion of phase 1 acquisition trials, mice were retrained with 4 trials using a hidden platform in the same location with all 4 visual cues. Probe trials were then continued over the next 4 days with 3, 2, 1, and 0 cues. Time spent in the target area was normalized to the mean time at the 0 cue trial. After memory extinction with a 0 cue probe trial, memory reversal trials were performed with 6 different visual cues. Briefly, the hidden platform was moved to a different quadrant and 4 trials per day were performed for 5 days. A probe trial was performed 24 hours after the last trial. Phase 2 acquisition trials and reversal trials were performed in a lightened room with different cues placed on the walls surrounding the tank. To test short-term working memory, the hidden platform was placed in a different location each day for 5 days. Each day, mice were released 3 times from a quadrant other than the quadrant where the platform was located in the hidden or reverse trials. A video camera was mounted on the ceiling in the center of the pool, and all trials were recorded and analyzed by ANY-maze software.
2.7. Immunohistochemical analysis of Aβ and pTau pathologies
For tissue analysis, half-brains (n = 12) were fixed using 4% paraformaldehyde in 0.1 M PBS overnight at 4 °C and then sequentially immersed in 0.1 M PBS containing 15% to 30% sucrose at 4 °C. The brains were cut into 30-μm coronal sections. Immunohistochemical staining was used to detect Aβ and pTau using methods described previously (Liu et al., 2010). Briefly, free-floating sections were incubated with rabbit polyclonal anti-human Aβ antibody (Cell Signaling, Beverly, MA, USA; clone #2454, 1:200) and mouse monoclonal anti-human PHF-tau antibody (Pierce Endogen, Rockford, IL, USA; clone#AT180,1:200) overnight at 4 °C followed by anti-rabbit or mouse-IgG in PBS for 1 hour. After washing in PBS, sections were incubated with streptavidin conjugated to horse-radish peroxidase in PBS for 1 hour. The reaction was terminated with a solution containing diaminobenzidine and hydrogen peroxide (0.001%). Sections were then mounted on gelatin-coated slides, heat-dried, dehydrated through a graded series of alcohols, cleared in xylene, and covered with Permount (Vector Laboratories, Burlingame, CA, USA). Digital images were captured and documented with an Olympus BX51 microscope (Tokyo, Japan). Images of hippocampus from at least 5 brain sections/mouse were captured. Numbers of CA1 neurons exhibiting Aβ immunoreactivity or pTau immunoreactivity were counted. All analyses were performed by an investigator blinded to the treatment history of the mice.
2.8. Measurements of D-β-hydroxybutyrate and glucose
Blood glucose and D-β-hydroxybutyrate levels were measured using glucose and ketone sticks and a Precision-Xtra meter (Abbott Labs, Abbott Park, IL, USA).
2.9. Statistical analysis
All values are presented as means ± standard error of the mean (SEM). The Student t test was used for body weights, blood glucose and ketone levels, and immunohistochemistry. Analyses of behavioral data were performed using either two-way ANOVA repeated measures with unbalanced sample volume (two-way ANOVARM) or a one-way ANOVA, as appropriate for the dataset analyzed.
3. Results
3.1. 3xTgAD mice fed a KET diet exhibit a lower average body weight and a higher β-hydroxybutyrate concentration
During the first 50 days on the experimental diets, the mice in both the KET and control (CHO) diet groups exhibited fluctuations in body weight as they adapted to the diets (Fig. 1). Thereafter, the mice on the KET diet maintained body weight approximately 10% to 12% lower than the body weight of mice in the CHO group (KET 38.0 ± 0.7 g, CHO 42.6 ± 1.9 g). There was no difference in the body weight of mice assigned to each group at the time of diet initiation (KET 38.8 g, CHO 37.9 g). Levels of β-hydroxybutyrate were significantly higher in mice in the KET group (0.71 mM vs. 0.14 mM in the CHO group). Blood glucose levels in the KET mice (152 mg/dL) were not different than glucose levels in the CHO mice (156 mg/dL) (Table 2).
Table 2.
Measurement of ketone concentration in the ketone ester (KET) and carbohydrate-enriched (CHO) diets
| Constituent | KET diet | CHO diet |
|---|---|---|
| β-Hydroxybutyrate (mM) | 0.71 ± 0.12a | 0.14 ± 0.01 |
| Glucose (mg/dL) | 152 ± 8.48 | 156 ± 6.95 |
Measurements of the concentration of ketone and glucose in the KET and CHO 3xTgAD mice resulted in a significantly increased β-hydroxybutyrate concentration in the KET group. The glucose concentration did not differ significantly between the 2 groups.
p < 0.001 compared to the value for mice in the CHO diet group.
3.2. Elevated plus maze and open field testing reveal an anxiolytic effect of ketone ester in 3xTgAD mice
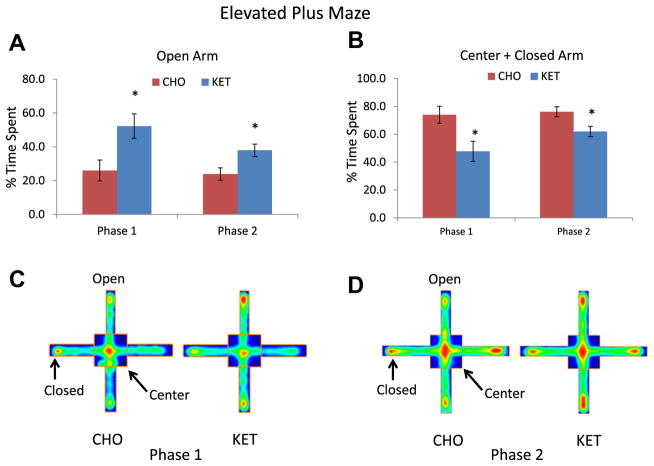
In elevated plus maze testing, mice in the KET group spent significantly more time in the open arms during both phase 1 (KET 52.2 seconds, CHO 26.0 seconds; p = 0.011) and phase 2 (KET 38.0 seconds, CHO 23.9 seconds; p = 0.016), whereas mice in the CHO group spent significantly more time in the center and closed arms (Fig. 2). Two-way ANOVARM testing demonstrated a significance between the KET and CHO groups in the time spent in the center and closed area for phase 1 (p = 0.001) and phase 2 (p = 0.002).
Fig. 2.
Ketone ester feeding exerts an anxiolytic action in 3xTgAD mice. Results of both phase 1 and phase 2 elevated plus maze testing of 3xTgAD mice in the carbohydrate-enriched diet (CHO) and ketone ester (KET) diet groups. (A and B) Results for time spent in the open arms (A) and the closed arms plus the center arm of the maze (B). (C and D) Heat maps for representative 3xTgAD mice from the CHO and KET diet groups from phase 1 (C) and phase 2 (D) tests showing the cumulative amounts of time that the mice spent in the different regions of the elevated plus maze. Green, yellow, and red colors correspond to low, medium, and high percentage of spent time in the indicated area, respectively. Values are the mean and SEM (n = 10–14 mice per group) *p < 0.05 by t test. #p < 0.05 by two-way ANOVARM.
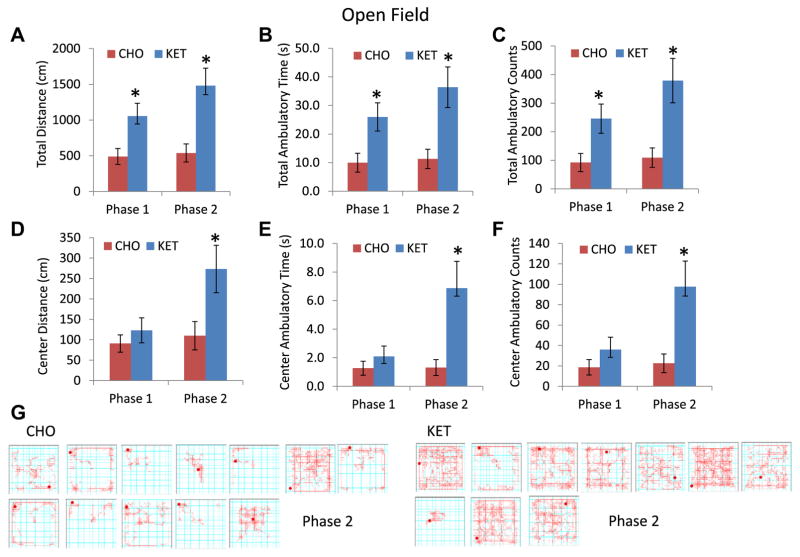
In open field testing, mice in the KET group exhibited twice the activity as indicated by greater total distance traveled (KET 1054 cm, CHO 490 cm; p = 0.019), time of ambulation (KET 26.0 seconds, CHO 10.0 seconds; p = 0.018), and ambulation counts (KET 246, CHO 91.9; p = 0.024) for phase 1. For phase 2, the values were as follows: total distance traveled (KET 1481 cm, CHO 539 cm; p = 0.003); ambulatory time (KET 36.4 seconds, CHO 11.3 seconds; p = 0.005); and ambulatory counts (KET 379, CHO 109; p = 0.005) (Fig. 3). Although there were differences in ambulation parameters, there was no time difference between the CHO and KET groups in the center versus peripheral zones during phase 1. However, during phase 2, the mice in the KET group spent significantly more time in the center area compared with mice in the CHO group (Fig. 3).
Fig. 3.
Ketone ester-fed 3xTgAD mice exhibit greater exploratory activity in the open field test. (A) Total distance traveled (cm) in phases 1 and 2. (B) Total ambulatory time (seconds) in phases 1 and 2. (C) Total ambulatory counts in phases 1 and 2. (D, E, and F) Distance traveled (D), ambulatory time (E), and ambulatory counts (F) in the open area. (G) Examples of the paths of individual mice in the open field in phase 2 tests. Values are the means and SEM (n = 10–14 mice per group) *p < 0.05 by t test. #p < 0.05 by two-way ANOVARM. CHO, carbohydrate-enriched diet; KET, ketone ester diet.
3.3. KET-fed 3xTgAD mice exhibit superior cognitive performance compared with CHO-fed 3xTgAD mice
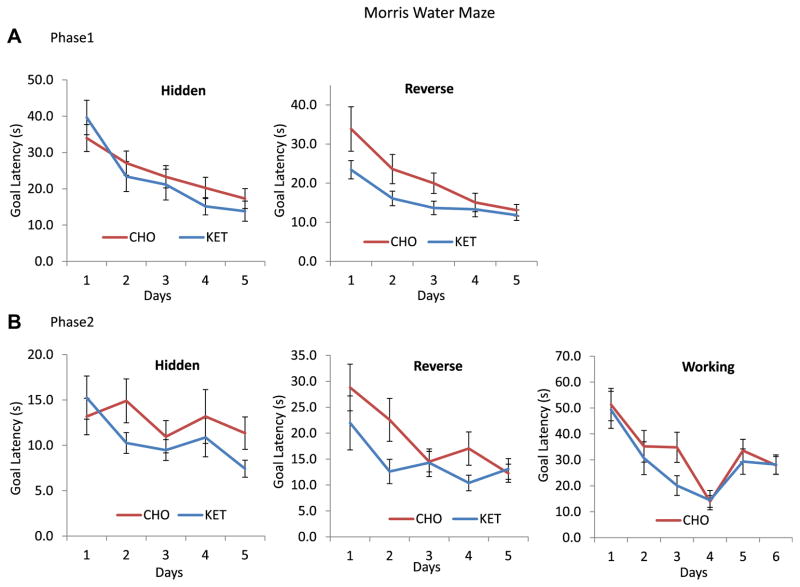
In the phase 1 hidden platform (memory acquisition) test in the Morris water maze, the mice in the KET and CHO diet groups exhibited similar goal latencies across the 5 days of testing, thus revealing no discernible effects of diet on the ability of the mice to learn the location of the platform (Fig. 4A, left graph). Mice in the KET group exhibited significantly lower goal latency in the reverse Morris water maze test compared with mice in the CHO group (Fig. 4A, right graph). Two-way ANOVARM testing for phase 1 reversal trials in the water maze revealed a significantly lower goal latency (time required to reach the platform) for mice in the KET group compared with those in the CHO group (p = 0.026) (Fig. 4A). In addition, for phase 1, when the hidden and reverse water maze were combined, two-way ANOVARM demonstrated a lower latency for mice in the KET group compared with those in the CHO group (p = 0.040). The results from the probe trials (amount of time spent in the target area) in phase 1 performed after the hidden platform test (latency: KET 39.5 seconds, CHO 50.5 seconds; p = 0.075; speed: KET 0.21 m/s, CHO 0.28 m/s, p = 0.27) and after the reverse test (latency: KET 28.7 seconds, CHO 26.2 seconds; p = 0.56; speed: KET 0.22 m/s, CHO 0.21 m/s, p = 0.40) were not significantly different, indicating that memory extinction had occurred in both groups, enabling transition to the next trials. Swimming speeds of mice during the probe tests were also not significantly different between the 2 diet groups (platform removal probe trial after the hidden platform test: KET 0.215 m/s, CHO 0.198 m/s, p = 0.125; after the reversal test: KET 0.225 m/s, CHO 0.215 m/s, p = 0.402). Shorter latency times were observed in phase 2 in both groups of mice, consistent with them remembering the task from the phase 1 testing. However, when data for hidden, reverse, and working memory tasks were combined and analyzed using two-way ANOVARM, mice in the KET group had significantly lower escape latency times (hidden + reverse, p = 0.040; and reverse alone, p = 0.026 in phase 1; hidden + reverse + working combined, p = 0.018 in phase 2) (Fig. 4B). There were no significant effects of diet on swimming speed or distance in these tests. The data for the probe trials (time spent in the target quadrant) after the hidden water maze (latency: KET 31.2 seconds, CHO 27.3 seconds; p = 0.200; speed: KET 0.22 m/s, CHO 0.21 m/s; p = 0.33) and the probe trials after the reverse water maze (latency: KET 30.3 seconds, CHO 26.2 seconds; p = 0.362; speed: KET 0.23 m/s, CHO 0.22 m/s; p = 0.50) were not significantly different, indicating that memory extinction had occurred in mice in both diet groups (Supplemental Figure 1). Swimming speeds during the visible platform test were as follows: KET 0.176 m/s, CHO 0.166 m/s (p = 0.182).
Fig. 4.
A ketone ester (KET) diet improves the performance of 3xTgAD mice in hippocampus-dependent water maze tests of spatial memory. (A, B) Results for goal latencies for memory acquisition trials (hidden platform) and reversal trials in phase 1 (12 months) and phase 2 (15 month) tests. The results of the working memory tests in phase 2 are also shown. Performance in the KET group proved significant using two-way ANOVARM measures analysis for phase 1 in Hidden and Reverse (p = 0.040) and Reverse alone (p = 0.026). For phase 2, significance was demonstrated for Hidden, Reverse, and Working (p = 0.018). Values are mean and SEM (n = 10–14).
3.4. Evidence that a KET diet differentially affects hippocampus-dependent and amygdala-dependent memory
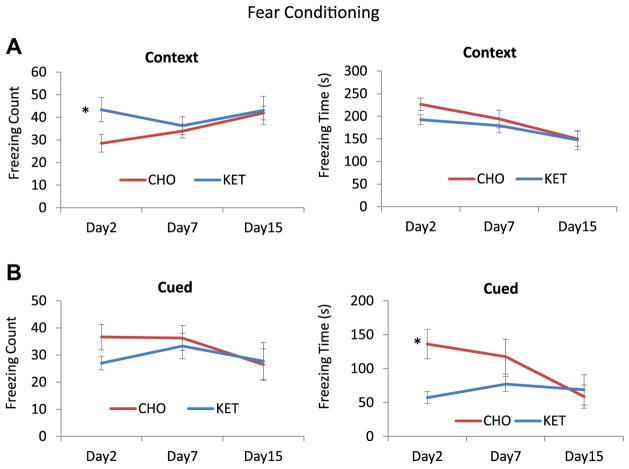
Fear conditioning testing was performed 1 week after water maze testing. Mice in the KET and CHO groups exhibited similar learning curves on day 1 of fear conditioning, demonstrating equal abilities to associate the audio tone with the foot shock (values for the KET group were 2.0, 7.66, and 13.1, and values for the CHO group were 1.3, 11.0, and 12.3; p values were 0.51, 0.30 and 0.70, respectively). In the contextual memory test, mice in the KET group exhibited significantly more freezing events on day 2 (KET 43.4, CHO 28.5; p = 0.033), but their total freezing time did not differ significantly from the CHO group (KET 226, CHO 192; p = 0.078) (Fig. 5). Cued memory testing demonstrated that total freezing counts for mice in the KET and CHO groups were similar (KET 27.0, CHO 36.6; p = 0.082), whereas the KET mice exhibited a lower freezing time (KET 57.0, CHO 136; p = 0.004). Data for the KET and CHO groups on days 7 and 15 were not significantly different for either contextual or cued memory.
Fig. 5.
Fear conditioning tests demonstrated that ketone ester (KET) fed 3xTgAD mice exhibit a stronger context-dependent fear response related to hippocampal memory and a more rapid extinction of amygdala-dependent tone-related conditioned fear compared with 3xTgAD mice fed the carbohydrate-enriched (CHO) control diet. (A) Freezing counts on days 2, 7, and 15 in a contextual conditioning test. (B) Freezing times on days 2, 7, and 15 in a contextual conditioning test. (C) Freezing counts on days 2, 7, and 15 in a cued tone conditioning test. (D) Freezing times on days 2, 7, and 15 in a cued tone conditioning test. *p < 0.05 by t test. Values are means and SEM (n = 10–13 mice per group). Two-way ANOVARM analysis (days 2, 7, and 15) did not show a significant difference between the KET and CHO groups.
3.5. A KET diet suppresses Aβ accumulation and pTau pathology in 3xTgAD mice
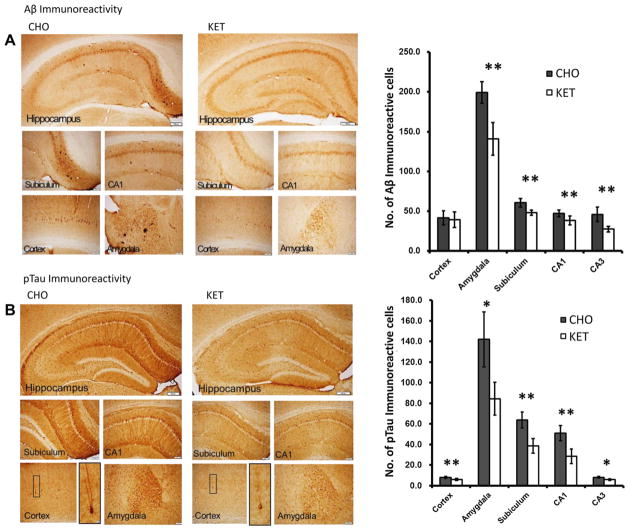
Immunohistochemical analysis of brain slices demonstrated a reduction in Aβ in the amygdala, subiculum, and the hippocampus in the 3xTgAD mice in the KET diet group compared with mice in the control CHO group (Fig. 6A). Images of immunostained brain sections revealed many neurons in the hippocampus, cerebral cortex, and amygdala that exhibited (intracellular) Aβ immunore-activity (Fig. 6A, left panel). The results of cell counting revealed that the number of Aβ immunoreactive neurons was significantly lower in mice in the KET group for all 5 brain regions evaluated (hippocampal CA and CA3 neurons, subiculum, amygdala, and cerebral cortex) compared with mice in the CHO group (Fig. 6A, right panel). Numbers of pTau-positive neurons were significantly lower in mice in the KET group compared with those in the CHO group for all 5 brain regions evaluated (Fig. 6B).
Fig. 6.
Ketone ester feeding reduces intracellular accumulations of amyloid β (Aβ) and phosphorylated tau (pTau) in the subiculum, CA1 and CA3 area of hippocampus, amygdala, and cerebral cortex of 3xTgAD mice. (A) Aβ immunoreactivity in brain sections from mice in the carbohydrate-enriched (CHO; left) and ketone ester (KET; right) diet groups. The upper panels are low magnification images of the regions of the hippocampus and the lower panels are high magnification images of the regions of the subiculum, CA1, cerebral cortex, and amygdala. The graph on the right shows the results of counts of Aβ immunoreactive cells in the indicated brain regions. (B) pTau immunoreactivity in brain sections from mice in the CHO (left) and KET (right) diet groups. The upper panels are low magnification images of the regions of the hippocampus and the lower panels are high magnification images of the regions of the subiculum, CA1, cerebral cortex, and amygdala respectively. The graph on the right shows the results of counts of pTau immunoreactive cells in the indicated brain regions. Scale bars: lower magnification images, 200 μm; high magnification images, 100 μm. Values are the mean ± SEM (n = 6–9 mice per group). *p < 0.05 and **p < 0.001 by the Student t test.
4. Discussion
A diet containing a specific ketone ester was found to ameliorate behavioral abnormalities (anxiety and memory deficits) and reduce the amounts of Aβ and pTau in brain regions known to mediate those behaviors in 3xTgAD mice. Previous studies have demonstrated a positive association between levels of endogenous ketone bodies and cognitive function in human subjects and animal models (see Maalouf et al., 2009 for a review). In addition, dietary interventions that increase levels of ketone bodies, including intermittent fasting and a ketogenic diet, can protect neurons against oxidative, metabolic, and excitotoxic insults relevant to the pathogenesis of AD (Bruce-Keller et al., 1999; Halagappa et al., 2007). However, previous studies did not determine whether ketone bodies were directly responsible for the neuroprotective effects of such diets. Our data establish the therapeutic efficacy of the synthetic ketone ester (R)-3-hydroxybutyrate-(R)-1,3-butanediol monoester, administered as a dietary supplement, in a mouse model of AD. Thus, we show that a ketone body is sufficient to suppress AD-like pathology and behavioral abnormalities.
Ketones can act as an energy source for the brain during prolonged periods of fasting or in situations of compromised glucose utilization, whereas in the fed state, glucose is the main energy source for neurons. During fasting, endogenous fat stores are metabolized to ketone bodies, which are then exported to other tissues where they enter the mitochondria and provide substrates for the tricarboxylic acid cycle producing NADH equivalents. Increasing circulating ketone bodies, via fasting or feeding a high-fat low-carbohydrate diet, is effective in treating epilepsy. However, the palatability of high-fat diets is generally poor and, when fed for prolonged periods, high-fat diets are likely to be atherogenic. In an effort to circumvent these problems, we synthesized a ketone ester that allows for an increase in circulating ketones. The ketone ester used in the present study provides 2 equivalents of D-β-hydroxybutyrate, the mammalian physiological enantiomer, in addition to generating reducing equivalents for the cell. Provision of ketones may allow the brain to maintain energy homeostasis in disease states where glucose is either limiting or cannot be used.
In the congenic line of 3xTgAD mice used in the present study, Aβ and pTau accumulations become evident at about 8 months of age and progressively increase thereafter, with anxiety evident by 9 months and hippocampal synaptic dysfunction and memory deficits detectable by 10 months (Nelson et al., 2007). 3xTgAD mice also exhibit an attentional deficit involving cholinergic dysfunction (Romberg et al., 2011), freezing behavior (Sterniczuk et al., 2010), and amygdala-related anxiety (Espana et al., 2010). We explored the effect of a ketone ester on 3xTgAD mice by elevated plus maze, open field, Morris water maze, and fear conditioning testing at the age of 12 months (phase 1) and 15 months (phase 2) along with immunohistochemical characterization to analyze cognitive as well as pathologic phenomena similar to those that occur in AD.
3xTgAD mice tend to have higher levels of anxiety and a lowered threshold for fear responses that correlate with Aβ pathology in the amygdala and hippocampus (Espana et al., 2010; Liu et al., 2010; Rothman et al., 2012). The result of this anxiety is generally decreased exploratory and ambulatory behavior in the open field and avoidance of the open arms in the elevated plus maze (Halagappa et al., 2007; Sterniczuk et al., 2010). Patients with AD generally exhibit such anxiety as well as agitation and other psychopathologic symptoms (Chung and Cummings, 2000). In the present study, the KET-fed 3xTgAD mice demonstrated significantly more spontaneous exploratory behavior in the elevated plus maze and the open field test compared with 3xTgAD mice on the control diet, thus establishing an anxiolytic effect of the ketone ester.
The fear conditioning test is a form of the classic Pavlovian memory test in which the mouse learns to associate a location (context) and a tone (conditioned stimulus) with an electric foot shock. The fear-related memory and anxiety manifests as freezing behavior. Contextual fear is associated with hippocampal and amygdala neural circuitry, whereas tone-associated fear is more closely associated with amygdala pathways (Phillips and LeDoux, 1992). It was previously reported that in 6-month-old 3xTgAD mice, 50% to 75% of neurons in the amygdala exhibit intracellular Aβ and 25% to 50% of CA1 neurons and 20% of CA2/3 neurons exhibit intracellular Aβ (Espana et al., 2010). We found a greater percentage of neurons in the amygdala were Aβ immunoreactive compared with the hippocampus. The KET diet had a large anxiolytic effect on the 3xTgAD mice, and a relatively lesser effect in ameliorating hippocampus-dependent memory deficits. In addition to ameliorating amygdala-dependent anxiety phenotypes in the elevated plus maze and open field tests, we found that 3xTgAD mice fed the KET diet exhibited significantly lower freezing times in tone-associated fear conditioning, demonstrating that amygdala neural circuitry is preserved in association with reduced Aβ and pTau pathology in amygdala neurons.
Hippocampus-dependent learning and memory is impaired in 3xTgAD mice. We found that 3xTgAD mice in the KET and CHO groups performed similarly in the hidden platform version of the water maze, although there was a trend towards shorter goal latencies for mice in the KET group over the 5-day testing period. However, the KET diet improved the performance of the 3xTgAD mice in the reversal learning version of the water maze test after mice in each diet group had learned the previous platform position at the same level of proficiency. The KET diet did not affect performance of the 3xTgAD mice in the visible platform version of the water maze, and swim speed was unaffected by diet, indicating that differences in anxiety levels and motor function did not contribute to the superior performance of 3xTgAD mice in the KET group in the reversal learning task. The reversal trial reveals whether or not animals can extinguish their initial memory of the platform position and learn its new position. It was previously reported that the latter type of cognitive flexibility is impaired in βAPP mutant mice (Tremml et al., 1998) and in an animal model of depression (Bessa et al., 2009), which is relevant to AD in that depression is a risk factor for AD (Aznar and Knudsen, 2011).
Mice in the KET diet group exhibited significantly fewer Aβ- and pTau-positive neurons in the CA1, CA3, and subiculum, consistent with suppression of the AD-like pathogenic molecular cascades as the mechanism by which the ketone ester preserves memory function in 3xTgAD mice.
Reduced cerebral glucose metabolism is widely recognized to precede clinical signs of MCI in AD (Mamelak, 2012). FDG PET studies have shown that the reductions in the metabolic rate for glucose utilization in the parietotemporal area at the MCI stage significantly predict decline to AD with an overall accuracy range of 75% to 100% (Mosconi, 2005). 3xTgAD mice that were 18 months old had significant reductions in FDG uptake in every brain region measured (Nicholson et al., 2010), and Aβ impairs glucose transport and mitochondrial function in neurons (Keller et al., 1997). The decline in the metabolic rate for glucose utilization preceding the clinical signs of cognitive impairment in young adult carriers of the apolipoprotein E ε4 allele has been attributed to decreased mitochondrial function (Valla et al., 2010).
In tissue culture models relevant to AD, impairment of mitochondrial pyruvate dehydrogenase activity (Hoshi et al., 1996) and associated cell death caused by addition of Aβ1-42 could be overcome by addition of 4 mM Na D-β-hydroxybutyrate(Kashiwaya et al., 2000), which provides an alternative source of mitochondrial substrate during impairment of glucose utilization (Sato et al., 1995). In an in vivo study, we showed that levels of malonyl-coenzyme A and mitochondrial uncoupling proteins were reduced in brain cells of rats fed a diet that contained the same ketone ester that was used in the present study (Kashiwaya et al., 2010). It has also been shown that ketone bodies can protect cultured cerebral cortical neurons by a mechanism involving increasing the NAD+/NADH ratio in the neurons (Maalouf et al., 2007). In addition, it was recently reported that dietary supplementation with 2-deoxyglucose increases the level of ketone bodies, enhances mitochondrial function and suppresses Aβ pathology in 3xTgAD mice (Yao et al., 2011). A preliminary study provided evidence that increased blood β-hydroxybutyrate levels through an oral dose of medium chain triglycerides can enhance cognition in memory-impaired human subjects (Reger et al., 2004).
Previous studies have demonstrated that manipulation of the carbohydrate and fat components of the diet can modify cognitive function and underlying processes of neuroplasticity in normal mice and in animal models of AD. Diets high in saturated fat and/or simple sugars such as fructose and glucose impair neuroplasticity and can accelerate the development of Aβ and pTau pathologies in AD mice (Refolo et al., 2000; Cao et al., 2007; Stranahan et al., 2008; Studzinski et al., 2009; Julien et al., 2010). It is therefore possible that the KET diet would be more or less effective if a control diet with a different composition was used. AIN-93 is a highly reproducible and nutrient-defined research diet that allowed us to modify 1 component while keeping other essential nutrients constant between the control and KET diet groups. The AIN-93 diet was introduced as an improved formula to replace the AIN-76A diet (Reeves et al.,1993). One major difference between the latter 2 diets is that the primary carbohydrate source in AIN-76A is sucrose, whereas the primary carbohydrate source in AIN-93 is cornstarch. We found that the AIN-93 diet is permissive for the development of AD pathology and cognitive deficits in 3xTgAD mice, which is consistent with a previous study with a different transgenic AD mouse model (Wang et al., 2009).
The present findings show that long-term feeding of ketone esters not only improved behavioral cognitive function but also decreased Aβ and pTau pathologic changes. The increase in blood ketone bodies, by either a ketogenic diet or by feeding a ketone ester, would be expected to alleviate the impaired brain glucose metabolism that precedes the onset of AD. Ketone bodies can bypass the block in glycolysis resulting from impairment of insulin function (Kashiwaya et al., 1997). Our preclinical findings suggest that a ketone ester-containing diet has the potential to retard the disease process and improve cognitive function of patients with AD.
Supplementary Material
Acknowledgments
The authors thank Andrew Holmes, Elizabeth Gratton, and Shireesh Srivastava for scientific advice and technical assistance. This research was supported by the Intramural Research Programs of the National Institute on Aging and the National Institute on Alcohol Abuse and Alcoholism.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neurobiolaging.2012.11.023.
Footnotes
Disclosure statement
The authors declare no competing financial interests.
References
- Aznar S, Knudsen GM. Depression and Alzheimer’s disease: is stress the initiating factor in a common neuropathological cascade? J Alzheimers Dis. 2011;23:177–193. doi: 10.3233/JAD-2010-100390. [DOI] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Mesquita AR, Oliveira M, Pego JM, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. A trans-dimensional approach to the behavioral aspects of depression. Front Behav Neurosci. 2009;3:1. doi: 10.3389/neuro.08.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer’s disease: characteristics and treatment. Neurol Clin. 2000;18:829–846. doi: 10.1016/s0733-8619(05)70228-0. [DOI] [PubMed] [Google Scholar]
- Conklin HW. Cause and treatment of epilepsy. J Am Osteopath Assoc. 1922;26:11–14. [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana J, Gimenez-Llort L, Valero J, Minano A, Rabano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol Psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, Tekin S, Burns A, Cummings J, del Ser T, Inzitari D, Orgogozo JM, Sauer H, Scheltens P, Scarpini E, Herrmann N, Farlow M, Potkin S, Charles HC, Fox NC, Lane R. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6:501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Takashima A, Noguchi K, Murayama M, Sato M, Kondo S, Saitoh Y, Ishiguro K, Hoshino T, Imahori K. Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase I/glycogen synthase kinase 3beta in brain. Proc Natl Acad Sci U S A. 1996;93:2719–2723. doi: 10.1073/pnas.93.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, King MT, Veech RL. Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Am J Cardiol. 1997;80:50A–64A. doi: 10.1016/s0002-9149(97)00458-x. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Pawlosky R, Markis W, King MT, Bergman C, Srivastava S, Murray A, Clarke K, Veech RL. A ketone ester diet increases brain malonyl-CoA and uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar rat. J Biol Chem. 2010;285:25950–25956. doi: 10.1074/jbc.M110.138198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Pitta M, Lee JH, Ray B, Lahiri DK, Furukawa K, Mughal M, Jiang H, Villarreal J, Cutler RG, Greig NH, Mattson MP. The KATP channel activator diazoxide ameliorates amyloid-beta and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;22:443–457. doi: 10.3233/JAD-2010-101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamelak M. Sporadic Alzheimer’s disease: the starving brain. J Alzheimers Dis. 2012;31:459–474. doi: 10.3233/JAD-2012-120370. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nuclear Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Nelson RL, Guo Z, Halagappa VM, Pearson M, Gray AJ, Matsuoka Y, Brown M, Martin B, Iyun T, Maudsley S, Clark RF, Mattson MP. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007;205:166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RM, Kusne Y, Nowak LA, LaFerla FM, Reiman EM, Valla J. Regional cerebral glucose uptake in the 3xTG model of Alzheimer’s disease highlights common regional vulnerability across AD mouse models. Brain Res. 2010;1347:179–185. doi: 10.1016/j.brainres.2010.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept) J Neurosci. 2011;31:3500–3507. doi: 10.1523/JNEUROSCI.5242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, Mattson MP. 3xTgAD mice exhibit altered behavior and elevated Abeta after chronic mild social stress. Neurobiol Aging. 2012;33:830 e1–12. doi: 10.1016/j.neurobiolaging.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- Sterniczuk R, Antle MC, Laferla FM, Dyck RH. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 2. Behavioral and cognitive changes. Brain Res. 2010;1348:149–155. doi: 10.1016/j.brainres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski CM, Li F, Bruce-Keller AJ, Fernandez-Kim SO, Zhang L, Weidner AM, Markesbery WR, Murphy MP, Keller JN. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP x PS1 knock-in mice. J Neurochem. 2009;108:860–866. doi: 10.1111/j.1471-4159.2008.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Tremml P, Lipp HP, Muller U, Ricceri L, Wolfer DP. Neurobehavioral development, adult openfield exploration and swimming navigation learning in mice with a modified beta-amyloid precursor protein gene. Behav Brain Res. 1998;95:65–76. doi: 10.1016/s0166-4328(97)00211-8. [DOI] [PubMed] [Google Scholar]
- Valla J, Yaari R, Wolf AB, Kusne Y, Beach TG, Roher AE, Corneveaux JJ, Huentelman MJ, Caselli RJ, Reiman EM. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab (Lond) 2005;2:28–35. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Thomas P, Zhong JH, Bi FF, Kosaraju S, Pollard A, Fenech M, Zhou XF. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotoxicity Res. 2009;15:3–14. doi: 10.1007/s12640-009-9000-x. [DOI] [PubMed] [Google Scholar]
- Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 1921;2:307–308. [Google Scholar]
- Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, Mayorga AJ, Wang D, Brashear HR, Nye JS. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Chen S, Mao Z, Cadenas E, Brinton RD. 2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer’s disease. PloS One. 2011;6:e21788. doi: 10.1371/journal.pone.0021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.