Abstract
Paracoccidioides brasiliensis and P. lutzii are thermodimorphic species that cause paracoccidioidomycosis. The cell wall is the outermost fungal organelle to form an interface with the host. A number of host effector compounds, including immunologically active molecules, circulate in the plasma. In the present work we extracted cell wall-associated proteins from the yeast pathogenic phase of P. brasiliensis, isolate Pb3, grown in the presence of human plasma, and analyzed bound plasma proteins by liquid chromatography-tandem mass spectrometry. Transport, complement activation/regulation and coagulation pathway were the most abundant functional groups identified. Proteins related to iron/copper acquisition, immunoglobulins, and protease inhibitors were also detected. Several human plasma proteins described here have not been previously reported as interacting with fungal components, specifically, clusterin, hemopexin, transthyretin, ceruloplasmin, alpha-1-antitrypsin, apolipoprotein A-I, and apolipoprotein B-100. Additionally, we observed increased phagocytosis by J774.16 macrophages of Pb3 grown in plasma, suggesting that plasma proteins interacting with P. brasiliensis cell wall might be interfering in the fungal relationship with the host.
Keywords: Paracoccidioides brasiliensis, cell wall, human plasma proteins
Introduction
Paracoccidioides brasiliensis and P. lutzii are thermodimorphic species responsible for paracoccidioidomycosis (PCM), a prevalent systemic granulomatous mycosis in Latin America. The active disease occurs in 1 to 2% of infected individuals, whose number is estimated in 10 million throughout endemic areas (San-Blas, et al., 2002). Once in the pulmonary alveolar epithelium, inhaled infectious particles can establish infection as long as they transform into the pathogenic yeast form.
The cell wall is the outermost fungal structure in contact with the host and its dynamic structure can rapidly change to adapt to the environment (Kapteyn, et al., 2000). The yeast phase of Paracoccidioides cell wall is composed mainly of α-1,3-glucan and chitin, with a small proportion of β-1,3-glucan and galactomannan (Kanetsuna, et al., 1972). Typical covalently linked structural proteins have not yet been described in Paracoccidioides; however, numerous non-covalently linked proteins have been shown in this compartment (Puccia, et al., 2011).
Human plasma is composed by a large number of proteins, including both typical plasma proteins, such as albumin and lipoproteins, and tissue molecules that can be used in diagnosis and therapeutic monitoring (Anderson & Anderson, 2002). Although 1,175 proteins have been described in human plasma (reviewed in (Anderson, et al., 2004)), 95% of protein abundance is represented by only ten (Putnam, 1984, Pieper, et al., 2003): albumin (54%), immunoglobulin G (17%), alpha-1-antitrypsin (3.8%), alpha-2-macroglobulin (3.6%), immunoglobulin A (3.5%), transferrin (3.3%), haptoglobin (3%), apolipoprotein A-1 (3%), immunoglobulin M (2%) and alpha-1-acid-glycoprotein (1.3%).
Many plasma compounds, such as complement components and immunoglobulins, are immunologically active molecules and compose major defense lines of the host against invading microbes (Zipfel, et al., 2007). Therefore, a better knowledge of the interactions between fungal cell wall and host plasma proteins may help us to understand infection development and host defense (Cottier & Pavelka, 2012).
The aim of the present work was to identify human plasma proteins that interact with P. brasiliensis yeast cell wall, since they might interfere in the host-pathogen relationship. For this we employed liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based proteomic analysis to identify proteins extracted with hot sodium dodecyl sulfate (SDS) from Pb3 cell wall, carefully isolated from yeasts cultivated in plasma-containing defined medium. We chose Pb3 as model because it represents P. brasiliensis cryptic species PS2, whose members are less virulent in B10.A mice (Carvalho, et al., 2005). In this model, Pb3 evokes a predominant Th1-type protective immune response enriched in IgG2a, IgG2b, and IgG3 and high amounts of INF-γ (unpublished data).
1. Materials and methods
1.1. P. brasiliensis isolate and growth conditions
P. brasiliensis isolate Pb3 was maintained in the yeast phase at 36°C in solid modified YPD medium (0.5% yeast extract, 0.5% casein peptone, 1.5% glucose, pH 6.5). For cell wall isolation, yeast cells were cultivated in defined Ham’s F12 medium (Invitrogen) added of 1.5% glucose (F12/Glc) and supplemented or not with 2% heat-inactivated (56°C, 30 min) human plasma, obtained from healthy donors of Hospital São Paulo (UNIFESP Ethics Committee, approval protocol number 0366/07). Although we started with 2% plasma, we observed protein precipitation, which was discarded by centrifugation (6,000xg, 30 min, 4°C). Cells were transferred from 7-day-old slants into F12/Glc (200 mL) and cultivated at 36°C for 4 days (pre-inoculum). Yeast cells from four pre-inoculums were transferred to 500 mL of fresh medium and cultivated for 2 days for cell wall purification. Yeast cells were analyzed for viability (>95%) with Trypan blue.
1.2. Cell wall purification
Yeast cells cultivated in the presence (Pb3pl) or absence (Pb3) of heat-inactivated human plasma were harvested by centrifugation, washed three times with phosphate saline buffer (PBS) and mechanically disrupted with glass beads (425-600 µm, Sigma Aldrich) in B. Braun (6 times for 10 min, alternating with 10 min in ice) in the presence of PBS with protease inhibitors (100 mM ethylenediamine tetraacetic acid, EDTA, 10 mM 1,10-phenanthroline, 1 mM phenylmethylsulfonyl fluoride, PMSF, 1 µM pepstatin A and 15 µM trans-epoxysuccinyl-L-leucylamido(4-guanidino)butane, E-64). Cell wall was isolated from cytoplasmic contents and membranous structures by three sequential centrifugations (8,000×g for 45 min at 25°C) in 85% sucrose (Kanetsuna, et al., 1969). Non-specifically bound components were eliminated by five sequential washes with each of the ice-cold solutions: deionized water, 5% NaCl, 2% NaCl, 1% NaCl, and 1 mM PMSF (Pitarch, et al., 2002); final cell wall preparation was lyophilized.
1.3. SDS-extraction of cell surface-associated proteins
Isolated cell wall (100 mg) was extracted twice by boiling with SDS for 5 min in extraction buffer (100 mM EDTA, 50 mM Tris-HCl pH 7.8, 2% SDS). The SDS extracts were centrifuged, filtrated through a sterile 0.22-micron filter, and precipitated in ice-cold acetone (1 h at -20°C). After a 30-min centrifugation (16,000×g at 4°C), the protein pellet was removed, washed in acetone, and dried at room temperature.
1.4. Proteomic analysis
Protein digestion was carried out using the ammonium bicarbonate/methanol method (Russell, et al., 2001). Tryptic peptides were desalted in POROS R2 microcolumns (Jurado, et al., 2007) and dried in an Eppendorf vacuum centrifuge concentrator. Peptides were then dissolved in 0.1% formic acid (FA), loaded onto a reversed-phase trap column (1 cm×75 µm, Luna C18, 5 µm, Phenomenex), and separated in a capillary column (20 cm×75 µm, Luna C18, 5 µm, Phenomenex) coupled to a nanoHPLC (1D Plus, Eksigent). Peptides were eluted in a linear gradient from 8.75% to 35% acetonitrile in 0.1% FA over 200 min and directly analyzed in an electrospray-linear ion trap-mass spectrometer (LTQ XL/ETD, Thermo Fisher Scientific) equipped with a TriVersa NanoMate nanospray source (Advion). The nanospray was set at 1.45 kV and 0.25 psi N2 pressure using a chip A (Advion). MS spectra were collected in centroid mode at the 400-1700 m/z range and the ten most intense ions were subjected twice to collision-induced dissociation with 35% normalized collision energy, before being dynamically excluded for 60s.
MS/MS spectra from peptides with 800 to 3,500 Da, more than 10 counts, and at least 15 fragments were converted into DTA files using Bioworks v.3.3.1 (Thermo Fisher) and searched against human (IPI v), porcine trypsin (GenBank) and Paracoccidioides (http://www.broadinstitute.org/annotation/genome/dimorph_collab.1/MultiHome) sequences, in both correct and reverse orientations, using TurboSequest (Bioworks 3.3.1, Thermo Fisher Scientific). The database search parameters included: i) trypsin cleavage in both peptide termini with one missed cleavage site allowed; ii) carbamidomethylation of cysteine residues as a fixed modification; iii) oxidation of methionine residues as a variable modification; and iv) 2.0 Da and 1.0 Da for peptide and fragment mass tolerance, respectively. TurboSequest outputs were filtered with DCn ≥ 0.05, peptide probability ≤ 0.05, and Xcorr ≥ 1.5, 2.0, and 2.5 for singly-, doubly-, and triply charged peptides, respectively. After filtering, the files were exported into XML formats and the peptide sequences were assembled into proteins using an in-house written script (Nakayasu, et al., 2012). The protein hits were refiltered with the sum of peptide Xcorr ≥ 3.5. The false-discovery rate (FDR) was estimated as described previously (Rodrigues, et al., 2008). Only proteins detected by at least two peptides exclusively in the Pb3pl cell wall were considered.
Functions and processes in which identified proteins are involved were analyzed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 (http://david.abcc.ncifcrf.gov) (Dennis, et al., 2003), and Blast2GO (http://www.blast2go.org/) (Conesa, et al., 2005) for Gene Ontology (GO). The exponentially modified protein abundance index (emPAI) (Ishihama, et al., 2005) was used for protein abundance comparison considering the protein molecular masses.
1.5. In vitro phagocytosis assay
Phagocytosis assays were carried out with macrophage cell lineage J774.16 cultured in DMEM/10% inactivated FBS. 2x105 cells were activated with 50 U/ml IFN-γ (PeproTech, Rock Hill, NJ) at 37°C overnight and incubated with P. brasiliensis yeasts at a ratio of 5:1 macrophages:fungi for 6 h at 37°C. Yeasts were cultivated in plasma-containing F12 medium. When grown in F12 alone, they were incubated with plasma (37°C, 1 h) before the assay. Fresh and heat-inactivated plasma (56°C, 1 h) were used. Three washes with 0.15 M α-methyl-mannopyranoside were performed to remove non-internalized yeasts bound via mannose receptor. Cells were fixed with methanol, stained with Giemsa (1:2 for 30 min) and phagocytosed yeasts were counted under light microscopy. Phagocytic index (PI) was defined as infected macrophages/counted macrophages and pairwise comparison between groups was done by the Student t-test.
2. Results and discussion
In order to identify human plasma proteins that interact with P. brasiliensis yeast surface, carefully isolated cell wall preparations were exhaustedly washed with salt to remove non-specifically bound proteins. Non-covalently interacting plasma proteins were extracted with hot SDS, and tryptic peptides were analyzed by LC-MS/MS (for raw data, see Supplemental Files). We identified 52 plasma proteins with two or more peptides present only in Pb3pl cell wall, annotated them into functional categories, and quantified them by relative emPAI (mass%) (Table 1). We chose the emPAI method for protein quantification since it provides an absolute abundance value that enabled us to compare our data with the literature. Proteins categorized as transport, complement activation/regulation and coagulation pathways were the most abundant. Proteins related to lipid metabolism, immune response, acute-phase response, and homeostasis were identified at lower relative amounts.
Table 1.
Plasma proteins detected by LC-MS/MS in P. brasiliensis (Pb3)-derived cell wall. Distribution into functional groups was performed according to Gene Ontology classification. Protein relative abundance in the sample (relative emPAI mass%) and mass percentage in plasma (Pieper, et al., 2003) are shown.
| Protein Code | Cellular Process | emPAI mass% cell wall |
Plasma mass% (as in Pieper, et al., 2003) |
|---|---|---|---|
| Complement activation / regulation | 38.6 | ||
| IPI00783987 | Complement C3 | 10 | |
| IPI00887739 | Similar to complement C3 | 7.4 | |
| IPI00739237 | Complement C3 | 9.3 | |
| IPI00478003 | Alpha-2-macroglobulin | 6.9 | 3.6 |
| IPI00887154 | Complement component 4B | 1.4 | |
| IPI00291262 | Clusterin | 1.3 | |
| IPI00921523 | Complement factor B | 1.1 | |
| IPI00021727 | C4b-binding protein alpha chain | 0.8 | |
| IPI00029739 | Complement factor H | 0.4 | |
| Transport | 19.3 | ||
| IPI00384697 | Serum albumin | 7.0 | 54 |
| IPI00022434 | Serum albumin | 6.0 | |
| IPI00022488 | Hemopexin | 2.6 | 1.1 |
| IPI00878282 | Serum albumin | 1 | |
| IPI00940791 | Transthyretin | 0.7 | 0.3 |
| IPI00017601 | Ceruloplasmin | 2.1 | |
| Coagulation pathway | 14.7 | ||
| IPI00790784 | Alpha-1-antitrypsin | 2.3 | 3.8 |
| IPI00032179 | Antithrombin-III | 1.4 | 0.3 |
| IPI00298971 | Vitronectin | 1.4 | |
| IPI00877703 | Fibrinogen gama chain | 1.1 | |
| IPI00298497 | Fibrinogen beta chain | 1.1 | |
| IPI00019568 | Prothrombin | 1.1 | |
| IPI00022418 | Fibronectin splice variant E | 1 | |
| IPI00339226 | Fibronectin | 3.4 | |
| IPI00022371 | Histidine-rich glycoprotein | 0.7 | |
| IPI00029717 | Fibrinogen alpha chain | 1 | |
| IPI00019580 | Plasminogen | 0.4 | |
| Immunoglobulins (immune response) | 9.7 | 22.5 | |
| IPI00852577 | Ig lambda-1 chain C regions | 0.7 | |
| IPI00154742 | Ig lambda-2 chain C regions | 0.6 | |
| IPI00386879 | Immunoglobulin heavy constant alpha 1 | 2.3 | |
| IPI00827560 | HRV Fab N28-VL | 0.5 | |
| IPI00896380 | Ig mu chain C region | 1.8 | |
| IPI00739205 | Ig heavy chain V-I region HG3 | 0.5 | |
| IPI00384407 | Myosin-reactive Ig heavy chain variable region | 0.4 | |
| IPI00384409 | Myosin-reactive Ig heavy chain variable region | 0.4 | |
| IPI00784950 | Immunoglobulin heavy constant alpha 2 | 1.1 | |
| IPI00785067 | Immunoglobulin heavy constant alpha 2 | 1.1 | |
| IPI00470652 | Single-chain Fv | 0.5 | |
| Lipid metabolism | 9.3 | ||
| IPI00021841 | Apolipoprotein A-I | 1.4 | 3 |
| IPI00847635 | Alpha-1-antichymotrypsin | 0.6 | 0.6 |
| IPI00022229 | Apolipoprotein B-100 | 6.8 | |
| IPI00218732 | Serum paraoxonase/arylesterase 1 | 0.5 | |
| Others/Unknown | 4.6 | ||
| IPI00796830 | UNKNOWN | 0.6 | |
| IPI00646384 | UNKNOWN | 0.5 | |
| IPI00940494 | Uncharacterized protein | 0.5 | |
| IPI00022895 | Alpha-1B-glycoprotein | 1.3 | |
| IPI00879931 | Serpin peptidase inhibitor | 0.9 | |
| IPI00292530 | Inter-alpha-trypsin inhibitor heavy chain H1 | 0.7 | |
| IPI00935352 | Uncharacterized protein | 0.2 | |
| Acute-phase response | 2 | ||
| IPI00218192 | Inter-alpha-trypsin inhibitor heavy chain H4 | 1.5 | |
| IPI00022431 | Alpha-2-HS-glycoprotein | 0.5 | 0.8 |
| Homeostasis | 1.7 | ||
| IPI00032220 | Angiotensinogen | 1.3 | |
| IPI00477597 | Haptoglobin-related protein | 0.4 | 3 |
| Protein Code | Cellular Process | emPAI | Mr (Da) | emPAI*Mr | emPAI mass% | emPAI mol% | Plasma mass% (as in [21]) | |
|---|---|---|---|---|---|---|---|---|
| Immunoglobulins (immune response) | 9.72 | 25.48 | 22.50 | |||||
| IPI00852577 | Ig lambda-1 chain C regions | P0CG04 | 0.70125428 | 11.35 | 7.96 | 0.67 | 3.92 | |
| IPI00154742 | Ig lambda-2 chain C regions | P0CG05 | 0.649648074 | 11.29 | 7.34 | 0.62 | 3.63 | |
| IPI00386879 | immunoglobulin heavy constant alpha 1 | Q96K68 | 0.505836354 | 53.09 | 26.85 | 2.27 | 2.83 | |
| IPI00827560 | HRV Fab N28-VL | A2IPI3 | 0.467799268 | 12.28 | 5.75 | 0.49 | 2.61 | |
| IPI00896380 | Ig mu chain C region | P01871 | 0.435035831 | 49.31 | 21.45 | 1.81 | 2.43 | |
| IPI00739205 | Ig heavy chain V-I region HG3 | P01743 | 0.42510267 | 12.95 | 5.50 | 0.47 | 2.37 | |
| IPI00384407 | Myosin-reactive immunoglobulin heavy chain variable region | Q9UL92 | 0.333521432 | 13.58 | 4.53 | 0.38 | 1.86 | |
| IPI00384409 | Myosin-reactive immunoglobulin heavy chain variable region |
Q9UL94 Q9UL92 |
0.333521432 | 13.21 | 4.40 | 0.37 | 1.86 | |
| IPI00784950 | immunoglobulin heavy constant alpha 2 |
Q6MZV6 Q9UL92 |
0.251875026 | 51.64 | 13.01 | 1.10 | 1.41 | |
| IPI00785067 | immunoglobulin heavy constant alpha 2 | Q6P089 | 0.245197085 | 52.00 | 12.75 | 1.08 | 1.37 | |
| IPI00470652 | Single-chain Fv | Q65ZC8 | 0.211527659 | 26.13 | 5.53 | 0.47 | 1.18 | |
| Transport | Q9UL92 | 19.34 | 22.02 | |||||
| IPI00384697 | Serum albumin | P02768 | 1.187761624 | 69.37 | 82.39 | 6.96 | 6.64 | 54 |
| IPI00022434 | Serum albumin | Q56G89 | 1.020949938 | 69.08 | 70.53 | 5.96 | 5.70 | |
| IPI00022488 | Hemopexin | P02790 | 0.59985872 | 51.68 | 31.00 | 2.62 | 3.35 | 1.1 |
| IPI00878282 | Serum albumin | Q9UL92 | 0.519911083 | 22.86 | 11.88 | 1.00 | 2.90 | |
| IPI00940791 | Transthyretin | E7EW61 | 0.412537545 | 20.29 | 8.37 | 0.71 | 2.30 | 0.3 |
| IPI00017601 | Ceruloplasmin | P00450 | 0.201284899 | 122.21 | 24.60 | 2.08 | 1.12 | |
| Complement activation / regulation | Q9UL92 | 38.63 | 18.42 | |||||
| IPI00783987 | Complement C3 | P01024 | 0.632172129 | 187.15 | 118.31 | 10.00 | 3.53 | |
| IPI00887739 | Similar to complement C3 | Q9UL92 | 0.605016318 | 144.81 | 87.61 | 7.40 | 3.38 | |
| IPI00739237 | Complement C3 | Q9UL92 | 0.584893192 | 187.15 | 109.46 | 9.25 | 3.27 | |
| IPI00478003 | Alpha-2-macroglobulin | P01023 | 0.502551161 | 163.29 | 82.06 | 6.94 | 2.81 | 3.6 |
| IPI00887154 | Complement component 4B | Q6U2L1 | 0.342078063 | 47.45 | 16.23 | 1.37 | 1.91 | |
| IPI00291262 | Clusterin | P10909 | 0.291549665 | 52.50 | 15.30 | 1.29 | 1.63 | |
| IPI00921523 | Complement factor B |
P00751 Q9UL92 |
0.154781985 | 85.53 | 13.24 | 1.12 | 0.86 | |
| IPI00021727 | C4b-binding protein alpha chain |
P04003 Q9UL92 |
0.149756995 | 67.03 | 10.04 | 0.85 | 0.84 | |
| IPI00029739 | Complement factor H |
P08603 Q9UL92 |
0.034700871 | 139.10 | 4.83 | 0.41 | 0.19 | |
| Coagulation pathway | Q9UL92 | 14.74 | 13.87 | |||||
| IPI00790784 | Alpha-1-antitrypsin |
P01009 Q9UL92 |
0.584893192 | 46.74 | 27.34 | 2.31 | 3.27 | 3.8 |
| IPI00032179 | Antithrombin-III | P01008 | 0.304321387 | 52.60 | 16.01 | 1.35 | 1.70 | 0.3 |
| IPI00298971 | Vitronectin | P04004 | 0.299081397 | 54.31 | 16.24 | 1.37 | 1.67 | |
| IPI00877703 | Fibrinogen gama chain | C9JC84 | 0.24782547 | 52.34 | 12.97 | 1.10 | 1.38 | |
| IPI00298497 | Fibrinogen beta chain | P02675 | 0.232846739 | 55.93 | 13.02 | 1.10 | 1.30 | |
| IPI00019568 | Prothrombin | P00734 | 0.184484581 | 70.04 | 12.92 | 1.09 | 1.03 | |
| IPI00022418 | Fibronectin splice variant E | A6YID6 | 0.163561851 | 70.22 | 11.48 | 0.97 | 0.91 | |
| IPI00339226 | Fibronectin | P02751 | 0.151650644 | 262.63 | 39.83 | 3.37 | 0.85 | |
| IPI00022371 | Histidine-rich glycoprotein | P04196 | 0.142068906 | 59.58 | 8.46 | 0.72 | 0.79 | |
| IPI00029717 | Fibrinogen alpha chain | P02671 | 0.119902793 | 94.97 | 11.39 | 0.96 | 0.67 | |
| IPI00019580 | Plasminogen | P00747 | 0.051908639 | 90.57 | 4.70 | 0.40 | 0.29 | |
| Lipid metabolism | 9.28 | 5.49 | ||||||
| IPI00021841 | Apolipoprotein A-I | P02647 | 0.528306733 | 30.78 | 16.26 | 1.37 | 2.95 | 3 |
| IPI00847635 | Alpha-1-antichymotrypsin | P01011 | 0.158323286 | 47.65 | 7.54 | 0.64 | 0.88 | 0.6 |
| IPI00022229 | Apolipoprotein B-100 | P04114 | 0.156051281 | 515.61 | 80.46 | 6.80 | 0.87 | |
| IPI00218732 | Serum paraoxonase/arylesterase 1 | P27169 | 0.140624924 | 39.73 | 5.59 | 0.47 | 0.79 | |
| Acute-phase response | 1.97 | 1.62 | ||||||
| IPI00218192 | Inter-alpha-trypsin inhibitor heavy chain H4 | Q14624 | 0.171190257 | 103.36 | 17.69 | 1.50 | 0.96 | |
| IPI00022431 | Alpha-2-HS-glycoprotein | F5H0Q5 | 0.118872212 | 46.60 | 5.54 | 0.47 | 0.66 | 0.8 |
| Homeostasis | 1.71 | 2.31 | ||||||
| IPI00032220 | Angiotensinogen | P01019 | 0.291549665 | 53.15 | 15.50 | 1.31 | 1.63 | |
| IPI00477597 | Haptoglobin-related protein | P00739 | 0.122018454 | 39.03 | 4.76 | 0.40 | 0.68 | 3 |
| Others/Unknown | 4.61 | 10.81 | ||||||
| IPI00796830 | UNKNOWN | 0.519911083 | 12.993 | 6.76 | 0.57 | 2.90 | ||
| IPI00646384 | UNKNOWN | 0.42510267 | 13.16 | 5.59 | 0.47 | 2.37 | ||
| IPI00940494 | Uncharacterized protein | F5GXM8 | 0.389495494 | 14.08 | 5.48 | 0.46 | 2.18 | |
| IPI00022895 | Alpha-1B-glycoprotein | P04217 | 0.291549665 | 54.25 | 15.82 | 1.34 | 1.63 | |
| IPI00879931 | Serpin peptidase inhibitor | E9PGN7 | 0.182298865 | 59.49 | 10.85 | 0.92 | 1.02 | |
| IPI00292530 | Inter-alpha-trypsin inhibitor heavy chain H1 | P19827 | 0.079775162 | 101.39 | 8.09 | 0.68 | 0.45 | |
| IPI00935352 | Uncharacterized protein | F8W967 | 0.047615753 | 41.90 | 2.00 | 0.17 | 0.27 | |
| Not identified | 1183.21 | |||||||
| transferrin | 3.3 | |||||||
| alpha-1-acid glycoprotein | 1.3 | |||||||
| Protein Code | Cellular Process | emPAI mass% | Plasma mass% (as in [21]) | cw/pl | pl/cw |
|---|---|---|---|---|---|
| Immunoglobulins (immune response) | 9.72 | 22.5 | 0.432 | 2.314815 | |
| IPI00852577 | Ig lambda-1 chain C regions | 0.672563076 | |||
| IPI00154742 | Ig lambda-2 chain C regions | 0.620103392 | |||
| IPI00386879 | immunoglobulin heavy constant alpha 1 | 2.269575171 | |||
| IPI00827560 | HRV Fab N28-VL | 0.485626254 | |||
| IPI00896380 | Ig mu chain C region | 1.81289135 | |||
| IPI00739205 | Ig heavy chain V-I region HG3 | 0.465122774 | |||
| IPI00384407 | Myosin-reactive immunoglobulin heavy chain variable region | 0.382790971 | |||
| IPI00384409 | Myosin-reactive immunoglobulin heavy chain variable region | 0.372220528 | |||
| IPI00784950 | immunoglobulin heavy constant alpha 2 | 1.099261708 | |||
| IPI00785067 | immunoglobulin heavy constant alpha 2 | 1.077556648 | |||
| IPI00470652 | Single-chain Fv | 0.467083877 | |||
| Transport | 19.34 | ||||
| IPI00384697 | Serum albumin | 6.963384401 | |||
| IPI00022434 | Serum albumin | 5.961013305 | |||
| IPI00878282 | Serum albumin | 1.004441092 | |||
| 3 serum albumin | 13.9288388 | 54 | 0.257941 | 3.876849 | |
| IPI00022488 | Hemopexin | 2.619847634 | 1.1 | 2.38168 | 0.419872 |
| IPI00940791 | Transthyretin | 0.707569825 | 0.3 | 2.358566 | 0.423986 |
| IPI00017601 | Ceruloplasmin | 2.078922683 | |||
| Complement activation / regulation | 38.63 | ||||
| IPI00783987 | Complement C3 | 9.999049156 | |||
| IPI00887739 | Similar to complement C3 | 7.404535366 | |||
| IPI00739237 | Complement C3 | 9.251239525 | |||
| IPI00478003 | Alpha-2-macroglobulin | 6.935546654 | 3.6 | 1.926541 | 0.519065 |
| IPI00887154 | Complement component 4B | 1.371943478 | |||
| IPI00291262 | Clusterin | 1.293506619 | |||
| IPI00921523 | Complement factor B | 1.118902604 | |||
| IPI00021727 | C4b-binding protein alpha chain | 0.848425949 | |||
| IPI00029739 | Complement factor H | 0.407937086 | |||
| Coagulation pathway | 14.74 | ||||
| IPI00790784 | Alpha-1-antitrypsin | 2.310338244 | 3.8 | 0.607984 | 1.644781 |
| IPI00032179 | Antithrombin-III | 1.352922438 | 0.3 | 4.509741 | 0.221742 |
| IPI00298971 | Vitronectin | 1.372699212 | |||
| IPI00877703 | Fibrinogen gama chain | 1.096228858 | |||
| IPI00298497 | Fibrinogen beta chain | 1.100620553 | |||
| Fibrinogen alpha chain | 0.962426618 | ||||
| 3.159276029 | |||||
| IPI00019568 | Prothrombin | 1.092007895 | |||
| IPI00022418 | Fibronectin splice variant E | 0.97063572 | |||
| IPI00339226 | Fibronectin | 3.366033953 | |||
| IPI00022371 | Histidine-rich glycoprotein | 0.715357485 | |||
| IPI00029717 | Fibrinogen alpha chain | 0.962426618 | |||
| IPI00019580 | Plasminogen | 0.397335516 | |||
| Lipid metabolism | 9.28 | ||||
| IPI00021841 | Apolipoprotein A-I | 1.37424672 | 3 | 0.458082 | 2.183014 |
| IPI00847635 | Alpha-1-antichymotrypsin | 0.6376098 | 0.6 | 1.062683 | 0.941014 |
| IPI00022229 | Apolipoprotein B-100 | 6.800214728 | |||
| IPI00218732 | Serum paraoxonase/arylesterase 1 | 0.47220433 | |||
| Acute-phase response | 1.97 | ||||
| IPI00218192 | Inter-alpha-trypsin inhibitor heavy chain H4 | 1.495399077 | |||
| IPI00022431 | Alpha-2-HS-glycoprotein | 0.46818096 | 0.8 | 0.585226 | 1.708741 |
| Homeostasis | 1.71 | ||||
| IPI00032220 | Angiotensinogen | 1.309744753 | |||
| IPI00477597 | Haptoglobin-related protein | 0.402496621 | 3 | 0.134166 | 7.453479 |
| Others/Unknown | 4.61 | ||||
| IPI00796830 | UNKNOWN | 0.570921874 | |||
| IPI00646384 | UNKNOWN | 0.472631708 | |||
| IPI00940494 | Uncharacterized protein | 0.46342725 | |||
| IPI00022895 | Alpha-1B-glycoprotein | 1.336849378 | |||
| IPI00879931 | Serpin peptidase inhibitor | 0.916601794 | |||
| IPI00292530 | Inter-alpha-trypsin inhibitor heavy chain H1 | 0.68359158 | |||
| IPI00935352 | Uncharacterized protein | 0.168609529 | |||
| Not identified | |||||
| transferrin | 3.3 | 0 | 3.3 | ||
| alpha-1-acid glycoprotein | 1.3 | 0 | 1.3 |
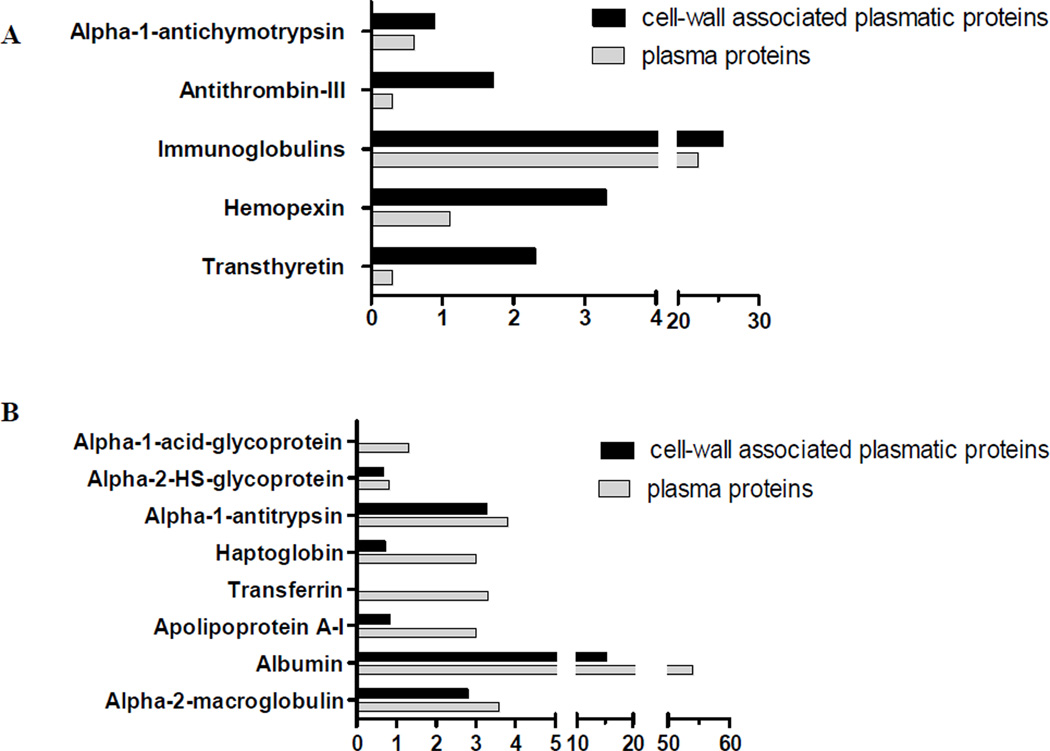
We also correlated the relative emPAI of cell wall-associated plasma proteins with their relative mass percentages in plasma (Pieper, et al., 2003), as shown in Table 1 and Fig. 1. Note that proteins of the coagulation pathway (antithrombin-III), transport (hemopexin and transthyretin), and complement activation/regulation (alpha-2-macroglobulin) were abundantly enriched in the fungal cell wall. Of them, only the latter is among the most abundant in plasma, representing 3.6% of total plasma proteins mass (Pieper, et al., 2003) versus 6.9% of cell wall-bound proteins (Fig. 1A).
Fig. 1.
Relative abundance (relative emPAI mass%) of plasma proteins presently identified in P. brasiliensis (Pb3) isolated cell wall. Their percentage relative to total plasma proteins (Pieper, et al., 2003) is shown in parallel. The figures show proteins relatively more abundant in the cell wall (A) or in plasma (B).
Albumin, which is the most abundant plasma protein (54%), was responsible for only 13.9% of cell wall-associated protein mass (Table 1; Fig. 1B). Alpha-1-acid and alpha-2-HS glycoproteins, haptoglobin, transferrin, apolipoprotein A-1, alpha-1-antitrypsin, and immunoglobulins were also relatively more abundant in plasma (Fig. 1B). Together, these observations suggest that plasma proteins have not randomly bound to the cell wall and that our analysis generally identified specifically bound proteins.
The presence of albumin interacting with cell wall components is speculative, and unspecific binding cannot be disregarded in this particular case. However, it has already been shown that Candida albicans Ala1/Ala5 adhesin is able to bind to BSA-coated beads, probably because of free threonine, serine, or alanine patches (Gaur, et al., 2002). Although an Ala1/Ala5 adhesin ortholog has not been found in Paracoccidioides genome, there could be other(s) albumin-binding protein(s) not yet described. In Paracoccidioides, many proteins colocalize to the surface and bind to extracellular matrix-associated proteins (reviewed in (Puccia, et al., 2011)), but none has apparently been tested to bind to BSA.
Many immunoglobulin chains were found on the cell wall; however, they were twice more abundant in plasma than among cell wall-associated plasma proteins (Fig. 1B). That is not surprising, considering that only a small amount of the total immunoglobulin repertoire would be able to recognize fungal surface antigens, leading to opsonization and activation of both the classical complement pathway and phagocytosis (Ehrnthaller, et al., 2011).
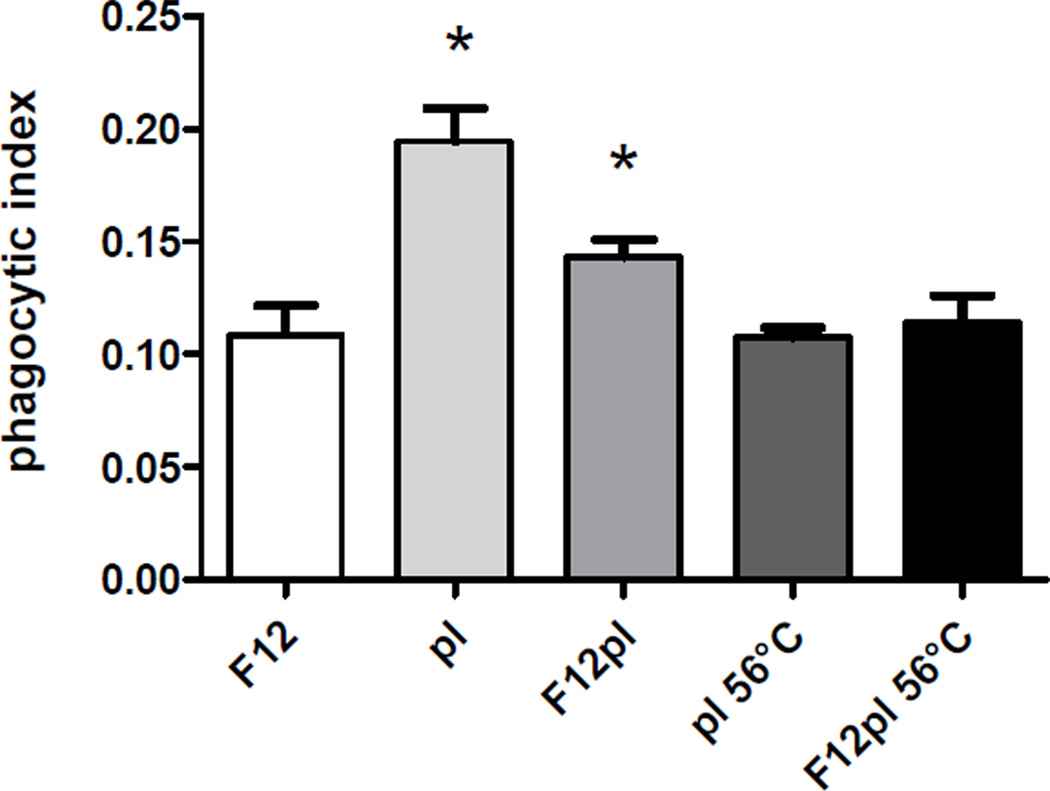
Complement activation/regulation components, such as C3, C4b-binding protein alpha chain (C4BP), factors B and H were responsible for 38.6% of the cell wall-bound plasma protein mass. That corroborates with previously reported immunofluorescence data showing that C3, C3a, C3d, C3g, C4, C5b-9, and factors H and B are present on the P. brasiliensis yeast cell surface (Munk & Da Silva, 1992). The results in Fig. 2 showed that Pb3 cultivated in plasma-containing medium was 31% more internalized by J774.16 macrophages than Pb3 grown in the absence of plasma, while incubation in pure plasma caused a 78% increase in phagocytosis, corroborating previous data about the effect of serum in phagocytosis of a distinct isolate [32]. The effect was probably related to complement binding, considering that controls with inactivated plasma (both to grow and to assay the yeasts) were similar to a negative control with medium alone.
Fig. 2.
Phagocytic index for Pb3 yeast cells after 6 h of incubation with J774.16 macrophages. The assay was carried out with yeasts grown in F12 (control), F12-containing either inactivated (F12pl 56°C) or fresh human plasma (F12pl), and also with yeasts grown in F12, but previously incubated for 1 h at 37°C in heat-inactivated (pl 56°C) or fresh (pl) human plasma. Values are averages of three measurements with standard deviations. *Significant differences (P < 0.05) comparing with F12 control.
In C. albicans, C3b binds directly to the yeast surface or via mannan-specific antibodies (Zhang & Kozel, 1998), opsonizing and mediating recognition by host immune effector cells for phagocytosis (van Lookeren Campagne, et al., 2007). To avoid an excessive response and subsequent self-damage to host tissues, the complement system is tightly regulated by soluble and membrane bound proteins, such as factor-I, factor-H, C4BP, vitronectin and clusterin (Carroll, 2004), presently identified. Complement regulators would help the pathogen to evade the immune system by down regulating complement activation. C4BP is a major plasma inhibitor of the classical and mannose-binding lectin-mediated complement pathways and its alpha-chain is responsible for binding to C. albicans cell wall (Meri, et al., 2004). Some microorganism surface ligands of complement factors have already been elucidated, such as Pra1 and Gpm1 in C. albicans (Zipfel, et al., 2007). In this fungus, interaction with vitronectin increased binding to and phagocytosis by macrophages (Limper & Standing, 1994).
The complement cascade is intimately connected to the blood coagulation system and their activation occurs simultaneously (Markiewski, et al., 2007), thus explaining why we identified members of the coagulation cascade on P. brasiliensis cell wall preparations. In C. albicans, plasminogen bound to surface CaGpm1p was accessible for activation and was converted to active plasmin, which is a key enzyme of intravascular fibrinolysis and acts in the degradation of the host extracellular matrix (Poltermann, et al., 2007). P. brasiliensis Pb3 has two CaGpm1p orthologs: fructose-2,6-biphosphatase (PABG_05093) and conserved hypothetical protein PABG_05096, whose localization and affinity for plasminogen remain unknown. Fibrinogen chains were detected in high abundance (3.1% emPAI mass%) among cell wall-associated plasma proteins. Als3p adhesin in C. albicans binds to fibrinogen (Nobbs, et al., 2010), and although an ortholog in P. brasiliensis has not been found, other protein(s) might have similar functions.
Transport proteins such as hemopexin (discussed below) and transthyretin were more represented in the cell wall than in plasma (Table 1 and Fig. 1A). Transthyretin, involved in thyroxine and retinol transport, had altered expression in plasma during experimental invasive pulmonary aspergillosis (Gonzales, et al., 2010). It presents adhesive properties and binds to many compounds including plant flavonoids (Green, et al., 2005). Possibly, transthyretin may bind to P. brasiliensis cell wall components via disulfide bridges (Ruiz-Herrera, et al., 2006), considering it can form disulfide bonds with a thiol-Sepharose 4B column (Fex, et al., 1977).
Extracellular proteases can play important roles in pathogenic fungal nutrition, tissue invasion, and host immune system evasion (Naglik, et al., 2003). Recently, Maza and coworkers (Maza, et al., 2012) showed that P. brasiliensis extracellular proteases degrade proinflammatory cytokines. Therefore, host protease inhibitors would be an obvious defense mechanism by neutralizing fungal proteases involved in infection. On the cell wall of P. brasiliensis grown in plasma-containing medium we identified plasma proteins with serine protease inhibitor activity, such as alpha-1-antitrypsin, inter-alpha-trypsin inhibitor, alpha-2-macroglobulin, and angiotensinogen (Table 1). P. brasiliensis extracellular thiol-dependent subtilysin-like protease (Carmona, et al., 1995) and a secreted 66-kDa serine protease (Parente, et al., 2010) could possibly be neutralized by the human plasma protease inhibitors during infection. These fungal serine protease activities cleave extracellular matrix-associated proteins in vitro and could play a role in tissue damage and dissemination.
Both iron and copper are key regulators of host-pathogen interactions (Doherty, 2007, Kim, et al., 2008). We presently identified hemopexin and ceruloplasmin bound to P. brasiliensis cell wall. Hemopexin tightly binds to heme groups and scavenges the free heme in order to protect the body from oxidative damage. Ceruloplasmin is responsible for carrying about 70% of the total copper in human plasma and exhibits a copper-dependent oxidase activity, which possibly oxidizes Fe2+ into Fe3+, thus participating in iron transport. Microorganism receptors for host Fe-binding proteins and ligands have been described (Nevitt, 2011). The presence of plasma iron and copper carriers in P. brasiliensis cell wall may be due to an attempt to accumulate these nutrients during growth. Iron availability is important for fungal growth (Arango & Restrepo, 1988), and the presence of siderophores has been demonstrated (Castaneda, et al., 1988). In silico analysis showed that P. brasiliensis also has a high-affinity copper transport protein (Ctr3p) ortholog (Silva, et al., 2011). The importance of copper homeostasis in Cryptococcus neoformans virulence was demonstrated, since it was linked to capsule production and inhibition of phagocytosis (Chun & Madhani, 2010).
In conclusion, by using a careful protocol employing sucrose centrifugation and successive washes with different NaCl concentrations, we isolated cell wall from Pb3 yeasts cultivated in the presence of human plasma. The non-covalently associated plasma proteins were extracted with boiling SDS and a proteomic analysis by LC/MS-MS was applied. Complement pathway components were identified, and their role in the phagocytosis was suggested. Several human plasma proteins described here have not been previously reported as interacting with fungal components, specifically, clusterin, hemopexin, transthyretin, ceruloplasmin, alpha-1-antitrypsin, apolipoprotein A-I, and apolipoprotein B-100. This report represents an initial step to understanding the P. brasiliensis cell wall interaction with host components and the possible role of plasma proteins in the host-parasite relationship and infection, especially in a low virulence isolate.
Data availability: Proteomic data will be available online upon the acceptance of the present manuscript
Supplementary Material
Acknowledgments
This work has been funded by FAPESP, CNPq, and NIH (grants # 5G12RR008124-16A1, 5G12RR008124-16A1S1, and 8G12MD007592). We thank the Biomolecule Analysis Core Facility at UTEP, supported by the Research Centers in Minority Institutions (RCMI) program, grant # 8G12MD007592, to the Border Biomedical Research Center (BBRC), from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the NIH, for the access to the LC-MS instrument.
References
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Polanski M, Pieper R, et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- Arango R, Restrepo A. Growth and production of iron chelants by Paracoccidioides brasiliensis mycelial and yeast forms. J Med Vet Mycol. 1988;26:113–118. [PubMed] [Google Scholar]
- Carmona AK, Puccia R, Oliveira MC, Rodrigues EG, Juliano L, Travassos LR. Characterization of an exocellular serine-thiol proteinase activity in Paracoccidioides brasiliensis. Biochem J. 1995;309 ( Pt 1):209–214. doi: 10.1042/bj3090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Carvalho KC, Ganiko L, Batista WL, et al. Virulence of Paracoccidioides brasiliensis and gp43 expression in isolates bearing known PbGP43 genotype. Microbes Infect. 2005;7:55–65. doi: 10.1016/j.micinf.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Castaneda E, Brummer E, Perlman AM, McEwen JG, Stevens DA. A culture medium for Paracoccidioides brasiliensis with high plating efficiency, and the effect of siderophores. J Med Vet Mycol. 1988;26:351–358. [PubMed] [Google Scholar]
- Chun CD, Madhani HD. Ctr2 links copper homeostasis to polysaccharide capsule formation and phagocytosis inhibition in the human fungal pathogen Cryptococcus neoformans. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Cottier F, Pavelka N. Complexity and dynamics of host-fungal interactions. Immunol Res. 2012;53:127–135. doi: 10.1007/s12026-012-8265-y. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–1344. doi: 10.1093/jn/137.5.1341. [DOI] [PubMed] [Google Scholar]
- Ehrnthaller C, Ignatius A, Gebhard F, Huber-Lang M. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011;17:317–329. doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex G, Laurell CB, Thulin E. Purification of prealbumin from human and canine serum using a two-step affinity chromatographic procedure. Eur J Biochem. 1977;75:181–186. doi: 10.1111/j.1432-1033.1977.tb11515.x. [DOI] [PubMed] [Google Scholar]
- Gaur NK, Smith RL, Klotz SA. Candida albicans and Saccharomyces cerevisiae expressing ALA1/ALS5 adhere to accessible threonine, serine, or alanine patches. Cell Commun Adhes. 2002;9:45–57. doi: 10.1080/15419060212187. [DOI] [PubMed] [Google Scholar]
- Gonzales DA, De Torre C, Wang H, et al. Protein expression profiles distinguish between experimental invasive pulmonary aspergillosis and Pseudomonas pneumonia. Proteomics. 2010;10:4270–4280. doi: 10.1002/pmic.200900768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green NS, Foss TR, Kelly JW. Genistein, a natural product from soy, is a potent inhibitor of transthyretin amyloidosis. Proc Natl Acad Sci U S A. 2005;102:14545–14550. doi: 10.1073/pnas.0501609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- Jurado JD, Rael ED, Lieb CS, Nakayasu E, Hayes WK, Bush SP, Ross JA. Complement inactivating proteins and intraspecies venom variation in Crotalus oreganus helleri. Toxicon. 2007;49:339–350. doi: 10.1016/j.toxicon.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kanetsuna F, Carbonell LM, Moreno RE, Rodriguez J. Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. J Bacteriol. 1969;97:1036–1041. doi: 10.1128/jb.97.3.1036-1041.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F, Carbonell LM, Azuma I, Yamamura Y. Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J Bacteriol. 1972;110:208–218. doi: 10.1128/jb.110.1.208-218.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapteyn JC, Hoyer LL, Hecht JE, et al. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol Microbiol. 2000;35:601–611. doi: 10.1046/j.1365-2958.2000.01729.x. [DOI] [PubMed] [Google Scholar]
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- Limper AH, Standing JE. Vitronectin interacts with Candida albicans and augments organism attachment to the NR8383 macrophage cell line. Immunol Lett. 1994;42:139–144. doi: 10.1016/0165-2478(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Maza PK, Oliveira P, Toledo MS, Paula DM, Takahashi HK, Straus AH, Suzuki E. Paracoccidioides brasiliensis induces secretion of IL-6 and IL-8 by lung epithelial cells. Modulation of host cytokine levels by fungal proteases. Microbes Infect. 2012 doi: 10.1016/j.micinf.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Meri T, Blom AM, Hartmann A, Lenk D, Meri S, Zipfel PF. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect Immun. 2004;72:6633–6641. doi: 10.1128/IAI.72.11.6633-6641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk ME, Da Silva WD. Activation of human complement system Paracoccidioides brasiliensis and its deposition on the yeast form cell surface. J Med Vet Mycol. 1992;30:481–484. [PubMed] [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu ES, Sobreira TJ, Torres R, Jr, Ganiko L, Oliveira PS, Marques AF, Almeida IC. Improved proteomic approach for the discovery of potential vaccine targets in Trypanosoma cruzi. J Proteome Res. 2012;11:237–246. doi: 10.1021/pr200806s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevitt T. War-Fe-re: iron at the core of fungal virulence and host immunity. Biometals. 2011;24:547–558. doi: 10.1007/s10534-011-9431-8. [DOI] [PubMed] [Google Scholar]
- Nobbs AH, Vickerman MM, Jenkinson HF. Heterologous expression of Candida albicans cell wall-associated adhesins in Saccharomyces cerevisiae Reveals differential specificities in adherence and biofilm formation and in binding oral Streptococcus gordonii. Eukaryot Cell. 2010;9:1622–1634. doi: 10.1128/EC.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente JA, Salem-Izacc SM, Santana JM, Pereira M, Borges CL, Bailao AM, Soares CM. A secreted serine protease of Paracoccidioides brasiliensis and its interactions with fungal proteins. BMC Microbiol. 2010;10:292. doi: 10.1186/1471-2180-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R, Su Q, Gatlin CL, Huang ST, Anderson NL, Steiner S. Multi-component immunoaffinity subtraction chromatography: an innovative step towards a comprehensive survey of the human plasma proteome. Proteomics. 2003;3:422–432. doi: 10.1002/pmic.200390057. [DOI] [PubMed] [Google Scholar]
- Pitarch A, Sanchez M, Nombela C, Gil C. Sequential fractionation and two-dimensional gel analysis unravels the complexity of the dimorphic fungus Candida albicans cell wall proteome. Mol Cell Proteomics. 2002;1:967–982. doi: 10.1074/mcp.m200062-mcp200. [DOI] [PubMed] [Google Scholar]
- Poltermann S, Kunert A, von der Heide M, Eck R, Hartmann A, Zipfel PF. Gpm1p is a factor H-, FHL-1-, and plasminogen-binding surface protein of Candida albicans. J Biol Chem. 2007;282:37537–37544. doi: 10.1074/jbc.M707280200. [DOI] [PubMed] [Google Scholar]
- Puccia R, Vallejo MC, Matsuo AL, Longo LV. The paracoccidioides cell wall: past and present layers toward understanding interaction with the host. Front Microbiol. 2011;2:257. doi: 10.3389/fmicb.2011.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam FR. The Plasma Proteins. Orlando, USA: Academic Press; 1984. [Google Scholar]
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J, Elorza MV, Valentin E, Sentandreu R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006;6:14–29. doi: 10.1111/j.1567-1364.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- Russell WK, Park ZY, Russell DH. Proteolysis in mixed organic-aqueous solvent systems: applications for peptide mass mapping using mass spectrometry. Anal Chem. 2001;73:2682–2685. doi: 10.1021/ac001332p. [DOI] [PubMed] [Google Scholar]
- San-Blas G, Nino-Vega G, Iturriaga T. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med Mycol. 2002;40:225–242. doi: 10.1080/mmy.40.3.225.242. [DOI] [PubMed] [Google Scholar]
- Silva MG, Schrank A, Bailao EF, et al. The homeostasis of iron, copper, and zinc in paracoccidioides brasiliensis, cryptococcus neoformans var. Grubii, and cryptococcus gattii: a comparative analysis. Front Microbiol. 2011;2:49. doi: 10.3389/fmicb.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9:2095–2102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Zhang MX, Kozel TR. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect Immun. 1998;66:4845–4850. doi: 10.1128/iai.66.10.4845-4850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Wurzner R, Skerka C. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol Immunol. 2007;44:3850–3857. doi: 10.1016/j.molimm.2007.06.149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.