Abstract
A systematic classification and accepted nomenclature of neuron types is much needed but is currently lacking. This article describes a possible taxonomical solution for classifying GABAergic interneurons of the cerebral cortex based on a novel, web-based interactive system that allows experts to classify neurons with pre-determined criteria. Using Bayesian analysis and clustering algorithms on the resulting data, we investigated the suitability of several anatomical terms and neuron names for cortical GABAergic interneurons. Moreover, we show that supervised classification models could automatically categorize interneurons in agreement with experts’ assignments. These results demonstrate a practical and objective approach to the naming, characterization and classification of neurons based on community consensus.
The problem of classifying and naming neurons has been a topic of debate for over 100 years. Nevertheless, a satisfactory consensus remains to be reached, even for restricted neuronal populations such as the GABAergic interneurons of the cerebral cortex. Over the past two decades, the amount of morphological, molecular, physiological and developmental data has grown rapidly, making classification harder rather than easier. A consistent neuronal classification and terminology will help researchers to manage this multidisciplinary knowledge, and is needed for specialists in neuroscience subfields to establish and maintain effective communication and data sharing1. As in other domains of science, taxonomies can be empirical or scientific. This distinction was well described by John Hughlings Jackson2 in 1874: “There are two ways of investigating diseases, and two kinds of classification corresponding thereto, the empirical and the scientific. The former is to be illustrated by the way in which a gardener classifies plants, the latter by the way in which a botanist classifies them. The former is, strictly speaking, only an arrangement. The gardener arranges his plants as they are fit for food, for ornament, etc. One of his classifications of ornamental plants is into trees, shrubs, and flowers. His object is the direct application of knowledge to utilitarian purposes. It is, so to speak, practical. The other kind of classification (the classification properly so-called) is rather for the better organization of existing knowledge, and for discovering the relations of new facts; its principles are methodical guides to further investigation. It is of great utilitarian value, but not directly.”
In spite of the many studies performed since the original findings of Santiago Ramón y Cajal, it is surprising that we still lack a catalogue of neuron types and names that is accepted by the general scientific community. Recognizing this problem, the International Neuroinformatics Coordinating Facility (INCF) has recently established a Neuron Registry within the Program on Ontologies of Neural Structures (PONS), with the aim to identify known neuron types on the basis of their defining properties3 (see the INCF Program on Ontologies of Neural Structures website). A collation of terms referring to neuron types is available as part of the Neuroscience Information Framework (NIF) from NeuroLex4 (see the NeuroLex website).
A milestone towards a future classification of GABAergic interneurons in the cerebral cortex was the standardization of the nomenclature of their properties1. However, at that time it was not possible to identify a set of anatomical traits that unambiguously define an interneuron class. In this Analysis article, we describe a new, community-based strategy for defining a morphological taxonomy. Our goal was to establish a list of terms that could be used by all researchers in the field to distinguish neuronal morphologies. Because the developmental and evolutionary processes that gave rise to these morphologies are incompletely understood, we sought a practical rather than a scientific classification: a ‘gardener’s approach’. To this end, we selected a limited number of neuron types and morphological properties based on studies performed over the years in many laboratories. These neuron types and morphological properties are not meant to be imposed but rather are proposed, with the goals of incorporating community feedback and reaching consensus.
In this article, we first provide an overview of historical and current issues involved in classifying cortical neurons and, in particular, interneurons. We then describe a novel, web-based interactive system (FIG. 1) that collected data about the terminological choices for a set of 320 cortical interneurons by 42 experts in the field. We used several analysis methods to empirically test the consistency, clarity and any emerging agreement on these terminological choices. This article deals primarily with neocortical GABAergic aspiny or sparsely spiny non-pyramidal neurons with non-projecting axons. Unless otherwise specified, we refer to these neurons, for simplicity, as ‘cortical interneurons’.
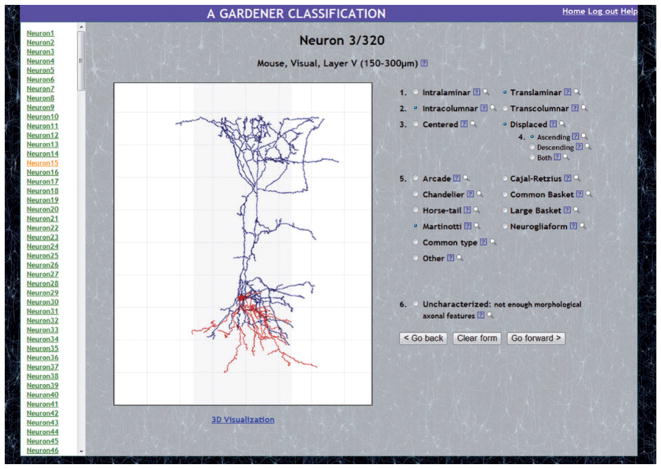
Figure 1. The web-based interactive system.
Screenshot of one of the 320 neurons included in the web-based interactive system. Also shown are the six axonal features and their categories (with possible values for each feature) displayed for the experts so they can select, for each feature, the category that is the most appropriate to describe the morphology of the neuron.
Historical overview
Two major classes of cortical neurons: principal cells and interneurons
Before the discovery of the Golgi method, the existence of different morphological types of cortical neurons was already recognized5. Since then, researchers have tried to deduce the functional role of neurons from their morphological characteristics. Observations of nerve tissue preparations stained with a carmine dye, a technique that was introduced by Joseph von Gerlach (1820–1896) and Rudolf Berlin (1833–1897), led to the suggestion that neurons could be classified into three main cell types6 (quoted in REF. 7) based on the shape of their somata: pyramidal cells (triangular somata), granular cells (small and irregular somata) and spindle-shaped cells (fusiform somata).This was the beginning of cytoarchitectural studies that were based mainly on the density and laminar distribution of different neuronal shapes. However, carmine staining and other methods available at that time only allowed visualization of neuronal cell bodies and a small portion of their proximal processes, making further characterization of cortical neurons difficult. By contrast, Golgi-stained preparations allowed much more complete staining of the neuron, including most of its parts (soma, dendrites and the unmyelinated axon), enabling visualization of their finer morphological details in young animals8. This led to a fuller characterization of neurons, allowing for the first time the exploration of their possible interconnections.
According to Cajal9, Golgi suggested that, in general, there were two morphologically and physiologically different types of neurons: motor (type I) neurons and sensory (type II) neurons. Motor neurons had long axons that not only gave rise to collaterals but also projected outside the grey matter. Sensory neurons had short axons that arborized near the parent cell and did not leave the grey matter. The former cells (with long axons) were thought to have a motor function because their axons were considered to be continuous with the motor roots, whereas the second type were thought to be sensory because their axonal branches were linked with afferent fibres. Cajal argued that it was not possible to maintain such a physiological distinction and designated Golgi’s two types as cells with a long axon (projection neurons) and as cells with a short axon (intrinsic neurons or interneurons), avoiding any consideration of their possible physiological roles. Since then, the term ‘interneuron’ has commonly been used as synonymous with short-axon cell10,11. Notably, some neurons are axonless, such as retina amacrine cells and olfactory granule cells.
Researchers soon realized that, in the cerebral cortex, most neurons were pyramidal cells with axons that were seen to enter or be directed towards the white matter (for example, see REF. 12). Therefore, pyramidal cells started being generally considered as both ‘principal cells’ and projection neurons (that is, cells with long axons).
Furthermore, from observations using the Golgi method, it was obvious that neurons showed a great diversity of morphologies. Thus, in addition to the terms based on the shape of the soma, neuroanatomists described neurons with names that were somewhat descriptive of their dendritic morphology and axonal arborization, alone or in combination. However, with few exceptions, no general consensus has emerged for naming cortical neurons. For example, at present most neuroscientists agree on the usage of terms such as pyramidal neuron, non-pyramidal neuron, interneuron and chandelier (or axo-axonic) cell. These cell types are readily distinguished by their clear morphological attributes. However, other common names, such as double bouquet cell, Martinotti cell, neurogliaform cell and basket cell, seem to lack a consensual definition. In these cases, the same name is often assigned to neurons of varying morphologies by different authors, and various terms are inconsistently adopted in different laboratories to represent the same cell classification. As a consequence, virtually every author has his or her own classification scheme and neuron terms, making the comparison and exchange of information among laboratories rather difficult, if not impossible.
What is a cortical interneuron?
By our definition, a cortical interneuron is a short-axon cell — that is, a neuron with an axon that does not leave the neocortex — and has a soma that is located in the cerebral cortex. Most cortical interneurons lack the typical somatodendritic morphological characteristics that are used to identify projection neurons, namely a pyramidal-shaped cell body and an apical dendritic tree that is distinct from and lies opposite to the basal dendritic arbor. However, the absence of these features should not be used to define interneurons, as they are neither necessary nor sufficient for distinguishing interneurons from projection neurons. Indeed, there are interneurons that have a somatodendritic morphology resembling that of pyramidal cells (for example, the so-called ‘pyramidal basket cells’13, and projection neurons that have a non-pyramidal appearance in their somata and dendrites14.
Traditionally, interneurons have been subdivided into two main groups14: spiny non-pyramidal cells and aspiny or sparsely spiny non-pyramidal cells. Spiny non-pyramidal cells are located in the middle cortical layers, especially in layer IV of primary sensory cortices. They comprise a morphologically heterogeneous group of interneurons with ovoid, fusiform and triangular somata. Most spiny non-pyramidal cells are excitatory (specifically, glutamatergic15), and their axons are distributed within layer IV or in the adjacent layers above or below the somatic location16. Aspiny or sparsely spiny non-pyramidal cells usually have axons that remain near the parent cell, although some run prominent collaterals in the horizontal dimension (that is, parallel to the cortical surface) or vertical dimension (that is, ascending and/or descending to reach other cortical layers). These interneurons appear to be mostly GABAergic and constitute ~10–30% of the total neuron population, with the percentage varying substantially between cortical layers, areas and species17,18. They are the main component of inhibitory cortical circuits.
Following the approach of the Petilla terminology1, we concentrate our effort on GABAergic cortical interneurons, thus excluding the majority of spiny non-pyramidal cells from the classification attempt. This choice is motivated by functional considerations, in the sense that the neurotransmitter released by a neuron is intimately linked to the role of this neuron in the circuitry. Moreover, restricting the scope of this classification to GABAergic interneurons has a practical reason, given the availability of reliable methods to identify GABA and related chemicals, such as its synthesizing enzymes (glutamate decarboxylase 65 (GAD65) and GAD67). Despite this relatively narrow definition, GABAergic cortical interneurons are located in all cortical layers and show a great variety of morphological, biochemical and physiological characteristics. Thus, rather than attempting a comprehensive classification of cortical interneurons, we focus on a group of less controversial cell types for which relatively more abundant experimental evidence converges on a limited number of defining properties within the anatomical domain.
Clarifications and remarks
In light of the above definitions, and before classifying specific interneuron types, it is useful to consider a number of points regarding the morphology and naming of cortical neurons raised by the collective work of many investigators.
First, over the years, the term interneuron has been most commonly used when referring to aspiny or sparsely spiny GABAergic non-pyramidal cells. These cells constitute the majority of interneurons and have come to epitomize the ‘typical’ cortical interneuron. As noted above, however, a minority of GABAergic interneurons are spiny19. Moreover, many interneurons that will become aspiny as they develop are spiny in the neonate14. For clarity, we propose to add the term ‘spiny’ to their name.
Second, some GABAergic non-pyramidal cortical cells (spiny and aspiny alike) project to other cortical areas20,21 and might not therefore be strictly considered as interneurons. We propose to add the term ‘projecting’ to their name.
Third, there are glutamatergic spiny non-pyramidal and pyramidal cells (mostly in layer IV of sensory cortices) with locally confined axons that are distributed near the parent cell soma and do not leave the cortical grey matter. Therefore, they might be considered to be short-axon neurons. However, because these cells are both morphologically and neurochemically rather distinct, we prefer to avoid the term ‘interneuron’ for glutamatergic spiny cells and propose to call them ‘intrinsic (or local) glutamatergic spiny cells’ instead.
Fourth, although most GABAergic interneurons have a non-pyramidal somatodendritic phenotype, some display a pyramidal (triangular) somatic shape. To minimize confusion, we propose to use the term ‘triangular’ to describe the somatic morphology of these interneurons.
Fifth, interneurons are highly diverse with regard to the morphology of their somata and of their dendritic and axonal arbors. For instance, interneurons displaying the same somatodendritic morphology may have different patterns of axonal arborization. Importantly, the axonal geometry is pivotal in establishing circuit connectivity. In several cases, axonal morphology is very distinct, facilitating comparisons of different interneurons. We therefore recommend, whenever historically tenable, using terms such as fusiform, stellate, multipolar, bitufted (neurons with two main dendrites running in opposite directions that, after a relatively short trajectory, resolve into two dendritic tufts) and bipolar (neurons with two principal long dendrites running in opposite directions and showing few dendritic collaterals) only to describe the somatic and/or dendritic morphology and not to name a particular interneuron type. Although these terms are useful descriptors of interneuron somatodendritic morphologies revealed by immunohistochemical staining against calcium-binding proteins and neuropeptides, such staining does not label the full extent of the axonal arbor and therefore does not allow one to unambiguously identify interneuron types. A good example is the double bouquet cell, a term adopted inconsistently in the literature. Some authors use this name for neurons with a bitufted dendritic morphology, regardless of the pattern of axonal arborization. Other authors use the term double bouquet cells for neurons with descending axons that form tightly intertwined bundles of long descending vertical collaterals resembling a horse tail11. Although these cells may have bitufted dendrites, interneurons with the same axonal patterns but with different somatodendritic morphologies also exist14. We propose that cortical interneurons identified by these characteristic axonal bundles be called ‘horse-tail’ cells.
Sixth, numerous neurons exist with axon collaterals that do not exhibit any orientation preferences. That is, they have more or less equal numbers of horizontal, oblique or vertical branches. In fact, most interneurons visualized in Golgi preparations or following intracellular labelling could match this description. We propose to introduce the term ‘common type’ to describe cells without any strikingly recognizable shape.
Seventh, an important morphological feature of cortical interneurons is the laminar and columnar reach of their axonal arbors. Following the Petilla terminology1, we propose to describe neurons with an axonal arbor that is confined to a single layer as ‘intralaminar’, and neurons with an axonal arbor that is not confined to a single layer as ‘translaminar’. Similarly, we refer to neurons with an axonal arbor that is confined to a single column as ‘intracolumnar’, whereas neurons with an axonal arbor that is not confined to a single column are referred to as ‘transcolumnar’ (FIG. 2).
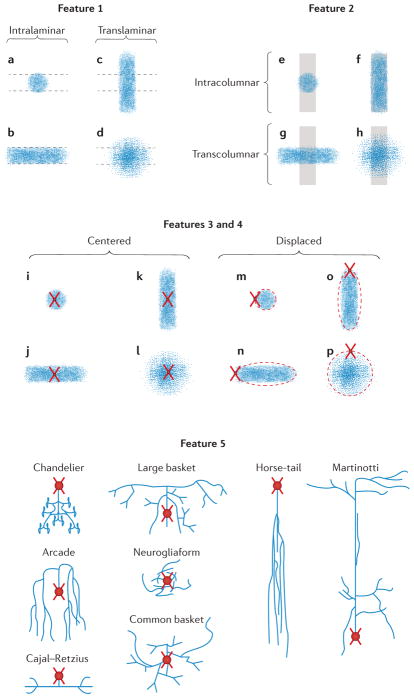
Figure 2. Schematics of the morphological features.
For each feature, the experts had to select the category that best described the neuron on display. For feature 1, the categories were intralaminar (a,b) versus translaminar (c,d). For feature 2, they were intracolumnar (e,f) versus transcolumnar (g,h). For feature 3, the categories were centered (i–l) versus displaced (m–p). For feature 4, they were ascending, descending or both. (This feature applied only when neurons were translaminar and displaced; o,p.) For feature 5 (interneuron types), the categories were arcade, common basket, large basket, Cajal–Retzius, chandelier, horse-tail, Martinotti, neurogliaform, common type (not shown) or other (not shown). When an insufficient number of morphological axonal features are visualized for a given interneuron the cell is considered anatomically uncharacterized (feature 6; not shown). Dashed horizontal lines indicate the extent of the cortical layer. Vertical grey shadows indicate the extent of the cortical column. Axonal arborization is represented by blue dots. Soma and dendritic arborization are represented as red circles and crosses, respectively. Possible variations on the relative position of the somata with respect to the axonal arborization of displaced neurons are represented by red dashed ovals.
Last, a relevant morphological feature of interneurons is the relative location of dendritic and axonal arbors. We propose to use the term ‘centered’ for neurons with dendritic and axonal arbors that are largely colocalized, and to use the term ‘displaced’ otherwise (FIG. 2). In the case of displaced neurons, axons of translaminar interneurons can be ‘ascending’ and/or ‘descending’ depending on whether, relative to the dendritic trees, they are distributed mostly towards the cortical surface, towards the white matter or approximately equally towards both.
Classification attempts
The Petilla terminology1 considered the characteristics that are suitable for describing GABAergic cortical interneurons and organized them into morphological, physiological and molecular properties. Although the identity of a neuron is characterized by all of its properties, a typical experimental identification of a given neuron is commonly limited to a subset of properties. Indeed, most studies primarily (if not exclusively) rely on detailed anatomical, physiological or molecular evidence, and few studies use a balanced combination of these characteristics. Consequently, on the basis of existing data, neurons could in principle be classified using any of these groups of criteria. Several initial attempts at neuronal classification formulated from the Petilla terminology effort1 are briefly summarized below.
Anatomical
The anatomical classification established in the Petilla terminology1 divides GABAergic cortical interneurons into those targeting pyramidal cells or displaying no target specificity and, at least in the hippocampus, those specifically targeting other interneurons. Interneurons targeting pyramidal cells were further subdivided on the basis of the target location and included interneurons targeting the axonal initial segment (axo-axonic or chandelier cells), interneurons targeting the perisomatic region (basket cells) and interneurons targeting the dendrites. Basket cells were further distinguished, on the basis of their axonal morphology, into interneurons with tangential (horizontal) axons, interneurons with radial (vertical) axons, interneurons with both tangential and radial axons and interneurons with axons that are too local to discern a tangential or radial orientation. Dendrite-targeting interneurons were subclassified on an even finer scale as having either a shaft bias or a spine bias, with both of these categories finally separated on the basis of their axonal morphology. Shaft-biased interneurons have radial axons that either descend towards the white matter (these were termed willow cells) or ascend towards the pia (these were termed Martinotti cells). Spine-biased interneurons were further divided on the basis of their axonal patterns and include horse-tail and neurogliaform cells.
Molecular
The molecular classification of the Petilla terminology1 divides cortical interneurons on the basis of the expression of specific molecular markers. In particular, five main groups of interneurons can be distinguished: those expressing parvalbumin (PV), including chandelier and basket cells; those expressing somatostatin (SOM), such as Martinotti cells; those expressing neuropeptide Y (NPY) but not SOM; those expressing vasoactive intestinal peptide (VIP); and those expressing cholecystokinin (CCK) but not SOM or VIP. These five groups can be further subdivided in multiple subtypes based on several molecular categories: transcription factors, neurotransmitters or their synthesizing enzymes, neuropeptides, calcium-binding proteins, neurotransmitter receptors, structural proteins, ion channels, connexins, pannexins and membrane transporters. For example, SOM-expressing interneurons can be subdivided depending on whether they also express NPY or calretinin (CR). Similarly, NPY-expressing interneurons and VIP-expressing interneurons can be subdivided depending on whether they also express CR22,23. A parallel effort to characterize interneurons based on transcription factors is also gaining traction24. This developmental classification separates cortical interneurons with an origin in the medial ganglionic eminence (MGE), lateral and dorsocaudal ganglionic eminence (CGE) and preoptic area (POA). The MGE group encompasses neocortical interneurons identified based on their molecular markers, including those expressing PV, SOM and, early in development, NPY. The CGE group includes the interneurons expressing both CR and VIP (horse-tail cells) and those expressing NPY later in development. The POA group expresses NPY. This mapping does not apply exactly to the hippocampus, as some expression differences have been reported in this area25.
Physiological
The physiological classification of the Petilla terminology1 identifies six main types of interneurons. Fast-spiking (FS) neurons show nonadapting spiking at steady-state, brief spikes and large fast after-hyperpolarizations and include continuous FS cells, delayed FS cells, stuttering FS cells and continuous stuttering FS cells. Non-adapting, non-fast spiking (NA-NFS) neurons display no apparent increase in the interspike interval at steady-state, and include continuous NA-NFS cells and burst-firing NA-NFS cells. Adapting (AD) neurons display a visually obvious increase in the interspike interval at steady-state and include continuous AD cells, bursting AD cells and delayed AD cells. Accelerating (AC) neurons display a decrease in the interspike interval at steady-state and include continuous AC cells and delayed AC cells. Irregular spiking (IS) neurons display an irregular interspike interval and include continuous IS cells and bursting IS cells. Lastly, intrinsic bursting (IB) neurons produce a stereotypical burst of two or more spikes riding on a depolarization envelope followed by a slow afterhyperpolarization potential and include rhythmic IB cells and initial IB cells.
Limitations of the Petilla terminology
Each of these classification schemes has limitations. For many cell types, the anatomical approach requires the identification of the subcellular postsynaptic target (or targets) in addition to the interneuron of interest. The molecular approach does not provide functional insight, as the functional roles of the most useful and commonly used markers are largely unknown. The physiological approach is greatly dependent on the experimental conditions and requires a complete specification and possibly standardization of experimental conditions to be widely acceptable. Thus, each of these complementary classifications provides only partial knowledge when taken individually, but a more comprehensive scheme involving multiple anatomical and functional criteria imposes considerable practical burdens.
Feature-based nomenclature proposal
As a pragmatic alternative and update to the anatomical characterization, we propose a taxonomic solution that is based mainly on axonal arborization patterns. We think that identification of these patterns may be among the most powerful tools available for the subclassification of interneurons.
Our classification design is based on six axonal features, numbered one to six (FIG. 2). These six features were selected as a representative subset of axonal morphological properties that may prove to be suitable for interneuron classification. After introducing all relevant definitions, we describe a web-based interactive system that is designed to evaluate this solution empirically, to test its potential for fostering consensus and to explore preliminary statistical patterns among the generated data (FIG. 1). Several statistical and pattern recognition techniques were used to achieve this goal, including the computation of agreement indices and the use of clustering and supervised classification algorithms.
First axonal feature
The first axonal feature refers to the distribution of the interneuron axonal arborization relative to cortical layers (FIG. 2). Within this feature, we propose two categories: intralaminar, which refers to interneurons with axonal arbors distributed predominantly in the layer of the parent soma; and translaminar, which refers to interneurons with axonal arbors distributed mainly above and/or below the cortical layer of the parent soma.
Second axonal feature
The second axonal feature refers to the distribution of the axonal arborization relative to the size of cortical ‘columns’ from a broad anatomical point of view. Certainly, the term column is vague26,27, as it can refer to small-scale mini-columns (with a diameter of 50 μm), to larger-scale macro-columns (with a diameter of 300–500 μm) and to multiple different structures within both these categories (including barrel columns and ocular dominance columns, the extent of arborization of single thalamic afferent fibres, cytochrome oxidase blobs, individual dendritic arbors of pyramidal cells and tangential widths of axonal patches originated from pyramidal cells). Thus, we have arbitrarily set the size of a cortical column at a diameter of 300 μm — a value that is similar across several species and cortical areas for many of these structures28,29. Within this feature, we propose two categories: intracolumnar, which refers to interneurons with axonal arbors primarily distributed at a distance from the parent soma that does not exceed 300 μm in the horizontal dimension (FIG. 2); and transcolumnar, which refers to interneurons with horizontal axonal collaterals exceeding a distance of 300 μm from the parent soma in the horizontal dimension.
Third axonal feature
The third axonal feature refers to the relative location of the axonal and dendritic arbors (FIG. 2). Within this feature, we propose the following categories: centered, which refers to interneurons with a dendritic arbor that is located mostly in the centre of the axonal arborization; and displaced, which refers to interneurons with a dendritic arbor that is shifted with respect to the axonal arborization (FIG. 2).
Fourth axonal feature
If a neuron is categorized as being both translaminar (for the first axonal feature) and displaced (for the third axonal feature), it can be further distinguished into the following categories1: ascending, which refers to interneurons with axonal arborization that is distributed mostly towards the cortical surface; descending, which refers to interneurons with axonal arborization that is distributed mostly towards the white matter; or ‘both’ (ascending and descending), which refers to interneurons with axonal arborization that is distributed towards both the cortical surface and the white matter (FIG. 2).
Fifth axonal feature: interneuron type
We defined a limited number of cell types for this classification step (see the Gardener Classification website) on the basis of recognizable morphological characteristics (FIG. 2) and the common usage of their name in the literature14. The first cell type — arcade or willow cells — denotes neurons with somata in layers II–VI, multipolar or bitufted dendrites and axons that give rise to axonal arcades, with predominantly vertical arbors and relatively long descending collaterals. The second cell type — common basket cells — denotes neurons with somata in layers II–VI, multipolar or bitufted dendritic arbors and axon collaterals that have numerous curved pre-terminal axon branches. The third cell type — large basket cells — denotes neurons with somata in layers II–VI, multipolar or bitufted dendrites and horizontally oriented axon collaterals that can reach a length of several hundred micrometres. These collaterals show numerous curved pre-terminal axon branches that innervate the somata and proximal dendrites of neurons. Frequently, these cells display one or several long descending axonal branches. The fourth cell type — Cajal–Retzius cells — denotes neurons with an axon plexus that is restricted to layer I and long dendrites with ascending branchlets to the pia. These neurons are not present in adult neocortex and in rodents persist only during the first two postnatal weeks30 (but see REF. 31). Cajal–Retzius cells proper do not contain GABA or express GABA-synthesizing enzymes GAD65 and GAD67 (REFS 32,33). There are also GABAergic neurons with somata in layer I and prominent long horizontal axon collaterals and/or dendrites32, and these are often also named Cajal–Retzius neurons in the developing neocortex, despite their different molecular characteristics from Cajal–Retzius neurons proper33. Given the purely morphological nature of the present study, most of the authors practically considered any GABAergic neuron in layer I with horizontally oriented axonal arborization as a putative Cajal–Retzius cell. The fifth cell type — chandelier cells — denotes neurons with somata in layers II–VI, multipolar or bitufted dendritic arbors and terminal axon branches that form short vertical rows of boutons resembling candlesticks. These interneurons are also referred to as axo-axonic cells as they synapse on the axonal initial segment of their pyramidal targets. The sixth cell type — horse-tail cells — denotes neurons with somata mostly in layers II–III, multipolar, bitufted or bipolar dendrites and axons forming tightly intertwined bundles of long descending vertical collaterals. The seventh cell type — Martinotti cells — denotes neurons with somata in layers II–VI, multipolar, bitufted or bipolar dendrites and ascending axons that give rise to two axonal arbors, one near the soma and another at a variable distance above. This second plexus may be dense (axonal tuft) or diffuse and it can be either in the same layer as the soma of origin or in the layers above (ascending axons can travel from layer VI to layer I). The eighth cell type — neuroglia-form cells — denotes neurons with somata in layers I–VI, multipolar dendritic arbors and that are characterized by very small and dense local axonal arborization around the parent cell body. Finally, we included the option common type to denote neurons with somata in layers I–VI, multipolar, bipolar or bitufted dendritic arbors and axon collaterals without any apparent target or orientation preference in the web-based interactive system (not shown in FIG. 2). Also, we added the option ‘other’ to label any given neuron with an alternative name in case the expert considered another term more appropriate.
Sixth axonal feature: uncharacterized versus characterized neurons
Interneurons that are uniquely characterized by peculiar morphological features can often be easily recognized, even when their axon is rather incompletely labelled. However, in many other cases, the axon needs to be fully labelled and reconstructed in order to distinguish the neuronal identity unequivocally. Thus, although it is not always necessary to visualize the full axonal and dendritic arborization to distinguish a given neuron, this is the preferred situation. Pragmatically, ‘sufficiently complete’ labelling simply means ‘clear enough’ to allow for the identification of a given morphological type. When an insufficient number of morphological axonal features are visualized for a given interneuron (because of incomplete staining, tissue slicing and so on), we propose that the cell should be deemed an anatomically ‘uncharacterized’ interneuron.
Study of inter-neuroscientist agreement
We designed and deployed an interactive web-based system (see the Gardener Classification website) to empirically test the level of agreement among 42 experts in the field in assigning the six features to individual cortical interneurons. The approach takes advantage of a common digital format to display, analyse and manipulate three-dimensional neuromorphological tracings reconstructed from light microscopy34. Images of the 320 interneurons included in the experiment were obtained either from the NeuroMorpho website35 or by scanning two-dimensional drawings from previous publications. Altogether, this pool includes interneurons from different areas and layers of the cerebral cortex of the mouse, rat, rabbit, cat, monkey and human (Supplementary information S1). The database does not necessarily constitute a representative sample from the neuron population in different areas, layers and species. Furthermore, most of the anatomy recovered from electrophysiological work in vitro is conditioned by both slice thickness and plane of cut, which may vary across laboratories. Nonetheless, these conditions reflect the typical experimental variability that confronts researchers in the field.
Experienced neuroscientists who are knowledgeable in this field were asked to ascribe the categories they considered most appropriate to each neuron (there were six features and 21 categories in total; see FIG. 2). So, for feature 1 (F1) they would either ascribe a neuron the category intralaminar or the category translaminar. For feature 2 (F2), either intracolumnar or transcolumnar; for feature 3 (F3), either centered or displaced; for feature 4 (F4), ascending, descending or both; for feature 5 (F5), arcade common basket, large basket, Cajal–Retzius, chandelier, horse-tail, Martinotti, neurogliaform, common type or other; and for feature 6 (F6), either characterized or uncharacterized.
To study the agreement regarding the assignment of the features between neuroscientists, we computed typical statistical measures of inter-expert concordance for each feature and for each category (a possible value for a feature). We also identified sets of similar neurons using clustering algorithms. Furthermore, we induced from the data a Bayesian network model for each expert to enable us to analyse their choices by comparing the network structures of different neuroscientists (Supplementary information S1). With this approach, the possible reasoning of the experts can be inferred from the probabilistic models. Finally, we built automatic classifiers to assign each neuron to one category for each of the six features (Supplementary information S1).
Analysis of the raw data
First, we performed a preliminary exploratory analysis of the raw data to study how the votes of the experts were distributed for the different features. We assessed the relative frequency of each category in the experiment. Less than 10% of neurons were rated as anatomically uncharacterized; as described above, this pertains to neurons with an insufficient number of morphological axonal features to allow classification. Thus, the vast majority of the neurons in the experiment were considered as characterized. The most frequently assigned categories of descriptive axonal features proposed in this study were translaminar, intracolumnar and displaced. The categories ascending and descending received a similar percentage of the ratings, whereas fewer neurons were assigned to the category both.
We then assessed the frequency with which interneurons were assigned to specific interneuron types. The most commonly assigned interneuron types were common type, common basket and large basket. The interneuron types Martinotti, neurogliaform and horse-tail received an intermediate percentage of ratings, whereas chandelier and arcade received the lowest percentage of ratings. Only three cells were classified as Cajal–Retzius by six experts; the remaining experts classified these neurons as uncharacterized, common type, common basket, large basket, Martinotti or other.
Finally, we checked whether the names given to the 79 neurons that were scanned from original publications were maintained in the present experiment by the authors of those publications. Interestingly, the authors were frequently inconsistent in naming certain neurons. For example, some neurons named neurogliaform cells in the original publication were classified as uncharacterized in the current experiment by the same author.
Experts’ agreement analysis
We computed statistical measures of inter-expert agreement to analyse the degree of concordance between the ratings given by the experts (Supplementary information S1). Here, the goal was to quantify the agreement among experts for each feature independently. We studied the agreement for both features and categories using the two most studied agreement indices: Fleiss’ pi and Cohen’s kappa indices.
We first analysed feature agreement. We found high levels of observed agreement between experts in the classification of neurons according to F1–F4 and F6 (observed agreement values exceeding 0.7; see FIG. 3b). The lowest level of inter-expert agreement (below 0.5) was found for F5.
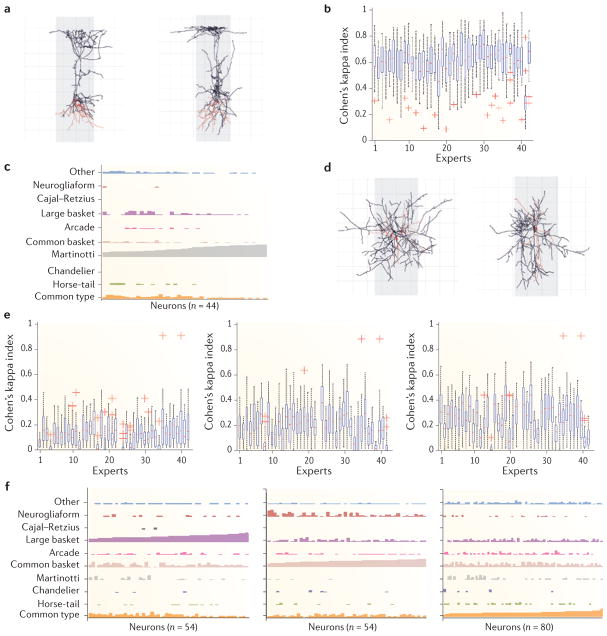
Figure 3. Agreement analysis.
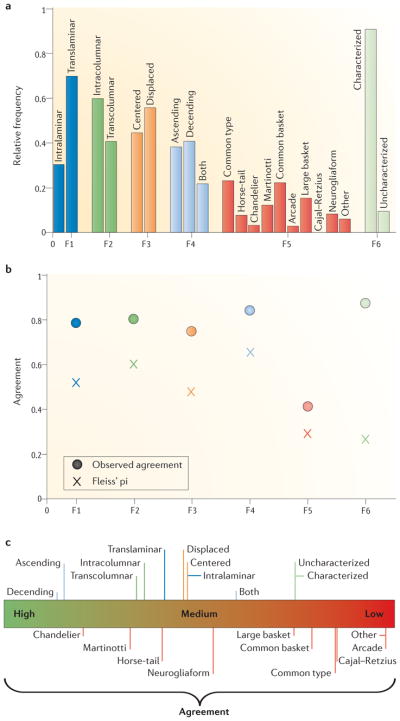
a | Relative frequency of each category for each feature (F1 to F6): that is, the number of times a category was selected divided by the total number of ratings for the relevant feature. b | Overall observed agreement (circles) and chance-corrected Fleiss’ pi index (crosses; see Supplementary information S1) for each feature, indicating the degree of concordance between the experts. c | Chance-corrected (Fleiss’ pi index) agreement achieved in each category of each feature. Categories of the same feature are shown using lines with the same colour; for example, the categories intracolumnar and transcolumnar (which correspond to the second axonal feature) are shown with dark green bars. Interneuron types that were easily distinguished by the experts yielded high agreement (for example, the categories chandelier and Martinotti), whereas confusing categories, such as common type, common basket and large basket, yielded low chance-corrected agreement values.
The observed agreement values were then corrected for chance agreement. Thus, when the inter-expert coincidence was above random levels, the chance-corrected agreement indices yielded values above 0. After correcting for chance agreement (see Supplementary information S1), the highest chance-corrected Fleiss’ pi inter-expert agreement was found for F4 (FIG. 3b). F1, F2 and F3 yielded intermediate chance-corrected agreement values, whereas F5 and F6 had low agreement. The difference between ‘observed agreement’ and Fleiss’ pi index was particularly high for F6; that is, for the decision on whether or not a neuron could be characterized, this feature had the highest observed agreement and the lowest Fleiss’ pi value. This was due to the fact that the category prevalence of this feature was very unbalanced, such that characterized neurons were much more frequent than uncharacterized ones, reducing the values of the agreement measures (Supplementary information S2).
We then calculated the chance-corrected agreement achieved for each category of every feature (Supplementary information S2). Ascending and descending were the two categories with the highest inter-expert agreement, as indicated by the high values obtained for the chance-corrected Fleiss’ pi index (see FIG. 3c and figure S7 in Supplementary information S2). Medium–high agreement levels were found for the categories intralaminar, translaminar, intracolumnar, transcolumnar, centered and displaced.
Regarding F5, we found that the category chandelier yielded the highest agreement (that is, there was little disagreement between all experts over whether a given neuron should be classified as a chandelier cell). The level of agreement was high or medium for Martinotti, horse-tail and neurogliaform cells, whereas it was lower for the rest of the proposed interneuron types (large basket, common basket, common type, Cajal–Retzius, arcade and other). As in the above agreement analysis for F6, characterized and uncharacterized were the categories with the lowest level of chance-corrected inter-expert agreement (Supplementary information S2). Moreover, an analysis of chance-corrected Fleiss’ pi index in which one or three experts were removed showed similar results, revealing those experts who contributed to the low agreement for some features (figure S8 in Supplementary information S2).
Additionally, we assessed whether Fleiss’ pi values changed if two categories of F5 were merged into one category. The rationale for this was that certain pairs of categories seemed to overlap in terms of the neurons that were assigned to them. In fact, Fleiss’ pi values increased when the categories common type, common basket and large basket were merged with each other (table S4 in Supplementary information S2); this reveals that these neuron types are ill-defined. By contrast, combining the Martinotti and/or chandelier categories with other categories yielded a lower chance-corrected agreement, suggesting that these neuron types are well defined. Furthermore, the above results were confirmed in a separate analysis using Cohen’s kappa index (FIG. 4b,e). This index is defined for scenarios with two experts and two categories. Thus, we assessed the level of agreement between all possible pairs of experts, resulting in a comparison of each expert with all the other experts (figures S9–S11 in Supplementary information S2). For example, the first blue box in FIG. 4b summarizes the agreement between the first expert and the other 41 experts regarding the categorization of a neuron as Martinotti. Thus, this high-valued box means that this expert categorized the same neurons as Martinotti as the majority of the remaining experts. Also, we can conclude that there was a high agreement between experts for the category Martinotti, as all box plots (excluding expert 41) showed high Cohen’s kappa index values (FIG. 4b). By contrast, there was a low level of agreement for common type, common basket and large basket cells, as reflected by the low values of the box plots (FIG. 4e). See Supplementary information S2 for further analyses regarding Cohen’s kappa index.
Figure 4. Examples of inter-expert agreement and disagreement.
a | Examples of neurons (neurons 3 and 272) categorized by 41 out of 42 experts as Martinotti. b | Box plots showing the agreement (quantified by Cohen’s kappa index) between pairs of experts when comparing cells categorized as Martinotti against all the other interneuron types. For example, the first blue box shows the agreement values between the expert 1 and the other 41 experts when classifying interneurons as Martinotti cells. High values of Cohen’s kappa index indicate high levels of inter-expert agreement. Apart from expert 41, the other experts yielded fairly high agreement when categorizing interneurons as Martinotti cells. The bottom and top of the blue boxes in the box plot are the lower and upper quartiles, respectively; the ends of the whiskers indicate the still considered typical values; the red crosses show outliers. c | A cluster of 44 neurons (shown from left to right) and the way they were assigned to one of the ten categories (each in different colour) of feature 5 by the experts. A vertical bar is shown for each category and each neuron, and the height of each bar indicates the number of experts who selected that category for that neuron. In the case of these 44 neurons, the neurons were classified as Martinotti by most of the experts. d | The left panel is an example of a neuron (neuron 31) that was categorized by 12 experts as common type, by 12 other experts as common basket, by 15 experts as large basket and by two experts as arcade. The right panel is another example of a neuron (neuron 274) that was categorized by 11 experts as common type, by 12 as common basket, by 14 as large basket, by one as chandelier, by one as arcade and by one as other. e | Low agreements between pairs of experts, as quantified by Cohen’s kappa index, when categorizing interneurons as common type (left), common basket (middle) and large basket (right) against all the other interneuron types. f | Examples of clusters of neurons (54, 54 and 80 neurons, respectively) that show no unique category with high bars (compared with panel c). The graphs show that, in each cluster, the neurons received a high number of votes for common type, common basket and large basket rather than mainly for one category. Thus, the categories that were selected most often — large basket (left), common basket (middle) and common type (left) — were nevertheless selected less often (shorter bars) than the category Martinotti in panel c (longer bars). Note that a high number of experts also categorized the neurons as neurogliaform or common basket (high bars in middle and right panels, respectively).
Neuron clustering
We used clustering algorithms on the classification data from the experts to find groups of interneurons (clusters) with similar morphological properties. The rationale for this analysis was not to define interneuron types but to check whether the experts’ votes for a given feature could separate neurons into clear groups. We performed the clustering analysis at two levels: neuron clustering for each feature and neuron clustering for all the features (Supplementary information S1).
First, we grouped the 320 neurons considering each feature independently. Thus, the clustering algorithm takes into account, for a given feature, which category was selected for each neuron by each individual expert. For F1–F3 and F6 (figures S12–S14 and S17 in Supplementary information S2), clear clusters of neurons could be identified for each category, whereas the clusters for F4 (figure S15 in Supplementary information S2) showed confusion about the category both. With regard to F5, we ran the algorithm to divide the set of 320 neurons into eight clusters (Supplementary information S1). FIG. 4c shows one cluster of neurons that clearly corresponds to Martinotti cells. By contrast, the panels in FIG. 4f show clusters that did not identify neurons corresponding to a single category; these clusters contained neurons that mainly corresponded to those categories for which no agreement was achieved by the experts. Results for the remaining clusters of F5 are reported in figure S16 in Supplementary information S2. These results indicate that although the scientific community was clear about some concepts (F1–F3 and F6), other categories (in F4 and F5) were controversial.
Next, we used another clustering algorithm to analyse the neurons, now taking into account all of the features at the same time. In this case, for a given neuron, the algorithm analyses the number of experts who selected each category of each feature without distinguishing between individual experts. This allowed us to study possible relationships between the features. FIG. 5 represents the clusters obtained in the analysis. We found some clusters containing neurons with clearly identified categories. For example, FIG. 5a shows a cluster of neurons that were clearly categorized as intralaminar, intra-columnar, centered and characterized. Furthermore, some of these neurons were mainly categorized as either common type, chandelier, common basket or neuroglia-form. Similarly, FIG. 5b shows neurons that were mainly categorized as translaminar, transcolumnar, displaced. ascending, Martinotti and characterized. By contrast, FIG. 5c shows a cluster of neurons that were mainly categorized as translaminar and intracolumnar but that were not clearly categorized for the rest of the features. Finally, FIG. 5f shows a cluster of neurons showing no clearly identified categories, corresponding mainly to uncharacterized neurons.
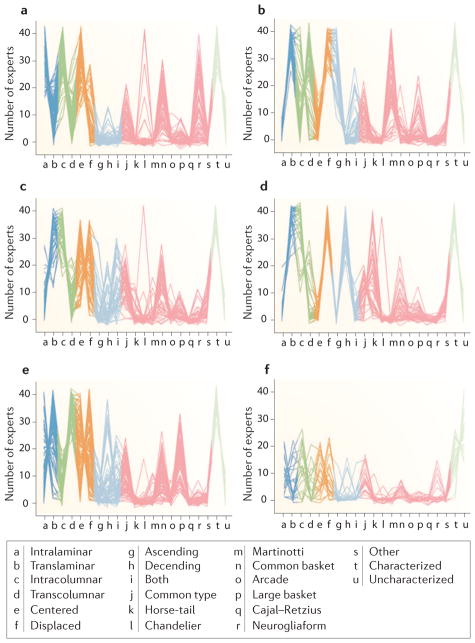
Figure 5. Clustering of neurons considering all features.
a–f | Parallel coordinate diagrams of clusters of neurons obtained with the k-means algorithm (k = 6) considering all of the features at the same time. Each line represents one neuron, showing the number of experts who selected each category of every feature when classifying that neuron. For example, panel b shows a cluster in which the majority of neurons were categorized by many experts as translaminar (dark blue), transcolumnar (dark green), displaced (orange), ascending (light blue), Martinotti (pink) and characterized (light green).
Bayesian networks for modelling experts’ opinions
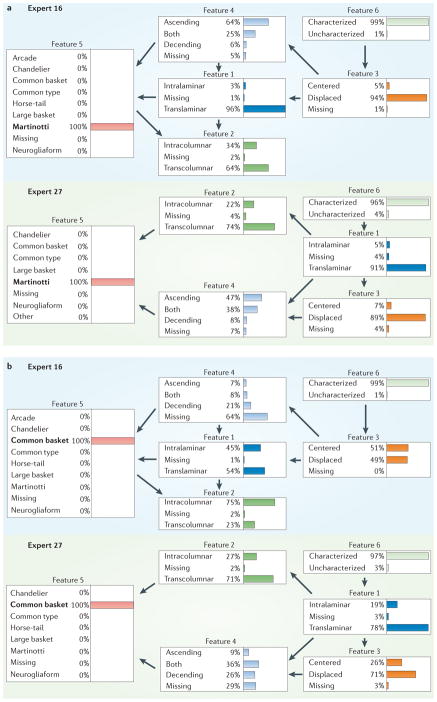
Bayesian network models can capture the way by which an expert understands the (probabilistic) relationships among all the features (see REFS 36,37 for an application to neuroanatomy). As opposed to the analyses above, which focused on studying each feature independently, Bayesian networks enable us to analyse the associations between a set of features. The graphical representation of Bayesian networks allows one to visualize and inspect the relationships between the features. Here, we trained a probabilistic graphical model for each expert and used these models to analyse the experts’ choice behaviours. In general, some Bayesian networks presented similar structures, whereas others showed different relationships between the features. For example, FIG. 6 shows the Bayesian networks ‘learned from’ experts 16 and 27 when they selected Martinotti or common basket. The two models had a different structure, as shown by the variations in the relationships between the features (see FIG. 6 and figure S18 in Supplementary information S2). Additionally, Bayesian networks allow us to draw probabilistic conclusions about the categories. On the basis of Bayes’ rule, we can also infer the likely reasoning of each expert and compare the behaviours of the different experts. Here, we selected some of the categories of F5 as evidence and inferred the most probable values for the rest of the features. This enabled us to identify the main properties for each interneuron type, allowing us to study the different conceptual thinking of the experts. In general, when we studied categories with high levels of agreement, the propagated probabilities were similar in all the Bayesian networks. For example, when the category Martinotti was analysed, Bayesian networks yielded similar propagated probabilities (for example, FIG. 6a and figure S18 in Supplementary information S2). By contrast, when we analysed a category with a low level of agreement, the propagated probabilities were clearly different (for example, common basket in FIG. 6b and in figure S19 in Supplementary information S2). That is, experts had a similar concept for Martinotti cells, whereas, for common basket cells, they rather differed in their reasoning for assigning this interneuron type (see Supplementary information S2 for further details).
Figure 6. Examples of Bayesian networks.
Examples of Bayesian network models of the choice behaviour of two experts (expert 16 and expert 27) when selecting the categories Martinotti (a) or common basket (b) in feature 5. In a Bayesian network structure, each feature is represented with a node (box) in the graph, and an arrow from one node X to another node Y in the graph represents the probabilistic dependence of Y on X (not shown here; see Supplementary information S1 for further details). Note that the direction of an arrow between two nodes does not necessarily reveal causality or hierarchy but merely shows a probabilistic relationship between the two corresponding features. When a category is selected (for example, Martinotti as neuron type in part a), probabilistic rules are used to propagate this information and to compute the conditional probability of any other node (for example, ascending as feature 4), shown by bar charts in this figure. Thus, the blue bar in feature 4 of part a means that if expert 16 called a neuron Martinotti, there was a 64% probability that he or she would consider it ascending. Similarities and differences between experts can be identified by comparing their Bayesian networks. For instance, arrows connecting feature 4 to feature 5 appear in both Bayesian networks, showing a common relationship for experts 16 and 27. Also, the propagated conditional probabilities can be used to compare experts’ opinions. When selecting Martinotti, the propagated probabilities (shown by percentages and coloured bars) are similar in the two Bayesian networks; for example, translaminar in feature 1 has 96% probability for expert 16 and 91% probability in the panel for expert 27 (a). By contrast, the propagated probabilities when selecting common basket differ greatly; for example, there is 75% probability that expert 16 will select intracolumnar in feature 2 and 27% probability that expert 27 will select intracolumnar in feature 2 (b).
Supervised classification of neurons: automatic classification
The ultimate goal of our experiment was to build a model that could automatically classify a neuron on the basis of its morphological characteristics and, more specifically, in terms of the six features defined in the present study. A supervised classifier is a model that can assign a category to a neuron based on its characteristics. Such a classifier must be trained with a dataset of neurons for which the true category is known. For this purpose, we used those neurons from the experiment (241) that had been reconstructed in three dimensions. We first measured 2,886 morphological parameters using Neurolucida Explorer. Then, we built mathematical models that could automatically classify these 241 neurons according to the values of their morphological parameters38,39. Because supervised classification tools require a single class value for each neuron, we used a naive approach of assigning to each neuron the category that received the highest number of votes40 (Supplementary information S1). As a first approach, we built six classifiers — one per feature (F1–F6). Moreover, we tested several different supervised classifier algorithms (Supplementary information S1). The easiest problem was classifying the neurons as either characterized or uncharacterized. This problem was solved with the highest accuracy, with only two misclassified neurons for this feature; that is, for this feature, the result of the classifier matched that of the (majority of the) experts. For F1, F2 and F3, the classifiers also yielded fairly high accuracies (table S6 in Supplementary information S2). By contrast, the accuracy for Feature F4 was much lower. There could be two main reasons for this low accuracy. First, the category ‘both’ was confusing for the experts, so the assigned category using the majority of votes might not capture the true morphological properties of the neurons for this feature. Second, none of the used morphological variables (Supplementary information S1) might adequately capture the vertical orientation of the axon with respect to the soma. Considering additional variables that specifically refer to the orientation of the axon might help improve the accuracy of the classifiers for this feature. The classifiers also yielded low accuracies when distinguishing the categories in F5 (Supplementary information S2). These results were not surprising because distinguishing the nine proposed neuronal types proved to be difficult for the experts.
Additionally, we further analysed F5 by training binary classifiers that distinguished one category against all the other categories that were considered together. We drew similar conclusions as those obtained in previous analyses (Supplementary information S2). Finally, we observed a frequent disagreement between the categories common type, common basket and large basket throughout the analyses of the supervised classification experiment, and we therefore merged these three categories and repeated the automatic classification experiment. This increased the accuracy of the classification (Supplementary information S2).
Discussion and future directions
This study empirically and quantitatively demonstrates that the gardener’s approach to neuron classification is untenable at this time and confirms the impression that different investigators use their own, mutually inconsistent schemes for classifying neurons based on morphological criteria. Many ambiguities are independent of the relative reconstruction quality and completeness of the tested neurons. A striking indication of the problem is that in several cases, experts assigned a different name to a neuron than the term they had chosen in their own original publication from which that same neuron was taken. This takes us back to the time of Cajal, who also inconsistently named various morphological types of interneurons. For example, Cajal termed neurons with different dendritic and axonal morphologies ‘double bouquet cells’ (células bipenachadas in Spanish; bitufted cells in English)11. In the present study, however, statistical analyses of inter-expert agreement, application of Bayesian networks and different clustering and supervised classification algorithms clearly separated readily distinguishable interneuron types from apparently confusing interneuron names. High-consensus terms included chandelier and Martinotti cells, indicating that these are more easily identifiable interneuron types. Low-consensus terms included arcade, basket cells and Cajal–Retzius cells, suggesting that these are potentially less useful names. Researchers generally agreed on specific morphological features, such as ascending versus descending and intracolumnar versus transcolumnar axonal arbors.
A solution: the Neuroclassifier
How might the situation be improved? On the basis of the supervised classification models described here, we have started the development of a computer tool for automatic classification of neurons, a ‘Neuroclassifier’. This machine will initially use probabilistic labels — based on the categories provided by experts — as neuron names and will evolve by combining supervised (known labels) and unsupervised (new labels) classification techniques. This may foster naming unification, robust classification and education of new students in the field through online learning techniques. As the scientific community uses the tool, more data will be incorporated into the Neuroclassifier, allowing model updates and increasing classification robustness and accuracy. Furthermore, other morphometric measurements encoding aspects of neuronal anatomy that are important for cortical circuit organization could be considered, including the percentage of axonal arbors that lie inside the cortical layer and column. Eventually, multiple correlative criteria — including molecular, physiological and synaptic connectivity attributes — would enable a more complete neuronal classification, which is a critical step towards better understanding of neuronal circuits.
Importantly, it should be kept in mind that the present analysis is limited to neurons from a small number of species, namely mammals commonly used in brain research. These include one lagomorph, two rodents, one felid and two primates. Although the results from our analysis may be consistent among these mammalian orders, the level of inter-expert agreement was not compared between species. Furthermore, the selection of interneurons from these species does not cover the probable variability of interneuronal morphologies among all mammalian families. In fact, except for the cat, the species in our study all belong to only one mammalian superorder — the Euarchontoglires. Although several ‘canonical’ neuronal morphologies are doubtlessly common to all placental mammals, some species (such as cetartiodactyls and xenarthrans) depart from the commonly observed neuron types41,42. Future inclusion of other species in the Neuroclassifier will allow detailed analysis of evolutionary conservation and species-specific neuron types.
Supplementary Material
Acknowledgments
We thank the experts who took part in testing neuron classification using the web-based interactive system (in addition to the authors of the article), in alphabetical order: L. Alonso-Nanclares, C. Dávid, H. Geoffroy, M. Inan, V. Garcia-Marín, Á. Merchán-Pérez, L. McGarry, A. Muñoz, C. Palazzetti, N. Povysheva, D. Rotaru, R. Scott, R. Tremblay and A. Zaitsev. This work was supported by funding from the Spanish Ministry of Economy and Competitiveness (grants TIN2010-20900-C04-04 (to P.L.), SAF2009-09394 (to J.DF.) and the Cajal Blue Brain Project, Spanish partner of the Blue Brain Project initiative from EPFL (to J.DF. and P.L.)) and the National Institutes of Health under Grant R01-39600 (to G.A.A.).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Petilla Interneuron Nomenclature Group. Petilla Terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. A representative group of researchers proposed a standardized nomenclature of interneuron properties, the Petilla terminology, which has been used as a recognized reference in the recent literature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson JH. On classification and on methods of investigation (1874) In: Taylor E, editor. Selected Writings. Hodder and Stoughton; 1931. [Google Scholar]
- 3.Hamilton D, Shepherd GM, Martone ME, Ascoli GA. An ontological approach to describing neurons and their relationships. Front Neuroinform. 2012;6(15) doi: 10.3389/fninf.2012.00015. This article provides a clear introduction to the neuroinformatics infrastructure requirements of neuronal classification, with a review of the outstanding technical, scientific and social challenges for the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson SD, Martone ME. Ontologies for neuroscience: what are they and what are they good for? Front Neurosci. 2009;3:60–67. doi: 10.3389/neuro.01.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Kölliker A. Handbuch der Gewebelehre des Menschen. Engelmann; German: 1852. [Google Scholar]
- 6.Berlin R. Beitrag zur Strukturlehre der Grosshirnwindungen. German: Junge. 1858. [Google Scholar]
- 7.Clarke E, O’Malley CD. The Human Brain and Spinal Cord. A Historical Study Illustrated by Writings from Antiquity to the Twentieth Century. Univ. of California Press; 1968. [Google Scholar]
- 8.Golgi C. Sulla struttura della sostanza grigia del cervello (Comunicazione preventiva) Gazz Med Ital Lombardia. 1873;33:244–246. (in Italian) [Google Scholar]
- 9.Cajal SR. Nuevo concepto de la histología de los centros nerviosos. Revista de Ciencias Médicas de Barcelona. 1892;18:361–376. English translation available in DeFelipe, J. & Jones, E. G. Cajal on the Cerebral Cortex (Oxford Univ. Press, 1988) [Google Scholar]
- 10.Rakic P. Local Circuit Neurons. The MIT Press; 1976. [PubMed] [Google Scholar]
- 11.DeFelipe J. Cortical interneurons: from Cajal to 2001. Prog Brain Res. 2002;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- 12.Meynert T. In: Handbuch der Lehre von den Geweben des Menschen und der Thiere. Stricker S, editor. Vol. 1. Verlag von Wilhelm Engelmann; German: 1871. pp. 694–808. [Google Scholar]
- 13.Amaral D, Lavenex P. In: The Hippocampus Book. Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. Oxford Univ. Press; 2006. pp. 37–114. [Google Scholar]
- 14.Jones EG, Peters A, editors. Cerebral Cortex: Volume 1: Cellular Components of the Cerebral Cortex. Plenum Press; 1984. This volume represents a standard reference work on the histology of cortical neurons. [Google Scholar]
- 15.Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J Physiol. 1999;521:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staiger JF, et al. Functional diversity of layer IV spiny neurons in rat somatosensory cortex: quantitative morphology of electrophysiologically characterized and biocytin labeled cells. Cereb Cortex. 2004;14:690–701. doi: 10.1093/cercor/bhh029. [DOI] [PubMed] [Google Scholar]
- 17.DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol. 2002;31:299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- 18.Meyer HS, et al. Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5A. Proc Natl Acad Sci USA. 2011;108:16807–16812. doi: 10.1073/pnas.1113648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubota Y, et al. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- 20.Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J Comp Neurol. 2007;505:526–538. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- 21.Melzer S, et al. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- 22.Karagiannis A, et al. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter JT, et al. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci. 1998;10:3617–3628. doi: 10.1046/j.1460-9568.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- 24.Welagen J, Anderson S. Origins of neocortical interneurons in mice. Dev Neurobiol. 2011;71:10–17. doi: 10.1002/dneu.20857. [DOI] [PubMed] [Google Scholar]
- 25.Tricoire L, et al. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakic P. Confusing cortical columns. Proc Natl Acad Sci USA. 2008;105:12099–12100. doi: 10.1073/pnas.0807271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockland KS. Five points on columns. Front Neuroanat. 2010;4:22. doi: 10.3389/fnana.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malach R. Cortical columns as devices for maximizing neuronal diversity. Trends Neurosci. 1994;17:101–104. doi: 10.1016/0166-2236(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 29.Mountcastle VB. Perceptual Neuroscience: The Cerebral Cortex. Harvard Univ. Press; 1998. [Google Scholar]
- 30.Chowdhury TG, et al. Fate of Cajal–Retzius neurons in the postnatal mouse neocortex. Front Neuroanat. 2010;4:10. doi: 10.3389/neuro.05.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marín-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- 32.Meyer G, Goffinet AM, Fairén A. What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex. Cereb Cortex. 1999;9:765–775. doi: 10.1093/cercor/9.8.765. [DOI] [PubMed] [Google Scholar]
- 33.Hevner RF, Neogi T, Englund C, Daza RA, Fink A. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- 34.Ascoli GA. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nature Rev Neurosci. 2006;7:318–324. doi: 10.1038/nrn1885. A broad perspective on the bioinformatics potential of digital neuronal morphology for the advancement of basic and computational neuroscience. [DOI] [PubMed] [Google Scholar]
- 35.Ascoli GA, Donohue DE, Halavi M. NeuroMorpho. Org: a central resource for neuronal morphologies. J Neurosci. 2007;27:9247–9251. doi: 10.1523/JNEUROSCI.2055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearl J. Probabilistic Reasoning in Intelligent Systems. Morgan Kaufmann; 1988. [Google Scholar]
- 37.López-Cruz PL, Bielza C, Larrañaga P, Benavides-Piccione R, DeFelipe J. Models and simulation of 3D neuronal dendritic trees using Bayesian networks. Neuroinformatics. 2011;9:347–369. doi: 10.1007/s12021-011-9103-4. [DOI] [PubMed] [Google Scholar]
- 38.Duda RO, Hart PE, Stork DG. Pattern Classification. 2. Wiley-Interscience; 2001. This textbook clearly describes the main supervised and unsupervised classification methods. [Google Scholar]
- 39.Jain AK, Duin RPW, Mao J. Statistical pattern recognition: a review. IEEE Trans Pattern Anal Mach Intell. 2000;22:4–37. [Google Scholar]
- 40.Raykar VC, et al. Learning from crowds. J Mach Learn Res. 2010;11:1297–1322. [Google Scholar]
- 41.Hof PR, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 42.Sherwood CC, et al. Neocortical neuron types in Xenarthra and Afrotheria: implications for brain evolution in mammals. Brain Struct Funct. 2009;213:301–328. doi: 10.1007/s00429-008-0198-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.