Abstract
Current guidelines recommend an implantable cardioverter-defibrillator (ICD) according to the left ventricular ejection fraction (LVEF). However, they do not mandate volumetric LVEF assessment. We sought to determine whether volumetric LVEF measurement using cardiovascular magnetic resonance imaging (CMR-LVEF) is superior to conventional LVEF measurement using 2-dimensional transthoracic echocardiography (Echo-LVEF) for risk stratifying patients referred for primary prevention ICD. Patients who underwent primary prevention ICD implantation at our institution and had undergone preimplantation CMR-LVEF from November 2001 to February 2011 were identified. Volumetric CMR-LVEF was determined from cine short-axis data sets. CMR-LVEF and Echo-LVEF were extracted from the clinical reports. The end point was appropriate ICD discharge (shock and/or antitachycardia pacing). Of 48 patients, appropriate ICD discharge occurred in 9 (19%) within 29 – 25 months (range 1 to 99, median 20). All patients met the Echo-LVEF criteria for ICD implantation; however 25% (95% confidence interval 13% to 37%) did not meet the CMR-LVEF criteria. None (0%) of these latter patients had received an appropriate ICD discharge. Using CMR-LVEF ≤30% as a threshold for ICD eligibility, 19 patients (40%) with a qualifying Echo-LVEF would not have been referred for ICD, and none (0%) received an ICD discharge. For primary prevention ICD implantation, volumetric CMR-LVEF might be superior to clinical Echo-LVEF for risk stratification and can identify a large minority of subjects in whom ICD implantation can be safely avoided. In conclusion, if confirmed by larger prospective series, volumetric methods such as CMR should be considered a superior “gatekeeper” for the identification of patients likely to benefit from primary prevention ICD implantation.
Current guidelines have resulted in many Medicare beneficiaries becoming eligible for primary prevention implantable cardioverter-defibrillator (ICD) implantation in the United States alone.1 In the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), most patients with ICD implantation for primary prevention never received a device discharge, with the average rate of appropriate ICD shocks estimated at 5.1% annually.2 Moreover, there appears to be a group of patients with a low left ventricular (LV) ejection fraction (LVEF) who remain at low risk of sudden cardiac death, although predictive measures have not been well validated.3–5 Currently, 2-dimensional transthoracic echocardiography is the most commonly used clinical method for serial measurement of the LV volumes and LVEF, because it is noninvasive and widely available. Cardiovascular magnetic resonance imaging (CMR) is also noninvasive and well-suited for assessment of the LV volumes and LVEF. Volumetric CMR methods have been shown to be both highly accurate and highly reproducible for the measurement of LV volumes and LVEF6 and superior to biplane echocardiographic and biplane CMR methods.7 It has also been previously recognized that transthoracic echocardiography (Echo-LVEF) underestimates the LVEF measured using CMR (CMR-LVEF), especially in patients with poor LVEF, with better correlation of 3-dimensional echocardiography with CMR.8 Given that current guidelines recommend ICD implantation for patients with a poor LVEF, without mandating a volumetric method, we hypothesized that the difference between 2-dimensional and volumetric assessment of LVEF would have a high effect on decision making and the outcome of patients considered for ICD implantation. Thus, we sought to determine the effect of volumetric CMR-LVEF compared to Echo-LVEF for risk stratification of patients referred for ICD implantation.
Methods
In the present retrospective cohort study, the Beth Israel Deaconess Medical Center clinical CMR database was queried to identify all patients undergoing ICD implantation for primary sudden cardiac death prevention from November 2001 to February 2011, who also had undergone preimplantation CMR. The patient demographics and clinical follow-up records from the hospital electronic medical records were reviewed.
All patients with ischemic cardiomyopathy had a clinical Echo-LVEF of ≤30% or Echo-LVEF of ≤35% with New York Heart Association class II or III heart failure. All patients with nonischemic dilated cardiomyopathy had an Echo-LVEF of ≤35% with New York Heart Association class II or III.
The clinical CMR-LVEF was measured for each patient, although the data were not applied to determine ICD implantation eligibility.
CMR and transthoracic echocardiography (without any contrast agent) were performed within 1 week of each other; otherwise, the subject had to have a second echocardiogram performed either before or after CMR, with the 2 Echo-LVEF values in agreement (i.e., the LVEF from both studies had to be on the same side of the guidelines classification). If no other echocardiographic study were available, we determined whether any possible interfering clinical events, such as myocardial infarction, hospitalization for heart failure exacerbation, and ICD shock, had occurred.
The study was performed with institutional review board approval of the Beth Israel Deaconess Medical Center. Written informed consent was waived.
CMR was performed using a Philips 1.5T (Philips Medical Systems, Amsterdam, The Netherlands) CMR scanner with a commercial 5-element cardiac-surface coil. Cine images were acquired in contiguous LV short-axis orientation with an electrocardiography-gated, breath-hold, steady-state, free-precession cine sequence with full LV coverage (8-mm slice thickness, 2-mm interslice gap, in-plane spatial resolution 2 × 2 mm, and 30-ms temporal resolution).
The endocardial borders at end-diastole and end-systole were manually traced using standard system software analysis tools. The end-diastolic volume and end-systolic volume were computed by the summation of the disks method, in which the sum of the cross-sectional areas was multiplied by the slice thickness. The LVEF was computed as 100% × (end-diastolic volume − end-systolic volume)/end-diastolic volume. The CMR-LVEF data were extracted from the clinical reports.
Clinical transthoracic echocardiography was performed using standard methods for each patient, and the findings were used to determine eligibility for ICD implantation.7 The Echo-LVEF data were extracted from the clinical reports.
All ICDs were implanted using the standard surgical technique2; the choice of device was at the discretion of the implanting physician, and the device was activated at completion of implantation. The devices were programmed for both antitachycardial pacing and shock, with 3 zones of therapy, including shock for ventricular fibrillation, antitachycardial pacing followed by shock for fast ventricular tachycardia, and a monitored zone for slower ventricular tachycardia. The exact therapy settings were adjusted at the discretion of the implanting physician.
The ICDs were interrogated at 1 and 3 months after implantation and every 6 months thereafter in the device clinic, during which appropriate sensing was confirmed, the device was interrogated, and the recorded events and ICD discharges were reviewed. An appropriate ICD discharge was defined as antitachycardia pacing or shocks delivered for ventricular tachyarrhythmias.9–12
Continuous variables are presented as the mean ± SD. Categorical variables are presented as numbers and percentages. The survival time for an appropriate ICD discharge outcome was defined as the interval (number of days) from ICD implantation to the appropriate ICD discharge. The end of the follow-up period was September 30, 2011. If the patient had not had an appropriate ICD discharge during the follow-up period, the patient’s outcome was considered censored. Univariate Cox regression analysis was used to assess the association between each variable of the baseline characteristics and the hazard function of the presence of an appropriate ICD discharge. The survival function of an appropriate ICD discharge was compared between the CMR-LVEF ≤30% and CMR-LVEF >30% cutoffs using Kaplan-Meier estimates and the log-rank test. All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina). The type I error was set at 0.05.
Results
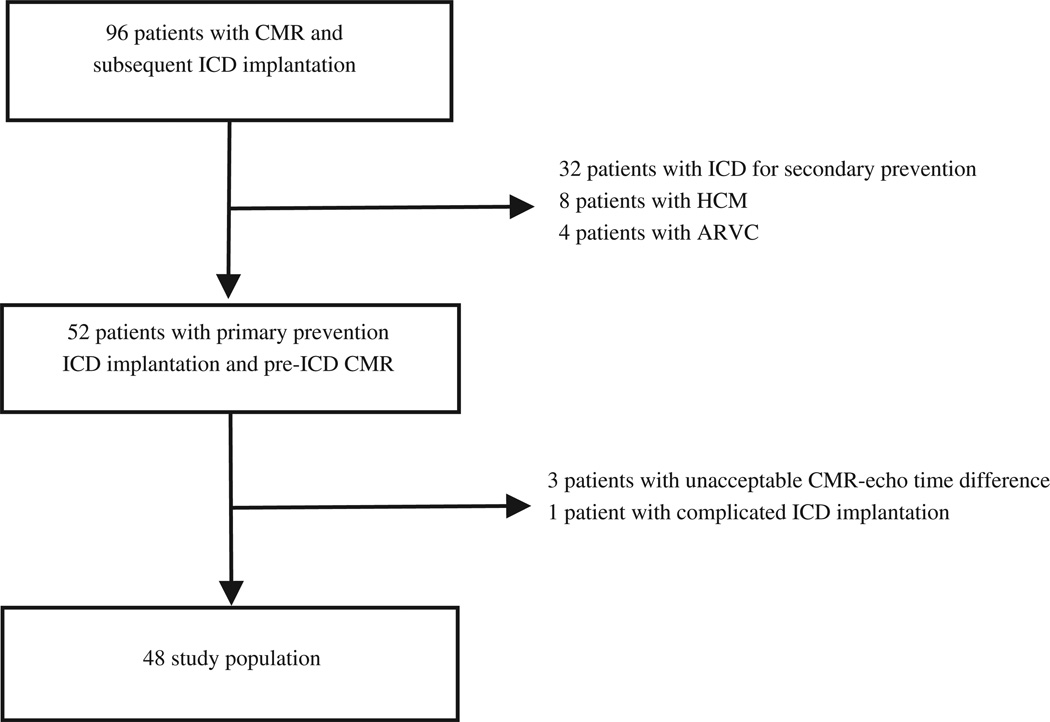
A total of 96 patients with CMR-LVEF data available before ICD implantation were identified, of whom 52 patients were referred for primary prevention of sudden cardiac death and underwent successful pre-ICD implantation CMR. Of these 52 patients, 3 had unacceptable CMR-Echo intervals and 1 had a complicated ICD implantation, which led to nonarrhythmic death 2 days after the procedure. These 4 patients were excluded from the analysis, leaving 48 patients in the present retrospective cohort study (Figure 1). The mean follow-up period was 29 × 25 months (range 1 to 99, median 20). The baseline characteristics of the entire cohort are summarized in Table 1.
Figure 1.
Flow diagram of the study. ARVC = arrhythmogenic right ventricular cardiomyopathy; CMR = cardiovascular magnetic resonance imaging; HCM = hypertrophic cardiomyopathy.
Table 1.
Baseline characteristics
| Variable | All (n = 48) | Appropriate ICD Discharge | p Value | |
|---|---|---|---|---|
| Yes (n = 9) | No (n = 39) | |||
| Age (yrs) | 63 ± 11 | 68 ± 10 | 63 ± 11 | 0.678 |
| Men | 34 (71%) | 8 (89%) | 26 (67%) | 0.212 |
| Ischemic cardiomyopathy | 25 (52%) | 6 (67%) | 19 (49%) | 0.640 |
| Biventricular pacing | 15 (31%) | 2 (22%) | 13 (33%) | 0.237 |
| Diabetes | 16 (33%) | 6 (67%) | 10 (26%) | 0.052 |
| Hypertension | 36 (75%) | 7 (78%) | 29 (74%) | 0.892 |
| Dyslipidemia | 27 (56%) | 5 (56%) | 22 (56%) | 0.461 |
| β Blocker | 41 (85%) | 7 (78%) | 34 (87%) | 0.700 |
| Angiotensin-converting enzyme inhibitor | 45 (94%) | 9 (100%) | 36 (92%) | 0.995 |
| Antiarrhythmic | 2 (4%) | 0 | 2 (5%) | 0.994 |
| Aspirin | 37 (77%) | 7 (78%) | 30 (77%) | 0.730 |
| New York Heart Association class before cardioverter-defibrillator implantation | 2.4 ± 0.6 | 2.6 ± 0.8 | 2.5 ± 0.6 | 0.741 |
| Inappropriate implantable cardioverter-defibrillator discharge | 3 (6%) | 0 | 3 (8%) | 0.994 |
| Echocardiography—left ventricular ejection fraction (%) | 23 ± 6 | 19 ± 5 | 24 ± 7 | 0.018 |
| Cardiac magnetic resonance—left ventricular ejection fraction (%) | 29 ± 9 | 22 ± 6 | 31 ± 9 | 0.007 |
| Cardiac magnetic resonance—end-diastolic volume (ml) | 268 ± 88 | 301 ± 79 | 260 ± 90 | 0.237 |
| Cardiac magnetic resonance—end-diastolic volume index | 138 ± 40 | 153 ± 40 | 135 ± 39 | 0.200 |
After a mean follow-up of 29 × 25 months, an appropriate ICD discharge had occurred in 9 patients (19%), including 4 who had received antitachycardial pacing and 5 who had received ICD shocks. In addition, 3 patients (6%) had received inappropriate shocks for atrial fibrillation.
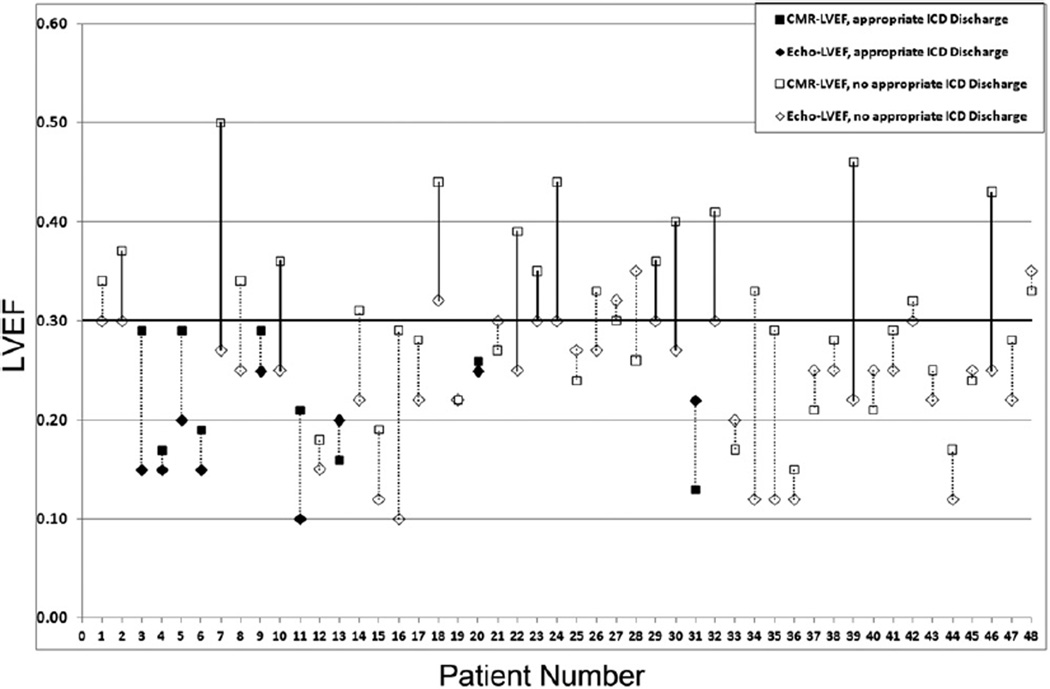
Although all patients (100%) met the Echo-LVEF criteria for ICD implantation, 25% (95% confidence interval 0.15% to 0.40%) of the subjects did not meet the criteria using the CMR-LVEF. Of these latter patients, none (0%) had received an appropriate ICD discharge (Figures 2 and 3).
Figure 2.
Individual Echo-LVEF and CMR-LVEF for each patient (each line corresponds to an individual patient). Patient numbers were sorted according to interval of ICD implantation; patient 1 received an ICD in 2001 and patient 48 received an ICD in 2011. Solid lines represent patient reclassification after applying CMR-LVEF. Solid markers represent those patients in whom an appropriate ICD discharge occurred.
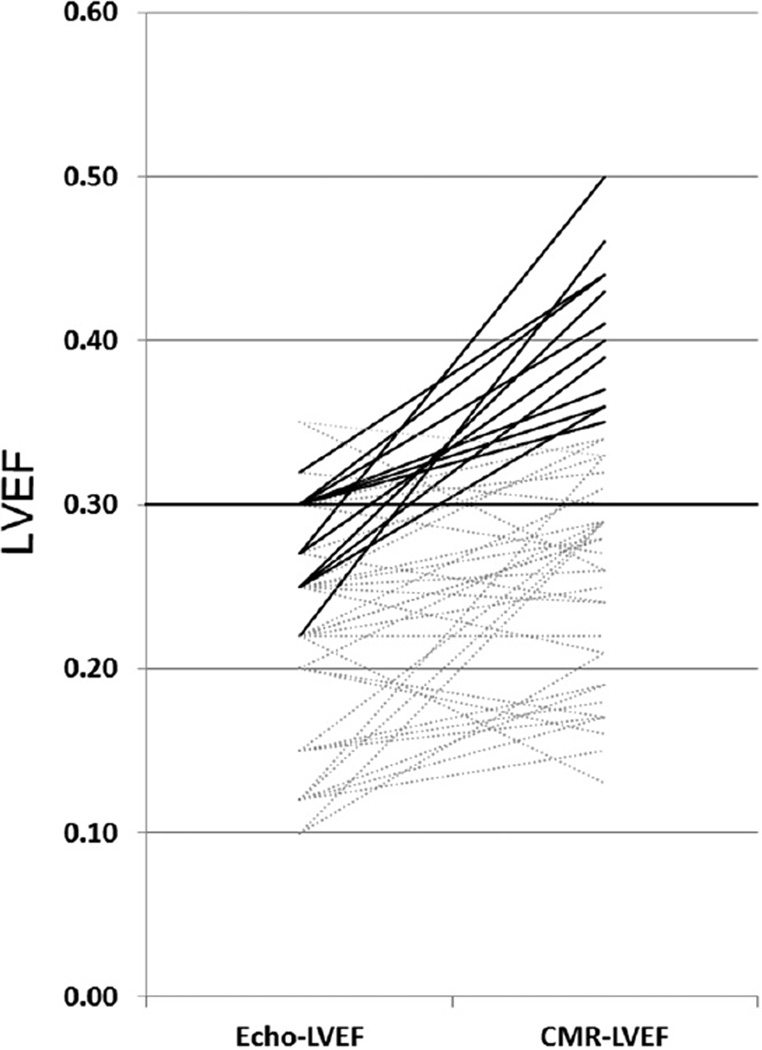
Figure 3.
Change in classification for ICD implantation using CMR-LVEF. Each line corresponds to 1 patient and left and right points demonstrate corresponding Echo-LVEF and CMR-LVEF, respectively. Solid lines represent those patients reclassified after applying CMR-LVEF.
The CMR-LVEF was ≤30% for all patients (ischemic, n = 6; nonischemic, n = 3) who had received an appropriate ICD discharge. In addition, 19 patients had a CMR-LVEF >30%, with appropriate ICD discharge occurring in none of these patients (0%). Using the CMR-LVEF threshold of ≤30% for ICD eligibility would have identified 19 of 48 (40%) of the Echo-LVEF–qualifying patients with a CMR-LVEF of ≥30% who did not require an ICD (Figure 2).
All patients with an appropriate ICD discharge had an Echo-LVEF of ≤30%; however, in the group with no appropriate ICD discharge, only 4 patients (8.3%) had an Echo-LVEF >30% compared to 19 patients with a CMR-LVEF >30%. Thus, applying a CMR-LVEF ≤30% threshold for ICD eligibility would safely spare 4.7 times (19 vs 4) more patients compared to using the Echo-LVEF ≤30% threshold, in whom an appropriate ICD discharge did not occur.
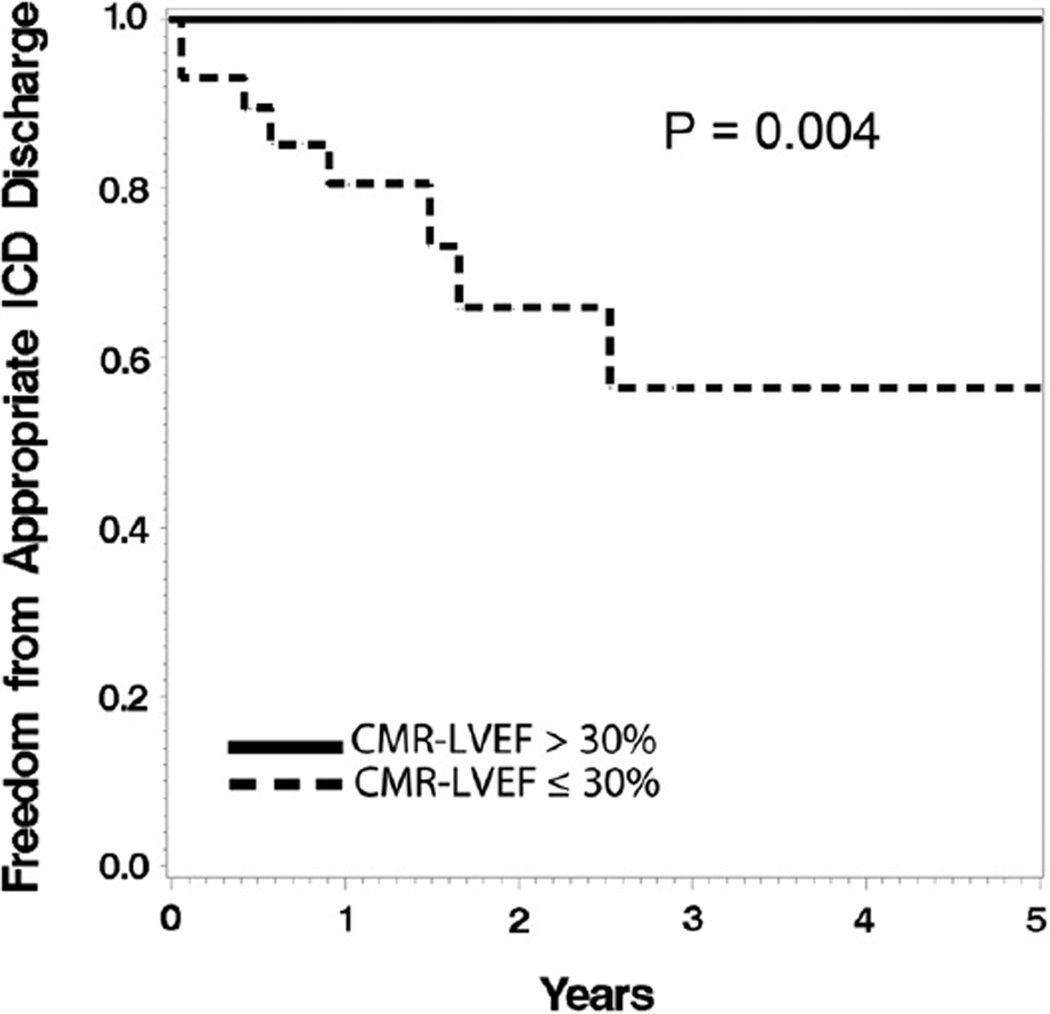
Survival free of an appropriate ICD discharge was significantly greater among the patients with a CMR-LVEF >30% than among those with a CMR-LVEF of ≤30% (p = 0.004; Figure 4). It was comparable between patients with an Echo-LVEF ≤30% and Echo-LVEF >30% (p = 0.55).
Figure 4.
Kaplan-Meier analysis. Patients with CMR-LVEF ≤30% had lower survival free of appropriate ICD discharge (log-rank p = 0.004).
Discussion
In the present retrospective study, we have demonstrated that volumetric CMR-LVEF is superior to Echo-LVEF for the classification of patients referred for primary prevention ICD implantation. In a mixed population of patients with ischemic and nonischemic cardiomyopathy, Echo-LVEF underestimated the LVEF compared to CMR-LVEF, and the greater value given by CMR led to reclassification of 25% (95% confidence interval 0% to 37%) of the patients qualifying using Echo-LVEF to low-risk status. Thus, ¼ of patients classified as high risk (ICD eligible) using Echo-LVEF were reclassified as low risk using CMR-LVEF and thus could have safely avoided ICD implantation. Additionally, our data suggest a lower threshold of CMR-LVEF for risk stratifying all patients at risk of sudden cardiac death; this would result in a 40% reduction in referrals for primary prevention ICD implantation, with none of these subjects receiving appropriate ICD therapy.
Our findings have demonstrated that volumetric LVEF assessment is superior to clinical transthoracic echocardiography for risk stratification of patients considered for primary prevention ICD implantation, confirming previous data that echocardiography underestimates the LVEF compared with volumetric CMR.8
In agreement with our study, Chuang et al8 reported that in 25 patients with dilated cardiomyopathy, volumetric CMR led to reclassification of the patients who had been stratified according to the Echo-LVEF.
Using a CMR-LVEF of ≤30% as the threshold for primary prevention ICD eligibility, 40% of Echo-LVEF–qualifying patients would have safely avoided the cost, inconvenience, and potential morbidity of ICD implantation. This finding is in accordance with recent observations that an ICD is more beneficial in patients with an LVEF of ≤30%. In a post hoc analysis of the SCF-HeFT,2 the benefit of an ICD was seen in patients with an LVEF of ≤30%, but not in those with an LVEF >30%. Also, in the Multicenter Automatic Defibrillator Implantation Trail I (MADIT-I),13 a survival benefit with an ICD was only seen in high-risk patients with more severe heart disease (i.e., LVEF <26%, heart failure requiring therapy, or a QRS duration of ≥0.12 second). The benefit increased progressively as a function of the number of risk factors. In addition, subgroup analysis of the MADIT-II showed no survival benefit in patients with an LVEF >25%.14 Although in subjects with an Echo-LVEF of ≤20%, the CMR-LVEF was greater than the Echo-LVEF, the difference was not significant enough for any reclassification.
Our study was relatively small and retrospective, with LVEF data extracted from the clinical reports and determined by multiple readers rather than by core laboratory analysis. However, this was likely more reflective of the “real world” experience. Although volumetric CMR methods were used for the present study, other methods, such as radionuclide ventriculography and cardiac computed tomography, could also be useful and remain to be explored. Larger prospective series using volumetric methods are needed to confirm these results before applying these findings in routine clinical practice.
Acknowledgment
We acknowledge Sebastian Weingärtner, BS, for his help in preparing some of the figures for our report.
This study was funded in part by grant R01EB008743-01 from the National Institutes of Health (Bethesda, Maryland) and grant 1 UL1 RR025758-01 from the National Center for Research Resources, National Institutes of Health (Bethesda, Maryland).
The contents of our report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or National Center for Research Resources (Bethesda, Maryland).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352:222–224. doi: 10.1056/NEJMp048354. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Sanders GD, Bigger JT, Buxton AE, Califf RM, Carlson M, Curtis A, Curtis J, Fain E, Gersh BJ, Gold MR, Haghighi-Mood A, Hammill SC, Healey J, Hlatky M, Hohnloser S, Kim RJ, Lee K, Mark D, Mianulli M, Mitchell B, Prystowsky EN, Smith J, Steinhaus D, Zareba W. Preventing tomorrow’s sudden cardiac death today: part I: current data on risk stratification for sudden cardiac death. Am Heart J. 2007;153:941–950. doi: 10.1016/j.ahj.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. MADIT-II Investigators. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 6.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 7.Bierig SM, Ehler D, Knoll ML, Waggoner AD. American Society of Echocardiography minimum standards for the cardiac sonographer: a position paper. J Am Soc Echocardiogr. 2006;19:471–474. doi: 10.1016/j.echo.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, Douglas PS, Manning WJ. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol. 2000;35:477–484. doi: 10.1016/s0735-1097(99)00551-3. [DOI] [PubMed] [Google Scholar]
- 9.Grimm W, Hoffmann JJ, Muller HH, Maisch B. Implantable defibrillator event rates in patients with idiopathic dilated cardiomyopathy, nonsustained ventricular tachycardia on Holter and a left ventricular ejection fraction below 30% J Am Coll Cardiol. 2002;39:780–787. doi: 10.1016/s0735-1097(01)01822-8. [DOI] [PubMed] [Google Scholar]
- 10.Hook BG, Marchlinski FE. Value of ventricular electrogram recordings in the diagnosis of arrhythmias precipitating electrical device shock therapy. J Am Coll Cardiol. 1991;17:985–990. doi: 10.1016/0735-1097(91)90884-c. [DOI] [PubMed] [Google Scholar]
- 11.Marchlinski FE, Gottlieb CD, Sarter B, Finkle J, Hook B, Callans D, Schwartzman D. ICD data storage: value in arrhythmia management. Pacing Clin Electrophysiol. 1993;16:527–534. doi: 10.1111/j.1540-8159.1993.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 12.Callans DJ, Hook BG, Marchlinski FE. Use of bipolar recordings from patch-patch and rate sensing leads to distinguish ventricular tachycardia from supraventricular rhythms in patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 1991;14:1917–1922. doi: 10.1111/j.1540-8159.1991.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 14.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]