Abstract
In a recent issue of Cell, Iliopoulos and colleagues demonstrate a novel and targetable epigenetic amplification loop in hepatocellular carcinoma involving HNF4α, miR-124, IL6-R, Stat3, miR-24 and miR-629. These results establish microRNAs as novel players in early stages of hepatocarcinogenesis and as potential targets for the treatment of hepatocellular carcinoma.
Liver cancer is the fifth leading cause of cancer mortality worldwide with an annual death toll of 700,000. In contrast to the decreasing mortality rates observed for many other types of cancers, liver cancer incidence and overall mortality have significantly increased in the United States over the past 20 years (El-Serag, 2011). Risk factors for hepatocellular carcinoma (HCC), the most common type of primary liver cancer, include chronic viral hepatitis, alcohol abuse and non-alcoholic fatty liver disease (El-Serag, 2011). Most HCC patients are diagnosed at intermediate or advanced stages, rendering only 15% of HCC patients eligible for curative therapies such as resection or liver transplantation. The introduction of the multi-kinase inhibitor sorafenib as the first drug with therapeutic efficacy against HCC represents the biggest therapeutic advance in the past decade (Llovet et al., 2008). Major hurdles in developing effective therapies against HCC have been its considerable heterogeneity and the absence of well-established and broadly targetable oncogene addiction. Although inflammation is a characteristic feature of the environment in which most HCCs arise, and inflammatory factors including IL-6 promote HCC in mice (Naugler et al., 2007) and negatively influence outcome in patients (Hoshida et al., 2008), few therapeutic efforts have been directed at these pathways.
In a recent paper published in Cell, Iliopoulos and colleagues demonstrate a novel and targetable proinflammatory pathway through which hepatocytes may acquire a transformed phenotype (Hatziapostolou et al., 2011). One of the outstanding features of this study is the discovery of an entirely epigenetic circuit that initiates transformation in the absence of genetic alterations. Another remarkable finding of the study is that the described epigenetic positive feedback circuit can maintain an inflammatory state through multiple generations of cells following removal of the initial trigger. This finding is similar to a previous study in which Iliopoulos et al. uncovered an epigenetic circuit in breast cancer that maintains inflammation through a Src-initiated NF-κB – Lin28 – Let-7 – IL-6 – NF-κB positive feedback loop (Iliopoulos et al., 2009).
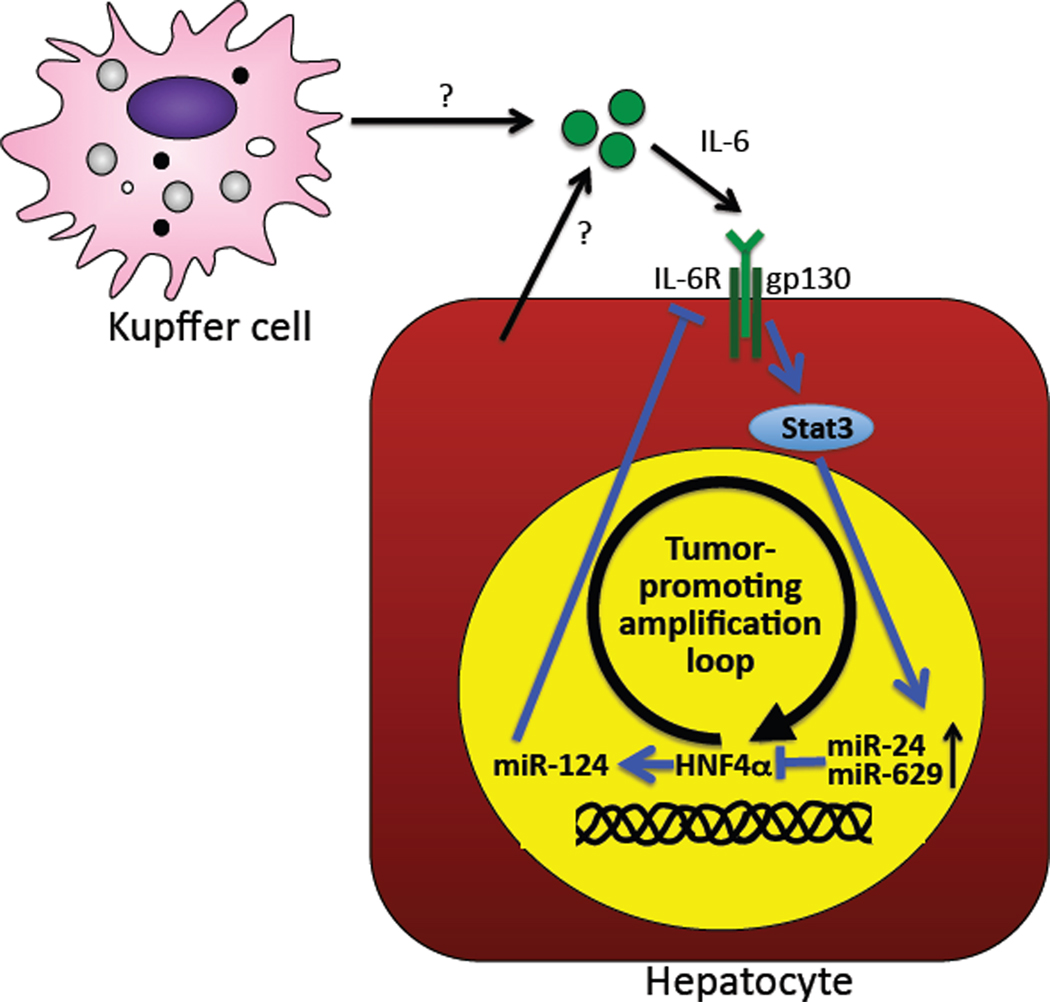
In the present study, the authors focus on HNF4α, a transcription factor that is essential for the development and differentiation of hepatocytes (Parviz et al., 2003). Knockdown of HNF4α in immortalized human hepatocytes led to cellular transformation and increased tumor formation after transplantation into immunosuppressed mice. As HNF4α expression was still suppressed after two months, the authors speculated that a continuous feedback loop must maintain HNF4α at low levels in these cells. Step-by-step, the authors meticulously uncovered several components of this feedback loop (Figure 1). First, the authors identified microRNAs miR-24 and miR-629 as potent regulators of HNF4α expression and confirmed the feedback loop by demonstrating increased miR-24 and miR-629 after HNF4α knockdown. In other words, knocking down HNF4α activates mechanisms that subsequently amplify or perpetuate the knockdown HNF4α. Overexpression of miR-24 and miR-629 virtually phenocopied the effects of HNF4α on gene expression, transformation and tumor formation. Common to both miR-24 and miR-629 was the presence of a Stat3 binding site in their promoter and their direct regulation by Stat3 as demonstrated by ChIP assays and Stat3-dependent induction of their expression by IL-6. Finally, the missing link between HNF4α and Stat3 was identified as miR-124, a microRNA that contains a HNF4α binding site in its promoter and directly targets IL-6R, thus closing the loop to IL-6-mediated Stat3 activation. The overexpression of Stat3 targets miR-24 and miR-629 or inhibition of HNF4α, the inducer of IL-6R repressing miR-124, all resulted in increased Stat3 phosphorylation, again confirming the presence of a positive feedback loop.
Figure 1. Transformation of hepatocytes by a HNF4α - miR-124 – IL6-R – Stat3 – miR-24/miR-629 amplification loop.
Temporary suppression of HNF4α induces permanent suppression of HNF4α by a feed-forward amplification loop that includes decreased miR-124 expression after HNF4α suppression, a subsequent increase in IL-6R expression and Stat3 signaling, and an upregulation of Stat3-inducible and HNF4α-targeting microRNAs miR-24 and miR-629.
To test the relevance of this novel inflammatory circuit in vivo, the authors delivered miR-124 to the liver during diethylnitrosamine (DEN)-induced hepatocarcinogenesis. Not only did miR-124 delivery decrease the number and size of DEN-induced tumors by 90% when given throughout the entire study, but it also led to a striking 80% decrease when treatment was started only 4 weeks before sacrifice. The authors also confirmed relevance to human hepatocarcinogenesis by demonstrating downregulation of HNF4α and its target miR-124, and upregulation of miR-24 and IL-6R in human HCC. Moreover, there were strong inverse correlations between miR-24 and miR-629 and their target HNF4α as well as between miR-124 and its target IL-6R, and a strong positive correlation between HNF4α and its target miR-124, thus suggesting the existence of the same epigenetic regulatory circuit in human HCC.
The data by Hatziapostolou et al. add significantly to the expanding data on microRNA control in cancer. In the liver, previous studies have shown that both up- and down-regulation of microRNAs contribute to liver cancer growth and invasiveness and predict survival (Ding et al., 2010; Hou et al., 2011; Ji et al., 2009). The two surprising findings of the current study are the key contribution of microRNAs to early stages of carcinogenesis and the astounding efficacy of microRNA therapy. The finding that transient suppression of a single transcription factor, HNF4α, or overexpression of miR-24 and miR-629 results in cellular transformation is unexpected and represents a major advance in the field. However, while the authors show clear relevance of HNF4α, miR-24 and miR-629 in established murine and human HCC, most of the data on transformation by transient HNF4α knockdown or miR-24 and miR-629 overexpression is based on studies in cell lines. Further in vivo models are needed to determine whether temporary knockdown of HNF4α or combined overexpression of miR-24 and miR-629 in the liver are indeed sufficient to trigger carcinogenesis. It is conceivable that factors generated in the tumor microenvironment, such as Kupffer cell-derived IL-6, might also serve to initiate the feed-forward loop in patients with chronic liver disease. The finding that miR-124 inhibits DEN hepatocarcinogenesis raises the prospect that microRNA-based therapies may represent a novel avenue to cancer treatment. While acknowledging that therapeutic success in mouse models is not always reproducible in clinical trials in patients, the demonstrated efficacy of miR-124 delivery appears unusually promising. Notably, the profound effects of miR-124 in advanced stages suggest that miR-124 may actually regress established tumors. These results should be further confirmed, preferentially in HCC models that incorporate the typical inflammatory and fibrotic microenvironment seen in patients with HCC. Since tumor cell populations are not homogeneous, it would also be important to investigate whether miR-124 based therapy affects all tumor cells equally, or whether subsets of cells, such as cancer stem cells, might escape from this treatment. Moreover, the key involvement of IL-6R in this circuit suggests that IL-6 antagonists may be able to interrupt this circuit and tumor growth.
In conjunction with their previous study (Iliopoulos et al., 2009), this new data by Iliopoulos and colleagues indicate that epigenetic circuits can establish permanent alterations of cellular phenotypes, and drive cancer initiation with a similar (or greater) potency as genetic alterations of oncogenes or tumor suppressors. This will without doubt reopen the debate as to whether genetic or epigenetic alterations represent the first hit in most human cancers. The recognition of a potential tumor-initiating role for microRNAs may render tumor cell biology even more complex - but also more exciting as it will undoubtedly open the door for new therapies, especially for difficult-to-treat cancers such as HCC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ding J, Huang S, Wu S, Zhao Y, Liang L, Yan M, Ge C, Yao J, Chen T, Wan D, et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol. 2010;12:390–399. doi: 10.1038/ncb2039. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, Ogata A, Karin M, Struhl K, Hadzopoulou-Cladaras M, Iliopoulos D. An HNF4α-miRNA Inflammatory Feedback Circuit regulates Hepatocellular Oncogenesis. Cell. 2011:147. doi: 10.1016/j.cell.2011.10.043. *bxs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–243. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 10.Parviz F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS, Duncan SA. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet. 2003;34:292–296. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]