Abstract
Significance: Pulmonary arterial hypertension (PAH) is a disorder characterized by increased pulmonary vascular resistance and mean pulmonary artery pressure leading to impaired function of the right ventricle, reduced cardiac output, and death. An imbalance between vasoconstrictors and vasodilators plays an important role in the pathobiology of PAH. Recent Advances: Nitric oxide (NO) is a potent vasodilator in the lung, whose bioavailability and signaling pathway are impaired in PAH. It is now appreciated that the oxidative product of NO metabolism, the inorganic anion nitrite (NO2−), functions as an intravascular endocrine reservoir of NO bioactivity that can be reduced back to NO under physiological and pathological hypoxia. Critical Issues: The conversion of nitrite to NO is controlled by coupled electron and proton transfer reactions between heme- and molybdenum-containing proteins, such as hemoglobin and xanthine oxidase, and by simple protonation and disproportionation, and possibly by catalyzed disproportionation. The two major sources of nitrite (and nitrate) are the endogenous l-arginine–NO pathway, by oxidation of NO, and the diet, with conversion of nitrate from diet into nitrite by oral commensal bacteria. In the current article, we review the enzymatic formation of nitrite and the available data regarding its use as a therapy for PAH and other cardiovascular diseases. Future Directions: The successful efficacy demonstrated in several animal models and safety in early clinical trials suggest that nitrite may represent a promising new therapy for PAH. Antioxid. Redox Signal. 18, 1797–1809.
Introduction
Pulmonary hypertension (PH) is a life-threatening progressive disorder characterized by persistent elevation in pulmonary artery pressure (>25 mmHg at rest), and increased pulmonary vascular resistance. With increasing pulmonary vascular resistance, there is a progressive increase in after-load on the right ventricle, leading to concentric hypertrophy, dilation, and decreased function (Fig. 1). This exerts significant stress on the right heart that will eventually fail if left untreated, leading to a drop in cardiac output. Right heart failure and a low cardiac output lead to the major symptoms of pulmonary arterial hypertension (PAH), such as dyspnea on exertion and syncope, and increasing risk of death.
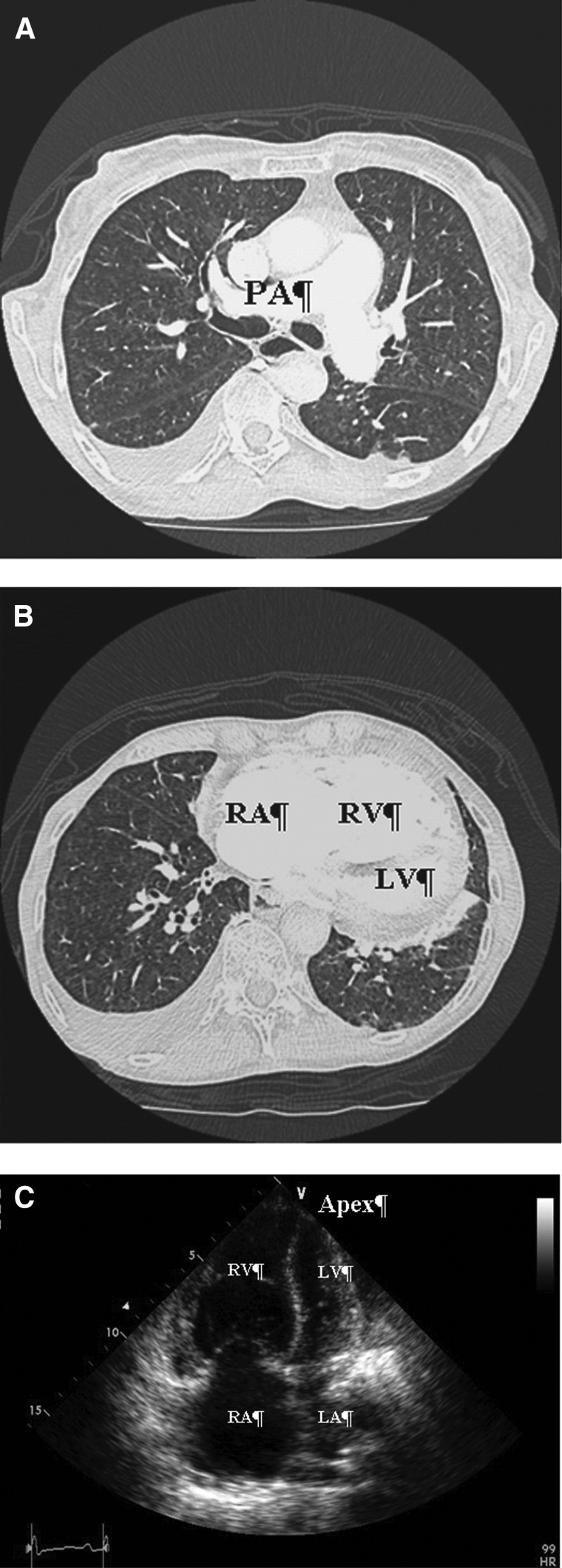
FIG. 1.
Radiological imaging in PH. (A) Contrast enhanced-CT image showing enlarged pulmonary artery in PAH patient. (B) CT image obtained from patient with severe PH. The right-sided chambers are dilated, the right ventricle hypertrophied. (C) Apical four-chamber two-dimensional echocardiography image showing enlarged right-sided chambers and small left ventricle. CT, computed tomography; LA, left atrium; LV, left ventricle; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RA: right atrium; RV, right ventricle.
PAH is a multifactorial process, but early disease is related to a dysregulation of critical vasodilator pathways (downregulation of nitric oxide [NO] and prostaglandin signaling) and vasoconstrictor pathways (upregulation of endothelin-1 and reactive oxygen species [ROS] signaling) (75). PAH is associated with decreased bioavailability and responsiveness of NO (14, 32). NO is produced in mammalian cells primarily in the metabolism of l-arginine. NO synthase (NOS) catalyzes the oxidation of l-arginine to produce l-citrulline in the presence of oxygen and NADPH (Fig. 2). Although the expression levels of endothelial NOS (eNOS) in patients with PAH can vary from high to normal, the formation of NO and eNOS activity have been found reduced in patients with idiopathic PAH (75). Recent studies suggest that eNOS uncoupling may relate to impaired NO production in PAH, a process by which the enzyme transfers electrons from the NOS reductase domain to the oxygenase domain and diverted to molecular oxygen forming superoxide rather than NO. This dysfunctional state of the eNOS enzyme in the presence of high levels of superoxide generation may increase the formation of peroxynitrite formation and enhance the vascular disease during PH.
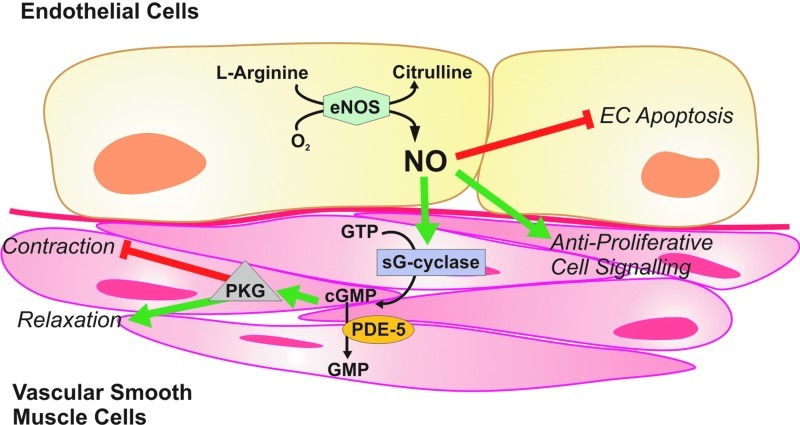
FIG. 2.
The classical l-arginine-NOS-NO signaling pathway. NO is produced in mammalian cells by an oxygen-dependent oxidation of a guanidine nitrogen of l-arginine (with citrulline as a side product). This multistep reaction is catalyzed by the heme-containing protein NOS, which also requires two flavin molecules and tetrahydrobiopterin as cofactors. In most endothelial cells, the type III isoform is expressed (or eNOS) as is regulated by calcium-dependent binding of calmodulin and by tyrosine phosphorylation. Target tissue effects of NO depend on its quantity. At higher concentrations, NO rapidly reacts with oxygen (and especially with superoxide), forming the highly reactive peroxynitrite. At lower concentrations, NO serves a regulatory role via the activation of soluble guanylate cyclase, resulting in increased cGMP levels in target cells. In vascular smooth muscle, cGMP causes relaxation by reducing the intracellular calcium concentration and by downregulating the contractile apparatus. These actions are mostly (although not exclusively) mediated by type I cGMP-dependent protein kinases. cGMP, cyclic guanosine monophosphate; eNOS, endothelial NOS; NO, nitric oxide; NOS, nitric oxide synthase. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Inhaled NO (iNO) is considered as a potential therapy targeting the NO pathway (33, 39), and a potent and selective pulmonary vasodilator (65). iNO therapy has been proposed for treatment of PAH, but also for persistent PH of the newborn (15), and bronchopulmonary dysplasia in prematurely born infants (21) and post-cardiac surgery (3). Other possible applications of iNO therapy include the treatment of pulmonary ischemia-reperfusion injury (9), the acute respiratory distress syndrome (18), and hypoxemia in the setting of severe chronic obstructive pulmonary disease (26). However, iNO gas has its limitations regarding dose and duration of the exposure, cumbersome and expensive delivery systems, off-target reactions of NO with oxygen to form reactive nitrogen species, and possible rebound PH when the intervention is interrupted (38). These limitations open the door to other molecules that can be a source of NO and nitrosative signaling, including the anion nitrite (NO2−) (55). Nitrite is a unique reactive nitrogen species, as it is relatively stable compared to NO [51.4 min (67) vs. 0.05–1.8 ms (68) half-life in whole blood] and can readily be reduced to NO, oxidized to nitrogen dioxide, and protonated to form S-nitrosothiol, providing NO-dependent and NO-independent signaling effects that have been reported to modulate vascular remodeling (2, 86).
Rediscovering Nitrite in Biology
Although in the ancient Chinese medicine, the sodium salts of nitrate and nitrite were used as a remedy for heart pains and ischemia (circa 800 AD) (54), only until recently, nitrate (NO3−) and nitrite (NO2−) were mostly considered undesired residues in the food chain with potential carcinogenic activity. Nitrate salts have also been used by early civilizations to cure meats, which not only improve their storage time and produce their reddish color but also kill pathogenic bacteria (69). The mechanism of the meat preservation by nitrate was characterized in the 19th century, when it was discovered to be converted to NO (bound to myoglobin). During the 1970s, public concern over toxicity increased, when nitrate and nitrite were associated to the endogenous formation of N-nitrosamines, which are carcinogenic. While remaining extremely controversial, the epidemiological links between nitrate/nitrite exposure, for example, in high-nitrate foods such as leafy green vegetables, remain unclear (25, 79).
The first relationship between nitrite formation and NO production was found by studying immune responses in activated macrophages. Analyses of bacterial-infected (74) or tumor-bearing (36) mice showed that macrophages use l-arginine to produce nitrite and l-citrulline. This was the first demonstration of the oxidation of a terminal nitrogen atom of l-arginine to an inorganic nitrogen oxide, an enzymatic activity later shown to be mediated by inducible NOS (iNOS). Marletta and colleagues define that a terminal guanidino nitrogen atom was the precursor of nitrite and nitrate synthesized by activated macrophages (41), and Tannenbaum et al. (76) confirmed that nitrate and nitrite are formed de novo in the intestine of the human body, reflecting in vivo NO formation and oxidation. These observations were followed by the demonstration of NO synthesis by mammalian endothelial cells. Moving forward another decade, NO was identified as a critical regulator of vascular homeostasis, neurotransmission, and host defense; and nitrite was considered a biomarker of NO levels or NO formation in vivo (40, 62).
More recently, increasing evidence suggests that nitrite not only is a biomarker of NO formation from NO synthesis but also represents a storage reservoir for NO that can be converted back to NO during physiological and pathological hypoxia (8, 16, 56). It is now known that nitrate and nitrite can be recycled to NO (or other bioactive forms of nitrogen) in blood and peripheral tissue (27, 55), representing an alternative to the classical l-arginine-NOS-NO signaling. In situations of hypoxia, when the oxygen-dependent function of the NOS may become compromised, nitrite reduction is enhanced. Chemically, nitrite shows a unique redox position between oxidative (NO2 radical) and reductive (NO radical) signaling and has a relatively long half-life in blood and tissue (67), representing a storage pool supporting NO signaling during hypoxic or metabolic stress.
The Nitrate-Nitrite-NO Signaling Pathway
The reductive mammalian nitrate-nitrite-NO pathway mediates important signaling events and complements the traditional oxidative l-arginine-NOS-NO pathway. The two major sources of nitrite (and nitrate) are the endogenous l-arginine-NO pathway and the diet. Our daily intake of exogenous nitrite mainly comes from food additives, preservatives, and drinking water, and nitrate from leafy greens and root vegetables. Upon intake of nitrate (Fig. 3), symbiotic bacteria in the oral cavity can reduce nitrate to nitrite producing a high concentration of nitrite (up to low millimolar) in saliva (8, 56). Salivary glands are able to concentrate and secrete nitrate (from plasma or diet), increasing the amount of nitrate available to the oral microbiome to be reduced to nitrite. When saliva is then swallowed and reaches the low pH milieu of the stomach, nitrite is converted nonenzymatically to NO via protonation and reduction (8) (Table 1). Nitrate and nitrite can be absorbed in the gastrointestinal tract into the circulation. Excess of nitrate will be excreted, and nitrite can be further reduced to NO by different enzymes through our body (55). NO can be oxidized to nitrate by oxyhemoglobin (29) or to nitrite by reaction with oxygen [hydrophobic NO autoxidation (53)] and by reaction with the copper-containing ceruloplasmin (73). This new pool of nitrite and nitrate is available again for uptake by the salivary glands, closing what is known as the enterosalivary circulation of nitrate (55).
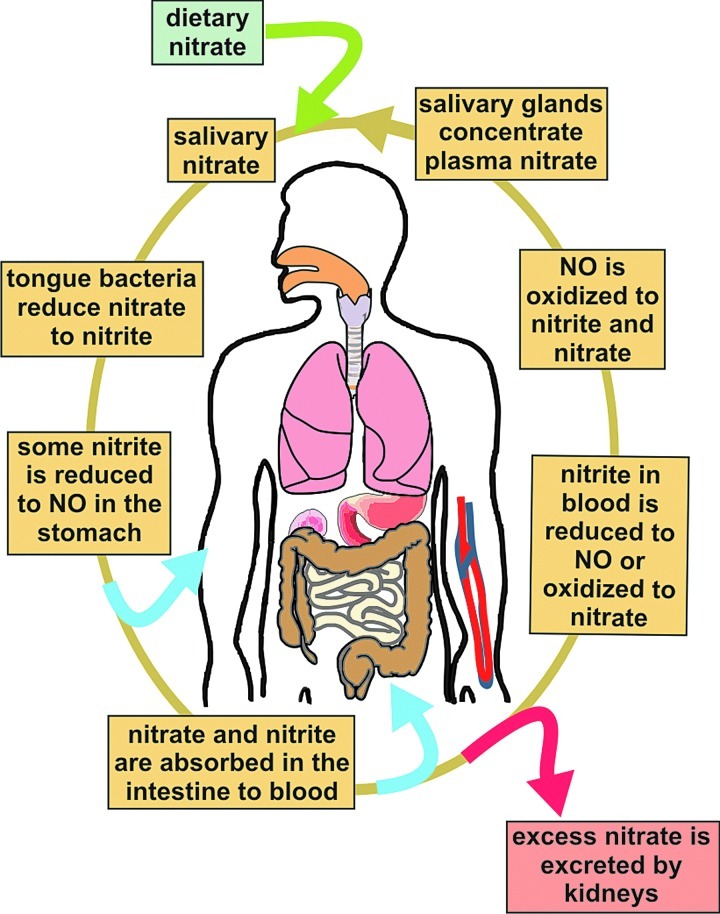
FIG. 3.
The enterosalivary circulation of nitrate in humans. The activity of orally ingested inorganic nitrate (from dietary sources—entry point as a green arrow) is thought to lie in its conversion to nitrite by facultative anaerobic bacteria found on the dorsal surface of the tongue. By swallowing, saliva enters the acidic stomach (around 1 L per day), where much of the nitrate is rapidly protonated to form nitrous acid, which decomposes further to form NO (absorption arrow in blue) and other bioactive nitrogen species. Nitrate and remaining nitrite are then absorbed from the intestine into the circulation and can be converted to bioactive NO in blood (absorption arrow in blue). Later on, NO can again be oxidized to nitrite and/or nitrate in tissue. Although much of the nitrate is excreted in the urine (excretion arrow in red), salivary glands actively concentrate nitrate from plasma that can go back again to the mouth to be reduced to nitrite. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Table 1.
Biological Reactions of the Reduction of Nitrite
| Agent | Reaction | |
|---|---|---|
| Protons, acidic milieu | NO2−+H+↔HNO2 | Eq. (1) |
| 2HNO2↔N2O3+H2O | Eq. (2) | |
| N2O3↔NO+NO2 | Eq. (3) | |
| Ascorbate | NO2−+H+↔HNO2 | |
| 2HNO2+Asc→2NO +dehydroAsc+2H2O | Eq. (4) | |
| Polyphenols | NO2−+H+↔HNO2 | |
| Ph-OH+HNO2→PH-O +NO+H2O | Eq. (5) | |
| Heme-containing proteins | NO2−+Fe2++H+→NO +Fe3++OH- | Eq. (6) |
| Molybdopterin-containing proteins | NO2−+Mo4++H+→NO +Mo5++OH- | Eq. (7) |
Mechanisms of Nitrite Bioactivation
Nitrite can be reduced to NO in vivo by both enzymatic and nonenzymatic processes (Fig. 4). Biological non-enzymatic NO formation was first reported by Zweier et al. (87) in an ischemic rat tissue where, at low pH, there was NOS-independent NO production. Benjamin et al. (8) showed that in the highly acidic environment (pH 3) of the stomach, nitrite is converted to NO gas. Nitrite production of NO at acidic pH is also enhanced by the presence of reducing compounds such as copper, ascorbate, and polyphenols (82) [Table 1, Eqs. (1)–(3)].
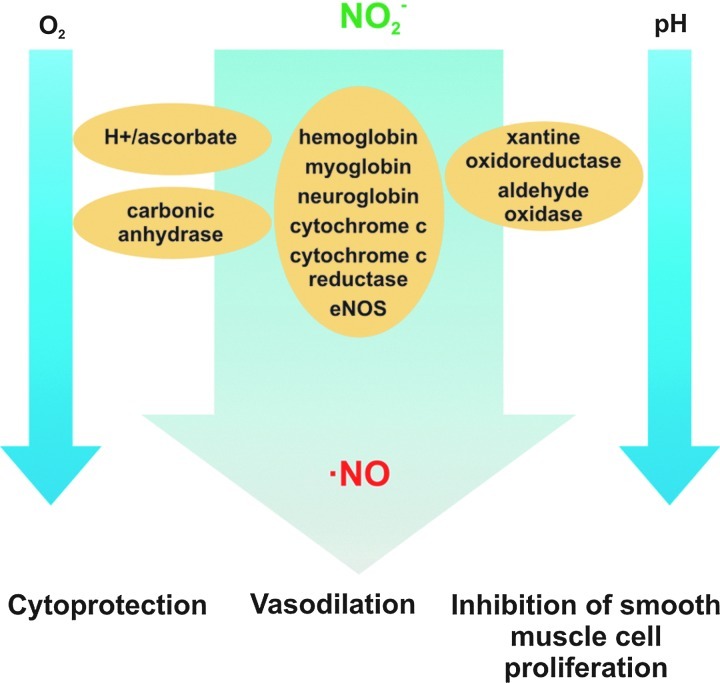
FIG. 4.
Nitrite chemistry, physiology, and therapeutics. Nitrite reduction to NO is favored by decreasing physiological oxygen tensions and low pH, via nonenzymatic pathways (in the presence of acid or reducing substrates) or enzymatic pathways catalyzed by metal-containing enzymes. The generated NO modulates critical signal transduction processes, inducing cytoprotection, vasodilation, and inhibition of SMC proliferation (30). SMC, smooth muscle cell. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Enzymatic NO formation from nitrite, via facilitated proton and electron transfer reactions, has been reported for a wide variety of metal-containing proteins, including hemoglobin (22), myoglobin (70), neuroglobin (77), cytochrome c (4), cytochrome c oxidase (12), eNOS (78), xanthine oxidoreductase (XOR) (84), aldehyde oxidase (AO) (50), and carbonic anhydrase (1).
Heme-containing proteins
Deoxyhemoglobin
Deoxyhemoglobin (deoxy-Hb) can catalyze nitrite reduction accompanied with NO generation and production of ferric heme (Fe+3). The formed NO can then bind to another deoxyheme to form iron-nitrosyl (Fe+2-NO) (Table 1) (22). As for other heme proteins, the reaction is faster at low pH, indicating the involvement of the protonated nitrite (nitrous acid—HNO2) [Table 1, Eq. (6)]. The reaction is allosterically regulated, being slow in the T-state, but much faster for the R-state deoxy-Hb (37). There is a balance between the faster reaction of nitrite with the R-state oxyhemoglobin and the availability of deoxyhemes to bind to nitrite, leading to a maximal rate of nitrite reduction at about 50% hemoglobin–oxygen saturation. These factors have suggested that the reaction can respond to change in pH and oxygen tension in blood and tissues, and potentially mediate hypoxic vasodilation (28, 29, 37).
Deoxymyoglobin
It reduces nitrite to NO at a faster rate than deoxy-Hb, and has been shown to modulate mitochondrial respiration via inhibition of cytochrome c oxidase (70, 72) (Fig. 5). Confirmatory studies in myoglobin knockout mice suggest a role for myoglobin in regulating respiration and cardiac energetics, and new studies suggest a contribution to hypoxic vasodilation (35). As indicated for hemoglobin, the reaction rates increase with low pH, which is usually linked to hypoxia. Therefore, myoglobin can use nitrite efficiently to produce NO during hypoxic or ischemic conditions (72).
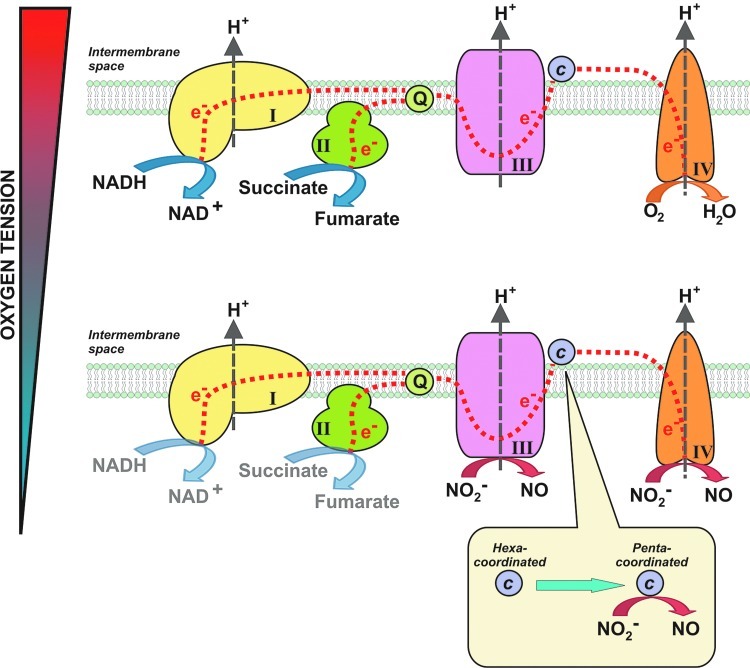
FIG. 5.
Sites of mitochondrial nitrite reduction. Upper panel: In normoxia, electrons enter the respiratory chain at complex I or II and are shuttled through the Q cycle to complex III. Electrons are then shuttled to cytochrome c and then to complex IV, where oxygen acts as the terminal electron acceptor. Protons are pumped from the matrix to the intermembrane space through the complexes to set up a proton gradient for ATP generation. Lower panel: During hypoxia, nitrite can be reduced at complex III (7) or cytochrome c oxidase (complex IV) (12). If cytochrome c is converted to its pentacoordinate form (through oxidation, nitration, or association with anionic lipid), it can reduce nitrite to NO (6, 72). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Cytochrome c
It is located at the intermembrane space of mitochondria, where it shuttles electrons from complex III of the mitochondrial respiratory chain to cytochrome c oxidase (complex IV). Under hypoxic conditions and those in which the cytochrome c heme becomes pentacoordinate, cytochrome c can bind nitrite and reduce nitrite to NO (4). The reduction of nitrite to NO by cytochrome c is vastly increased in the five-coordinate state, which is promoted by cardiolipin liposomes (6). This reductive reaction occurs between ferrous cytochrome c (Fe2+) and nitrite and represents a typical electron–proton transfer reaction [Table 1, Eq. (6)] (4).
Cytochrome c oxidase (complex IV)
Nitrite can react with cytochrome c oxidase to form NO (12). This reductive reaction occurs between ferrous cytochrome c oxidase (Fe2+) and nitrite and represents a typical electron–proton transfer reaction [Table 1, Eq. (6)]. Note that nitrite can also be reduced by other proteins, such as myoglobin, to form NO, which in turn can bind to ferrous cytochrome c oxidase and inhibit respiration. The formation of NO and inhibition of cytochrome c oxidase have been proposed as a pathway to reduce oxygen consumption at low oxygen levels, thus promoting oxygen diffusion deeper into ischemic tissues (35, 70, 71). In summary, cytochrome c oxidase has been proposed to function as both a nitrite reductase that generates NO, as well as a target for nitrite-NO-dependent regulation of respiration and oxygen consumption.
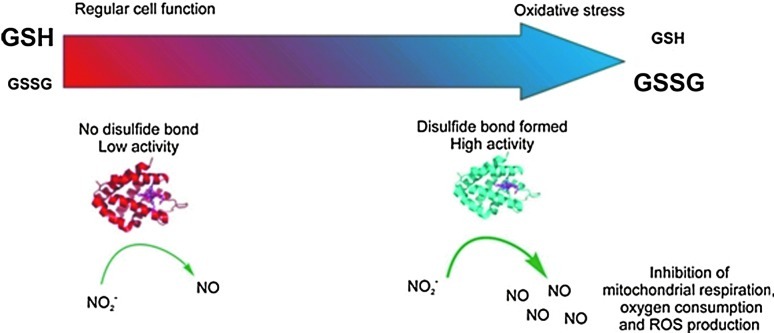
Neuroglobin
Evolved from a common ancestor shared by hemoglobin and myoglobin, human neuroglobin is found in brain neurons and also possess nitrite reductase activity. Neuroglobin, like cytochrome c, is a six-coordinate heme protein, with two histidines bound to the heme iron. The binding and reduction of nitrite to NO are favored when the heme is five coordinated. Tiso et al. (77) reported that deoxyneuroglobins where the sixth ligand (Histidine 64) is mutated to a nonheme-binding side chain (H64L and H64Q) can reduce nitrite at a very high rate. The wild-type neuroglobin six-to-five coordination is also regulated in a redox-dependent fashion, with oxidation of two surface redox-sensitive thiols to disulfides increasing the open probability of the heme and increasing the rate of nitrite binding and reduction to NO. Tiso et al. showed in these studies that the nitrite reductase reaction of neuroglobin could modulate mitochondrial respiration, similar to that shown for myoglobin (Table 2) (77). This pathway suggests a possible redox sensor mechanism for neuroglobin-mediated nitrite reduction. (Fig. 6)
Table 2.
Summary of Bimolecular Reaction Rates of Heme-Containing Proteins with Nitrite
FIG. 6.
Neuroglobin acts as a nitrite reductase under oxidative stress conditions. The thiol state of the glutathione is a good indicator of the oxidative stress in vivo conditions and can be modulated in vitro. In normal conditions, cells keep a high concentration of reduced glutathione (GSH) and low oxidized glutathione (GSSG). In these circumstances, the disulfide bond of Ngb is not formed, and the protein has a low nitrite reductase activity (left). As oxidative stress conditions develop (right), reduced glutathione is consumed, and the number of neuroglobin molecules with formed disulfide bonds increases. This leads to increased production of NO from nitrite, causing the inhibition of respiratory enzymes and limiting oxygen consumption and reactive oxygen species-producing reactions [reproduced with permission from (77)]. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Nitric oxide synthase
While though NOS is responsible for normoxic NO generation from arginine oxidation, it has been recently reported that NOS also catalyzes nitrite reduction to NO under anoxic conditions. Vanin et al. reported that eNOS catalyzes anoxic NO formation in murine microvascular brain endothelial cells, which is abolished by the eNOS inhibitors Nω-nitro-l-arginine or Nω-nitroarginine methyl ester (L-NAME) (78). This pathway may represent an alternative NO source during severe hypoxia in tissues. Webb et al. have shown that eNOS exists on whole red blood cells (81). When nitrite reacted with red blood cells in the presence of L-NAME or l-arginine at low oxygen tensions, NO production was inhibited by about 60%, while d-arginine, the inactive isomer, showed no significant effects, leading them to propose that this pathway may have some relevance to vasodilation. Nevertheless, further studies are needed to reconcile how this pathway may function in vivo, because it requires extremely low oxygen tensions.
Molybdopterin-containing proteins
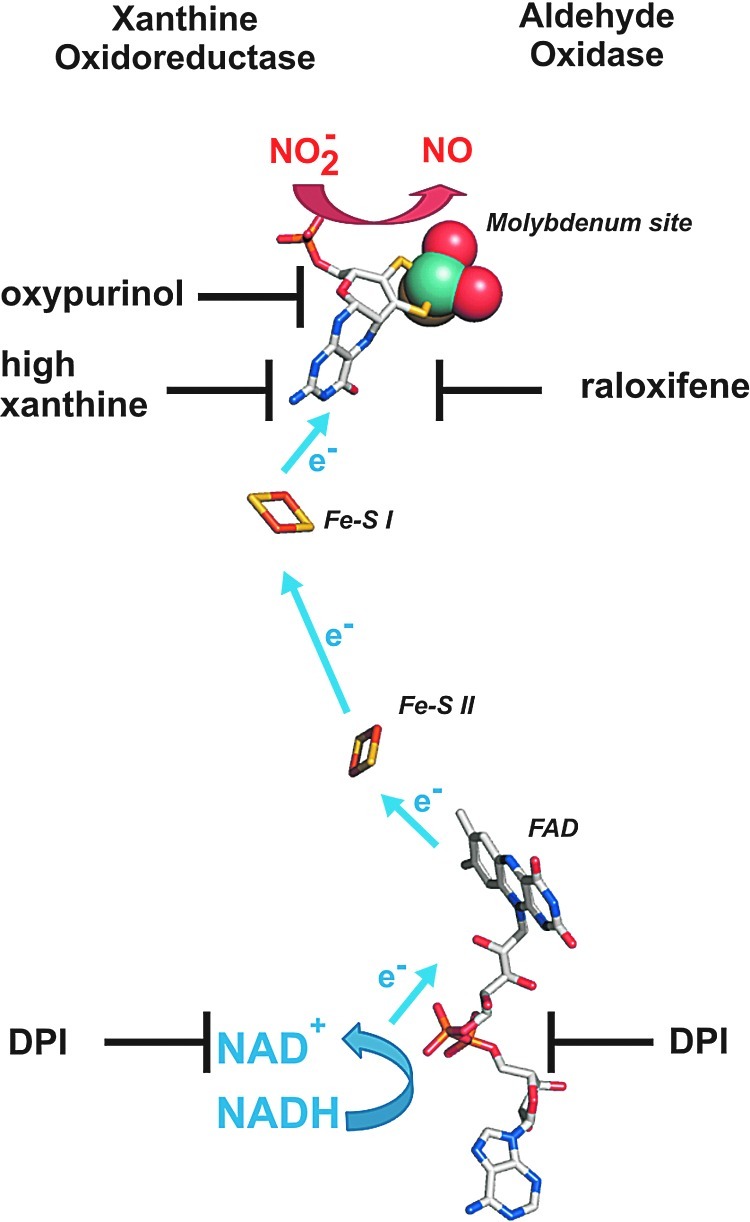
XOR and AO are two dimeric molybdopterin enzymes ubiquitously expressed in human tissues, containing one molybdenum (Mo) center, two nonidentical Fe2S2 clusters, and a flavin adenine dinucleotide cofactor. XOR catalyzes the terminal two steps in purine degradation and exists in cells primarily as a dehydrogenase (xanthine dehydrogenase [XDH]) where substrate-derived electrons reduce NAD+ to NADH. During inflammatory conditions, oxidation of critical cysteine residues or limited proteolysis converts XDH to xanthine oxidase (XO) (80). XO transfers substrate-derived electrons to O2, generating O2•− and H2O2. However, conversion to XO is not a requisite for ROS production, as XDH displays partial oxidase activity under conditions in which the NADH/NAD+ ratio is increased such as the hypoxemia (34). It is also under hypoxic (0–1% O2) conditions that both XO- and XDH-mediated nitrite reductase activities have been reported (47, 51). This is evidenced by inhibition of XO- and XDH-mediated reduction of nitrite to NO by the XOR-specific inhibitor, oxypurinol, and/or high concentrations of xanthine. This oxypurinol- and xanthine-induced inhibition of NO generation rates results from their binding to the Mo site, which is also the catalytic center for nitrite binding and reduction. A similar effect on nitrite reduction was observed for AO, which is inhibited by raloxifene. As the inhibitor for the flavin site, diphenylene iodonium chloride does not affect nitrite reduction when xanthine or aldehyde served as the electron donor, but considerably reduces rates of NO formation rates when NADH is the reducing substrate. These experiments indicate that nitrite is reduced at the Mo center of XO or AO, but electrons can be provided either at the Mo or the flavin site by various substrates (Fig. 7). It also suggests that electron withdrawal from XOR or AO by nitrite may serve not only to produce NO but also to reduce ROS formation and as such elicit salutary actions regarding NO derived from alternative sources.
FIG. 7.
The nitrite reductase activity of XOR and AO can be inhibited at either molybdenum or flavin site. Oxypurinol or high concentrations of xanthine inhibit nitrite reduction by binding to the molybdenum center of XOR. AO can be inhibited specifically by raloxifene, which binds to the molybdenum site. Electron can be provided at the flavin site, which is transferred via Fe2S2 clusters to the molybdenum site. DPI blocks the electron transfer at the flavin site, thus inhibiting the nitrite reductase activity of XOR or AO. The relative distances of the four redox-active centers were taken from the crystal structure of the bovine XOR (PDB code: 1fo4). AO, aldehyde oxidase; DPI, diphenylene iodonium chloride; XOR, xanthine oxidoreductase. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Nitrite disproportionation
Another mechanism for nitrite bioactivation is disproportionation, with nitrous acid or NO+ reacting with nitrite to form N2O3 [Table 1, Eqs. (2) and (3)]. This reaction has been proposed to be catalyzed by ferric hemoglobin (5) as well as carbonic anhydrase. Aamand et al. (1) recently demonstrated that NO was generated by nitrite reactions with carbonic anhydrase under both normoxia and hypoxia, which was stimulated by dorzolamide and acetazolamide, two specific inhibitors of carbonic anhydrase (Table 3). They propose that nitrite is structurally similar to bicarbonate and may react with histidines in the enzyme catalytic site to disproportionate to NO. However, more work is needed to fully explore this newly described pathway.
Table 3.
Kinetic Parameters for Other Enzymes That Catalyze Nitrite Reduction
Therapeutic Application of Nitrite for PH
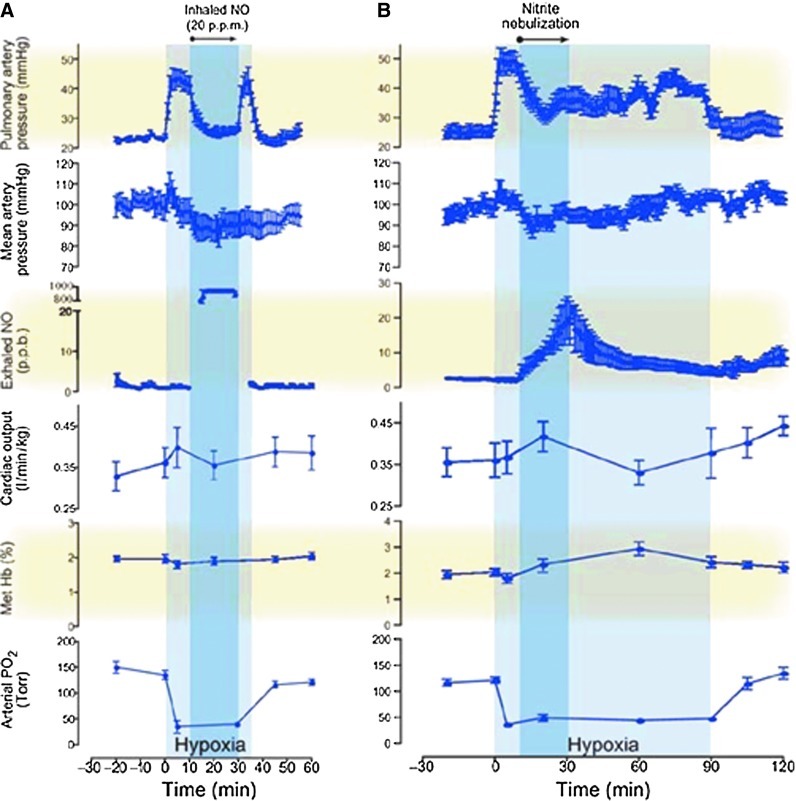
Recent studies have investigated the therapeutic effects of nitrite in PH models (85). Hunter et al. (38) first reported the effects of nebulized nitrite as a selective pulmonary vasodilator in an ovine hypoxia model. In this study, vasoconstriction induced by hypoxia was reduced by inhalation of NO gas (20 ppm) or nebulized nitrite (300 mg in 5 ml of phosphate-buffered saline for 20 min). Nebulized nitrite significantly reduced the pulmonary arterial pressures with lesser effects on systemic mean arterial blood pressure. The kinetic therapeutic responses to nitrite and NO were very different: The nitrite response was slower and lasted longer (over 60 min after inhalation), whereas the effect of NO response was faster, but returns rapidly to the hypoxic baseline when the treatment was discontinued (Fig. 8). The nitrite effect appears to be mediated by its slow conversion to NO, since measurements of exhaled NO gas increased, with values above baseline maintained after the end of the treatment. In addition, consistent with a possible role of deoxyhemoglobin as the nitrite reductase protein, formation of iron-nitrosyl-hemoglobin was also reported (38). More recently, using an ovine model of hemolysis-induced pulmonary vasoconstriction, the effect of nebulized nitrite (0.87 M), iNO (20 ppm), and intravenous sodium nitrite was compared. Both iNO and inhaled nitrite were able to reduce PH, whereas intravenous nitrite was ineffective at the concentrations studied. Nebulized sodium nitrite formulations were also studied in a rabbit model by Egemnazarov et al. (24). In this study, nitrite solutions acidified with ascorbic and citric acid (pH 5.3–5.4) showed a longer-lasting vasodilatory effect, although all formulations were effective in reducing hypoxic pulmonary vasoconstriction.
FIG. 8.
Comparison of the physiological effects of nebulized nitrite versus NO. Duration of effect of NO gas inhalation (A) or nitrite nebulization (B) on hemodynamic and metabolic measurements during hypoxic-induced PH. Treatment with nitrite aerosol resulted in a rapid sustained reduction in hypoxic-induced pulmonary vasoconstriction and a graded increase in exhaled NO gas concentration with no change in mean arterial blood pressure. These results are contrasted to the rapid return of pulmonary artery pressure to hypoxic baseline after termination of inhaled NO gas. Methemoglobin concentrations increased after nitrite nebulization from 2.1±0.1% to 2.8±0.8%. Note that exhalated NO concentrations reach the limit of detection during NO inhalation. [reproduced with permission from (38)]. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Repeated treatment with nebulized inhaled nitrite has been studied by Zuckerbraun et al. (86) in two animal models of PH: a rat, monocrotaline-induced PH model and a mouse hypoxia-induced PH model. They demonstrated that inhaled nebulized nitrite can prevent and reverse establish PH. In the hypoxic mouse model, intervention after 2 weeks of hypoxia, after the establishment of the PAH, halted its progression and reversed high right ventricular pressures. In the monocrotaline-induced PAH rat model, nebulized nitrite was able to diminish monocrotaline-induced muscularization and hyperplasia of the small pulmonary arteries. Nitrite effects in both models were blocked by the XO inhibitor allopurinol (in vitro) and by tungsten-enriched diet (in vivo), which replaces Mo in the active sites of enzymes such as XO and AO, inhibiting their activity. These data suggest that the protective effect of nitrite against the development and progression of hypoxia-induced PAH is mediated by XOR (86). Additional work suggested that the nitrite effect was mediated by NO–cyclic guanosine monophosphate signaling and downstream induction of the cell cycle checkpoint inhibitor p21, which inhibits smooth muscle cell proliferation (Fig. 9).
FIG. 9.
Nitrite-induced SMC proliferation is dependent on XOR. Hypoxia-induced PAH is inhibited by NO generation from nitrite, which is catalyzed by XOR. NO production from nitrite increases expression of p21, which inhibits SMC proliferation. Allopurinol or tungsten diet treatment inhibits XOR activity (85, 86).
Intravenous administration of sodium nitrite has been also explored in the context of PH. Casey et al. (11) investigated the effect of intravenous sodium nitrite (10–100 μmol/kg) as a pulmonary vasodilator in rats under hypoxic conditions. A vasodilatory effect in both pulmonary and systemic arterial pressure was observed, and these effects were inhibited by allopurinol, consistent with other rodent PAH models (86). While these studies suggest a role for XO as a nitrite reductase in rodent models, human studies have been performed testing a role for XO in nitrite-dependent vasodilation. Infusions of oxypurinol for 30 min, followed by coinfusions of nitrite, did not block nitrite-dependent vasodilation in humans (17). Paradoxically, oxypurinol increased nitrite-dependent vasodilation by about 10%. Future studies are required to reconcile findings in rodent disease models compared with human physiological studies.
Other Therapeutic Opportunities for Nitrite in Vascular Disease
In any pathological condition where NO bioavailability is compromised, nitrite administration may provide a therapeutic approach to restore NO levels through the nitrate-nitrite-NO signaling pathway. This approach has a direct relevance for cardiovascular disease, given the well-known vasodilatory effect of NO, and now appreciated vasodilatory effects of nitrite. We will summarize here some of the current evaluations of nitrite in vascular disease.
High blood pressure
As discussed earlier, plasma nitrite levels can be modified by dietary nitrate intake. Larsen et al. (49) studied the effect of dietary nitrate supplementation on healthy volunteers and found a significant reduction in blood pressure and increases in plasma nitrate and nitrite. Other studies have confirmed that dietary nitrate can decrease arterial blood pressure and enhance other parameters linked to NO metabolism, such as platelet aggregation and endothelial function during ischemia. Interestingly, these effects may be greater in men than that in women (46). In general, the dietary effects of nitrate are comparable to effects of vegetable-rich diet, suggesting that the high nitrate content of vegetables can be a part of their observed effects on blood pressure (59). Low doses of intraperitoneal, inhaled, or oral sodium nitrite have been shown to generate NO in blood vessels and to inhibit proliferative responses of smooth muscle cells in murine models of carotid injury.
Angiogenesis
Vascular endothelial growth factor-induced proliferation and organization of human endothelial cells have been shown to be dependent on eNOS and NO production (63), suggesting that nitrite-based therapies could be used to promote angiogenesis. Consistently, chronic intravenous administration of sodium nitrite has been shown to induce angiogenesis in a mouse model of hindlimb ischemia (48), and sodium nitrate therapy stimulated ischemic vasodilation and angiogenic activity after permanent femoral artery ligation (64).
Sickle cell disease
The pathology of sickle cell disease relates to vaso-occlusion by sickled erythrocytes and vasoconstriction due to increase NO scavenging associated with hemolytic anemia (83). Patients with sickle cell disease develop PH as they age, suggesting a potential role for nitrite therapy. A phase Ib clinical study by Mack et al. has shown that infused sodium nitrite was safe and increased forearm blood flow in patients with sickle cell disease (57), although sickle cell patients show a somewhat reduced response to nitrite as compared to healthy volunteers (16, 58).
Myocardial ischemia
Nitrite has protective effects in different ischemia reperfusion injury models (19). The effects are at least partly due to the ability of nitrite to reversibly inhibit mitochondrial metabolism, via S-nitrosation of complex I, and reduce reperfusion ROS formation (20, 60, 72). This reduction in reperfusion ROS is associated with a prevention of opening of the mitochondrial permeability transition pore and release of cytochrome c (72) (Fig. 10). Several studies have focused on the effects of nitrite treatment on myocardial ischemia. Duranski et al. showed a large effect in decrease of the infarct size when nitrite was applied during ischemia (23). The beneficial effect of nitrite in cardiac infarct has been found be independent of the time of administration during the ischemic phase and can be obtained by dietary intervention (31). The use of intravenous sodium nitrite to ameliorate the consequences of acute myocardial infarction is currently being evaluated in phase I–II clinical trials.
FIG. 10.
Nitrite mediates cytoprotection in ischemia/reperfusion injury. Nitrite potently mediates cytoprotection after ischemia/reperfusion (I/R) through the transient inhibition of complex I (via S-nitrosation) and subsequent limitation of oxidative damage. Because oxidants have been shown to sensitize the permeability transition pore, cytoprotective effects of nitrite could in part be caused by nitrite-dependent protection against pore opening after I/R (72). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Stroke
Several studies have investigated the use of nitrite on stroke therapy in preclinical models. Early administration of nitrite during reperfusion shows favorable effects in rats (42), although responses depended on the nitrite dosage. High amounts of nitrite provided no protection, and low doses reduced the infarction size and enhanced local cerebral blood flow and functional recovery (44). Long-term, high-dose (100 μg/kg) nitrite treatment showed improvement in the recovery after stroke in a rat ischemia model (45). Some positive effects have been also observed in a rat intracranial hemorrhage model (43).
Nitrite therapy has also been applied in the treatment of delayed cerebral vasospasm after subarachnoid hemorrhage, a subtype of hemorrhagic stroke. This condition is characterized by decreased middle cerebral artery flow due to the narrowing of large-capacitance arteries and often develops days after subarachnoid hemorrhage. Several studies indicate that the vasospasm is related to decreased bioavailability of NO, at least in part due to inhibition of eNOS and NO scavenging by hemoglobin from the subarachnoid clot. Pluta et al. (66) studied the effect of nitrite in a primate model of subarachnoid hemorrhage, and showed that nitrite prevented the development of cerebral vasospasm.
Therapeutic Developments
Nitrite has been proposed and tested successfully in several animal models and patients, with a variety of delivery systems and formulations. A mixture of sodium nitrite and acidifying agents such as ascorbic acid can rapidly release NO. This topical administration has been evaluated for its antimicrobial activity in various skin infections (61) and, with some modifications, in preventing catheter-associated urinary tract infections (10). It is also well established that nitrite can be given orally, but its bioavailability can be difficult to assess, because it is extremely dependent on the oral microbiome and its variable metabolism within the gastrointestinal tract. However, the use of organic allylic nitrocompounds as nitrite donors may overcome this issue (13). A third possibility is the much anticipated use of intravenous infusions of nitrite, but the dose and the duration of the treatment still need to be adjusted (67). One of the adverse effects of inorganic nitrite is the formation of methemoglobin. Nitrite can oxidize the iron at the heme group, modifying it from a ferrous (Fe2+) oxygen-binding form to a ferric (Fe3+) nonoxygen-binding state. If the increase of methemoglobin in circulating blood is higher than 5%, it can lead to cyanosis. However, studies with nitrite intravenous studies only report undetectable or modest increase (67).
As described above, inhaled sodium nitrite is a pulmonary vasodilator that can effectively prevent or reverse PAH in animal models. Studies suggest that it can be delivered safely, and it is ready for clinical translation for PAH patients. Animal toxicology studies with inhaled nitrite in rodents and dogs have been completed and phase Ia and Ib studies in normal volunteers completed. In a phase Ia study, inhaled nebulized sodium nitrite, at doses >17 mg, increases exhaled NO, whereas methemoglobin levels remained <3.5% in all subjects (Bradley et al., unpublished data). A proof-of-concept phase II trial of inhaled nitrite in patients with PAH is currently enrolling sites in the United States and Europe.
Summary and Conclusions
PAH is associated with decreased bioavailability and responsiveness of NO. Despite the potential therapeutic effect of iNO as a selective pulmonary vasodilator, its administration is inconvenient and difficult. The discovery that nitrite is a naturally occurring molecule in the body and may act endogenously as a reservoir of NO has suggested the idea that this anion may represent an alternative strategy for an effective NO-based therapy. NO can only be administered as a gas and cannot be mixed with oxygen, or it reacts to NO2. Nitrite (as a soluble salt) can be effectively delivered as a nebulized liquid, dry powder, intravenous solution, or oral formulation. Also, when compared with iNO, nitrite has a longer half-life. However, two caveats arise for either iNO or nitrite therapies: (i) both may have effects in conditions with soluble guanylate cyclase dysfunction (oxidation or downregulation); and (ii) potential harmful side products may be formed by reaction with ROS (NO2, peroxynitrite, or methemoglobin formation). Nitrite has demonstrated efficacy in multiple animal models not only of cardiovascular disorders but also of inflammatory diseases and bacterial infections. Clinical trials are ongoing and more planned in the near future to demonstrate the therapeutic efficacy of nitrite in patients with PAH.
Abbreviations Used
- AO
aldehyde oxidase
- cGMP
cyclic guanosine monophosphate
- CT
computed tomography
- deoxy-Hb
deoxyhemoglobin
- DPI
diphenylene iodonium chloride
- eNOS
endothelial NOS
- GSNO
S-nitroso glutathione
- iNO
inhaled NO
- iNOS
inducible NOS
- LA
left atrium
- L-NAME
Nω-nitroarginine methyl ester
- LV
left ventricle
- Mo
molybdenum
- NO
nitric oxide
- NOS
nitric oxide synthase
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- RA
right atrium
- ROS
reactive oxygen species
- RV
right ventricle
- sGC
soluble guanylate cyclase
- SMC
smooth muscle cell
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
- XOR
xanthine oxidoreductase
Acknowledgments
We thank Dr. Jesus Tejero, Dr. Courtney Sparacino-Watkins, and Dr. Eric Kelley (University of Pittsburgh). Dr. Gladwin receives research support from the NIH grants R01HL098032, RO1HL096973, and PO1HL103455, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania. Dr. Bueno, Dr. Mora, and Dr. Wang are supported by the Vascular Medicine Institute of the University of Pittsburgh.
Author Disclosure Statement
Dr. Gladwin is listed as a coinventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases. Dr. Gladwin consults with Aires Pharmaceuticals on the development of a phase II proof-of-concept trial using inhaled nitrite for pulmonary arterial hypertension.
References
- 1.Aamand R. Dalsgaard T. Jensen FB. Simonsen U. Roepstorff A. Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol. 2009;297:H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 2.Alef MJ. Vallabhaneni R. Carchman E. Morris SM., Jr. Shiva S. Wang Y. Kelley EE. Tarpey MM. Gladwin MT. Tzeng E. Zuckerbraun BS. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest. 2011;121:1646–1656. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardehali A. Hughes K. Sadeghi A. Esmailian F. Marelli D. Moriguchi J. Hamilton MA. Kobashigawa J. Laks H. Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation. 2001;72:638–641. doi: 10.1097/00007890-200108270-00013. [DOI] [PubMed] [Google Scholar]
- 4.Basu S. Azarova NA. Font MD. King SB. Hogg N. Gladwin MT. Shiva S. Kim-Shapiro DB. Nitrite reductase activity of cytochrome c. J Biol Chem. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu S. Grubina R. Huang J. Conradie J. Huang Z. Jeffers A. Jiang A. He X. Azarov I. Seibert R. Mehta A. Patel R. King SB. Hogg N. Ghosh A. Gladwin MT. Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 6.Belikova NA. Vladimirov YA. Osipov AN. Kapralov AA. Tyurin VA. Potapovich MV. Basova LV. Peterson J. Kurnikov IV. Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benamar A. Rolletschek H. Borisjuk L. Avelange-Macherel MH. Curien G. Mostefai HA. Andriantsitohaina R. Macherel D. Nitrite-nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochim Biophys Acta. 2008;1777:1268–1275. doi: 10.1016/j.bbabio.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin N. O'Driscoll F. Dougall H. Duncan C. Smith L. Golden M. McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 9.Botha P. Jeyakanthan M. Rao JN. Fisher AJ. Prabhu M. Dark JH. Clark SC. Inhaled nitric oxide for modulation of ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant. 2007;26:1199–1205. doi: 10.1016/j.healun.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson S. Weitzberg E. Wiklund P. Lundberg JO. Intravesical nitric oxide delivery for prevention of catheter-associated urinary tract infections. Antimicrob Agents Chemother. 2005;49:2352–2355. doi: 10.1128/AAC.49.6.2352-2355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey DB. Badejo AM., Jr. Dhaliwal JS. Murthy SN. Hyman AL. Nossaman BD. Kadowitz PJ. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. Am J Physiol Heart Circ Physiol. 2009;296:H524–H533. doi: 10.1152/ajpheart.00543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castello PR. David PS. McClure T. Crook Z. Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Chakrapani H. Gorczynski MJ. King SB. Allylic nitro compounds as nitrite donors. J Am Chem Soc. 2006;128:16332–16337. doi: 10.1021/ja066011v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champion HC. Bivalacqua TJ. Greenberg SS. Giles TD. Hyman AL. Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc Natl Acad Sci U S A. 2002;99:13248–13253. doi: 10.1073/pnas.182225899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark RH. Kueser TJ. Walker MW. Southgate WM. Huckaby JL. Perez JA. Roy BJ. Keszler M. Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 16.Cosby K. Partovi KS. Crawford JH. Patel RP. Reiter CD. Martyr S. Yang BK. Waclawiw MA. Zalos G. Xu X. Huang KT. Shields H. Kim-Shapiro DB. Schechter AN. Cannon RO., 3rd Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 17.Dejam A. Hunter CJ. Tremonti C. Pluta RM. Hon YY. Grimes G. Partovi K. Pelletier MM. Oldfield EH. Cannon RO., 3rd Schechter AN. Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger RP. Trzeciak SW. Criner GJ. Zimmerman JL. Taylor RW. Usansky H. Young J. Goldstein B. Association between inhaled nitric oxide treatment and long-term pulmonary function in survivors of acute respiratory distress syndrome. Crit Care. 2012;16:R36. doi: 10.1186/cc11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dezfulian C. Raat N. Shiva S. Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dezfulian C. Shiva S. Alekseyenko A. Pendyal A. Beiser DG. Munasinghe JP. Anderson SA. Chesley CF. Vanden Hoek TL. Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donohue PK. Gilmore MM. Cristofalo E. Wilson RF. Weiner JZ. Lau BD. Robinson KA. Allen MC. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127:e414–e422. doi: 10.1542/peds.2010-3428. [DOI] [PubMed] [Google Scholar]
- 22.Doyle MP. Pickering RA. DeWeert TM. Hoekstra JW. Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 23.Duranski MR. Greer JJ. Dejam A. Jaganmohan S. Hogg N. Langston W. Patel RP. Yet SF. Wang X. Kevil CG. Gladwin MT. Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egemnazarov B. Schermuly RT. Dahal BK. Elliott GT. Hoglen NC. Surber MW. Weissmann N. Grimminger F. Seeger W. Ghofrani HA. Nebulization of the acidified sodium nitrite formulation attenuates acute hypoxic pulmonary vasoconstriction. Respir Res. 2010;11:81. doi: 10.1186/1465-9921-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman D. Aldabbagh S. Doll R. Nitrates, nitrites and gastric-cancer in Great-Britain. Nature. 1985;313:620–625. doi: 10.1038/313620a0. [DOI] [PubMed] [Google Scholar]
- 26.Germann P. Ziesche R. Leitner C. Roeder G. Urak G. Zimpfer M. Sladen R. Addition of nitric oxide to oxygen improves cardiopulmonary function in patients with severe COPD. Chest. 1998;114:29–35. doi: 10.1378/chest.114.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Gilchrist M. Shore AC. Benjamin N. Inorganic nitrate and nitrite and control of blood pressure. Cardiovasc Res. 2011;89:492–498. doi: 10.1093/cvr/cvq309. [DOI] [PubMed] [Google Scholar]
- 28.Gladwin MT. Grubina R. Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res. 2009;42:157–167. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 29.Gladwin MT. Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladwin MT. Schechter AN. Kim-Shapiro DB. Patel RP. Hogg N. Shiva S. Cannon RO., 3rd Kelm M. Wink DA. Espey MG. Oldfield EH. Pluta RM. Freeman BA. Lancaster JR., Jr. Feelisch M. Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez FM. Shiva S. Vincent PS. Ringwood LA. Hsu LY. Hon YY. Aletras AH. Cannon RO., 3rd Gladwin MT. Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goret L. Tanguy S. Guiraud I. Dauzat M. Obert P. Acute administration of L-arginine restores nitric oxide-mediated relaxation in isolated pulmonary arteries from pulmonary hypertensive exercise trained rats. Eur J Pharmacol. 2008;581:148–156. doi: 10.1016/j.ejphar.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths MJ. Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 34.Harris CM. Massey V. The oxidative half-reaction of xanthine dehydrogenase with NAD; reaction kinetics and steady-state mechanism. J Biol Chem. 1997;272:28335–28341. doi: 10.1074/jbc.272.45.28335. [DOI] [PubMed] [Google Scholar]
- 35.Hendgen-Cotta UB. Merx MW. Shiva S. Schmitz J. Becher S. Klare JP. Steinhoff HJ. Goedecke A. Schrader J. Gladwin MT. Kelm M. Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hibbs JB., Jr. Taintor RR. Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 37.Huang Z. Shiva S. Kim-Shapiro DB. Patel RP. Ringwood LA. Irby CE. Huang KT. Ho C. Hogg N. Schechter AN. Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter CJ. Dejam A. Blood AB. Shields H. Kim-Shapiro DB. Machado RF. Tarekegn S. Mulla N. Hopper AO. Schechter AN. Power GG. Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 39.Ichinose F. Roberts JD., Jr. Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 40.Ignarro LJ. Buga GM. Wood KS. Byrns RE. Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iyengar R. Stuehr DJ. Marletta MA. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987;84:6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung KH. Chu K. Ko SY. Lee ST. Sinn DI. Park DK. Kim JM. Song EC. Kim M. Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 43.Jung KH. Chu K. Lee ST. Kim JM. Park DK. Kim M. Lee SK. Roh JK. Tolerated nitrite therapy in experimental intracerebral hemorrhage: Rationale of nitrite therapy in a broad range of hyperacute strokes. Neurochem Int. 2011;59:5–9. doi: 10.1016/j.neuint.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Jung KH. Chu K. Lee ST. Park HK. Kim JH. Kang KM. Kim M. Lee SK. Roh JK. Augmentation of nitrite therapy in cerebral ischemia by NMDA receptor inhibition. Biochem Biophys Res Commun. 2009;378:507–512. doi: 10.1016/j.bbrc.2008.11.081. [DOI] [PubMed] [Google Scholar]
- 45.Jung KH. Chu K. Lee ST. Sunwoo JS. Park DK. Kim JH. Kim S. Lee SK. Kim M. Roh JK. Effects of long term nitrite therapy on functional recovery in experimental ischemia model. Biochem Biophys Res Commun. 2010;403:66–72. doi: 10.1016/j.bbrc.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 46.Kapil V. Milsom AB. Okorie M. Maleki-Toyserkani S. Akram F. Rehman F. Arghandawi S. Pearl V. Benjamin N. Loukogeorgakis S. Macallister R. Hobbs AJ. Webb AJ. Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 47.Kelley EE. Hundley NJ. Tarpey MM. Oxygen dependence of XOR nitrite reductase activity. Free Radic Biol Med. 2009;47:S32. [Google Scholar]
- 48.Kumar D. Branch BG. Pattillo CB. Hood J. Thoma S. Simpson S. Illum S. Arora N. Chidlow JH., Jr. Langston W. Teng X. Lefer DJ. Patel RP. Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsen FJ. Ekblom B. Sahlin K. Lundberg JO. Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 50.Li H. Kundu TK. Zweier JL. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J Biol Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H. Samouilov A. Liu X. Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 52.Li H. Samouilov A. Liu X. Zweier JL. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J Biol Chem. 2004;279:16939–16946. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 53.Liu X. Miller MJ. Joshi MS. Thomas DD. Lancaster JR., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lo V, editor; Cullen C, editor. Medieval Chinese Medicine: the Dunhuang Medical Manuscripts. New York: Routledge; 2004. [Google Scholar]
- 55.Lundberg JO. Weitzberg E. Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 56.Lundberg JO. Weitzberg E. Lundberg JM. Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mack AK. McGowan Ii VR. Tremonti CK. Ackah D. Barnett C. Machado RF. Gladwin MT. Kato GJ. Sodium nitrite promotes regional blood flow in patients with sickle cell disease: A phase I/II study. Br J Haematol. 2008;142:971–978. doi: 10.1111/j.1365-2141.2008.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarty MF. Potential utility of full-spectrum antioxidant therapy, citrulline, and dietary nitrate in the management of sickle cell disease. Med Hypotheses. 2010;74:1055–1058. doi: 10.1016/j.mehy.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Moore LL. Bradlee ML. Singer MR. Qureshi MM. Buendia JR. Daniels SR. Dietary Approaches to Stop Hypertension (DASH) eating pattern and risk of elevated blood pressure in adolescent girls. Br J. 2012;Nutr:1–8. doi: 10.1017/S000711451100715X. [DOI] [PubMed] [Google Scholar]
- 60.Murillo D. Kamga C. Mo L. Shiva S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide. 2011;25:70–80. doi: 10.1016/j.niox.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ormerod AD. Shah AA. Li H. Benjamin NB. Ferguson GP. Leifert C. An observational prospective study of topical acidified nitrite for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds. BMC Res Notes. 2011;4:458. doi: 10.1186/1756-0500-4-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer RM. Ferrige AG. Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 63.Papapetropoulos A. Garcia-Cardena G. Madri JA. Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pattillo CB. Bir S. Rajaram V. Kevil CG. Inorganic nitrite and chronic tissue ischaemia: a novel therapeutic modality for peripheral vascular diseases. Cardiovasc Res. 2011;89:533–541. doi: 10.1093/cvr/cvq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pepke-Zaba J. Higenbottam TW. Dinh-Xuan AT. Stone D. Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991;338:1173–1174. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- 66.Pluta RM. Dejam A. Grimes G. Gladwin MT. Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 67.Pluta RM. Oldfield EH. Bakhtian KD. Fathi AR. Smith RK. Devroom HL. Nahavandi M. Woo S. Figg WD. Lonser RR. Safety and feasibility of long-term intravenous sodium nitrite infusion in healthy volunteers. PLoS One. 2011;6:e14504. doi: 10.1371/journal.pone.0014504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rassaf T. Preik M. Kleinbongard P. Lauer T. Heiss C. Strauer BE. Feelisch M. Kelm M. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest. 2002;109:1241–1248. doi: 10.1172/JCI14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reddy D. Lancaster JR., Jr. Cornforth DP. Nitrite inhibition of Clostridium botulinum: electron spin resonance detection of iron-nitric oxide complexes. Science. 1983;221:769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- 70.Shiva S. Huang Z. Grubina R. Sun J. Ringwood LA. MacArthur PH. Xu X. Murphy E. Darley-Usmar VM. Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 71.Shiva S. Rassaf T. Patel RP. Gladwin MT. The detection of the nitrite reductase and NO-generating properties of haemoglobin by mitochondrial inhibition. Cardiovasc Res. 2011;89:566–573. doi: 10.1093/cvr/cvq327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shiva S. Sack MN. Greer JJ. Duranski M. Ringwood LA. Burwell L. Wang X. MacArthur PH. Shoja A. Raghavachari N. Calvert JW. Brookes PS. Lefer DJ. Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiva S. Wang X. Ringwood LA. Xu X. Yuditskaya S. Annavajjhala V. Miyajima H. Hogg N. Harris ZL. Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 74.Stuehr DJ. Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tabima DM. Frizzell S. Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med. 2012;52:1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tannenbaum SR. Fett D. Young VR. Land PD. Bruce WR. Nitrite and nitrate are formed by endogenous synthesis in the human intestine. Science. 1978;200:1487–1489. doi: 10.1126/science.663630. [DOI] [PubMed] [Google Scholar]
- 77.Tiso M. Tejero J. Basu S. Azarov I. Wang X. Simplaceanu V. Frizzell S. Jayaraman T. Geary L. Shapiro C. Ho C. Shiva S. Kim-Shapiro DB. Gladwin MT. Human neuroglobin functions as a redox-regulated nitrite reductase. J Biol Chem. 2011;286:18277–18289. doi: 10.1074/jbc.M110.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanin AF. Bevers LM. Slama-Schwok A. van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ward MH. deKok TM. Levallois P. Brender J. Gulis G. Nolan BT. VanDerslice J International Society for Environmental E. Workgroup report: Drinking-water nitrate and health—recent findings and research needs. Environ Health Perspect. 2005;113:1607–1614. doi: 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waud WR. Rajagopalan KV. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O) Arch Biochem Biophys. 1976;172:365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- 81.Webb AJ. Milsom AB. Rathod KS. Chu WL. Qureshi S. Lovell MJ. Lecomte FM. Perrett D. Raimondo C. Khoshbin E. Ahmed Z. Uppal R. Benjamin N. Hobbs AJ. Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ Res. 2008;103:957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weitzberg E. Lundberg JO. Nonenzymatic nitric oxide production in humans. Nitric Oxide. 1998;2:1–7. doi: 10.1006/niox.1997.0162. [DOI] [PubMed] [Google Scholar]
- 83.Wood KC. Hsu LL. Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z. Naughton DP. Blake DR. Benjamin N. Stevens CR. Winyard PG. Symons MC. Harrison R. Human xanthine oxidase converts nitrite ions into nitric oxide (NO) Biochem Soc Trans. 1997;25:524S. doi: 10.1042/bst025524s. [DOI] [PubMed] [Google Scholar]
- 85.Zuckerbraun BS. George P. Gladwin MT. Nitrite in pulmonary arterial hypertension: therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovasc Res. 2011;89:542–552. doi: 10.1093/cvr/cvq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuckerbraun BS. Shiva S. Ifedigbo E. Mathier MA. Mollen KP. Rao J. Bauer PM. Choi JJ. Curtis E. Choi AM. Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 87.Zweier JL. Wang P. Samouilov A. Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]