Abstract
Necrotizing enterocolitis (NEC) is a devastating disease of premature infants. Probiotics decrease the risk of NEC in clinical and experimental studies. Antimicrobial peptides protect the gut against noxious microbes and shape the commensal microbiota, but their role in NEC remains unclear. We report that like in human ontogeny, the rat pup has low expression of Paneth cell antimicrobials, which increases rapidly during normal development. To investigate the expression of antimicrobial peptides in experimental NEC and the impact of probiotics on their expression, premature rats were divided into three groups: dam fed (DF), hand fed with formula (FF), or hand fed with formula containing Bifidobacterium bifidum (FF+BIF). All groups were exposed to asphyxia and cold stress. The expression of lysozyme, secretory phospholipase A2, pancreatic-associated proteins 1 and 3 mRNA was elevated in the FF (NEC) group, compared to the DF and FF+BIF groups where disease was attenuated. We conclude that induction of antimicrobial peptides occurs in experimental NEC similar to that reported in human disease and is attenuated when disease is averted by probiotic B. bifidum. The induction of antimicrobial peptides is likely an adaptive mucosal response that is often not sufficient to prevent disease in the premature gut.
Introduction
Necrotizing enterocolitis (NEC) is common in premature infants with an incidence of 3–10 % and a mortality rate for severe disease of 25–40 % (1, 2). The pathophysiology of this devastating disease remains poorly understood (3). The major risk factors underscore the central role of host-microbe interactions: immaturity of the intestine (structural, functional, and immunologic), enteral feedings, and dysbiosis (imbalance in the intestinal microbiota) (3, 4). Probiotics are dietary supplements containing live health-promoting bacteria. Several well-designed clinical trials have demonstrated a decreased incidence of NEC in premature infants receiving probiotic microorganisms (5). In the neonatal rat model of NEC, oral administration of the probiotic Bifidobacterium bifidum protects the small intestine against the disease (6, 7). This protective effect is associated with reduction of inflammation, stabilization of the mucus layer, improvement in intestinal integrity, and decreased epithelial apoptosis (7, 8).
A variety of immune mechanisms protect the intestinal mucosa both by mediating homeostatic interactions with the lumenal microbiota and by protecting from pathogenic microbes. Central to the innate immune defenses are physical and chemical barrier components, multiple pathways for microbe detection and response, and a collection of constitutive and inducible antimicrobial peptides and small proteins (henceforth referred to as simply antimicrobial peptides). Antimicrobial peptides are important in the immune function of phagocytic leukocytes and many epithelial cells. In the small intestine, antimicrobial peptides are expressed by Paneth cells, enterocytes, and leukocytes of the lamina propria (9).
Paneth cells, located at the base of the crypts of Lieberkühn in the small intestine, sense bacteria that penetrate the mucus layer, and in response, secrete large amounts of antimicrobial peptides that protect the intestinal mucosa, shape the intestinal microbiota, and protect the adjacent intestinal stem cells (9). Given the importance of the chemical barrier provided by Paneth cell antimicrobial peptides (4), and the involvement of intestinal microbes in disease pathogenesis, Paneth cell antimicrobial secretions likely play a role in protecting the intestinal mucosa from NEC (10).
In the human fetus, Paneth cells express antimicrobial peptides very early in gestation, long before exposure to bacteria, although at relatively low levels that increase with gestation (11). These observations generated the hypothesis that immaturity of Paneth cell function contributes to the increased risk of NEC in premature infants. Infants with NEC had higher numbers of Paneth cells and greater antimicrobial peptide mRNA expression at time of surgery than control infants with ileal atresia, suggesting that Paneth cell antimicrobials were increased in response to NEC (12), but that the fledgling response was inadequate in severe NEC. A contemporary study reported that some infants with NEC had very few lysozyme-positive Paneth cells, consistent with Paneth cell destruction, degranulation, or deficiency (13). A larger more recent analysis demonstrated no change in Paneth cell numbers or expression of Paneth cell antimicrobials during acute NEC in premature infants, but marked increases in Paneth cell numbers and expression of human defensin (HD)5 during recovery from NEC (14). Paneth cell metaplasia in the colon was also noted during recovery, a finding that has been reported previously in inflammatory bowel disease, but not NEC (14).
The aim of this study was two-fold: first, to define the development of intestinal antimicrobial molecule expression in the healthy rat pup, and second, to analyze the expression of these products in a well-established rat model of NEC with and without probiotic B. bifidum. We report that the rat pup, similar to premature infants, has low expression of Paneth cell antimicrobials that increases during development. Furthermore, we report an induction of antimicrobial peptide expression in experimental NEC that is attenuated when disease is averted by probiotic administration.
Methods
Antimicrobial expression in the rat pup
This protocol was approved by the Animal Care and Use Committee of the University of California Davis. To evaluate the mRNA expression in the developing rat pup, healthy Sprague-Dawley rat pups were raised with their dams and euthanized at days of life 3, 5, 10, 14, and 21 (n = 6 at each time point) and compared with healthy adult rats (n = 2).
Experimental NEC Model
This protocol was approved by the Animal Care and Use Committee of the University of Arizona. Neonatal Sprague-Dawley rats (Charles River Labs, Pontage, MI) were collected by Caesarean section 1 day before scheduled birth and their first feeding started two hours after delivery. Rat pups were hand-fed every 6 hours with rat milk substitute formula (FF, n = 30), formula containing 5×106 colony forming units per day of Bifidobacterium bifidum OLB6378 (NITE BP-31, Meiji Dairies, Odawara, Japan) (FF+BIF, n = 30), or dam-fed by surrogate mothers (DF, n = 16). All animals were exposed to asphyxia (breathing 100 % nitrogen gas for 60 seconds) and cold stress (4 degrees C for 10 minutes) twice daily (7, 8). After 96 hours, all of the surviving animals were euthanized by decapitation.
RNA Preparation and Real-time Polymerase Chain Reaction
For the healthy developmental rat studies (UC Davis), total RNA was isolated from ileal tissue using cesium chloride gradient ultracentrifugation (15). For the rat NEC studies (University of Arizona), total RNA was isolated from ileal tissue using the RNeasy Mini Kit (Qiagen, Santa Clarita, CA) (16). Real-time PCR for all specimens was performed using single-stranded cDNA from tissue with specific oligonucleotide primer pairs (Table 1) in a temperature cycler equipped with a fluorescence detection monitor (LightCycler, Roche Diagnostics, Mannheim, Germany) as previously described (17, 18). A negative control reaction that omitted template cDNA was included with each set of reactions to check for possible contamination. Gene-specific plasmid standards were included with each set of reactions. The PCR conditions were initial denaturation at 95 degrees C for 10 min, followed by 45 cycles with each cycle consisting of denaturation (95 degrees C for 15 s), annealing (60 degrees C for 5 s), and extension (72 degrees C for 10 s).
Table 1.
Primer Sets for quantitative Reverse Transcription Polymerase Chain Reaction
| Gene Target | Sequence |
|---|---|
| Lysozyme sense | 5'-CAAGCCATACAATGTGCGAAGAGAG-3' |
| Lysozyme antisense | 5'-TGTTGGTTTGAGGGGAAAGCAAG-3' |
| sPLA2 sense | 5'-CATTGTGGTGTGGGTGGCAGAG-3' |
| sPLA2 antisense | 5'-TGGTTTGTAGAGCAGGAGATTTGGC-3' |
| Cryptdin 5 sense | 5'-GACCAGGTTGTTTCTGTCTCCATTG-3 |
| Cryptdin 5 antisense | 5'-TGAGGCTTCCGTATCTCTTGTTGC-3' |
| Cryptdin 6 sense | 5'-AGCAACCATCAGATGAGGACCAGG-3' |
| Cryptdin 6 antisense | 5'-ACCTTGAGCACAGAACGCAGTGG-3' |
| PAP 1 sense | 5'-TGCCAGAAGAGACCTGAAGGACAC-3' |
| PAP 1 antisense | 5'-TTGTTACTCCACTCCCATCCACCTC-3' |
| PAP 3 sense | 5'-CCAAGAACCCAACAGAGGTGGATG-3' |
| PAP 3 antisense | 5'-GGTCCCACAGTGACTTCCAGAGACAG-3' |
| PSP Reg sense | 5'-GTTTCTCTACAAATCCTGGGACACTGG-3' |
| PSP Reg antisense | 5'-TTGGGCATCACAACTGTTATCTCTCC-3' |
| NP3 sense | 5'-TTTGGAGGGGATAAAGGCACTGC-3’ |
| NP3 antisense | 5'-TCAGCAACAGAGTCGGTAGATGCG-3’ |
| MMP7 sense | 5’-CGGCGGAGATGCTCACTTTGAC-3’ |
| MMP7 antisense | 5’-TGGCTCAGGAAGGGCGTTTGC-3’ |
| CRAMP sense | 5'-TGCCTCTAACCGTTTCCCAGACC-3' |
| CRAMP antisense | 5'-TGCTCAGGTAACTGCTGTGATGCC-3' |
| BD2 sense | 5'-TTTCTCCTGGTGCTGCTGTCGC-3' |
| BD2 antisense | 5'-CCACAAGTGCCAATCTGTCGAAAAC-3' |
| GAPDH sense | 5'-TGACCACAGTCCATGCCATCACTG-3' |
| GAPDH antisense | 5'-ATGACCTTGCCCACAGCCTTGG-3' |
NEC evaluation
After termination, a 2 cm piece of distal ileum was removed and fixed in 70% ethanol, paraffin embedded, and stained with hematoxylin and eosin for histological evaluation of NEC. Pathological changes in intestinal architecture were evaluated by a blinded investigator and a score of 0 to 4 assigned according to a published NEC scoring system with scores of 2 and above defined as NEC (19).
Immunohistochemistry
A 1–2 cm section of distal ileum was collected from each animal and fixed in either 70 % ethanol or 10 % formalin, paraffin embedded, sectioned at 4–6 µm, and processed as described (12, 20). The sections were blocked with 1.5 % normal goat serum (Vector Laboratories, Burlingame, CA) in PBS for 20 min, and then incubated with either rabbit anti-Lysozyme polyclonal antibody (Zymed, Invitrogen, Carlsbad, CA) or mouse anti-rat islet regenerating protein (Reg)IIIα monoclonal antibody (R&D system, Minneapolis, MN) in 1.5 % blocking serum overnight at 4° C then washed with PBS and incubated with either biotinylated anti-rabbit or anti-mouse secondary antibody (Vector Laboratories) in 1.5 % blocking serum for 20 min at room temperature. Vectastain Elite ABC reagent (Vector Laboratories) was applied and sections were incubated for another 20 min followed by 3,3'-diaminobenzidine substrate for 5 min, then light green counterstain.
Statistics
Pearson correlations were used to determine how closely the rise of antimicrobial peptides approximated exponential growth. Analysis of variance was used to compare antimicrobial mRNA expression levels among experimental groups. For each animal and antimicrobial molecule, the mean of two replicate templates was used as the absolute expression level, which was then log-transformed to improve the validity of statistical inferences. To address the primary research hypothesis that expression levels are increased in NEC and that the probiotics can reduce these levels, the main analysis of variance models included pairwise contrasts estimated between the NEC group and each of the other two groups.
Results
The rat pup is born with an immature intestinal tract that matures during the first 21 days of extra-uterine life, modeling the changing intestinal tract of the premature infant. The rat small intestine contains Paneth cells and secretes antimicrobial peptides that are orthologous to those of the human (Table 2).
Table 2.
Human and Rat Intestinal Antimicrobial Peptides and Proteins
| Species | Defensinsa | C-type Lectinsb | Enzymesc |
|---|---|---|---|
| Human | HNP 1-4 HD 5-6 BD 1-4 |
HIP/PAP PSP/Reg |
Lysozyme sPLA2 |
| Rat | NP 1-4 Cryptdins 5-6 rBD1-3 |
PAP1 PAP3 PSP/Reg |
Lysozyme sPLA2 |
Humans produce α-defensins in neutrophils (human neutrophil peptides (HNP) 1-4) and in Paneth cells (human defensin (HD) 5 and HD6) and β-defensins (BD) in skin and all mucosal surfaces. The rat produces similar α-defensins in neutrophils (NP) and Paneth cells (cryptdins) and β-defensins (rBD). In addition, the rat α-defensin NP3 is expressed in both Paneth cells and circulating neutrophils.
A family of C-type lectins that bind to peptidoglycan, and thus are highly selective against Gram positive bacteria, are expressed in the small intestine. Members of this family are named PSP/Reg (pancreatic secretory peptide/islet regenerating peptide) and PAP/RegIII (pancreatitis associated protein/islet regenerating peptide). In the human gut, PSP/Reg is expressed in Paneth cells, while PAP/RegIII (also known in the human as hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein or HIP/PAP) is expressed in both enterocytes and Paneth cells. The rat intestine expresses one PSP/Reg ortholog and two PAP/RegIII paralogs.
The enzymes lysozyme and secretory phospholipase A2 (sPLA2) are also expressed in Paneth cells. Lysozyme is also expressed in the intestine in macrophages and dendritic cells.
Antimicrobial gene expression increases exponentially in the developing rat pup
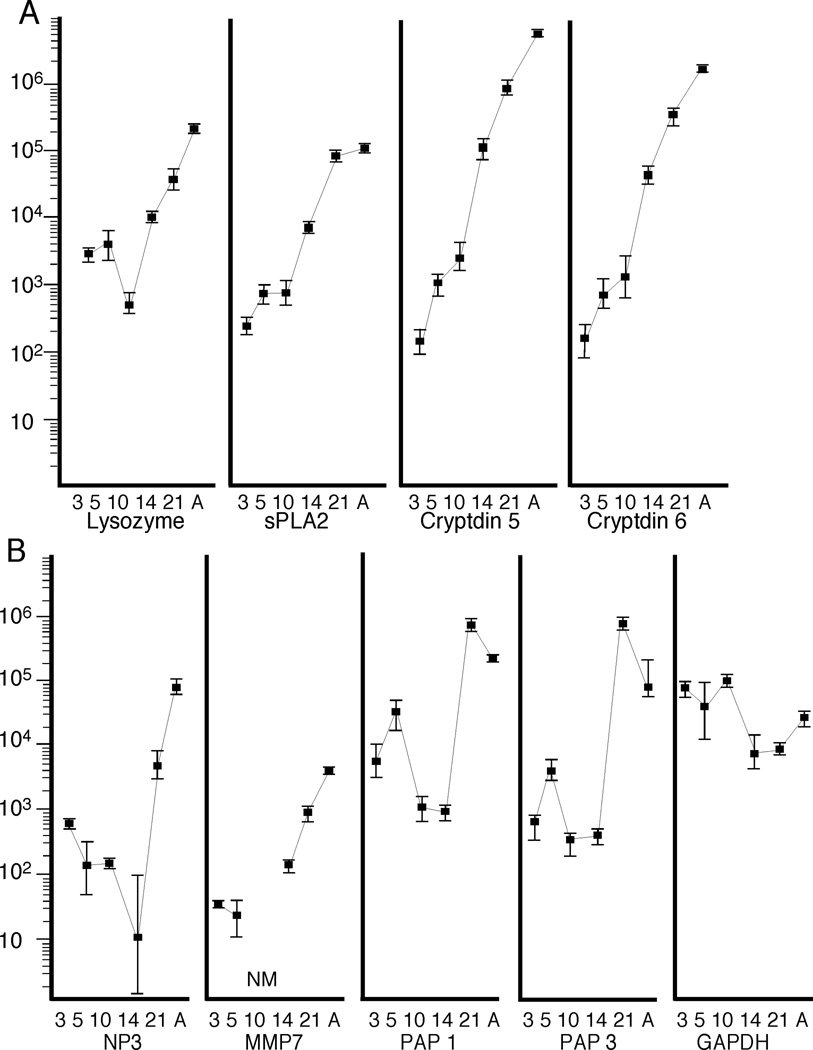
Figure 1 summarizes mRNA expression of antimicrobial peptides in development from three days of age through adult. Note that in order to display the wide range of expression (>10,000 fold), the Y-axis is a log scale. For the enzymes lysozyme and secretory phospholipase A2 (sPLA2), the α-defensins (cryptdins) 5 and 6, and the defensin processing enzyme matrix metalloproteinase (MMP) 7, the rapid change approximated a logarithmic increase with correlation co-efficients that ranged from 0.71 for lysozyme to 0.94 for MMP7. For the C-type lectins pancreatic associated proteins (PAP) 1 and 3 and the α-defensin neutrophil peptide (NP)3 expression changed minimally in the rat pup, but dramatically increased at the time of weaning (day 21) and remained high in the adult. The housekeeping gene GAPDH did not vary significantly during development. Expression of cathelicidin-related antimicrobial peptides (CRAMP) (21), pancreatic secretory peptide/islet regenerating peptide (PSP-Reg), and BD2 (a β defensin) (22) were at or below the level of reliable detection (100 mRNA copies /10 ng total RNA) throughout development (data not shown).
Figure 1. Antimicrobial Peptide mRNA in Development.
mRNA expression of antimicrobial peptides and the housekeeping gene GAPDH in the developing rat. Y-axis is mRNA copies per 10 ng RNA. X-axis is days of age. (A) N = 6 rats at 3, 5, 10, 14, and 21 days and 2 adults (38 days), (B) n = 3 rats at 3, 5, 10, 14, and 21 days and 2 adults (38 days). Values are geometric means; error bars are standard errors of the means. NM = not measured. A = adult.
Antimicrobial peptides are variably expressed in the developing rat pup
Sections of ileum from rat pups of varying ages stained with antibodies to lysozyme, NP3, and PAP/RegIII (stains both PAP1 and PAP3) are presented in Figure 2. Lysozyme and NP3 were strongly expressed in adult Paneth cells, but were not detectable in the youngest rat pups. There has been some controversy regarding the localization of PAP/RegIII in the small intestine. In the mouse, the orthologous molecule RegIIIγ is expressed in the secretory granules of the Paneth cell (23). In the rat, early studies immunolocalized PAP/RegIII to the enterocytes of the lower villus (24). We found that PAP/RegIII was present in the enterocytes even in the youngest rats, with minimal signal noted in the lower villi and crypts.
Figure 2. Antimicrobial Peptides in Development.
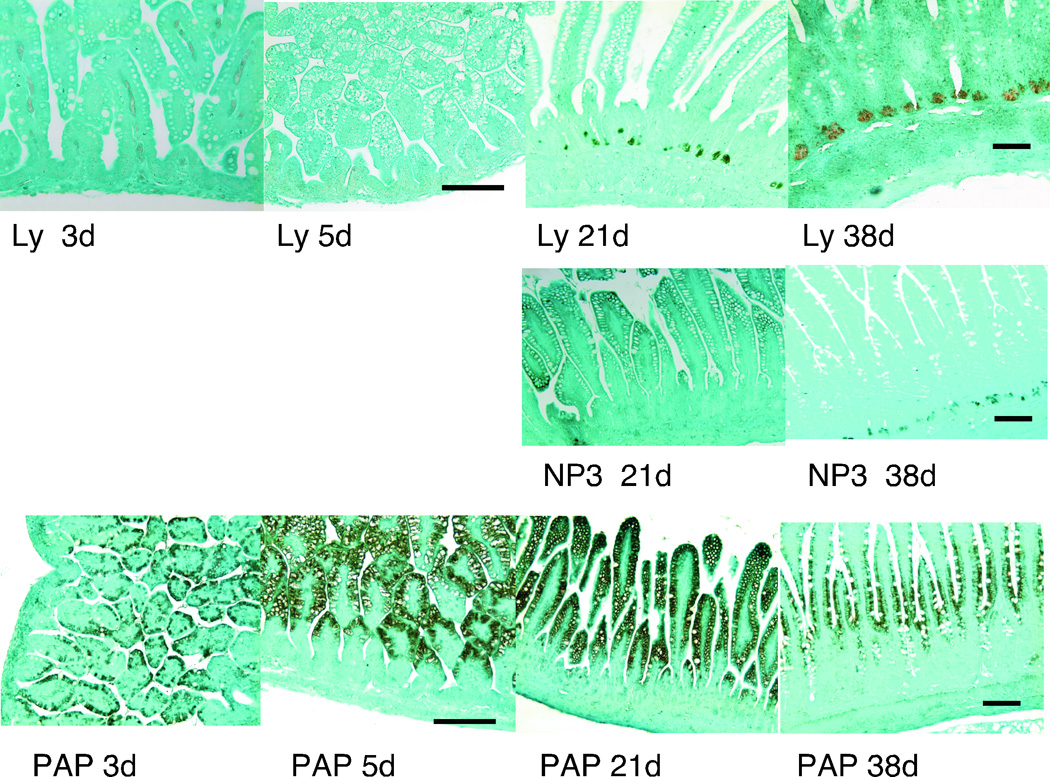
Immunohistochemistry analyses of healthy rat ileum at 3, 5, 21, and 38 (adult) days utilizing antibodies against lysozyme (Ly) and PAP/RegIII (PAP) and at days 21 and 38 against NP3. Magnification is 40X for 3 and 5 day specimens and 20X for 21 and 38 days. Measurement bar = 100 µm
Antimicrobial gene expression is increased in the NEC rat model and attenuated by B. bifidum
As shown previously (7), the incidence of histologic NEC was 0% in the DF group, 57% in the FF group, and 17% in the FF+Bif group (p < 0.01 χ2 analysis). Administration of the B. bifidum reduced the median histologic score to 1.0 compared to 2.0 in the FF group (p ≤ 0.01) (7).
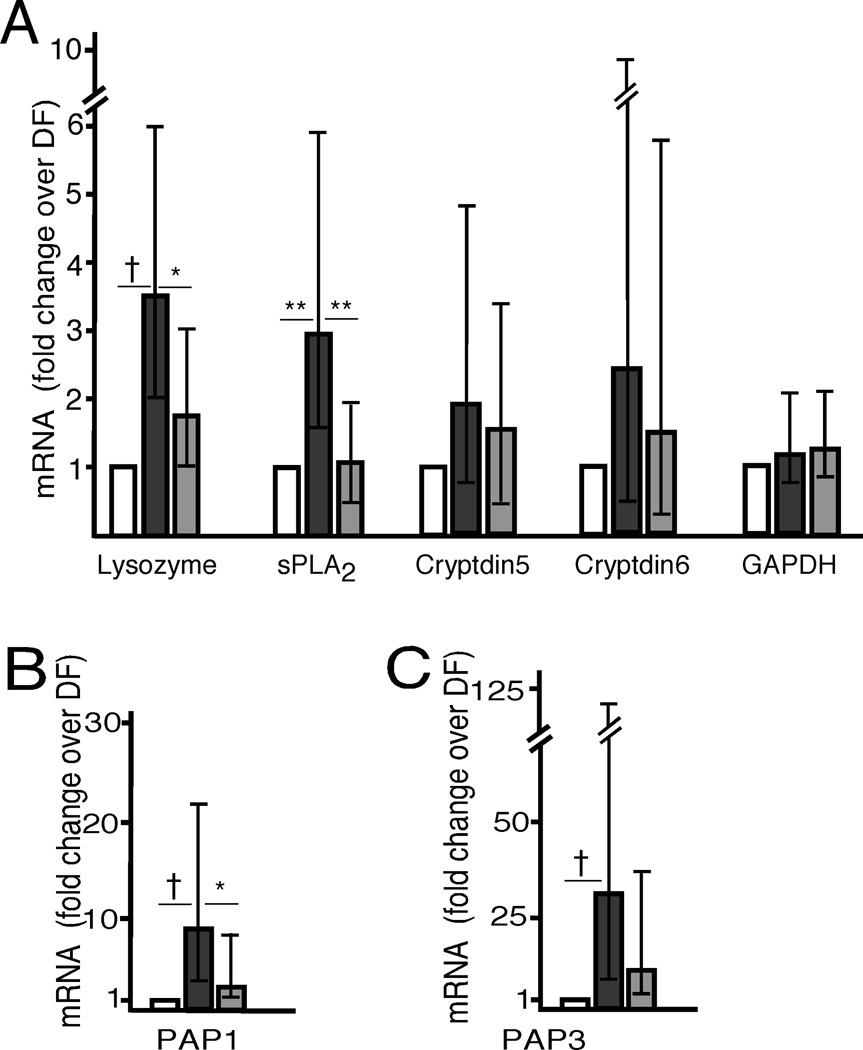
The small intestinal mRNA expression of antimicrobial enzymes, defensins, and C-type lectins is summarized for the three groups of rat pups in Figure 3. The comparisons between rat pups developing NEC (FF) and the other groups that demonstrated significant differences are denoted with asterisks (* = p < 0.05, ** = p < 0.01, † = p < 0.001). Significant increases in expression of lysozyme, sPLA2, PAP1, and PAP3 occurred in the FF group compared to the DF group and of lysozyme, sPLA2 and PAP1 in the FF group compared to the FF+BIF group. Cryptdins 5 and 6 demonstrated a similar pattern that did not reach statistical significance. The error bars in Figure 3 represent the 95% confidence intervals for fold change of expression of the FF and FF+BIF groups over the DF group and allow an additional comparison (i.e. if the 5% confidence interval is greater than 1, the difference is significant). Expression of PSP-Reg, MMP7, CRAMP, and NP3 was low in all 3 groups with no statistically significant differences between groups (data not shown). Rat pups born by Caesarian section one day early and fed by surrogate dams but not exposed to cold stress and asphyxia were similar to the dam-fed stressed group in expression of all antimicrobial molecules tested (data not shown).
Figure 3. Antimicrobial Peptide mRNA in NEC.
Fold increases above the dam-fed group in mRNA expression of Paneth cell products: enzymes, defensins and the housekeeping gene GAPDH (A), PAP1 (B) and PAP3 (C). All rat pups were exposed to asphyxia and cold stress. White bars = dam-fed, black bars = formula fed, gray bars = fed with formula supplemented with Bifidobacteria bifidum. N = 8 for each group. Error bars are 95% confidence intervals for the fold-change. * = p < 0.05, ** = p < 0.01, † = p < 0.001.
Detection of antimicrobial molecules in the NEC rat model
Sections of ileum from rat pups in the DF and FF groups showed positive staining for lysozyme in discrete cells in the lamina propria, with no difference between groups, but no staining of epithelial cells (data not shown). We could not detect staining of NP3 in either group suggesting that expression is too low for detection at this developmental stage. In spite of the dramatic increase in PAP1 and PAP3 in the FF group at the mRNA level, attempts to demonstrate differences between DF and FF tissue at the protein level with immunohistochemistry and western blotting using three different antibodies were unsuccessful. Whether this reflects no true change at the protein level or challenges specific to the antibodies selected is unclear.
Discussion
NEC remains a devastating disease of premature infants. Immaturity of the integrity of the intestinal barrier (physical, chemical, and functional) and differences in the intestinal microbiota appear to be important predisposing factors (3, 4, 25). Clinical studies indicate that probiotics are currently among the most promising preventive interventions (5, 26, 27). Our previous studies with B. bifidum OLB6378 have shown decreased intestinal injury and improved integrity of the intestinal barrier (7) and down-regulation of intestinal apoptosis (8) in the rat NEC model.
The present study was designed to investigate a central component of the mucosal innate immune system of the rat pup: antimicrobial peptides in development and in the rat NEC model. Our results show that as the rat pup matures toward weaning, mRNA expression of several intestinal antimicrobials increases 1000 to 10,000-fold. These changes are most marked for lysozyme, sPLA2, cryptdin 5 and cryptdin 6, all of which are produced in Paneth cells, but not enterocytes. PAP1 is highly expressed even in the very young rat pup; both PAP1 and PAP3 increase more than 100-fold near the time of weaning. NP3 is an α-defensin that is produced in both the neutrophil and the Paneth cell in the rat. MMP7 is a metalloproteinase that cleaves the inactive forms of the Paneth cell cryptdins to the active forms in rodents. Both of these Paneth cell products increase dramatically near the time of weaning.
Several differences in intestinal antimicrobial development between the mouse, the rat, and the human are noteworthy. In the human fetus, Paneth cells are identifiable by ultrastructural analysis and reverse transcriptase-PCR detection of HD5 and HD6 as early as 14–17 weeks gestation with increases in mRNA by Northern analysis of 40–250 fold between premature infants and adults (11). In mice, the appearance of lysozyme-staining Paneth cells between 4 and 7 days of life correlates with the development of resistance to infection with pathogenic Shigella (28). Expression of mouse intestinal defensins increases 100–1000 fold with development from the newborn to adult (29). The low expression of CRAMP in the young rat pup is in striking contrast to the mouse where constitutive expression is observed for the first 2 weeks after birth and then gradually decreases (29). In the mouse, Paneth cells are first identifiable at 7–14 days of life (28, 30). Our ability to identify the initial appearance of Paneth cells, the sources of antimicrobial peptides in the young rat pup, and changes in Paneth cell numbers in the rat NEC model is hampered by the lack of antibodies to Paneth cell specific peptides such as cryptdin 5 and cryptdin 6.
It is likely that expression of these different antimicrobial products in the rat reflect multiple mechanisms of control, as reported in the mouse. RegIIIγ (the mouse PAP/RegIII ortholog) and MMP7 appear to be inducibly expressed with minimal expression in germ-free mice and marked increases upon exposure to the intestinal microbiota (23, 31). In contrast, the mouse α-defensins (cryptdins) appear to be expressed constitutively with little or no differences between germ-free and conventionally raised mice (32). Our data in the rat support the notion that lysozyme, sPLA2, cryptdin 5, and cryptdin 6 increase rapidly with development, while PAP1, PAP3, NP3, and MMP7 increase at about the time of weaning, likely in response to changes in the intestinal microbiota.
The dramatic increases during development of rat intestinal antimicrobial expression parallel the more limited observations in human fetuses and premature infants. These observations suggest a possible association between the diminished Paneth cell products in premature infants and susceptibility of these infants to NEC. Specifically, low levels of antimicrobial peptide expression in premature infants (and young rat pups) may contribute to NEC susceptibility by mechanisms that involve either an inadequate response to opportunistic pathogens within the microbiota, and/or an inability to shape the developing microbiota in an ex-utero environment that is not physiologic (the “normal” milieu for the premature intestinal mucosa is to be bathed by swallowed amniotic fluid that is generally free from bacteria and bacterial antigens (33, 34)).
To further explore this hypothesis, we have utilized a well-established rat model of NEC. In this model, experimental NEC was attenuated by concurrent administration of Bifidobacteria bifidum (6), and completely obviated in rats remaining with and fed by surrogate dams but exposed to the same levels of asphyxia and cold stress. Our analyses demonstrate that mRNA expression of several antimicrobials is significantly increased in the formula-fed rat pups that develop NEC, compared to dam-fed controls exposed to the same asphyxia and cold stress that do not develop NEC. The pups that received formula supplemented with B. bifidum were relatively protected from NEC and had correspondingly minimal increases in expression of the antimicrobial peptides. Intestinal antimicrobial peptides are induced in NEC at the mRNA level, but the levels are low and difficult to detect at the protein level with available reagents, and thus may be inadequate to defend the immature gut from invasion and disruption by microorganisms.
It is possible that the increase in antimicrobial peptides in the FF pups is a response to mucosal inflammation. Indeed, in the colon, β-defensins 2 and 3 are induced by pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α (35). Likewise, adults with ankylosing spondylitis have increased expression of the α-defensin HD5, lysozyme and sPLA2 in the presence of chronic inflammation, but not in the presence of acute inflammation or normal histology (36). On the other hand, similar changes have not been demonstrated in other diseases of chronic intestinal inflammation (37). In ileal Crohn’s disease, decreased expression of HD5 and HD6 is observed, and more significant reductions are closely related to a specific mutation in the nucleotide-binding oligomerization domain containing 2 receptor; these changes did not correlate with inflammation (38). In colonic Crohn’s disease and ulcerative colitis, Paneth cell metaplasia results in increased expression of α-defensins that is isolated to the colon. Since in these various investigations there is a lack of evidence for induction of Paneth cell antimicrobial peptides in acute inflammation, we favor the explanation that the noted increases observed in this study are a response to disease (albeit insufficient for prevention) rather than a response to inflammation.
One proposed mechanism of probiotic action is stimulation of innate antimicrobial production. The impact of probiotics on expression of antimicrobial peptides has been studied for one of the inducible β-defensins expressed in the colon with mixed results (39–41). Our results suggest that B. bifidum does not increase expression of small intestinal antimicrobial products.
In conclusion, these studies show ontogenesis of antimicrobial peptides in the small intestine of neonatal rats under normal physiologic conditions. The development of experimental NEC is associated with increases in mRNA expression of several of these antimicrobial molecules at the site of injury. Oral administration of probiotic B. bifidum OLB6378 can avert both NEC and the associated increase in expression of antimicrobial peptides.
Acknowledgments
Statement of Financial Support:
MAU received funding from NIH HD059127 and the Children’s Miracle Network; BD from NIH HD039657 and a gift from Meiji Dairies Corporation; CLB from NIH AI32738; AK from The Development and Promotion of Science and Technology Talents Project,Thailand; HC and BCL from NIH T32AI060555; and MPS from NIH HD057744 and Gerber Foundation. This study was also made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and NIH Roadmap for Medical Research
References
- 1.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358:1700–1711. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 2.Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med. 2009;60:111–124. doi: 10.1146/annurev.med.60.050207.092824. [DOI] [PubMed] [Google Scholar]
- 3.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125:777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 5.Guthmann F, Kluthe C, Buhrer C. Probiotics for prevention of necrotising enterocolitis: an updated meta-analysis. Klin Padiatr. 2010;222:284–290. doi: 10.1055/s-0030-1254113. [DOI] [PubMed] [Google Scholar]
- 6.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R Jr. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 7.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G940–G949. doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1118–G1127. doi: 10.1152/ajpgi.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 10.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, Bevins CL. Human enteric defensins. Gene structure and developmental expression. J Biol Chem. 1996;271:4038–4045. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- 12.Salzman NH, Polin RA, Harris MC, Ruchelli E, Hebra A, Zirin-Butler S, Jawad A, Martin Porter E, Bevins CL. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res. 1998;44:20–26. doi: 10.1203/00006450-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Coutinho HB, da Mota HC, Coutinho VB, Robalinho TI, Furtado AF, Walker E, King G, Mahida YR, Sewell HF, Wakelin D. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J Clin Pathol. 1998;51:512–514. doi: 10.1136/jcp.51.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puiman PJ, Burger-Van Paassen N, Schaart MW, De Bruijn AC, De Krijger RR, Tibboel D, Van Goudoever JB, Renes IB. Paneth cell hyperplasia and metaplasia in necrotizing enterocolitis. Pediatr Res. 2011;69:217–223. doi: 10.1203/PDR.0b013e3182092a9a. [DOI] [PubMed] [Google Scholar]
- 15.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 16.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 18.Wehkamp J, Chu H, Shen B, Feathers RW, Kays RJ, Lee SK, Bevins CL. Paneth cell antimicrobial peptides: Topographical distribution and quantification in human gastrointestinal tissues. FEBS Letters. 2006;580:5344–5350. doi: 10.1016/j.febslet.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 20.Moore KS, Bevins CL, Tomassini N, Huttner KM, Sadler K, Moreira JE, Reynolds J, Zasloff M. A novel peptide-producing cell in Xenopus: multinucleated gastric mucosal cell strikingly similar to the granular gland of the skin. J Histochem Cytochem. 1992;40:367–378. doi: 10.1177/40.3.1552176. [DOI] [PubMed] [Google Scholar]
- 21.Termen S, Tollin M, Olsson B, Svenberg T, Agerberth B, Gudmundsson GH. Phylogeny, processing and expression of the rat cathelicidin rCRAMP: a model for innate antimicrobial peptides. Cell Mol Life Sci. 2003;60:536–549. doi: 10.1007/s000180300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia HP, Mills JN, Barahmand-Pour F, Nishimura D, Mallampali RK, Wang G, Wiles K, Tack BF, Bevins CL, McCray PB Jr. Molecular cloning and characterization of rat genes encoding homologues of human beta-defensins. Infect Immun. 1999;67:4827–4833. doi: 10.1128/iai.67.9.4827-4833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iovanna JL, Keim V, Bosshard A, Orelle B, Frigerio JM, Dusetti N, Dagorn JC. PAP, a pancreatic secretory protein induced during acute pancreatitis, is expressed in rat intestine. Am J Physiol. 1993;265:G611–G618. doi: 10.1152/ajpgi.1993.265.4.G611. [DOI] [PubMed] [Google Scholar]
- 25.Sharma R, Tepas JJ., 3rd Microecology, intestinal epithelial barrier and necrotizing enterocolitis. Pediatr Surg Int. 2010;26:11–21. doi: 10.1007/s00383-009-2536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szajewska H. Probiotics and prebiotics in preterm infants: Where are we? Where are we going? Early Hum Dev 86 Suppl. 2010;1:81–86. doi: 10.1016/j.earlhumdev.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Sherman MP. New concepts of microbial translocation in the neonatal intestine: mechanisms and prevention. Clin Perinatol. 2010;37:565–579. doi: 10.1016/j.clp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez MI, Regnault B, Mulet C, Tanguy M, Jay P, Sansonetti PJ, Pedron T. Maturation of paneth cells induces the refractory state of newborn mice to Shigella infection. J Immunol. 2008;180:4924–4930. doi: 10.4049/jimmunol.180.7.4924. [DOI] [PubMed] [Google Scholar]
- 29.Menard S, Forster V, Lotz M, Gutle D, Duerr CU, Gallo RL, Henriques-Normark B, Putsep K, Andersson M, Glocker EO, Hornef MW. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med. 2008;205:183–193. doi: 10.1084/jem.20071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bry L, Falk P, Huttner K, Ouellette A, Midtvedt T, Gordon JI. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci U S A. 1994;91:10335–10339. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65:3019–3027. doi: 10.1007/s00018-008-8182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putsep K, Axelsson LG, Boman A, Midtvedt T, Normark S, Boman HG, Andersson M. Germ-free and colonized mice generate the same products from enteric prodefensins. J Biol Chem. 2000;275:40478–40482. doi: 10.1074/jbc.M007816200. [DOI] [PubMed] [Google Scholar]
- 33.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25:341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 35.Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–651. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 36.Ciccia F, Bombardieri M, Rizzo A, Principato A, Giardina AR, Raiata F, Peralta S, Ferrante A, Drago S, Cottone M, Pitzalis C, Triolo G. Over-expression of paneth cell-derived anti-microbial peptides in the gut of patients with ankylosing spondylitis and subclinical intestinal inflammation. Rheumatology (Oxford) 2010;49:2076–2083. doi: 10.1093/rheumatology/keq239. [DOI] [PubMed] [Google Scholar]
- 37.Jager S, Stange EF, Wehkamp J. Antimicrobial peptides in gastrointestinal inflammation. Int J Inflam. 2010 doi: 10.4061/2010/910283. 2010:910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn's disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58:882–883. discussion 883-884. [PubMed] [Google Scholar]
- 39.Schlee M, Harder J, Koten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol. 2008;151:528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amit-Romach E, Uni Z, Reifen R. Multistep mechanism of probiotic bacterium, the effect on innate immune system. Mol Nutr Food Res. 2010;54:277–284. doi: 10.1002/mnfr.200800591. [DOI] [PubMed] [Google Scholar]
- 41.Mondel M, Schroeder BO, Zimmermann K, Huber H, Nuding S, Beisner J, Fellermann K, Stange EF, Wehkamp J. Probiotic E. coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2:166–172. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]