Abstract
Aims: The mitochondrial dysfunction in our lamb model of congenital heart disease with increased pulmonary blood flow (PBF) (Shunt) is associated with disrupted carnitine metabolism. Our recent studies have also shown that asymmetric dimethylarginine (ADMA) levels are increased in Shunt lambs and ADMA increases the nitration of mitochondrial proteins in lamb pulmonary arterial endothelial cells (PAEC) in a nitric oxide synthase (NOS)-dependent manner. Thus, we determined whether there was a mechanistic link between endothelial nitric oxide synthase (eNOS), ADMA, and the disruption of carnitine homeostasis in PAEC. Results: Exposure of PAEC to ADMA induced the redistribution of eNOS to the mitochondria, resulting in an increase in carnitine acetyl transferase (CrAT) nitration and decreased CrAT activity. The resulting increase in acyl-carnitine levels resulted in mitochondrial dysfunction and the disruption of mitochondrial bioenergetics. Since the addition of l-arginine prevented these pathologic changes, we examined the effect of l-arginine supplementation on carnitine homeostasis, mitochondrial function, and nitric oxide (NO) signaling in Shunt lambs. We found that the treatment of Shunt lambs with l-arginine prevented the ADMA-mediated mitochondrial redistribution of eNOS, the nitration-mediated inhibition of CrAT, and maintained carnitine homeostasis. In turn, adenosine-5′-triphosphate levels and eNOS/heat shock protein 90 interactions were preserved, and this decreased NOS uncoupling and enhanced NO generation. Innovation: Our data link alterations in cellular l-arginine metabolism with the disruption of mitochondrial bioenergetics and implicate altered carnitine homeostasis as a key player in this process. Conclusion: l-arginine supplementation may be a useful therapy to prevent the mitochondrial dysfunction involved in the pulmonary vascular alterations secondary to increased PBF. Antioxid. Redox Signal. 18, 1739–1752.
Introduction
Disruption of mitochondrial function is a critical event in a number of pathologic conditions, including hypoxia-ischemic injuries (5), stroke (54), diabetes (15), and hypertension (34). Under conditions of metabolic stress, mitochondria accumulate acyl-coenzyme A (acyl-CoA), which can inhibit oxidative phosphorylation (12). There is a decline in mitochondrial function associated with aging (30, 31), and oxidative damage to the mitochondrial enzymes regulating carnitine homeostasis is an important mediator in this process (30, 31). The principal enzyme affected has been identified as carnitine acetyltransferase (CrAT), which catalyzes a reversible equilibrium reaction between acyl-CoA and CoA, and acylcarnitine and carnitine (59). Pulmonary mitochondrial function is attenuated in our lamb model of a congenital heart defect with increased pulmonary blood flow (PBF) (Shunt), and this correlates with a disruption of carnitine metabolism (42). One of the major correlations with the disrupted carnitine homeostasis was a reduction in CrAT activity associated with increased nitration (42). However, the mechanism by which CrAT becomes nitrated was not elucidated. Asymmetric dimethylarginine (ADMA) is an endogenous competitive inhibitor of nitric oxide synthase (NOS). Increased ADMA levels are implicated in a number of conditions affecting the cardiovascular system. Our recent studies have shown that the ADMA levels are increased in Shunt lambs secondary to a decrease in dimethylarginine hydrolases (DDAH) activity (47) and that ADMA increases the nitration of mitochondrial proteins in cultured lamb pulmonary arterial endothelial cells (PAEC) (46). Thus, the purpose of this study was to determine whether there was a mechanistic link between increases in ADMA and the disruption of carnitine metabolism; and if so, whether l-arginine supplementation could prevent the mitochondrial dysfunction in Shunt lambs. In cultured PAEC, we found that ADMA increased CrAT nitration and decreased CrAT activity via the redistribution of endothelial nitric oxide synthase (eNOS) from the plasma membrane to the mitochondria, which resulted in a disruption in carnitine metabolism and mitochondrial bioenergetics. In Shunt lambs, we found that l-arginine supplementation prevented the ADMA-mediated translocation of eNOS to the mitochondria and this attenuated the nitration-mediated inhibition of CrAT associated with increased PBF. This, in turn, preserved carnitine homeostasis, adenosine-5′-triphosphate (ATP) levels, and eNOS/heat shock protein 90 (Hsp90) interactions. This resulted in a decrease in NOS uncoupling and enhanced nitric oxide (NO) generation in l-arginine supplemented Shunt lambs. Taken together, our data suggest that there is a link between cellular arginine metabolism and mitochondrial dysfunction through the disruption of carnitine homeostasis, indicating that l-arginine supplementation may be a useful therapy for the endothelial dysfunction associated with various cardiovascular disorders, including pulmonary hypertension.
Innovation.
Our study provides a novel insight into the role of endothelial nitric oxide synthase mitochondrial targeting and the disruption of endothelial mitochondrial bioenergetics in pulmonary hypertension. Further, our data implicate increases in asymmetric dimethylarginine and the disruption of carnitine homeostasis as key players in this process. We speculate that l-arginine supplementation may have therapeutic potential in the treatment of the pulmonary endothelial dysfunction in patients with increased pulmonary blood flow.
Results
The ADMA-induced redistribution of eNOS to the mitochondria disrupts mitochondrial bioenergetics
PAEC were transiently transfected with an eNOS-green fluorescent protein (GFP) construct (48), treated with Mitotracker, and then exposed to ADMA (5 μM) in the presence or absence of l-arginine (75 μM). The co-localization of the GFP-tagged eNOS with the red-labeled mitochondria was determined over a 60-min period, and the Pearson product-moment correlation coefficient was determined to estimate the redistribution of eNOS to the mitochondria. ADMA induced a time-dependent increase in the localization of eNOS to the mitochondria (Fig. 1A). Western blotting of isolated mitochondria was also used to confirm the ADMA-mediated increase in eNOS localized to the mitochondria (Fig. 1B). The redistribution of eNOS to the mitochondria correlated with a decrease in mitochondrial membrane potential (Fig. 1C, D), a decrease in superoxide dismutase (SOD) 2 (Fig. 1E), and an increase in uncoupling protein 2-protein levels (Fig. 1F). Consistent with these results, the NOS inhibitors, NG-methyl-l-arginine acetate (l-NMMA) and Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) increased protein nitration levels (Fig. 1G) and decreased mitochondrial membrane potential (Fig. 1H, I). Further, ADMA treatment increased both the level of uncoupled NOS associated with the mitochondria (Fig. 1J) and the levels of peroxynitrite within the mitochondria (Fig. 1K). Although basal respiration was unaffected (Fig. 2A, B), both reserve respiratory capacity (Fig. 2A, C) and maximal respiratory capacity (Fig. 2A, D) were significantly reduced by ADMA. In all cases, changes were prevented by the addition of l-arginine (Figs. 1 and 2). Treatment of PAEC with ADMA in the presence or absence of l-arginine did not alter either DDAH1- (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars) or DDAH2-protein levels (Supplementary Fig. S1B). These treatments did not alter cellular DDAH activity (Supplementary Fig. S1C) or appear to induce apoptotic events in PAEC (Supplementary Fig. S2).
FIG. 1.
l-Arginine attenuates the ADMA-mediated eNOS mitochondrial translocation and mitochondrial dysfunction in PAEC. Cells were transfected with eNOS-GFP. The mitochondria of these cells were then labeled with MitoTracker (red), and the cells were exposed to ADMA (5 μM) in the presence or absence of l-arginine (75 μM). The extent of mitochondrial localization of eNOS was then determined by measuring the intensity of yellow fluorescence (overlap of red fluorescence of MitoTracker and green fluorescence of eNOS-GFP). There is a time-dependent increase in yellow fluorescence in the ADMA treated cells, and this is blocked by the addition of l-arginine (A). Mitochondrial fractions were also isolated, and the level of eNOS protein accumulation in mitochondria was determined by Western blotting (B). ADMA increases eNOS accumulation in the mitochondria, and this is blocked by l-arginine (B). Loading was normalized by reprobing with the mitochondrial protein, VDAC and reprobed with antibodies raised against NaKATPase, Calnexin, 58K, or laminB1 to demonstrate no cross-contamination with the plasma membrane, ER membrane, Golgi, or nuclear fractions, respectively (B). A separate gel was run using a PAEC cell lysate (20 μg) to demonstrate that each antibody recognizes the ovine protein (B). ADMA also reduces the mitochondrial membrane potential as estimated by the increase in the green, momomeric form and the decrease in the aggregated red form of the DePsipher compound (C, D) Western blot analysis also revealed that ADMA decreased the levels of SOD2 (E) and increased the levels of UCP2 (F). l-Arginine treatment prevented these changes (E, F). The NOS inhibitors, l-NMMA (100 μM) and l-NAME (100 μM) also increased PAEC protein nitration (G) and reduced the mitochondrial membrane potential (H, I). The translocation of eNOS to the mitochondria was associated with NOS uncoupling as shown by an increase in NOS-derived superoxide (J) as well as an increase in mitochondrial peroxynitrite levels (K). Values are means±SEM; n=4–8. *p<0.05 versus untreated, †p<0.05 versus ADMA alone. 3-NT, 3-nitrotyrosine; ADMA, asymmetric dimethylarginine; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; GFP, green fluorescent protein; l-NAME, Nω-nitro-l-arginine methyl ester hydrochloride; l-NMMA, NG-methyl-L-arginine acetate; NOS, nitric oxide synthase; PAEC, pulmonary arterial endothelial cells; SEM, standard error of the mean; SOD, superoxide dismutase; UCP2, uncoupling protein 2; VDAC, voltage-dependent anion channel. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
FIG. 2.
ADMA inhibits mitochondrial respiration in PAEC. PAEC (75,000 cells/0.32 cm2) were exposed to ADMA (5 μM, 2 h) in the presence or absence of l-arginine (75 μM); then, the Seahorse XF24 analyzer was used to take measurements. Oligomycin (1 μM), FCCP (1 μM), and rotenone and antimycin A (1 μM each) were added at the indicated points (A). ADMA did not significantly alter basal mitochondrial respiration as estimated by measuring the rate of OCR (B). However, both the reserve respiratory capacity (C) and the maximal respiratory capacity (D) in PAEC were significantly attenuated by ADMA, and these changes were prevented by l-arginine (C, D). Values are means±SEM; n=5–6. *p<0.05 versus untreated; †p<0.05 versus ADMA only. FCCP, carbonilcyanide p-triflouromethoxyphenylhydrazone; OCR, oxygen consumption rate. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
ADMA disrupts carnitine metabolism in PAEC
Our previous studies demonstrated that increased levels of ADMA in Shunt lambs (47) correlated with a disruption in carnitine homeostasis and a loss of carnitine acetyl transferase (CrAT) activity (42). Carnitine homeostasis is of critical importance for maintaining oxidative phosphorylation (41). To determine whether ADMA disrupts mitochondrial bioenergetics in PAEC via the disruption of carnitine homeostasis, we first examined the effect of ADMA (5 μM, 2 h) on CrAT activity and found it to be significantly reduced (Fig. 3A), although protein levels were unaffected (Fig. 3B). The reduction in CrAT activity correlated with an increase in CrAT nitration (Fig. 3C), and increased levels of acyl carnitine (Fig. 3D) and the acyl carnitine:free carnitine ratio (Fig. 3E), indicating that carnitine homeostasis was disrupted. Again, the addition of l-arginine preserved CrAT activity and maintained carnitine homeostasis (Fig. 3). No changes in the protein levels of the other carnitine homeostasis enzymes (carnitine palmitoyltransferase I [CPT1] and carnitine palmitoyltransferase II [CPT2]) were observed (data not shown).
FIG. 3.
ADMA disrupts carnitine homeostasis in PAEC. PAEC were treated with ADMA (5 μM, 2 h) in the presence or absence of l-arginine (75 μM), and CrAT activity was determined. ADMA decreases CrAT activity in PAEC, and this is prevented by l-arginine (A). Western blot analysis indicates that ADMA does not change CrAT protein levels (B). Protein extracts (1 mg) were also subjected to IP using an antibody that was specific to 3-NT and then analyzed by Western blot analysis using a specific antiserum raised against CrAT. A representative IB is shown. Data were normalized by carrying out Western blot analysis on IP input (20 μg) of the protein extracts probed with an antibody to CrAT. ADMA significantly increases CrAT nitration (C), and this is prevented by l-arginine (C). The affect of ADMA on acylcarnitine levels (D) and the acylcarnitine:free carnitine ratio (E) was also determined. ADMA significantly increases acylcarnitine levels and the acylcarnitine:free carnitine ratio. Again, l-arginine treatment prevented these changes (D, E). Values are means±SEM; n=4–6. *p<0.05 versus untreated; †p<0.05 versus ADMA alone. CrAT, carnitine acetyl transferase; IB, immunoblot; IP, immunoprecipitation.
l-Arginine supplementation prevents the mitochondrial redistribution of eNOS in Shunt lambs
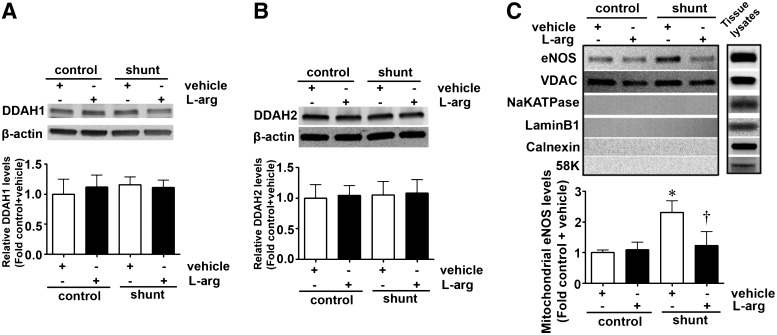
Our previous studies have demonstrated that in Shunt lambs, ADMA levels are increased due to a reduction in DDAH activity (47). Interestingly, this reduction in DDAH activity does not correlate with a reduction in either DDAH1- (Fig. 4A) or DDAH2- (Fig. 4B) protein levels. However, as in PAEC exposed to ADMA, there is an increase in eNOS in the mitochondrial fraction of Shunt lambs (Fig. 4C). This increase is not observed in Shunt lambs supplemented with l-arginine (500 mg/kg/day, Fig. 4C). l-Arginine had no affect on the mitochondrial distribution of eNOS in control lambs (Fig. 4C).
FIG. 4.
l-Arginine supplementation prevents eNOS mitochondrial translocation in lambs with increased PBF. Total protein extracts prepared from the peripheral lung of Shunt or age-matched control lambs supplemented or not with l-arginine at 3 weeks of age and subjected to Western blotting using antibodies raised against DDAH1 (A) or DDAH2 (B). Representative images are shown. Blots were reprobed with β-actin to normalize loading. No changes in DDAH1- or DDAH2-protein levels are observed between the four groups. Mitochondrial protein extracts (5 μg) were also prepared from each of the four groups and subjected to Western blotting using an antibody raised against eNOS. Loading was normalized by reprobing the membranes with an antibody raised against the mitochondrial protein, VDAC and reprobed with antibodies raised against NaKATPase, Calnexin, 58K, or laminB1 to demonstrate no cross-contamination with the plasma membrane, ER membrane, Golgi, or nuclear fractions, respectively. A representative image is shown. The levels of eNOS in the mitochondria are significantly increased in Shunt lambs, and this was prevented by l-arginine supplementation (C). Values are means±SEM; n=4–9. *p<0.05 versus control+vehicle; †p<0.05 versus Shunt+vehicle. PBF, pulmonary blood flow.
l-Arginine supplementation preserves carnitine homeostasis and lung ATP levels in Shunt lambs
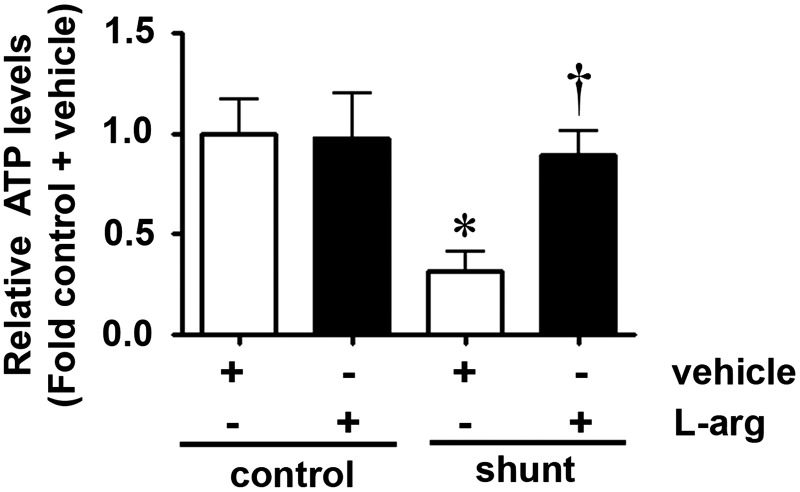
Our recent studies have shown that increased CrAT nitration and decreased CrAT activity correlates with a disruption in carnitine homeostasis and NO signaling in Shunt lambs (42). To determine whether l-arginine supplementation could preserve carnitine homeostasis and mitochondrial function in Shunt lambs, we examined the effect of l-arginine supplementation on the levels of total protein nitration, CrAT activity, and CrAT nitration in control and Shunt lambs exposed to l-arginine (500 mg/kg/day) or vehicle for 3 weeks. We found that SOD2 activity is decreased in Shunt lambs (Fig. 5A) but even though there is a trend toward preservation, l-arginine supplementation did not prevent the decrease in SOD2 activity (Fig. 5A). l-Arginine had no affect on SOD2 activity in control lambs (Fig. 5A). Our data also indicate that in Shunt lambs, total protein nitration (Fig. 5B) as well as CrAT nitration (Fig. 5C) are increased whereas CrAT activity (Fig. 5D) is decreased compared with age-matched control lambs. l-Arginine supplementation decreased the levels of both total protein- (Fig. 5B) and CrAT-nitration (Fig. 5C) and preserved CrAT activity (Fig. 5D) without altering CrAT protein levels (Fig. 5E). l-Arginine supplementation had no effect on total protein- (Fig. 5B) or CrAT nitration (Fig. 5C) or CrAT activity (Fig. 5D) in control lambs. l-Arginine supplementation in either control or Shunt lambs did not significantly change the levels of CPT1 or CPT2 (data not shown). Consistent with our in vitro data, in Shunt lambs, both acylcarnitine levels and the acylcarnitine:free carnitine ratios were significantly increased compared with control lambs (Fig. 5F, G), while l-arginine supplementation completely abolished these increases (Fig. 5F, G). l-Arginine supplementation had no affect on carnitine homeostasis in control lambs (Fig. 5F, G). Since carnitine homeostasis is of critical importance for maintaining oxidative phosphorylation (41) and ATP generation (59), we determined ATP levels in control and Shunt lambs with and without l-arginine administration. l-Arginine supplementation prevented the reduction of ATP levels observed in Shunt lambs (Fig. 6) but had no affect on control lambs (Fig. 6).
FIG. 5.
l-Arginine supplementation preserves CrAT activity in lambs with increased PBF. SOD2 activity is decreased in Shunt lambs (A), but l-arginine supplementation failed to preserve SOD2 activity (A). Protein extracts (30 μg) prepared from peripheral lungs of Shunt lambs or age-matched controls with either l-arginine or vehicle administration were subjected to dot-blot analysis using an antibody raised against 3-NT. Protein levels were normalized by re-probing with an antibody that was specific to (-actin. There is an increase in total protein nitration in Shunt lambs that is attenuated by l-arginine supplementation (B). Protein extracts (1 mg) prepared from peripheral lungs of control and Shunt lambs with l-arginine or vehicle administration were subjected to IP using an antibody that was specific to 3-NT and then analyzed by Western blot analysis using a specific antiserum raised against CrAT. A representative IB is shown. Data were normalized by carrying out Western blot analysis on IP input (20 μg) of the protein extracts probed with an antibody to CrAT. CrAT nitration is significantly increased in Shunt lambs, and this is prevented by l-arginine supplementation (C). CrAT activity (D) and CrAT protein levels (E) were also determined in peripheral lung lysates. The decrease in CrAT activity in Shunt lambs is prevented by l-arginine supplementation (D). l-Arginine supplementation does not alter CrAT protein levels in either Shunt or control lambs (E). The affect of l-arginine supplementation on acylcarnitine levels (F) and the acylcarnitine:free carnitine ratio (G) was also determined. The increase in acylcarnitine levels (F) and the acylcarnitine:free carnitine ratio (G) in Shunt lambs is prevented by l-arginine supplementation. l-Arginine supplementation did not alter SOD2 activity (A), total CrAT or CrAT nitration levels (B, C), acylcarnitine levels (F) or the acylcarnitine:free carnitine ratio (G) in control lambs. Values are means±SEM; n=6–9. *p<0.05 versus control+vehicle; †p<0.05 versus Shunt+vehicle.
FIG. 6.
l-Arginine supplementation preserves ATP levels in lambs with increased PBF. ATP levels were determined in peripheral lung lysates prepared from Shunt or age-matched control lamb with either l-arginine or vehicle administration. The decrease in ATP levels in Shunt lambs is prevented by l-arginine supplementation. l-Arginine supplementation does not alter ATP levels in control lambs. Values are means±SEM; n=6–9. *p<0.05 versus control+vehicle; †p<0.05 versus Shunt+vehicle. ATP, adenosine-5′-triphosphate.
l-arginine supplementation preserves NO signaling in Shunt lambs
Consistent with our previous studies (3), we found that eNOS protein levels were significantly increased in Shunt, compared with control lambs (Fig. 7A). However, this increase was ablated by l-arginine supplementation (Fig. 7A). l-Arginine alone had no effect on eNOS protein levels in control lambs (Fig. 7A). The eNOS dimer:monomer ratio is also decreased in Shunt lambs (Fig. 7B), and this was again prevented by l-arginine supplementation (Fig. 7B). l-Arginine had no affect on the eNOS dimer:monomer ratio in control lambs (Fig. 7B). Since ATP levels are decreased in Shunt lambs (Fig. 6) and Hsp90 chaperone activity is ATP dependent (42), we also determined the affect on Hsp90/eNOS interactions in control and Shunt lambs with and without l-arginine supplementation. Consistent with our previous studies (42), our data indicate that, when normalized by total eNOS protein levels, Hsp90/eNOS interactions are disrupted in Shunt lambs (Fig. 7C), while l-arginine treatment prevented the decrease in Hsp90/eNOS interactions in Shunt lambs (Fig. 7C). Again, l-arginine supplementation alone had no affect on Hsp90/eNOS interactions in control lambs (Fig. 7C). Finally, we tested the effect of l-arginine on NOS uncoupling and nitric oxide metabolites (NOx) generation. Our data indicate that NOS uncoupling, as estimated by an increase in NOS-derived superoxide, is significantly increased in Shunt lambs, while l-arginine treatment completely abolished this increase (Fig. 7D). We also found that l-arginine supplementation preserved NOx generation in Shunt lambs (Fig. 7E). Again, l-arginine supplementation alone had no effect on NOS-derived superoxide (Fig. 7D) or NOx generation in control lambs (Fig. 7E).
FIG. 7.
l-Arginine supplementation preserves NO signaling in lambs with increased PBF. Protein extracts (30 μg) prepared from peripheral lungs of Shunt lambs or age-matched controls with either l-arginine or vehicle administration was subjected to Western blot analysis using an antibody raised against eNOS. Loading was normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown. eNOS protein levels are significantly increased in Shunt lambs, and this is prevented by l-arginine supplementation (A). Protein extracts (60 μg) were also subjected to LT-PAGE to evaluate dimeric and monomeric forms of eNOS. A representative IB is shown. The eNOS dimer:monomer ratio is significantly decreased in Shunt lambs, and this is prevented by l-arginine supplementation (B). The interaction of eNOS with Hsp90 was determined by IP using a specific antiserum raised against eNOS in peripheral lung lysates (1 mg). IP extracts were analyzed using antisera against either eNOS or Hsp90. A representative image is shown (C). The levels of eNOS protein associated with Hsp90 relative to total eNOS protein were then calculated to account for the differences in the levels of eNOS protein between groups. There is a significant decrease in the association of eNOS with Hsp90 in Shunt lambs (C), while l-arginine supplementation preserves the eNOS–Hsp90 interaction (C). NOS-derived superoxide generation in the peripheral lung is increased in Shunt lambs, and this is attenuated by l-arginine supplementation (D). Peripheral lung NOx levels are decreased in Shunt lambs and increased by l-arginine supplementation (E). l-Arginine supplementation affected eNOS protein levels (A), eNOS dimer:monomer ratio (B), eNOS–Hsp90 interaction (C), NOS-derived superoxide (D), or NOx levels (E) in control lambs. Values are means±SEM; n=6–12. *p<0.05 versus control+vehicle, †p<0.05 versus Shunt+vehicle. Hsp90, heat shock protein 90; NO, nitric oxide; LT-PAGE, low-temperature polyacrylamide gel electrophoresis.
Discussion
The carnitine “shuttle” is critical for maintaining normal mitochondrial function, and its disruption is associated with disrupted mitochondrial bioenergetics (9, 41). CrAT plays a central role in this process. CrAT reconverts the short- and medium-chain acyl-CoAs into acylcarnitines. Through this mechanism of reversible acylation, the cell is able to modulate the intracellular concentrations of free-CoA and acyl-CoA (50). Oxidative damage to CrAT can result in the disruption of carnitine homeostasis (30, 31), indicating that the activity of CrAT may be more important than changes in its expression. Indeed, in 2-week-old Shunt lambs, the decrease in CrAT activity is greater than the overall decrease in CrAT protein (42). This decrease in CrAT activity is associated with increased CrAT nitration (42). Consistent with these results, we found that at 3 weeks of age, total protein nitration and CrAT nitration levels were also significantly increased in Shunt lambs, which corresponded with reduced CrAT activity. Further, the increase in CrAT nitration appears to be driven by the redistribution of eNOS to the mitochondria. Although the mechanism for this translocation has not been resolved, it requires the presence of the mitochondrial targeting sequence previously identified in eNOS (17). However, it has been proposed that eNOS redistribution from the plasma membrane to other sub-cellular compartments may be a mechanism for regulating the enzymatic activity of eNOS (16), and plasma membrane-bound eNOS and Golgi-bound eNOS generate more NO than cytosolic eNOS (10, 22, 60). Alternatively, since NO has been shown to regulate the electron transport chain (8) in the mitochondria, it may be involved in regulating ATP generation. However, further studies will be required to evaluate these possibilities.
Recent data have shown that in pulmonary hypertension there is a phenotypic switch in the smooth muscle cell (SMC) that results in the appearance of a glycolytic phenotype or “Warburg effect” to generate the ATP required to maintain cell viability (40). In SMC, this reduction in oxidative phosphorylation correlates with an increase in mitochondrial membrane potential, a reduction in mitochondrial-derived reactive oxygen species (ROS), and an anti-apoptotic phenotype (14). In PAEC, we observe a similar reduction in oxygen consumption and a decrease in both reserve and maximal respiratory capacity, indicative of an attenuation of oxidative phosphorylation. However, we also observe a reduction in mitochondrial membrane potential and, as we have, previously published, an increase in mitochondrial-derived ROS and a reduction in ATP levels (46). Thus, unlike SMC, PAEC exposed to a “stress milieu” do not appear to be able to maintain their ATP levels, suggesting that they are incapable of enhancing their glycolytic rate. Thus, our data suggest that strategies which target the glycolytic phenotype in SMC (49) could result in even greater injury to the endothelium and further attenuate NO signaling.
There also appears to be developmental regulation of the carnitine homeostasis enzymes in lambs exposed to increased PBF. At 2 weeks of age, we have previously shown that CPT1, CPT2, and CrAT protein levels are decreased in Shunt lambs (42). However, our Western blot analyses indicate that these differences are lost in 3-week-old Shunt lambs, suggesting that there are compensatory signaling pathways which normalize the expression of the carnitine homeostasis enzymes. Although this is possible, as we are examining protein levels in peripheral lung tissue containing multiple cell types, we are missing changes in CPT1, CPT2, and CrAT that are occurring only in the endothelium. However, in support of our data, we have observed similar developmental changes in the expression of a number of genes in Shunt lambs over the first 2 months of life, including eNOS (3), prepro-endothelin-1 (2, 36), endothelin type A receptor and endothelin type B receptors (4), and transforming growth factor β1 (33). Further, we speculate that it is the inhibition of CrAT activity which is key to the mitochondrial dysfunction we observe in Shunt lambs. Indeed, our data demonstrate that the level of a protein does not necessarily reflect its actual activity and emphasizes the need to evaluate the role of post-translational protein modifications in the disease process. Examining post-translational modifications is a complex process, as our data indicate that CrAT activity is decreased ∼2–3-fold at 3 weeks of age, at 2 weeks of age relative CrAT activity (normalized to changes in CrAT protein levels) are decreased ∼8–10-fold, suggesting that in addition to modifications which inhibit CrAT activity (nitration), there are also potential post-translational events that may stimulate activity. Although these events are unresolved, carnitine homeostasis can be regulated by malonyl-CoA binding to the N-terminal domain of CPT-1 (25, 43, 44). In turn, changes in the levels of malonyl-CoA are regulated by the activity of acetyl-CoA carboxylase mediated by 5′-adenosine 5′-monophosphate-activated protein kinase (26, 32, 56). Direct phosphorylation of the carnitine homeostasis enzymes by Ca2+/calmodulin-dependent protein kinase II (51, 53) or alterations in the interactions of cytoskeletal components with the mitochondrial outer membrane have also been proposed (52). However, future studies will be required to elucidate these events.
The ATP-dependent molecular chaperone, Hsp90 interacts with proteins that are required for efficient NO biosynthesis, including eNOS (23), soluble guanylate cyclase (58), guanosine-5′-triphosphate cyclohydrolase I (47), 3-phosphoinostide-dependent kinase-1 (55), and Akt1 (7). Reductions in Hsp90–eNOS attenuate NO production (18, 19, 42, 46), leading to eNOS uncoupling (37, 42, 46). eNOS uncoupling is a complex process that is mediated by decreases in the availability of the substrate, l-arginine, the co-factor tetrahydrobiopterin or, as we have recently shown, increases in the cellular levels of ADMA (46). Consistent with these studies, we demonstrate that decreased ATP levels in Shunt lambs correlate with disturbed Hsp90–eNOS interactions and increased eNOS uncoupling, while l-arginine supplementation preserves Hsp90–eNOS and NOx production. l-Arginine supplementation did not enhance NOx levels in control lambs. The reasons for this are unclear. However, ADMA inhibits NO generation by competing with l-arginine for the substrate-binding site of NOS. Thus, the eNOS coupling/uncoupling decision is a competition between l-arginine and ADMA for the eNOS active site. Thus, the preservation of NO generation Shunt lambs supplemented with l-arginine my be due, in part, to an increased likelihood that l-arginine rather than ADMA will be in the active site. However, under physiologic conditions, there is already sufficient l-arginine present to keep eNOS tightly coupled so that increasing its levels has no significant effect on its ability to generate NO. It is also unclear why l-arginine supplementation prevents the increase in eNOS expression in Shunt lambs. However, it is possible that the increase in NO signaling observed in l-arginine-treated Shunt lambs induces vasodilation and reduces the exposure of pulmonary endothelium to shear stress. Since shear stress is a major stimulus of increased eNOS gene expression, this would result in less eNOS transcription and a reduction in eNOS protein levels as observed. Alternatively, it is possible that l-arginine is exerting a positive effect independent of its affects on the ADMA:l-arginine ratio and NO signaling in Shunt lambs. This possibility is supported by a recent work in which l-arginine was shown to prevent d-glucose-mediated increases in arginase expression, oxidative stress, endothelial dysfunction, and advanced glycation endproducts (AGE) formation in an NO-independent manner (13).
Materials and Methods
Surgical preparations and care
This is described in the online Supplementary Data. All protocols and procedures were approved by the Committees on Animal Research at University of California, San Francisco, Georgia Health Sciences University, and the German Heart Center.
Antibodies and chemicals
Anti-CrAT antibody was purchased from Proteintech; anti-Hsp90 and anti-eNOS antibodies were purchased from BD Transduction laboratories. The anti-nitrotyrosine antibody was obtained from Calbiochem. sodium potassium ATPase (NaKATPase; cat# ab7671), LaminB1 (cat# ab8982), Calnexin (cat# ab119202), and 58K (ab115832) were purchased from Abcam, and an antibody that specifically recognizes cleaved, but not full length, caspase-3 (Cell signaling Technology; cat#9661S). DDAH1 and DDAH2 rabbit polyclonal antibodies were purchased from Biosynthesis, Inc. For the cleaved caspase Western blots, cell extracts (5 μl) prepared from Jurkat cells exposed or not to etoposide (25 μM, 5 h) were purchased from Cell Signaling Technology (cat#2043S) and used as positive and negative controls, respectively. β-Actin was purchased from Sigma, and voltage-dependent anion channel was bought from Cell Signaling. ADMA was purchased from Calbiochem. l-NMMA, and l-NAME was purchased from Sigma. l-[4,5-3H]-NMMA was purchased from American Radiolabeled Chemicals, Inc.
Cell culture and treatment
Primary cultures of ovine fetal PAEC were isolated and cultured as previously described (24). Cells were utilized between passages 9 and 14. At least 24 h before ADMA treatment, standard medium was replaced with l-arginine and phenol-red-free Dulbecco's modified Eagle medium (Athena Enzyme System). Before all treatments, PAEC were serum starved for 2 h, then exposed or not to ADMA (5 μM), l-NMMA (100 μM), or l-NAME (100 μM) for 2 h. Cells were also treated with l-arginine (75 μM; Sigma) as indicated.
Live cell imaging of eNOS redistribution to the mitochondria
This was accomplished using an eNOS-tagged GFP as described in the online Supplementary Data.
Analysis of mitochondrial membrane potential
Mitochondrial membrane potential was analyzed using the lipophilic cation 5,5′6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide as previously described (11, 57).
Mitochondrial isolation
The mitochondria from tissue or cells were isolated using Pierce Mitochondria isolation kits (Pierce) according to the manufacturer's guidelines.
Analysis of mitochondrial bioenergetics
The XF24 Analyzer (Seahorse Biosciences) was used to estimate the affect of ADMA on mitochondrial bioenergetics in PAEC as described in the online Supplementary Data.
Quantification of total and free carnitine levels by high-performance liquid chromatography
Detection of carnitines was performed using a Shimadzu UFLC system with a 5 μm Omnispher C18 column (250×4.6 mm OD) and equipped with an RF-10AXL fluorescence detector (Shimadzu USA Manufacturing Corporation). Total and free carnitine levels were quantified by fluorescence detection at 248 nm (excitation) and 418 nm (emission). The acyl carnitines were calculated by subtracting the free carnitine values from the total carnitine values for all the samples as previously described (42).
Measurement of peroxynitrite levels in isolated mitochondria
The formation of peroxynitrite was determined by the oxidation of dihydrorhodamine 123 (EMD Millipore) to rhodamine 123, as previously described (1).
Measurement of eNOS-derived superoxide production in isolated mitochondria
To detect superoxide generation in isolated mitochondria, we performed electron paramagnetic resonance (EPR) measurements in freshly isolated mitochondria as described in the online Supplementary Data.
Measurement of CrAT activity
Cells or peripheral lung tissue were lysed in 50 mM Tris·HCl, pH 7.5, 2 mM ethylenediaminetetraacetic acid, 5 mM MgCl2, 0.8 mM dithiothreitol, and protease inhibitor cocktail. Samples were briefly sonicated and centrifuged at 3000g for 5 min. CrAT activity was immediately determined using a modification of the method described by Liu et al. (31). The μmoles of product formed was calculated using an NADH standard curve measured at 340 nm.
Measurement of SOD2 activity
In lung tissue homogenates, the activities of SOD1 and SOD3 were inhibited by incubating samples on ice in the presence of 2 mM potassium cyanide for 1 h (27). SOD2 activity was then measured using an SOD activity kit (Enzo Life Sciences, cat #ADI-900-157). SOD2 activity was reported as units/μg protein.
Western blot analysis
Total protein prepared from cells (20 μg), peripheral tissue (30 μg) or mitochondria isolated from cells (3 μg), or peripheral lung tissue (5 μg) were separated on 4%–20% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (PVDF). Immunoblotting was carried out using the appropriate antibodies in Tris-base buffered saline with 0.1% Tween 20 and 5% bovine serum albumin. After washing, the membranes were probed with horseradish peroxidase-conjugated goat antiserum to rabbit or mouse. Reactive bands were visualized using chemiluminescence (Super Signal West Femto; Pierce) on a Kodak 440CF image station. Bands were quantified using Kodak Image Station software (Kodak 1D 3.6). Loading was normalized by reprobing the membranes with an antibody that was specific to β-actin.
Measurement of eNOS dimer levels
The detection of dimeric and monomeric forms of eNOS in lung homogenates was carried out using low-temperature polyacrylamide gel electrophoresis (LT-PAGE) as previously described (21). Subsequent to LT-PAGE, the gels were transferred to PVDF and probed with an anti-eNOS antibody. A cell lysate with a 5× Laemmli buffer, was boiled for 5 min and was used as an eNOS monomer control.
Immunoprecipitation analysis
The interactions of eNOS/Hsp90 or nitrated CrAT levels were analyzed by immunoprecipitation (IP) analysis as described (47). The efficiency of each IP was normalized by reprobing the membranes with the IP antibody (IP:Hsp90) or by Western blot analysis of 30 μg of the IP protein extract probed using a specific antibody for CrAT (IP:3-nitrotyrosine [3-NT]).
Determination of total protein nitration
In order to examine global protein nitration cell or tissue, lysates were analyzed for 3-NT levels using a dot blot procedure as described (1).
Determination of ATP levels
ATP levels were estimated using the firefly luciferin–luciferase method utilizing a commercially available kit (Invitrogen). ATP is consumed, and light is emitted when firefly luciferase catalyzes the oxidation of luciferin. The amount of light emitted during the reaction is proportional to the availability of ATP. Luminescence was measured using a Fluoroscan Ascent FL luminometer (Thermo Electron).
Determination of NOx levels
To measure NO levels in peripheral lung tissue, lysates were initially treated with cold ethanol to remove proteins; then, we utilized a Sievers 280i Nitric Oxide Analyzer (GE) to determine NOx levels as described earlier (42).
Measurement of superoxide levels
To detect superoxide generation in peripheral lung tissue, EPR measurements were performed using the spin trap, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine.HCl as already described (35, 45). NOS-derived superoxide levels were determined by subtracting the superoxide values in the presence of the NOS inhibitor, ethylisothiourea (100 μM, ETU) from the superoxide values in the absence of ETU.
Assay for dimethylarginine dimethylaminohydrolase activity
The dimethylarginine dimethylaminohydrolase (DDAH) activity was determined by monitoring the conversion of l-[4,5-3H]-NMMA (American Radiolabeled Chemicals, Inc.) to l-[3H]-citrulline as previously described (28) using the modifications described in the online Supplementary Data.
Statistical analyses
The mean±standard error of the mean were calculated, and comparisons were made by the unpaired t-test (for two groups) or analysis of variance followed by Newman–Keuls or Bonferroni post-hoc testing (for ≥3groups). The statistical significance of differences was set at p<0.05. Statistical analysis was performed using GraphPad Prism version 4.01 for Windows (GraphPad Software). Data >2 standard deviation from the mean were not analyzed.
Supplementary Material
Abbreviations Used
- 3-NT
3-nitrotyrosine
- acyl-CoA
acyl-coenzyme A
- ADMA
asymmetric dimethylarginine
- ATP
adenosine-5′-triphosphate
- CC-3
cleaved caspase-3
- CMH
1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine.HCl
- CPT1
carnitine palmitoyltransferase I
- CPT2
carnitine palmitoyltransferase II
- CrAT
carnitine acetyl transferase
- DDAH
dimethylarginine hydrolases
- DHR
dihydrorhodamine
- DMEM
Dulbecco's modified Eagle's medium
- eNOS
endothelial nitric oxide synthase
- EPR
electron paramagnetic resonance
- ER
endoplasmic reticulum
- ETU
ethylisothiourea
- FCCP
carbonilcyanide p-triflouromethoxyphenylhydrazone
- GFP
green fluorescent protein
- Hsp90
heat shock protein 90
- IP
immunoprecipitation
- L-NAME
Nω-Nitro-l-arginine methyl ester hydrochloride
- L-NMMA
NG-methyl-l-arginine acetate
- LT-PAGE
low-temperature polyacrylamide gel electrophoresis
- NaCl
sodium chloride
- NAD
nicotinamide adenine dinucleotide
- NaKATPase
sodium potassium ATPase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOx
nitric oxide metabolites
- OCR
oxygen consumption rate
- PAEC
pulmonary arterial endothelial cells
- PBF
pulmonary blood flow
- PVDF
polyvinylidene difluoride membranes
- ROS
reactive oxygen species
- SEM
standard error of the mean
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- SPB
sodium phosphate buffer
- UCP2
uncoupling protein 2
- VDAC
voltage-dependent anion channel
Acknowledgments
This research was supported in part by grants HL60190 (to S.M.B.), HL67841 (to S.M.B.), HL084739 (to S.M.B.), R21HD057406 (to S.M.B.), and HL61284 (to J.R.F.), all of which were from the National Institutes of Health, by a grant from the Fondation Leducq (to S.M.B., S.F. and J.R.F.), a Scientist Development Grant from the American Heart Association National Office (to S.S.), and Cardiovascular Discovery Institute Seed Awards (to S.S. and S.K.). O.R. and R.R. were supported in part by the National Institutes of Health Training Grant 5T32-HL06699. O.R. was also supported in part by F32HL103136.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aggarwal S. Gross CM. Kumar S. Datar S. Oishi P. Kalkan G. Schreiber C. Fratz S. Fineman JR. Black SM. Attenuated vasodilatation in lambs with endogenous and exogenous activation of cGMP signaling: role of protein kinase G nitration. J Cell Physiol. 2011;226:3104–3113. doi: 10.1002/jcp.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black SM. Bristow J. Soifer SJ. Fineman JR. Altered regulation of the ET-1 cascade in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res. 2000;47:97–106. doi: 10.1203/00006450-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Black SM. Fineman JR. Steinhorn RH. Bristow J. Soifer SJ. Increased endothelial NOS in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol. 1998;275:H1643–H1651. doi: 10.1152/ajpheart.1998.275.5.H1643. [DOI] [PubMed] [Google Scholar]
- 4.Black SM. Mata-Greenwood E. Dettman RW. Ovadia B. Fitzgerald RK. Reinhartz O. Thelitz S. Steinhorn RH. Gerrets R. Hendricks-Munoz K. Ross GA. Bekker JM. Johengen MJ. Fineman JR. Emergence of smooth muscle cell endothelin B-mediated vasoconstriction in lambs with experimental congenital heart disease and increased pulmonary blood flow. Circulation. 2003;108:1646–1654. doi: 10.1161/01.CIR.0000087596.01416.2F. [DOI] [PubMed] [Google Scholar]
- 5.Blomgren K. Zhu C. Hallin U. Hagberg H. Mitochondria and ischemic reperfusion damage in the adult and in the developing brain. Biochem Biophys Res Commun. 2003;304:551–559. doi: 10.1016/s0006-291x(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 6.Bolte S. Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 7.Brouet A. Sonveaux P. Dessy C. Moniotte S. Balligand JL. Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 8.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 9.Calo LA. Pagnin E. Davis PA. Semplicini A. Nicolai R. Calvani M. Pessina AC. Antioxidant effect of L-carnitine and its short chain esters: relevance for the protection from oxidative stress related cardiovascular damage. Int J Cardiol. 2006;107:54–60. doi: 10.1016/j.ijcard.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 10.Church JE. Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem. 2006;281:1477–1488. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- 11.Cossarizza A. Baccarani-Contri M. Kalashnikova G. Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- 12.DeVivo Da IT. Primary and secondary disorders of carnitine metabolism. Int J Pediatr. 1990;5:135–141. [Google Scholar]
- 13.Dhar I. Dhar A. Wu L. Desai K. Arginine attenuates methylglyoxal- and high glucose-induced endothelial dysfunction and oxidative stress by an endothelial nitric-oxide synthase-independent mechanism. J Pharmacol Exp Ther. 2012;342:196–204. doi: 10.1124/jpet.112.192112. [DOI] [PubMed] [Google Scholar]
- 14.Dromparis P. Sutendra G. Michelakis ED. The role of mitochondria in pulmonary vascular remodeling. J Mol Med (Berl) 2010;88:1003–1010. doi: 10.1007/s00109-010-0670-x. [DOI] [PubMed] [Google Scholar]
- 15.Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Duran WN. Breslin JW. Sanchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res. 2010;87:254–261. doi: 10.1093/cvr/cvq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao S. Chen J. Brodsky SV. Huang H. Adler S. Lee JH. Dhadwal N. Cohen-Gould L. Gross SS. Goligorsky MS. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem. 2004;279:15968–15974. doi: 10.1074/jbc.M308504200. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Cardena G. Fan R. Shah V. Sorrentino R. Cirino G. Papapetropoulos A. Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 19.Gratton JP. Fontana J. O'Connor DS. Garcia-Cardena G. McCabe TJ. Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez JA. Parry AJ. McMullan DM. Chapin CJ. Fineman JR. Decreased surfactant proteins in lambs with pulmonary hypertension secondary to increased blood flow. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1264–L1270. doi: 10.1152/ajplung.2001.281.5.L1264. [DOI] [PubMed] [Google Scholar]
- 21.Hallmark OG. Phung YT. Black SM. Chimeric forms of neuronal nitric oxide synthase identify different regions of the reductase domain that are essential for dimerization and activity. DNA Cell Biol. 1999;18:397–407. doi: 10.1089/104454999315286. [DOI] [PubMed] [Google Scholar]
- 22.Iwakiri Y. Satoh A. Chatterjee S. Toomre DK. Chalouni CM. Fulton D. Groszmann RJ. Shah VH. Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci U S A. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang J. Cyr D. Babbitt RW. Sessa WC. Patterson C. Chaperone-dependent regulation of endothelial nitric-oxide synthase intracellular trafficking by the co-chaperone/ubiquitin ligase CHIP. J Biol Chem. 2003;278:49332–49341. doi: 10.1074/jbc.M304738200. [DOI] [PubMed] [Google Scholar]
- 24.Kelly LK. Wedgwood S. Steinhorn RH. Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004;286:L984–L991. doi: 10.1152/ajplung.00224.2003. [DOI] [PubMed] [Google Scholar]
- 25.Kerner J. Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486:1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 26.Kudo N. Barr AJ. Barr RL. Desai S. Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 27.Kuthan H. Haussmann HJ. Werringloer J. A spectrophotometric assay for superoxide dismutase activities in crude tissue fractions. Biochem J. 1986;237:175–180. doi: 10.1042/bj2370175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai YL. Aoyama S. Nagai R. Miyoshi N. Ohshima H. Inhibition of L-arginine metabolizing enzymes by L-arginine-derived advanced glycation end products. J Clin Biochem Nutr. 2010;46:177–185. doi: 10.3164/jcbn.09-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. This reference has been deleted.
- 30.Liu J. Head E. Gharib AM. Yuan W. Ingersoll RT. Hagen TM. Cotman CW. Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J. Killilea DW. Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makinde AO. Gamble J. Lopaschuk GD. Upregulation of 5′-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res. 1997;80:482–489. doi: 10.1161/01.res.80.4.482. [DOI] [PubMed] [Google Scholar]
- 33.Mata-Greenwood E. Meyrick B. Fineman JR. Black SM. Alterations in TGF-b1 expression in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol. 2003;285:L209–L221. doi: 10.1152/ajplung.00171.2002. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien D. Chunduri P. Iyer A. Brown L. L-carnitine attenuates cardiac remodelling rather than vascular remodelling in deoxycorticosterone acetate-salt hypertensive rats. Basic Clin Pharmacol Toxicol. 2010;106:296–301. doi: 10.1111/j.1742-7843.2009.00480.x. [DOI] [PubMed] [Google Scholar]
- 35.Oishi PE. Wiseman DA. Sharma S. Kumar S. Hou Y. Datar SA. Azakie A. Johengen MJ. Harmon C. Fratz S. Fineman JR. Black SM. Progressive dysfunction of nitric oxide synthase in a lamb model of chronically increased pulmonary blood flow: a role for oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2008;295:L756–L766. doi: 10.1152/ajplung.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovadia B. Reinhartz O. Fitzgerald R. Bekker JM. Johengen MJ. Azakie A. Thelitz S. Black SM. Fineman JR. Alterations in ET-1, not nitric oxide, in 1-week-old lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol. 2003;284:H480–H490. doi: 10.1152/ajpheart.00493.2002. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard KA., Jr. Ackerman AW. Gross ER. Stepp DW. Shi Y. Fontana JT. Baker JE. Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem. 2001;276:17621–17624. doi: 10.1074/jbc.C100084200. [DOI] [PubMed] [Google Scholar]
- 38.Rafikova O. Rafikov R. Kumar S. Sharma S. Aggarwal S. Schneider F. Jonigk D. Black SM. Tofovic SP. Bosentan inhibits oxidative and nitrosative stress and rescues occlusive pulmonary hypertension. Free Radic Biol Med. 2012;56C:28–43. doi: 10.1016/j.freeradbiomed.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy VM. Meyrick B. Wong J. Khoor A. Liddicoat JR. Hanley FL. Fineman JR. In utero placement of aortopulmonary shunts: a model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92:1–8. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 40.Rehman J. Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol. 661:171–185. doi: 10.1007/978-1-60761-500-2_11. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S. Black SM. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov Today Dis Mech. 2009;6:e31–e39. doi: 10.1016/j.ddmec.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma S. Sud N. Wiseman DA. Carter AL. Kumar S. Hou Y. Rau T. Wilham J. Harmon C. Oishi P. Fineman JR. Black SM. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L46–L56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi J. Zhu H. Arvidson DN. Cregg JM. Woldegiorgis G. Deletion of the conserved first 18 N-terminal amino acid residues in rat liver carnitine palmitoyltransferase I abolishes malonyl-CoA sensitivity and binding. Biochemistry (Mosc) 1998;37:11033–11038. doi: 10.1021/bi9803426. [DOI] [PubMed] [Google Scholar]
- 44.Shi J. Zhu H. Arvidson DN. Woldegiorgis G. A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver carnitine palmitoyltransferase I abolishes malonyl-CoA inhibition and high affinity binding. J Biol Chem. 1999;274:9421–9426. doi: 10.1074/jbc.274.14.9421. [DOI] [PubMed] [Google Scholar]
- 45.Sud N. Sharma S. Wiseman DA. Harmon C. Kumar S. Venema RC. Fineman JR. Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1444–L1453. doi: 10.1152/ajplung.00175.2007. [DOI] [PubMed] [Google Scholar]
- 46.Sud N. Wells SM. Sharma S. Wiseman DA. Wilham J. Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X. Fratz S. Sharma S. Hou Y. Rafikov R. Kumar S. Rehmani I. Tian J. Smith A. Schreiber C. Reiser J. Naumann S. Haag S. Hess J. Catravas JD. Patterson C. Fineman JR. Black SM. CHIP-dependent GTP cyclohydrolase I degradation in lambs with increased pulmonary blood flow. Am J Respir Cell Mol Biol. 2011;45:163–171. doi: 10.1165/rcmb.2009-0467OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian J. Hou Y. Lu Q. Wiseman DA. Vasconcelos Fonsesca F. Elms S. Fulton DJ. Black SM. A novel role for caveolin-1 in regulating endothelial nitric oxide synthase activation in response to H2O2 and shear stress. Free Radic Biol Med. 2010;49:159–170. doi: 10.1016/j.freeradbiomed.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuder RM. Davis LA. Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 185:260–266. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaz FM. Wanders RJ. Carnitine biosynthesis in mammals. Biochem J. 2002;361:417–429. doi: 10.1042/0264-6021:3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velasco G. Geelen MJ. Gomez del Pulgar T. Guzman M. Malonyl-CoA-independent acute control of hepatic carnitine palmitoyltransferase I activity. Role of Ca2+/calmodulin-dependent protein kinase II and cytoskeletal components. J Biol Chem. 1998;273:21497–21504. doi: 10.1074/jbc.273.34.21497. [DOI] [PubMed] [Google Scholar]
- 52.Velasco G. Gomez del Pulgar T. Carling D. Guzman M. Evidence that the AMP-activated protein kinase stimulates rat liver carnitine palmitoyltransferase I by phosphorylating cytoskeletal components. FEBS Lett. 1998;439:317–320. doi: 10.1016/s0014-5793(98)01400-8. [DOI] [PubMed] [Google Scholar]
- 53.Velasco G. Guzman M. Zammit VA. Geelen MJ. Involvement of Ca2+/calmodulin-dependent protein kinase II in the activation of carnitine palmitoyltransferase I by okadaic acid in rat hepatocytes. Biochem J. 1997;321(Pt 1):211–216. doi: 10.1042/bj3210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vosler PS. Graham SH. Wechsler LR. Chen J. Mitochondrial targets for stroke: focusing basic science research toward development of clinically translatable therapeutics. Stroke. 2009;40:3149–3155. doi: 10.1161/STROKEAHA.108.543769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei Q. Xia Y. Roles of 3-phosphoinositide-dependent kinase 1 in the regulation of endothelial nitric-oxide synthase phosphorylation and function by heat shock protein 90. J Biol Chem. 2005;280:18081–18086. doi: 10.1074/jbc.M413607200. [DOI] [PubMed] [Google Scholar]
- 56.Winder WW. Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 57.Wiseman DA. Wells SM. Hubbard M. Welker JE. Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L165–L177. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- 58.Xia T. Dimitropoulou C. Zeng J. Antonova GN. Snead C. Venema RC. Fulton D. Qian S. Patterson C. Papapetropoulos A. Catravas JD. Chaperone-dependent E3 ligase CHIP ubiquitinates and mediates proteasomal degradation of soluble guanylyl cyclase. Am J Physiol Heart Circ Physiol. 2007;293:H3080–H3087. doi: 10.1152/ajpheart.00579.2007. [DOI] [PubMed] [Google Scholar]
- 59.Zammit VA. Ramsay RR. Bonomini M. Arduini A. Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev. 2009;61:1353–1362. doi: 10.1016/j.addr.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q. Church JE. Jagnandan D. Catravas JD. Sessa WC. Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1015–1021. doi: 10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.