Abstract
The cytoskeletal protein Shroom3 is a potent inducer of epithelial cell shape change and is required for lens and neural plate morphogenesis. Analysis of gut morphogenesis in Shroom3 deficient mouse embryos revealed that the direction of gut rotation is also disrupted. It was recently established that Pitx2-dependent, asymmetrical cellular behaviors in the dorsal mesentery (DM) of the early mid-gut, a structure connecting the gut-tube to the rest of the embryo, contribute to the direction of gut rotation in chicken embryos by influencing the direction of the dorsal mesenteric tilt. Asymmetric cell shapes in the DM epithelium are hypothesized to contribute to the tilt, however, it is unclear what lies downstream of Pitx2 to alter epithelial cell shape. The cells of the left DM epithelium in either Pitx2 or Shroom3 deficient embryos are shorter and wider than those in control embryos and resemble the shape of those on the right, demonstrating that like Pitx2, Shroom3 is required for cell shape asymmetry and the leftward DM tilt. Because N-cadherin expression is specific to the left side and is Pitx2 dependent, we determined whether Shroom3 and N-cadherin function together to regulate cell shape in the left DM epithelium. Analysis of mouse embryos lacking one allele of both Shroom3 and N-cadherin revealed that they possess shorter and wider left epithelial DM cells when compared with Shroom3 or N-cadherin heterozygous embryos. This indicates a genetic interaction. Together these data provide evidence that Shroom3 and N-cadherin function cooperatively downstream of Pitx2 to directly regulate cell shape changes necessary for early gut tube morphogenesis.
Introduction
Early establishment of left-right asymmetry in the embryo leads to the leftward expression of Pitx2, a transcription factor and major regulator of asymmetric morphogenesis (Yoshioka et al., 1998; Shiratori et al., 2001). Perturbation of Pitx2 expression in vertebrate model systems causes a wide range of laterality defects in several organs including those of the gut (Logan et al., 1998; Ryan et al., 1998; Campione et al., 1999; Gage et al., 1999; Lin et al., 1999; Lu et al., 1999; Ai et al., 2006). The direction of gut coiling is reversed in both the chick and frog when Pitx2 is ectopically expressed on the embryo's right side (Logan et al., 1998; Ryan et al., 1998; Campione et al., 1999). Similarly, it was observed that gut-coiling reversals occur in Pitx2-deficient mice and that the phenotype was particularly sensitive to gene dose (Liu et al., 2001; Shiratori et al., 2006). Although Pitx2 is required for the directionality of gut coiling, the mechanisms acting downstream have yet to be fully characterized.
An early sign of gut asymmetry is observed within the dorsal mesentery (DM), a mesodermally derived structure that ventrally suspends the gut tube and its surrounding tissue along the anterior/posterior axis of the embryo (Davis et al., 2008). The DM is initially rectangular in shape and consists of two symmetrical epithelial layers comprising cuboidally shaped cells that flank mesenchyme. Subsequently, asymmetry is generated by changes in cellular architecture on the left side that include a narrowing and lengthening of epithelial cells into columnar and bottle-cell like morphology, and an increase in mesenchymal density. Together, these events drive leftward tilting of the DM along the A/P axis of the gut tube and convert the DM into a trapezoidal shape (Davis et al., 2008). When the DM tilt is inhibited through the ectopic, rightward expression of Pitx2, which is normally restricted to the left epithelium and mesenchyme, gut situs is randomized, suggesting that the DM tilt biases the direction of the ensuing gut coiling (Davis et al., 2008). Pitx2 regulates mesenchymal density by inducing expression of N-cadherin, an adhesion molecule normally restricted to the cells of the left DM and that causes asymmetrical mesenchymal packing (Kurpios et al., 2008). Although asymmetric mesenchymal density contributes to DM tilting, computational modeling predicts that it cannot fully account for the DM tilt and mechanisms shaping the DM epithelia also contribute (Kurpios et al., 2008). What lies downstream of Pitx2 that regulates epithelial cell shape change remains unclear.
A molecule that is both necessary for and sufficient to induce epithelial cell shape changes during morphogenesis is the cytoskeletal protein Shroom3. Ectopic expression of Shroom3 in Xenopus embryos and in cultured cells can induce both epithelial cell lengthening and apical constriction, a process by which the apical circumference is reduced causing cells to adopt a bottle-cell like morphology (Haigo et al., 2003; Hildebrand, 2005). Deficiency of Shroom3 in mice, frogs, and chick embryos prevents these cell shape changes, which result in severe morphogenetic defects in the neural tube and lens (Hildebrand and Soriano, 1999; Haigo et al., 2003; Lee et al., 2007; Nishimura and Takeichi, 2008; Plageman et al., 2010). Shroom3 induces apical constriction by regulating apical actin filament localization and interacting with the Rhokinases Rock1, and Rock2. (Nishimura and Takeichi, 2008; Plageman et al., 2010) Apical constriction is often accompanied by an increase in epithelial cell length but the mechanism by which cells elongate is unknown (Hardin and Keller, 1988; Leptin and Grunewald, 1990; Lee et al., 2009). Identifying molecules that act in concert with Shroom3 will aid in our understanding of mechanisms regulating cell shape change during morphogenesis. The data presented in this article describe a role for Shroom3 during mouse gut morphogenesis and demonstrate Shroom3 cooperatively functions with N-cadherin downstream of Pitx2.
Materials and Methods
Animal maintenance and use
Mice were housed in a pathogen-free vivarium in accordance with institutional policies. Embryos were extracted by performing hysterectomies on isofluorane anesthetized, pregnant females at specific gestational ages. Embryos were fixed in 4% paraformaldehyde >1 hour and stored. Shroom3Gt/Gt (Hildebrand and Soriano, 1999), Pitx2-/- (Gage et al., 1999), N-cadherin+/- (Radice et al., 1997), N-cadherinflox/flox (Kostetskii et al., 2005), and Z/EG (Novak et al., 2000) mouse lines have been described previously.
Immunofluorescence and quantification
Immunofluorescent labeling of cryosections was performed as described (Smith et al., 2005) using the following antibodies: α-Shroom3 (1:1000) (Hildebrand, 2005), α-Pitx2 (1:500) (a gift from P. Gage), α -N-cadherin (1:300, Abcam, ab18203), α-myosin IIB (1:5000, Covance, PRB445P), and α-β-catenin (1:500, Santa Cruz, sc-7199). F-actin and nuclei were visualized using Phalloidin (Invitrogen, A12379, 1:1000) and Hoechst 33342 (Sigma, B-2261, 1:1000). N-cadherin labeling in the DM required pre-treatment with 3% acetic acid for 5 minutes prior to antibody incubation. The quantification of apical/basal phalloidin and myosin II immunofluorescent intensities was performed as previously described (Plageman et al., 2010). In short, cryosections from >3 embryos of each genotype were utilized, and the intensities were measured using ImageJ v1.33 and normalized to the average intensity of the entire field.
Cell shape quantification
Outlines of individual cells were traced in Photoshop CS4 (Adobe) using spatial cues from β-catenin and F-actin labeling similar to previously described methods (Plageman et al., 2010). Width and height measurements of the traces were quantified from approximately 10-15 left and right DM epithelial cells per section from 3-8 embryos.
Statistics
All statistical analysis was performed using the statistical software SPSS 13. The data sets analyzed were determined as normally distributed based on the results of the Kolmogorov-Smirnov and Shapiro-Wilk normality tests. The means of each data set were analyzed using one-way ANOVA with the post-hoc Tukey's test. Statistical significance was assumed when p<0.05.
Results and Discussion
Shroom3 and Pitx2 control gut morphogenesis through regulation of cell shape
The cell shape changes of the left DM epithelium, which include epithelial lengthening and apical constriction, are reminiscent of those elicited by Shroom3 (Haigo et al., 2003; Plageman et al., 2010). Because Pitx2 is required for this asymmetry and Pitx transcription factors can regulate Shroom3 expression in Xenopus (Chung et al., 2010), we determined whether Pitx2 and Shroom3 deficient embryos share similar phenotypes during mouse gut morphogenesis (Fig. 1). At E11.5, the midgut, at the level of the duodenum, begins to rotate to the right forming a C-like bend (Fig 1A). In contrast, Shroom3Gt/Gt (n=3) and Pitx2-/- (n=2) embryos have a duodenum that fails to form the C-like bend at E11.5/E12.5 (Fig. 1B,J) (Liu et al., 2001). By E12.5 the proximal midgut, relative to the stomach, normally coils in a clockwise direction (Fig. 1C) which is later positioned at E15.5 such that the proximal coil passes behind and dorsal to the duodenum (Fig. 1E, G). In 86% of Shroom3Gt/Gt embryos (6/7) the proximal mid-gut coils in a counter-clockwise direction at E12.5 (Fig. 1D) and by E15.5 the coil is positioned to the left of the duodenum (Fig. 1F, H). Similarly, the midgut of 2/3 Pitx2+/- embryos examined at E12.5 coiled in a counter-clockwise direction (Fig. 1I). The similarity in phenotypes suggests that Pitx2 and Shroom3 may be functioning in the same pathway during gut morphogenesis. The distinct phenotypes observed in Pitx2 heterozygous versus homozygous mutants (Fig. 1I-J) are consistent with another gene dosage study where an increase in Pitx2 isoform dosage changed the lack of rotation phenotype in Pitx2 nulls into one where the direction of gut coiling was reversed (Liu et al., 2001). The Shroom3Gt/Gt phenotype at E11.5 initially resembles Pitx2-/- embryos but appears to recover and then resemble the Pitx2+/- phenotype (E12.5-E15.5).
Figure 1. Shroom3 and Pitx2 deficient mice have similar gut coiling defects.
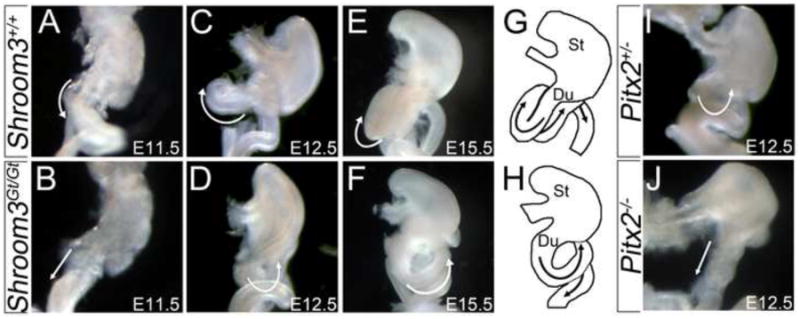
(A-J) Ventral view of digestive tracts from embryos of the indicated age and genotype were dissected from the liver and pancreas in order to visualize the direction of gut coiling (white arrows). The outlines of the Stomach (St), Duodenum (Du), and proximal intestines are diagrammed at E15.5 in G and H to illustrate directional coiling.
Once it was established that Shroom3 and Pitx2 mutant embryos have similar gut coiling defects, younger embryos were analyzed to compare morphology of the DM. At E10.75, the DM normally tilts to the left side of the embryo along the anterior/posterior axis both caudally (Fig. 2A) and more rostrally near the duodenum (Fig. 2B). In contrast, the DM of Shroom3Gt/Gt and Pitx2-/- embryos tilts to the left caudally (Fig. 2C, E), but rostrally, the Shroom3Gt/Gt (Fig. 2D) and Pitx2-/- DM (Fig. 2F) does not tilt to the left and the flanking epithelia are parallel. The rostral specific DM phenotype is consistent with the location of the coiling defect observed in the proximal gut of older embryos (Fig. 1), however, it is unclear why the phenotype is limited to this region.
Figure 2. Shroom3 and Pitx2 are required for cell shape asymmetries during gut morphogenesis.
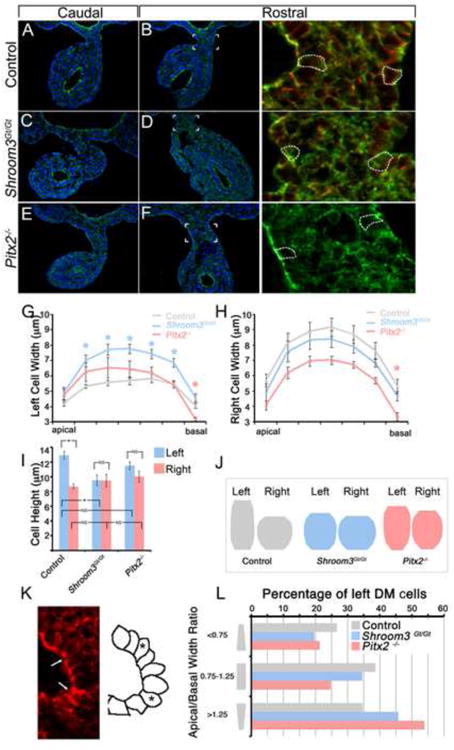
(A-F) E10.75 embryos of the indicated genotype were transversely cyrosectioned caudally, approximately halfway between the fore- and hind-limbs (A,C,E), or rostrally near the duodenum (B,D,F) and immunofluorescently labeled with F-actin (green) and/or β-catenin (red). The area indicated in B, D, and F is magnified and displayed in the right-most panels. The dashed white outlines are traces of β-catenin signal demarcating individual cell borders. (G-I) Traces of the left and right dorsal mesentery epithelial cell outlines were measured and the average widths along the apical-basal axis (G,H) and height (I) were calculated. Asterisks represent statistical significance compared with the control (wt) averages (p<0.05). (J) The average widths and height of left and right dorsal mesentery epithelial cells from the indicated genotypes was quantified and used to generate diagrams representing the average cell shape. (K) Representative image of wild-type left dorsal mesentery epithelium containing apically constricted cells (arrows). The diagram on the right outlines the shape of the cells pictured with asterisks marking apically constricted cells. (L) The number of left DM epithelial cells from embryos of the indicated genotypes, defined by those with an apical/basal width ratio of <0.75, 0.75-1.25, or >1.25 indicating apically constricted, columnar, or basally constricted shapes, respectively, were counted and the total percentage of each type of cell was determined.
To determine whether the failure to tilt is accompanied by differences in cell shape, analysis of individual cells from the left and right DM epithelia was performed. Cell boundaries were traced based on a combination of β-catenin and F-actin staining (Fig. 2B, D, F) and the width along the apical/basal axis (Fig. 2G,H) and the height (Fig. 2I) of the traces were quantified. In wild-type embryos, the cells on the left DM epithelium are thinner and taller than the cells in the right epithelium (Fig. 2G-I). The cellular dimensions and their asymmetry in the mouse are consistent with what was previously observed in the chick (Davis et al., 2008; Kurpios et al., 2008) demonstrating that the mechanisms governing the DM tilt and subsequent gut morphogenesis are conserved.
The cells of the left DM in Shroom3Gt/Gt embryos are wider (Fig. 2G,H) and shorter (Fig. 2I) compared with the wild-type while the cells on the right and are not significantly different than the wild-type in width or height. Notably, the average shape of the left DM epithelial cells of Shroom3Gt/Gt embryos resembles the average shape on the right (Fig. 2J). These data suggest that Shroom3 functions to regulate gut morphogenesis by elongating and thinning the cells of the left DM epithelium, which is consistent with its role in lens and neural tube morphogenesis (Haigo et al., 2003; Plageman et al., 2010). Similarly, shape analysis of DM epithelial cells from Pitx2-/- embryos demonstrated that the cell height is not significantly different between the right and the left (Fig. 2I). Unlike the Shroom3 mutants, the average cell width of the Pitx2-/-DM epithelia is not significantly different than control embryos on both the right and left side along most of the apical/basal axis except at the basal most point (Fig. 2G,H). Although not significantly different than the control, the widths between the right and left Pitx2-/- DM epithelia are similar and the average cell shape is close to symmetric (Fig. 2J). The striking resemblance of the Pitx2 and Shroom3 mutant phenotypes suggests that these molecules are acting in the same pathway to regulate cell shape during DM morphogenesis.
It was noted that the left DM epithelium of the chick contained cells with a bottle-cell morphology (Davis et al., 2008). While the average shape of the left DM epithelial cells is not bottle-shaped, cells that have narrower apical widths than basal widths are present, often at the point of greatest bend (Fig. 2K, arrows). Because Shroom3 is known to induce apical constriction, we determined whether the percentage of apically constricted cells in the left DM epithelium was significantly different in both the Shroom3 and Pitx2 mutant embryos. Both mutants had a smaller percentage of cells that were apically constricted (Fig 2L) suggesting that both Shroom3 and Pitx2 facilitate this shape change in left DM epithelium thus contributing to the DM tilt.
Shroom3 regulates F-actin and Myosin II localization in the dorsal mesentery
One of the molecular characteristics of the DM as it tilts is an accumulation of apical F-actin on the left DM epithelium (Fig. 3A) (Davis et al., 2008). Because Shroom3 regulates the accumulation of cytoskeletal components such as F-actin and Myosin II in the lens and neural tube (Hildebrand and Soriano, 1999; Hildebrand, 2005; Plageman et al., 2010), we determined whether the same was true for the DM. We observed that both F-actin and Myosin II labeling is strong at the apical end of left DM epithelial cells compared with those on the right (Fig. 3A,B). Shroom3Gt/Gt embryos do not have a strong apical signal of F-actin or Myosin II in the left DM (Fig. 3C,D) and the apical/basal ratios are quantifiably distinct from the wild-type (Fig. 3E,F). The apical accumulation of F-actin and Myosin II observed in the left cells is a feature indicative of cells undergoing cell shape changes such as apical constriction and cell elongation (Pilot and Lecuit, 2005). The reduction of apical F-actin and myosin II in Shroom3 mutants also suggests that Shroom3 initiates cell shape changes in the left DM epithelium by apically localizing cytoskeletal components associated with cell shape change.
Figure 3. F-actin and Myosin II are mislocalized in the dorsal mesentery of Shroom3 deficient embryos.
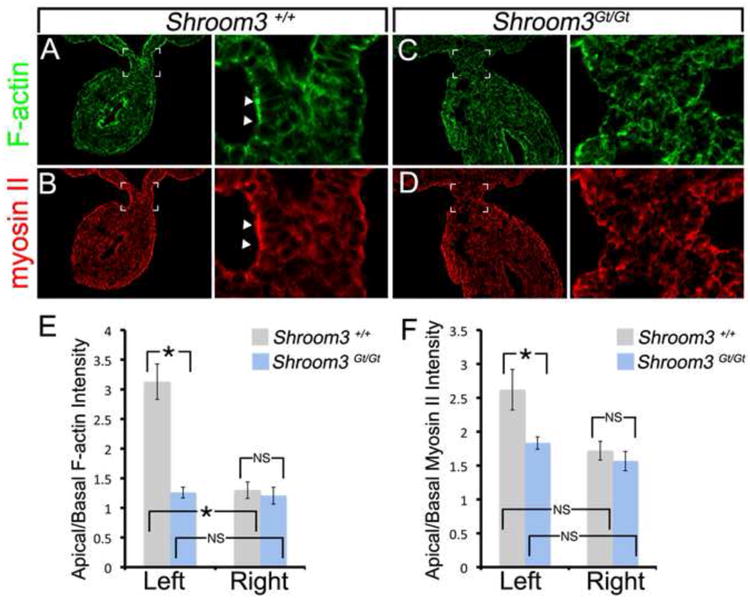
(A-D) E10.75 wild-type or Shroom3Gt/Gt embryos were cryosectioned just caudal to the duodenum and the sections were F-actin and β-catenin immunofluorescently labeled. The boxed region indicates the region magnified in the adjacent panel. The arrowheads indicate regions of intense labeling. (E-F) The average apical/basal F-actin and Myosin II intensity ratios from wild-type and Shroom3Gt/Gt embryos were calculated from the left and right dorsal mesentery epithelium. Asterisks and brackets indicate means that are significantly different (p<0.05). NS=not significant
Shroom3 expression is not dependent on Pitx2 in the dorsal mesentery
In the Xenopus gut, embryonic Shroom3 expression is dependent on Pitx1 (Chung et al., 2010). Furthermore, all three Pitx transcription factors (Pitx1, 2, and 3) can induce the formation of elongated, apically constricted cells, a response that is dependent on Shroom3 (Chung et al., 2010). For these reasons we hypothesized that in the mouse DM, Shroom3 expression is dependent on Pitx2 and that Pitx2 regulates cell shape through direct regulation of Shroom3. However, upon X-gal staining of Shroom3+/Gt embryos, a method previously utilized to report Shroom3 transcription (Hildebrand and Soriano, 1999; Plageman et al., 2010), we observed that Shroom3 expression is not asymmetric like Pitx2 and is instead expressed throughout the DM (Fig. 4A). Similarly, immunofluorescent antibody labeling of Shroom3 revealed that Shroom3 protein is apically localized on both the left and right DM epithelium in both wild-type (Fig. 4C) and Pitx2-/- (Fig. 4D) embryos. Neither the wild-type expression pattern, nor the pattern observed in Pitx2 mutants is consistent with a role for Pitx2 directly regulating Shroom3 expression in the mouse DM. Additionally, Shroom3 deficiency does not affect Pitx2 expression (compare Fig. 4E, F) ruling out the possibility that Shroom3Gt/Gt and Pitx2-/- embryos share phenotypes because one gene affects expression of the other.
Figure 4. Pitx2 regulates N-cadherin but not Shroom3 in the left dorsal mesentery epithelium.
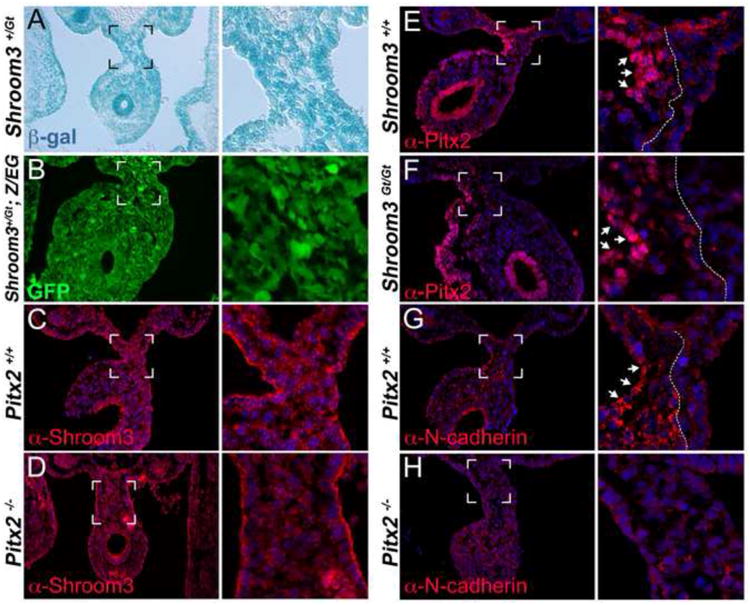
(A) X-gal staining of the dorsal mesentery from E10.75 Shroom3+/Gt embryos. (B) Shroom3+/Gt mice were crossed with the Z/EG line, which reports cre-recombinase activity via GFP expression. E10.75 embryos from this cross were visualized for GFP to observe areas of the dorsal mesentery expressing Cre under control of the Shroom3 locus. (C-H) E10.75 embryos of the indicated genotyped were cryosectioned and immunofluorescently labeled with the indicated antibody. The bracketed region indicates the area magnified in the adjacent panel. The arrows indicate regions of strong labeling intensity and the dotted line indicates the border of strong expression.
Shroom3 and N-cadherin function cooperatively downstream of Pitx2 in left DM
Given that Pitx2 does not appear to regulate Shroom3 expression, yet they seem to be in the same pathway, we surmised that Pitx2 is regulating the expression of a gene that cooperatively functions with Shroom3 to modulate cell shape. In the chick DM, asymmetric N-cadherin expression is induced by Pitx2 (Kurpios et al., 2008). In the mouse, we found that N-cadherin is asymmetrically expressed in the left DM with particularly strong staining in the left epithelium (arrows Fig. 4G). The strong, asymmetric localization of N-cadherin is greatly reduced in Pitx2-/- embryos (Fig. 4H) suggesting that in the mouse DM, Pitx2 regulates N-cadherin.
Because N-cadherin is asymmetrically expressed and regulated by Pitx2 in the mouse DM we determined whether N-cadherin and Shroom3 function together to regulate morphogenesis and cell shape. To do this, N-cadherin and Shroom3-deficient mice were intercrossed to produce doubly heterozygous embryos that were analyzed for phenotypes. Neither Shroom3+/Gt (Fig. 5A), nor N-cadherin+/- (Fig. 5B) embryos had an obvious phenotype, except at a low frequency (3/33, 9.1% and 1/33, 3.0%, respectively). However, Shroom3+/Gt; N-cadherin+/- embryos displayed exencephaly in over 1/3 of the embryos examined (14/38) (Fig. 5C) demonstrating that N-cadherin and Shroom3 genetically interact during neural tube morphogenesis. The Shroom3Gt allele expresses cre-recombinase wherever Shroom3 is expressed and can faithfully recombine alleles in these regions (Fig. 4B). Intercrossing a conditional N-cadherin line with Shroom3+/Gt mice also produces doubly heterozygous embryos with exencephaly at a similar frequency (5/13, 38.4%) (Fig. 5D) and suggests that the phenotype is dependent on cells that express both Shroom3 and N-cadherin.
Figure 5. N-cadherin and Shroom3 cooperatively function during neural tube and gut morphogenesis.
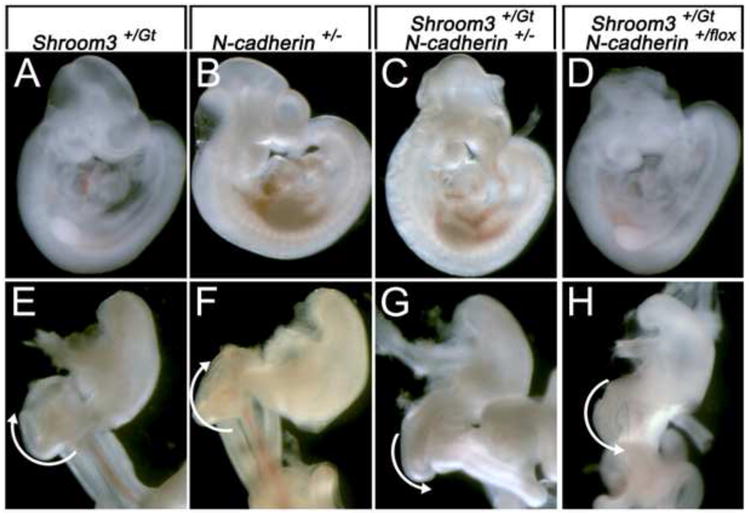
(A-D) Whole embryos with the indicated genotypes are indicated. Note the presence of exencephaly in embryos in panels C and D. (E-H) Ventral view of digestive tracts dissected from E14.5 embryos of the indicated genotype dissected from the liver and pancreas. The white arrows indicate the direction of gut coiling.
Analysis of the gut from exencephalic, doubly heterozygous embryos reveals, that similar to Shroom3Gt/Gt mice, gut coiling is reversed and rotates counterclockwise at the level of the duodenum (compare Fig. 5E,F with 5G,H). In addition, the DM of Shroom3+/Gt; N-cadherin+/flox embryos is rectangular and the gut tube does not tilt (Fig. 6B) while the control embryos are normal (Fig. 6A). To determine if this phenotype is dependent on cell shape abnormalities in the DM, the left and right epithelial cell shapes were examined and their dimensions quantified (Fig. 6C-E). The average cell width along the apical basal axis in the left and right DM epithelium is not significantly different in Shroom3+/Gt; N-cadherin+/flox embryos when compared with control animals (Fig. 6C,D). In contrast, the average cell height in the left DM of mutants is significantly shorter than control embryos (Fig. 6E) and the left and right cell heights of Shroom3+/Gt; N-cadherin+/flox embryos are similar. Combining these measurements and generating an average cell shape demonstrated that the asymmetry observed in control embryos is attenuated in the Shroom3+/Gt; N-cadherin+/flox embryos (Fig. 6F) a result akin to Shroom3+/Gt; N-cadherin+/- embryos (data not shown). When the percentage of apically constricted cells in the left and right epithelium was quantified, we found that doubly heterozygous embryos have fewer than the control (Fig. 6G). Furthermore, the double heterozygotes had more basally constricted cells than the controls (Fig. 6G). Together these data indicate that Shroom3 and N-cadherin cooperatively function to regulate cell shape in the DM.
Figure 6. N-cadherin and Shroom3 cooperatively regulate cell shape in the dorsal mesentery.
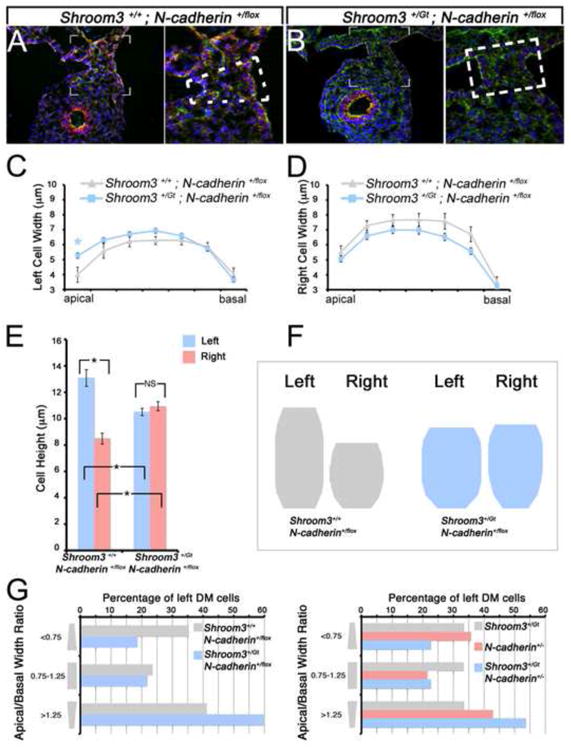
(A-B) Representative cryosections from E10.75 embryos with the indicated genotype were immunofluorescently labeled with β-catenin (red), F-actin (green), and Hoechst (blue). The hatched lines indicate the general shape of the dorsal mesentery; note the trapezoidal shape in the control (A) and the rectangular shape in the mutant embryo (B). (C-E) Individual cell shapes of E10.75 left and right dorsal mesentery epithelia of the indicated genotypes were outlined and their average height and width were quantified. Asterisks and brackets represent a significant difference compared with the control (wt) averages (p<0.05). NS=not significant (F) The average cell shape of dorsal mesentery epithelial cells from the indicated genotype was generated utilizing the averages quantified in C-E and depicted. (G) The number of left DM epithelial cells from embryos of the indicated genotypes, defined by those with an apical/basal width ratio of <0.75, 0.75-1.25, or >1.25 indicating apically constricted, columnar, or basally constricted shapes, respectively, were counted and the total percentage of each type of cell was determined.
The mechanism by which Shroom3 and N-cadherin cooperate is still unclear. However, like Shroom3, a role for apical constriction (Nandadasa et al., 2009; Morita et al., 2010) and cell elongation have already been attributed to N-cadherin (Leonard et al., 2010). In addition, a cytoskeletal connection of the apical constriction machinery and the adherens junctions is necessary for apical constriction (Sawyer et al., 2009). Given that both Shroom3 and N-cadherin deficient epithelial cells also have reduced apical F-actin accumulation (Nandadasa et al., 2009; Plageman et al., 2010), it is reasonable to speculate that Shroom3 and N-cadherin are working together to modulate the cytoskeletal machinery driving apical constriction and cell elongation.
Conclusions
The data presented here suggest that Pitx2, Shroom3, and N-cadherin function in the same pathway to regulate asymmetric cell shape within the DM during gut morphogenesis. We propose a model where Pitx2 regulates DM asymmetry by affecting mesenchymal density as previously described (Kurpios et al., 2008) as well as cell shape of the left epithelium through the regulation of N-cadherin (Fig. 7). The DM contains elongated and apically constricted cells only within the left epithelium because of the overlap of Shroom3 and N-cadherin expression. Cells on the right do not have this cell shape because they lack the appropriate levels of N-cadherin to trigger Shroom3 induced cell shape changes. Consequently, the DM adopts a trapezoidal shape, causing leftward tilting of the primitive gut, and biases the direction of gut looping. When epithelial cell shape asymmetry is disrupted the bias is removed and gut coiling defects ensue. A similar Shroom3/N-cadherin dependent mechanism may be in place in both neural tube and lens morphogenesis as development of both structures are affected by the lack of N-cadherin or Shroom3 (Radice et al., 1997; Nandadasa et al., 2009; Pontoriero et al., 2009; Plageman et al., 2010). Further dissection of the mechanism by which Shroom3 induced cell shape changes requires N-cadherin containing adherens junctions is required and will no doubt aid in our understanding of morphogenetic mechanisms in many contexts.
Figure 7. Shroom3 and a Pitx2-N-cadherin pathway function cooperatively to generate asymmetric cell shape changes during gut morphogenesis.
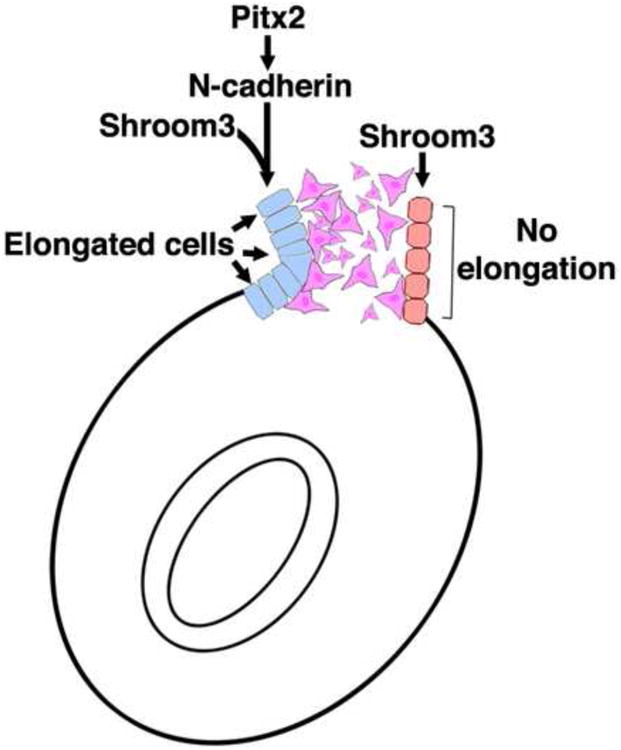
The model illustrates the proposed mechanism where Shroom3 regulates cell shape specifically in the left dorsal mesentery downstream of Pitx2. The Pitx2 regulation of Shroom3 activity is achieved by directing asymmetric, leftward N-cadherin expression. The elongation and apical constriction of left epithelial dorsal mesentery cells together with asymmetrical mesenchymal packing causes leftward tilting biasing the direction of gut coiling.
Highlights.
>Shroom3 directs gut morphogenesis via cell shape changes in the dorsal mesentery. >Pitx2 regulates asymmetric N-cadherin expression to direct epithelial cell shape. >Shroom3 and N-cadherin cooperatively regulate cell shape.
Acknowledgments
We thank Paul Speeg for excellent technical assistance and Jeff Hildebrand for providing the Shroom3 antibody. We acknowledge grant support from the NIH (R01 EY17848) and from the Abrahamson Pediatric Eye Institute Endowment at Children's Hospital Medical Center of Cincinnati to R.A.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai D, Liu W, Ma L, Dong F, Lu MF, Wang D, Verzi MP, Cai C, Gage PJ, Evans S, et al. Pitx2 regulates cardiac left-right asymmetry by patterning second cardiac lineage-derived myocardium. Dev Biol. 2006;296(2):437–49. doi: 10.1016/j.ydbio.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, Nowotschin S, Viebahn C, Haffter P, Kuehn MR, et al. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 1999;126(6):1225–34. doi: 10.1242/dev.126.6.1225. [DOI] [PubMed] [Google Scholar]
- Chung MI, Nascone-Yoder NM, Grover SA, Drysdale TA, Wallingford JB. Direct activation of Shroom3 transcription by Pitx proteins drives epithelial morphogenesis in the developing gut. Development. 2010;137(8):1339–49. doi: 10.1242/dev.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NM, Kurpios NA, Sun X, Gros J, Martin JF, Tabin CJ. The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev Cell. 2008;15(1):134–45. doi: 10.1016/j.devcel.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126(20):4643–51. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13(24):2125–37. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Hardin J, Keller R. The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development. 1988;103(1):211–30. doi: 10.1242/dev.103.1.211. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118(Pt 22):5191–203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99(5):485–97. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, Molkentin JD, Radice GL. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res. 2005;96(3):346–54. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Izpisua Belmonte JC, Tabin CJ. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc Natl Acad Sci U S A. 2008;105(25):8499–506. doi: 10.1073/pnas.0803578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Le MP, Wallingford JB. The shroom family proteins play broad roles in the morphogenesis of thickened epithelial sheets. Dev Dyn. 2009;238(6):1480–91. doi: 10.1002/dvdy.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Scherr HM, Wallingford JB. Shroom family proteins regulate gamma-tubulin distribution and microtubule architecture during epithelial cell shape change. Development. 2007;134(7):1431–41. doi: 10.1242/dev.02828. [DOI] [PubMed] [Google Scholar]
- Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110(1):73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401(6750):279–82. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Liu C, Liu W, Lu MF, Brown NA, Martin JF. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development. 2001;128(11):2039–48. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- Logan M, Pagan-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 1998;94(3):307–17. doi: 10.1016/s0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401(6750):276–8. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Morita H, Nandadasa S, Yamamoto TS, Terasaka-Iioka C, Wylie C, Ueno N. Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis. Development. 2010;137(8):1315–25. doi: 10.1242/dev.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandadasa S, Tao Q, Menon NR, Heasman J, Wylie C. N- and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development. 2009;136(8):1327–38. doi: 10.1242/dev.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135(8):1493–502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28(3-4):147–55. [PubMed] [Google Scholar]
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232(3):685–94. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr, Chung MI, Lou M, Smith AN, Hildebrand JD, Wallingford JB, Lang RA. Pax6-dependent Shroom3 expression regulates apical constriction during lens placode invagination. Development. 2010;137(3):405–15. doi: 10.1242/dev.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326(2):403–17. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181(1):64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, de la Pena J, Sabbagh W, Greenwald J, Choe S, et al. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394(6693):545–51. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol. 2009;186(1):57–73. doi: 10.1083/jcb.200904001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori H, Sakuma R, Watanabe M, Hashiguchi H, Mochida K, Sakai Y, Nishino J, Saijoh Y, Whitman M, Hamada H. Two-step regulation of left-right asymmetric expression of Pitx2: initiation by nodal signaling and maintenance by Nkx2. Mol Cell. 2001;7(1):137–49. doi: 10.1016/s1097-2765(01)00162-9. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Yashiro K, Shen MM, Hamada H. Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development. 2006;133(15):3015–25. doi: 10.1242/dev.02470. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285(2):477–89. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, et al. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell. 1998;94(3):299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]


