Abstract
Objective
Previous work demonstrated that intestinal cholesterol absorption and regulated expression of intestinal Npc1L1 and ABCA1 are all required for liver X agonist (LXR)-mediated increases in HDL biogenesis. We reexamined those conclusions in mice with intestine-specific deletion of the microsomal triglyceride transfer protein (MTTP-IKO), where chylomicron (CM) formation is eliminated.
Methods and Results
MTTP-IKO mice demonstrated sustained ~90% reduction in cholesterol absorption and >80% reduction in NPC1L1 expression, yet LXR agonist treatment increased serum HDL and upregulated intestinal ABCA1 expression. Hepatic lipogenesis and triglyceride content increased with LXR agonist treatment in both genotypes. Biliary cholesterol secretion was increased in MTTP-IKO mice without further increase upon LXR agonist administration. LXR agonist treatment caused a paradoxical increase in cholesterol absorption in MTTP-IKO mice and decreased fecal neutral sterol (FNS) excretion, but to levels that still exceeded FNS excretion in LXR agonist treated control mice. Finally, MTTP-IKO mice demonstrated indistinguishable patterns of increased cholesterol turnover and efflux following intravenous radiolabeled cholesterol administration, with or without LXR agonist treatment.
Conclusion
Both intestinal and hepatic cholesterol efflux pathways are basally upregulated in MTTP-IKO mice. Moreover, LXR dependent pathways modulate intestinal cholesterol absorption, transport, efflux and HDL production independent of CM assembly and secretion.
Keywords: Chylomicron secretion, Niemann-Pick C1-Like1, HDL, Reverse cholesterol transport
There is major interest in therapeutic strategies that raise the levels of serum high density lipoprotein (HDL) cholesterol as an approach to attenuate and reverse atherosclerotic vascular disease 1. While the core concepts of reverse cholesterol transport have been discussed for decades 2, there is renewed interest in the tissue-specific sources of HDL and in particular the regulation of intracellular transport pathways involved in promoting cholesterol efflux 3.
HDL formation is dynamically regulated by liver X receptors (LXRα and LXRβ) 4, 5 via transcriptional activation of downstream targets including ATP-binding cassette proteins ABCA1, ABCG1 and ABCG5/G8, each of which modulate key cholesterol efflux pathways and in turn participate in the regulation of intestinal cholesterol absorption as well as in reverse cholesterol transport 6, 7. The therapeutic potential of this approach was highlighted by studies demonstrating that pharmacologic LXR agonist activation promotes reverse cholesterol transport 7. Further studies established a critical role for intestinal (versus hepatic) ABCA1 in HDL biogenesis 8, while more recent studies established a key role of intestinal (versus hepatic and macrophage) LXRα in reverse cholesterol transport 9, 10, findings that together highlight the importance of intestine-specific cholesterol absorption and efflux pathways in regulating HDL biogenesis and cholesterol homeostasis.
However, several features of LXR agonist-mediated increases in HDL formation and intestinal cholesterol transport and absorption are incompletely understood. Prominent among these is the suggestion that cholesterol absorption per se seems to be required for the HDL-raising effects of LXR agonist treatment, as evidenced by the attenuated responses in mice lacking the recycling transporter NPC1L1, where cholesterol absorption was greatly reduced and which exhibit both reduced baseline expression of intestinal ABCA1 and a diminished response to LXR agonist treatment 11–13.
In the current study, we have explored the role of chylomicron-dependent cholesterol trafficking in the response to LXR agonist treatment. Earlier studies demonstrated that patients with abetalipoproteinemia exhibit dramatically reduced cholesterol absorption 14, findings phenocopied in mice with conditional intestine-specific deletion of the abetalipoproteinemia gene product, microsomal triglyceride transfer protein (MTTP-IKO)15. However, the singular importance of chylomicron-dependent intestinal cholesterol transport was called into question by studies demonstrating that MTTP inhibition decreased but did not eliminate intestinal cholesterol transport from enterocytes, with up to 30% of intestinal cholesterol secretion from enterocytes being accounted for by a non-chylomicron (ie HDL) route 16.
Here we demonstrate that MTTP-IKO mice exhibit LXR dependent modulation of intestinal cholesterol absorption, transport, efflux and HDL production independent of CM assembly and secretion.
METHODS
Please see SUPPLEMENTAL METHODS for details of diets, cholesterol absorption, biliary cannulation, cholesterol turnover, analysis of serum, tissue, bile and fecal lipids, RNA extraction and Q-PCR quantitation.
Animals- MTTP-IKO mice were generated as described, with conditional activation of a Villin-Cre–ERT2 fusion gene 17 triggered by intraperitoneal injection of 1mg Tamoxifen (Sigma, T5648) for 5 consecutive days 15, 18. MTTP-floxed littermates were used as controls. Animals were studied at the indicated intervals in the figure legends following tamoxifen treatment. All animal protocols were approved by the Washington University Animal Studies Committee and conformed to criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
LXR agonist treatment- Mice were fed standard mouse chow and at the indicated ages gavaged daily for 7 days with a solution containing either vehicle alone (SUPPLEMENTAL METHODS) or with 25 mg/kg body weight T0901317 (Cayman Chemical Company).
Statistical Analyses- All data are shown as mean ± standard error unless otherwise noted. Statistical comparisons were made using GraphPad Prism 4.0 (GraphPad, San Diego, CA) with one way ANOVA for multiple group comparisons and t testing as a post-hoc test for comparing different pairs. A value of p< 0.05 was considered significant.
RESULTS
No adaptation in intestinal cholesterol absorption in Mttp-IKO mice
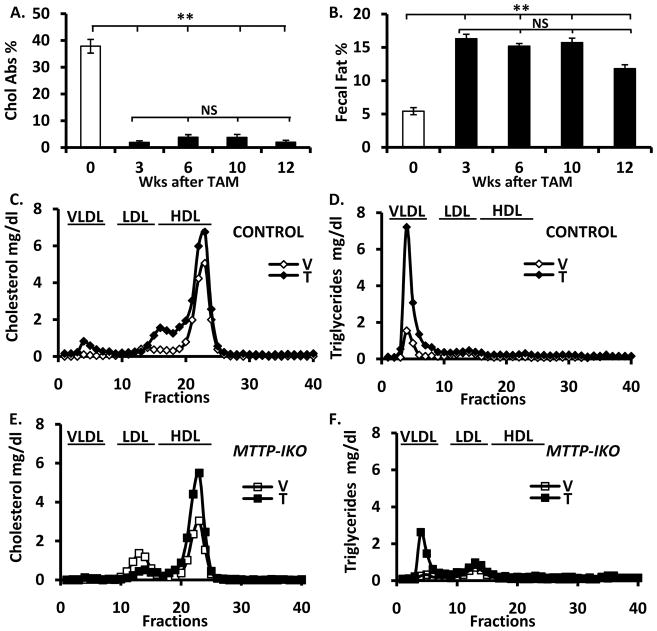
Since intestinal cholesterol absorption, at least as modeled in vitro, may occur independent of chylomicron secretion 16, we sought to demonstrate evidence for compensatory adaptation in vivo following intestinal MTTP deletion. However, we found less than 5% cholesterol absorption at all time points examined (Figure 1A) along with consistently elevated fecal fat excretion (Figure 1B), suggesting that the phenotypes of defective intestinal lipid transport are maintained for at least 12 weeks following a single cycle of tamoxifen induction. These findings together suggest that intestinal cholesterol transport from the lumen into the systemic circulation is almost completely abrogated in MTTP-IKO mice.
Figure 1. LXR agonist administration increases HDL concentration in MTTP-IKO mice.
(A). Sustained >90% reduction in cholesterol absorption in MTTP-IKO mice (data from 5–8 mice per group). (B). Sustained fat malabsorption in MTTP-IKO mice over the same time period. (C). Increased HDL cholesterol and (D) increased VLDL triglyceride in control mice treated with LXR agonist (data from 4 mice per group). (E). Increased HDL cholesterol and (F) increased VLDL triglyceride in MTTP-IKO mice treated with LXR agonist (data from 4–5 mice per group). Symbols ** indicate p<0.01.
LXR activation increases serum HDL cholesterol in MTTP-IKO mice
As predicted 7, LXR agonist administration to control mice increased serum triglyceride and cholesterol concentrations (Table 1), in association with increased cholesterol content of large HDL (Figure 1C and Supplemental Figure 1 for apolipoprotein content), as well as VLDL triglyceride enrichment (Figure 1D, right panel). There was a qualitatively similar response to LXR agonist treatment in MTTP-IKO mice, with increased serum cholesterol and triglyceride concentrations (Table 1), an increase in HDL cholesterol (Figure 1E) and an increase in VLDL triglyceride (Figure 1F). LXR agonist administration resulted in enrichment of the HDL fractions with apolipoprotein E in both genotypes (Supplemental Figure 1). These findings suggest that the HDL raising effect of LXR agonist administration is preserved in mice with virtually no cholesterol absorption.
Table 1.
Serum lipid concentrations
| Genotype | TG | TC | CE | FC | PL | FAA |
|---|---|---|---|---|---|---|
| Control-V | 57.7±6.5a | 95.9±8.0a | 65.1±6.8a | 30.8±9.7a | 184.6±20.1a | 0.54±0.07a |
| Control-T | 200.3±40.9b | 166.2±11.8b | 116.3±8.0b | 49.9±4.3b | 306.1±21.9b | 0.35±0.03b |
| MttpIKO-V | 60.1±5.0a | 63.7±8.5c | 41.2±5.9c | 22.5±2.9c | 134.3±37.9c | 0.33±0.08b |
| MttpIKO-T | 97.3±17.3c | 98.1±4.2 a | 65.5±4.0a | 32.6±3.1a | 200.0±11.1a | 0.3±0.03b |
Control (Mttp wild type) and MttpIKO mice (n=6–11) were treated either vehicle (V) or T0901317 (T). Serum total cholesterol (TC), free cholesterol (FC), phospholipids (PL), triglyceride (TG) and free fatty acid (FFA) concentrations were determined (data are mg/dL except for FAA, mmol/L). The difference between values associated with different superscript letters for the parameters indicated in each vertical column is statistically significant (p<0.05).
Conserved, LXR-dependent regulation of genetic pathways for intestinal and hepatic cholesterol transport in MTTP-IKO mice
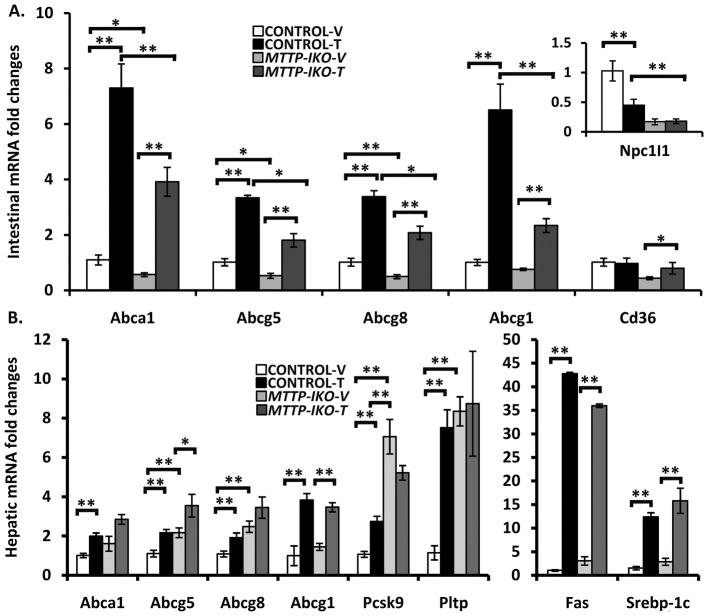
We next sought to understand some of the mechanisms underlying the response to LXR agonist treatment in MTTP-IKO mice, particularly in the face of attenuated cholesterol absorption. We focused on the expression of NPC1L1 and of ABC type sterol transporters in the small intestine and liver since these have been previously implicated in HDL formation and in the response to LXR agonist administration 8, 11, 19. We found that intestinal NPC1L1 mRNA expression was decreased by >80% in MTTP-IKO mice with no change following LXR agonist treatment (Figure 2A, inset). Expression of ABC transporters (Abca1, Abcg5/8, Abcg1) involved in cholesterol efflux was also suppressed in vehicle treated MTTP-IKO mice (Figure 2A), but each of these transporters demonstrated a robust increase in mRNA abundance following LXR agonist administration (Figure 2A). It is worth noting the comparable fold-induction (average ~3–7 fold) between the genotypes of these ABC transporters in response to LXR agonist treatment, despite the reduced overall abundance in treated MTTP-IKO mice compared to treated control mice, findings reminiscent of the observations in NPC1L1 knockout mice following LXR agonist treatment 11. These data suggest that the regulation of intestinal LXR target genes (including canonical targets such as PPARα and SREBP1-c, Supplemental Table 1) 20 was for the most part preserved in MTTP-IKO mice.
Figure 2. Gene expression changes in intestine and liver of control and MTTP-IKO mice following LXR administration.
(A). mRNA abundance was measured by Q-RT-PCR for the indicated transcripts on intestinal mucosal extracts from 4 mice per group. Inset illustrates the expression levels of NPC1L1. (B). mRNA abundance was measured by Q-RT-PCR for the indicated transcripts on hepatic extracts from 4 mice per group. Symbols * and ** indicate p<0.05 or <0.01, respectively.
We also examined the same panel of genes in hepatic extracts from the same groups, which revealed LXR dependent upregulation of Abca1, Abcg5/8, Abcg1 and Pcsk9 mRNA in control mice (Figure 2B). Hepatic expression of Abca1 and Abcg5/8 was upregulated in untreated MTTP-IKO mice and increased only slightly with LXR agonist treatment, suggesting distinctive regulation of hepatic cholesterol efflux pathways in the setting of defective chylomicron formation and which was not further modified by LXR agonist administration. A different pattern was observed with hepatic Abcg1, Fas and Srebp1-c mRNA, in which baseline expression was indistinguishable in both control and MTTP-IKO mice and LXR agonist treatment induced comparable expression of both targets in both genotypes (Figure 2B and Supplemental Table 2). These findings again emphasize that the response of selected lipid metabolic pathways to LXR agonist administration is preserved in the absence of cholesterol absorption. Among the notable exceptions to this general paradigm was the pattern observed for Pcsk9 and the phospholipid transfer protein (Pltp), where MTTP-IKO mice demonstrated striking upregulation which was equal to or exceeded that observed in LXR treated control mice and which was not further increased by LXR agonist treatment (Figure 2B).
Intestinal MTTP deletion modifies LXR agonist modulation of intestinal and hepatic lipid content
In order to understand how LXR agonist treatment led to increased HDL levels in mice exhibiting defective chylomicron assembly and severely attenuated cholesterol absorption, we examined intestinal and hepatic lipid content as well as biliary lipid secretion in the various groups of mice. We demonstrated the expected increases in intestinal triglyceride, phospholipid and free fatty acid content in MTTP-IKO mice, none of which were influenced by LXR agonist treatment (Table 2A). Total intestinal cholesterol content was unchanged with LXR agonist treatment in either genotype (Table 2A) but we found a striking and unanticipated increase in esterified cholesterol in MTTP-IKO mice, an observation we will return to in a later section.
Table 2.
Tissue lipid content
| A) INTESTINAL MUCOSA
| ||||||
|---|---|---|---|---|---|---|
| Genotype | TG | TC | FC | CE | PL | FFA |
| Control-V | 66.2±7.4a | 49.7±7.4a | 45.5±5.2a | 4.3±2.3a | 88.1±2.2a | 25.11±1.96a |
| Control-T | 74.0±14.5a | 55.0±5.2a | 46.9±3.3a | 8.1±2.2a | 102.4±5.9a | 45.5±17.7a |
| MttpIKO-V | 704.9±80.9b | 76.1±5b | 51.4±1.6a | 27.7±3.8b | 149.8±9.6b | 192.8±43.1b |
| MttpIKO-T | 704.6±45.9b | 78.5±8.1b | 55.3±4.7a | 23.2±3.6b | 169.5±9.3b | 159.2±22.1b |
| B). HEPATIC
| ||||||
|---|---|---|---|---|---|---|
| Genotype | TG | TC | FC | CE | PL | FFA |
| Control-V | 71.3±10.3a | 27.3±1.1a | 10.7±1.3a | 16.6±1.9a | 99.1±5.5a | 0.17±0.01a |
| Control-T | 260.4±18.5b | 17.6±1.9b | 11.4±1.3a | 6.1±0.9b | 105.7±4.5a | 0.34±0.03b |
| MttpIKO-V | 39.5±6.3c | 21.9±1.1b | 9.6±1.3a | 12.4±1.6ab | 94.1±8.3a | 0.14±0.01a |
| MttpIKO-T | 246.7±21.7b | 19.3±1.7b | 9.1±0.8a | 10.2±1.8ab | 103.4±3.4a | 0.27±0.02b |
Control and MttpIKO mice (n=6–11) were treated either vehicle (V) or T0901317 (T). Tissue total cholesterol (TC), free cholesterol (FC), phospholipids (PL), and triglyceride (TG) were analyzed (Methods). Hepatic (A) and mucosal (B) lipid content was expressed as mean ±SE ug/mg protein, except FFA nmol/mg protein. The difference between values associated with different superscript letters for the parameters indicated in each vertical column is statistically significant (p<0.05).
Hepatic triglyceride content was decreased in untreated MTTP-IKO mice compared to control mice (Table 2B), as noted previously 15. LXR agonist treatment increased hepatic lipogenesis and increased triglyceride content in both genotypes, reaching indistinguishable values in treated control and MTTP-IKO mice (Table 2B). These observations are an important departure from findings in LXR agonist treated NPC1L1−/− mice, which demonstrated attenuated hepatic steatosis and decreased induction of lipogenic mRNAs 11. The current findings suggest that decreased intestinal cholesterol absorption in the setting of defective chylomicron secretion does not afford protection against hepatic steatosis in LXR agonist treated MTTP-IKO mice.
Intestinal MTTP deletion modifies both basal and LXR agonist modulation of biliary lipid secretion and fecal neutral sterol (FNS) excretion
We next turned to an analysis of potential cholesterol efflux pathways to account for the HDL raising effects of LXR agonist treatment in MTTP-IKO mice. Biliary cholesterol secretion was increased in LXR agonist treated control mice, but not to levels observed in untreated MTTP-IKO mice (Table 3). Moreover, LXR agonist treatment of MTTP-IKO mice yielded no further increase in biliary cholesterol secretion, findings in accord with the mRNA expression data for Abcg5/8 alluded to above (Figure 2B). We considered the possibility that altered content of an endogenous oxysterol might account for the basal increase in biliary cholesterol secretion, possibly as a result of increased liganding of LXR 21. To begin to explore this possibility we examined oxysterol content in control and MTTP-IKO mice, the findings demonstrating increased 25 hydroxycholesterol in livers of MTTP-IKO mice (Supplemental Table 3). These findings, considered together with the increased mRNA expression of hepatic Abcg5/8, raise the possibility that basal alterations in oxysterol content may modulate canalicular cholesterol transporter expression and in turn lead to increased biliary cholesterol secretion in MTTP-IKO mice. These changes in hepatic oxysterol content may also explain in part the basal upregulation of hepatic Pltp mRNA expression alluded to above.
Table 3.
Biliary lipid secretion
| Flow rate | Cholesterol | Phospholipids | Bile acid | |
|---|---|---|---|---|
| Control-V | 42.4±4.3a | 24.2±2.9a | 246.9±23.4a | 1455.3±362.6a |
| Control-T | 86.8±16.0b | 74.4±17.9b | 251.2±31.9a | 2069.6±94.8a |
| MttpIKO-V | 88.2±4.6b | 104.6±17.3b | 315.1±26.7a | 2101.8±414.6a |
| MttpIKO-T | 86.4±7.6b | 92.98±6.1b | 235.4±12.6a | 2550.2±301.8a |
Control and MttpIKO mice (n=7–17) were treated either vehicle (V) or T0901317 (T) for 7 days. Bile samples were collected for 1 hour. The flow rate was expressed as μl/min/kg body weight. Biliary cholesterol, phospholipids and bile acids were analyzed (Methods). Biliary lipid secretion was expressed as nmol/min/Kg body weight. The difference between values associated with different superscript letters for the parameters indicated in each vertical column is statistically significant (p<0.05).
We next evaluated FNS output in the various groups to ascertain whether LXR agonist treatment modified net cholesterol efflux in MTTP-IKO mice. LXR agonist treatment increased FNS excretion in control mice, although not to the levels found in untreated MTTP-IKO mice (Figure 3A). By contrast, LXR agonist treatment of MTTP-IKO mice led to a significant decrease in FNS excretion (Figure 3A). To understand the mechanism for this decrease, we examined cholesterol absorption in the various groups of mice. We found that LXR agonist treatment reduced cholesterol absorption in control mice (Figure 3B), as previously demonstrated 22–24. By contrast, LXR agonist treatment of MTTP-IKO mice induced a paradoxical increase in cholesterol absorption, to levels comparable to those observed in LXR agonist treated control mice (Figure 3B). Thus, despite the virtual elimination of luminal cholesterol absorption under basal conditions, LXR agonist administration to MTTP-IKO mice leads to increased HDL biogenesis and modulates intestinal cholesterol absorption, presumably via chylomicron independent pathways.
Figure 3. LXR modulation of fecal neutral sterol excretion, cholesterol absorption and efflux in control and MTTP-IKO mice.
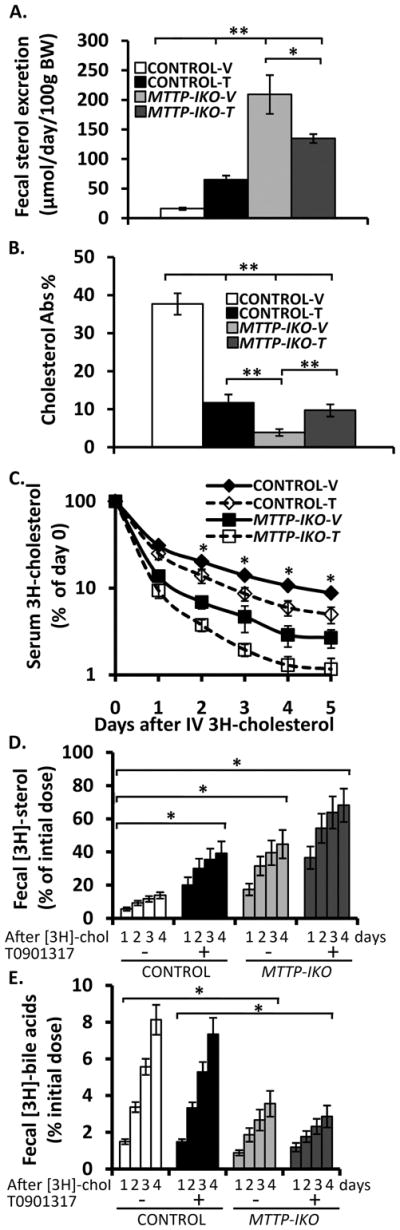
(A). Fecal neutral sterol excretion was quantified (SUPPLEMENTAL METHODS) from samples obtained in 7–13 mice per group both at baseline and following LXR administration. (B). Cholesterol absorption was measured by the dual fecal isotope ratio method in the same groups (n=7 per group) as above. (C). Mice (4–5 per group) were administered an intravenous bolus of 3H cholesterol and serum cholesterol specific activity determined at the indicated intervals over the following 5 days. (B). Stool was collected from each of the animals in the groups above and samples saponified and extracted (SUPPLEMENTAL METHODS) for quantification of both radiolabeled neutral sterol (B) and bile acid (C) species. Symbols * and ** indicate p<0.05 or <0.01, respectively.
To understand the impact of the changes induced by LXR administration on net cholesterol efflux in the various groups of mice, we administered an intravenous bolus of radiolabeled cholesterol and examined the serum kinetics and cumulative radiolabel recovery in neutral and acidic fractions in feces. The distribution of radiolabeled cholesterol in serum was largely (~70%) within HDL by one day after administration (Supplemental Figure 2A). LXR administration accelerated the turnover of cholesterol in control mice (Figure 3C) and augmented recovery of radiolabeled fecal neutral sterol (Figure 3D), findings consistent with other studies 25. However, despite the increase in cholesterol absorption and decreased FNS excretion in LXR treated MTTP-IKO mice (Figure 3A,B), cholesterol turnover and cumulative recovery of radiolabeled FNS were similarly increased in MTTP-IKO mice, with or without LXR agonist treatment and if anything, the recovery of radiolabeled FNS tended to be slightly higher in the treated group (Figure 3C,D). The recovery of radiolabeled bile acids was higher in control compared to MTTP-IKO mice and was unchanged by LXR agonist treatment (Figure 3E). Residual radiolabeled cholesterol and bile acid was determined in the liver at sacrifice, the findings again reflecting decreased cholesterol and bile acid in LXR agonist treated control mice and in MTTP-IKO mice with or without LXR agonist treatment (Supplemental Figure 2B, C).
Taken together, the findings suggest that augmented cholesterol efflux in MTTP-IKO mice is mediated at least in part through upregulation of canalicular cholesterol export, coupled with the almost complete elimination of lumen to serum cholesterol transport. The findings further suggest that LXR agonists increase HDL biogenesis and modulate intestinal cholesterol absorption and flux in MTTP-IKO mice through pathways that are independent of chylomicron assembly and secretion.
DISCUSSION
A confluence of recent studies has highlighted the importance of intestine-specific LXR activation in promoting cholesterol efflux 9, 10, 26. While it is clear that the small intestine plays a critical role in both cholesterol absorption and efflux as well as in HDL biogenesis, the precise interactions of the various pathways involved along with their metabolic regulation is incompletely understood. Here we show that LXR activation increases HDL biogenesis in MTTP-IKO mice through mechanisms independent of intestinal chylomicron assembly and despite the virtual elimination of cholesterol absorption. These findings were unanticipated in view of studies showing that intestinal cholesterol absorption per se seems to be required for the HDL-raising effects of LXR agonist treatment, as evidenced by the attenuated responses in NPC1L1 knockout mice, where cholesterol absorption was greatly reduced 11.
The observation that LXR agonist treatment augments HDL biogenesis in MTTP-IKO mice raises some important questions, including just how LXR activation might promote HDL production in the virtual absence of intestinal cholesterol absorption and from exactly which tissue and from which metabolic pool does this cholesterol arise? It is worth noting that our studies were conducted using a general rather than intestine-specific LXR agonist 10, 27, but several findings point to an effect mediated through intestinal LXR in MTTP-IKO mice. These include the significant upregulation of intestinal Abca1 and Abcg1 expression in LXR agonist treated MTTP-IKO mice, which would be anticipated to augment cholesterol delivery into the systemic compartment; the small yet significant increase in apparent cholesterol absorption in LXR agonist treated MTTP-IKO mice inferred from the dual fecal isotope data along with decreased FNS excretion in these animals. It is worth pointing out that the expression of Abcg1 mRNA (as inferred from the QPCR crossing threshold in our study) in enterocytes and hepatocytes is quite low, findings in line with other observations28, and thus the upregulation of Abcg1 mRNA following LXR agonist treatment should be interpreted with caution. Nevertheless, the findings together strongly imply that elements of intestinal cholesterol uptake and efflux are indeed regulated by LXR agonist administration, yet in a manner that is independent from chylomicron assembly and secretion. Earlier studies, using MTTP inhibitor treated rat enterocytes and intestine-like cell lines, indicated the possibility of non-chylomicron and apoB-independent pathways of cholesterol transport might account for up to 30% of intestinal cholesterol secretion, and further suggested that these pathways may be independently regulated 16, 29. The current findings offer insights that both extend and contrast with those studies. Specifically, our results do not support the suggestion that intestinal cholesterol absorption occurs to any measurable extent in vivo (<5% likely representing the lower detectable limit of this method) in the absence of chylomicron formation. Nevertheless, the findings in LXR agonist treated MTTP-IKO mice are broadly consistent with findings that suggest a non-chylomicron route for cholesterol transport from the enterocyte to the systemic circulation.
In considering the mechanisms whereby LXR agonist administration leads to intestinal cholesterol mobilization and augmented HDL biogenesis in MTTP-IKO mice, one possibility is the small increase in de-novo or newly absorbed cholesterol noted above in these animals as evidenced by the apparent increase in cholesterol absorption in LXR agonist treated MTTP-IKO mice (Figure 3B). Another possibility is that LXR administration leads to mobilization of an existing or “pre-absorbed” pool of intestinal cholesterol. One of the unanticipated findings in the current studies was the basal increase in intestinal mucosal cholesterol content in MTTP-IKO mice which was almost completely accounted for by an ~6-fold increase in cholesterol ester accumulation (Table 2A). These findings are an important departure from other studies in which intestinal MTTP was inducibly deleted in vivo, where those authors demonstrated a 60% reduction in intestinal cholesterol ester content and a 29% increase in free cholesterol 29. It is worth pointing out, however, that those studies were conducted using the Mx1 Cre line (which deletes hepatic and to some extent intestinal MTTP) in an LDL receptor knockout background, rather than the model used in the current studies 29. Nevertheless, while the explanation for the discrepancy in intestinal cholesterol ester accumulation between the two lines of mice is yet to be explored, it might be reasonable to speculate that LXR administration augments mobilization of a preformed and expanded enterocyte pool of cholesterol in our line of MTTP-IKO mice. While not measured directly in our studies, intestinal cholesterol synthesis as inferred by the expression of HMGCoA reductase mRNA, was slightly reduced in MTTP-IKO mice both at baseline and following LXR administration (Supplemental Table 1), making it less likely that upregulation of newly synthesized intestinal cholesterol might account for the HDL-cholesterol raising effects of LXR administration.
It is also worth considering the possibility that LXR agonist administration led to systemic mobilization of hepatic cholesterol in MTTP-IKO mice, in addition to the sustained increase in canalicular cholesterol output. Our prior studies indicated that hepatic lipogenesis and VLDL secretion was increased in MTTP-IKO mice 15 and the current studies suggest that some of the pathways involved may share LXR-dependent regulation. Examples include the baseline upregulation of the canalicular cholesterol export heterodimeric subunits Abcg5/8 as well as SREBP1-c and Pltp 30, 31. Our studies also suggest that the adaptive alterations in hepatic lipid metabolism in MTTP-IKO mice include increased abundance of oxysterols, including known LXR ligands 21. LXR agonist administration led to a further increase in some (Abca1, Abcg1, Abcg5/8,) of the genes involved in cellular cholesterol efflux, consistent with previous studies demonstrating that the synthetic LXR agonist is a more potent ligand than the endogenous oxysterols examined. However, while not discounting the formal possibility that the increase in HDL might be accounted at least in part from hepatic sources it is worth noting that the dominant pathway for increased cholesterol efflux in MTTP-IKO mice, in both basal and LXR treated conditions, appears to be via canalicular secretion. The overwhelming importance of the biliary secretion pathway is emphasized by the negligible effects of LXR agonist administration on the kinetics of cholesterol turnover and efflux in MTTP-IKO mice, suggesting that the small increase in cholesterol absorption and decreased fecal neutral sterol excretion is more than offset by the continued increase in canalicular cholesterol secretion coupled with decreased cholesterol absorption in the setting of defective intestinal chylomicron secretion and lipid export.
In summary, the current findings illustrate the complex and interrelated pathways that modulate cholesterol efflux pathways in vivo. The observation that canalicular cholesterol secretion is dramatically enhanced in the setting of defective intestinal chylomicron assembly and the virtual elimination of cholesterol absorption, suggests the possibility that strategies for intestine-specific MTTP inhibition may be attractive considerations by which to promote cholesterol efflux. This strategy would have direct appeal in the setting of elevated LDL cholesterol levels, and it would be interesting to examine whether a similar response to that observed in the current study would be observed in LDL receptor null mice. The additional observation that LXR agonist administration leads to increased HDL biogenesis in the absence of chylomicron secretion reinforces the notion that intestine-specific LXR agonists may have utility even in the absence of cholesterol absorption.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by grants HL38180, DK56260, DK52574 to NOD; HL067773 to DSO; and by the Washington University Metabolomics Facility (P60 DK020579).
The authors thank Alex Lehman and Joyce Fung for their outstanding technical assistance.
Footnotes
DISCLOSURES
NONE.
References
- 1.Shah PK. Atherosclerosis: Targeting endogenous apo a-i--a new approach for raising hdl. Nat Rev Cardiol. 2011;8:187–188. doi: 10.1038/nrcardio.2011.37. [DOI] [PubMed] [Google Scholar]
- 2.Verdery RB. Reverse cholesterol transport from fibroblasts to high density lipoproteins: Computer solutions of a kinetic model. Can J Biochem. 1981;59:586–592. doi: 10.1139/o81-081. [DOI] [PubMed] [Google Scholar]
- 3.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England journal of medicine. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, Angelin B, Bjorkhem I, Pettersson S, Gustafsson JA. Hepatic cholesterol metabolism and resistance to dietary cholesterol in lxrbeta-deficient mice. The Journal of clinical investigation. 2001;107:565–573. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor lxr alpha. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and abc1-mediated efflux of cholesterol by rxr heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 7.Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver x receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 8.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal abca1 directly contributes to hdl biogenesis in vivo. The Journal of clinical investigation. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo Sasso G, Murzilli S, Salvatore L, D’Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P, Moschetta A. Intestinal specific lxr activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell metabolism. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda T, Grillot D, Billheimer JT, Briand F, Delerive P, Huet S, Rader DJ. Tissue-specific liver x receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2010;30:781–786. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Niemann-pick c1-like 1 is required for an lxr agonist to raise plasma hdl cholesterol in mice. Arterioscler Thromb Vasc Biol. 2008;28:448–454. doi: 10.1161/ATVBAHA.107.160465. [DOI] [PubMed] [Google Scholar]
- 12.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-pick c1 like 1 (npc1l1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. The Journal of biological chemistry. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 13.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-pick c1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 14.Illingworth DR, Connor WE, Lin DS, Diliberti J. Lipid metabolism in abetalipoproteinemia: A study of cholesterol absorption and sterol balance in two patients. Gastroenterology. 1980;78:68–75. [PubMed] [Google Scholar]
- 15.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific mttp deficiency. The Journal of biological chemistry. 2006;281:4075–4086. doi: 10.1074/jbc.M510622200. [DOI] [PubMed] [Google Scholar]
- 16.Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. The Journal of biological chemistry. 2003;278:31610–31620. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- 17.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y, Luo J, Kennedy S, Davidson NO. Conditional intestinal lipotoxicity in apobec-1−/− mttp-iko mice: A survival advantage for mammalian intestinal apolipoprotein b mrna editing. The Journal of biological chemistry. 2007;282:33043–33051. doi: 10.1074/jbc.M705386200. [DOI] [PubMed] [Google Scholar]
- 19.Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue-specific induction of intestinal abca1 expression with a liver x receptor agonist raises plasma hdl cholesterol levels. Circ Res. 2006;99:672–674. doi: 10.1161/01.RES.0000244014.19589.8e. [DOI] [PubMed] [Google Scholar]
- 20.Colin S, Bourguignon E, Boullay AB, Tousaint JJ, Huet S, Caira F, Staels B, Lestavel S, Lobaccaro JM, Delerive P. Intestine-specific regulation of pparalpha gene transcription by liver x receptors. Endocrinology. 2008;149:5128–5135. doi: 10.1210/en.2008-0637. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs lxr signaling in cultured cells and the livers of mice. Cell metabolism. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver x receptor agonist requires atp-binding cassette transporters g5 and g8. The Journal of biological chemistry. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 23.Plosch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, Kema IP, Groen AK, Shan B, Kuipers F, Schwarz M. Sitosterolemia in abc-transporter g5-deficient mice is aggravated on activation of the liver-x receptor. Gastroenterology. 2004;126:290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 24.Kruit JK, Plosch T, Havinga R, Boverhof R, Groot PH, Groen AK, Kuipers F. Increased fecal neutral sterol loss upon liver x receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147–156. doi: 10.1053/j.gastro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver x receptor stimulates trans-intestinal excretion of plasma cholesterol. The Journal of biological chemistry. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temel RE, Sawyer JK, Yu L, Lord C, Degirolamo C, McDaniel A, Marshall S, Wang N, Shah R, Rudel LL, Brown JM. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell metabolism. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng D, Hiipakka RA, Dai Q, Guo J, Reardon CA, Getz GS, Liao S. Antiatherosclerotic effects of a novel synthetic tissue-selective steroidal liver x receptor agonist in low-density lipoprotein receptor-deficient mice. J Pharmacol Exp Ther. 2008;327:332–342. doi: 10.1124/jpet.108.142687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bojanic DD, Tarr PT, Gale GD, Smith DJ, Bok D, Chen B, Nusinowitz S, Lovgren-Sandblom A, Bjorkhem I, Edwards PA. Differential expression and function of abcg1 and abcg4 during development and aging. Journal of lipid research. 2010;51:169–181. doi: 10.1194/jlr.M900250-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iqbal J, Rudel LL, Hussain MM. Microsomal triglyceride transfer protein enhances cellular cholesteryl esterification by relieving product inhibition. The Journal of biological chemistry. 2008;283:19967–19980. doi: 10.1074/jbc.M800398200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver x receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. The Journal of biological chemistry. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki H, Goldstein JL, Brown MS, Liang G. Lxr-srebp-1c-phospholipid transfer protein axis controls very low density lipoprotein (vldl) particle size. The Journal of biological chemistry. 2010;285:6801–6810. doi: 10.1074/jbc.M109.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.