Abstract
Prefrontal cortical dendritic spine remodeling during adolescence may open a window of vulnerability to pathological stimuli that impact long-term behavioral outcomes, but causal mechanisms remain unclear. We administered the Rho-kinase inhibitor HA-1077 during three adolescent periods in mice to destabilize dendritic spines. In adulthood, cocaine-induced locomotor activity was exaggerated. By contrast, when administered in adulthood, HA-1077 had no psychomotor consequences and normalized food-reinforced instrumental responding after orbitofrontal-selective knockdown of Brain-derived neurotrophic factor, a potential factor in addiction. Thus, early-life Rho-kinase inhibition confers cocaine vulnerability, but may actually protect against pathological reward-seeking—particularly in cases of diminished neurotrophic support—in adulthood.
Keywords: orbitofrontal, fasudil, learning, ROCK, dependence, sensitization
Prefrontal cortical dendritic spines remodel in adolescence
Adolescence marks a neurobiologically distinct developmental period characterized by high rates of experimental drug use and vulnerability to the development of substance dependence. Adolescent substance exposure markedly increases the likelihood of developing lifelong dependence, defined by a loss of control over drug intake and drug use despite adverse consequences [1,2]. Cocaine dependence emerges with particular virulence; for example, approximately 15–16% of adolescent cocaine users will develop dependence within 10 years of first exposure, but only 12–13% of alcohol users and 8% of marijuana users will develop dependence in the same period [3]. Thus, identifying mechanisms of cocaine vulnerability is a critical research imperative.
It is widely appreciated that amphetamine and amphetamine-like psychostimulants such as cocaine are potent regulators of dendritic spines within the prefrontal cortex [4]. In the orbitofrontal prefrontal cortex (oPFC)1, a structure widely associated with addiction vulnerability [5], exposure to psychostimulants decreases dendritic spine density [6–8]. Moreover, cocaine exposure in adolescence causes oPFC dendritic spine elimination that is sustained into adulthood [8], suggesting early-life structural modifications may contribute to lifelong vulnerability to cocaine.
Recent work with genetic knockout models supports this perspective: Mice deficient in beta1-integrin, p190RhoGAP, and Arg kinase, all of which are expressed in the prefrontal cortex and organize or are implicated in postnatal dendritic spine stability, show exaggerated cocaine-induced psychomotor sensitization in adulthood [8–11]. In the case of Arg, knockout also confers vulnerability to cocaine-induced oPFC-dependent cognitive deficits and increases sensitivity to reward-predictive cues [8,9]. A limitation of these models, however, is that unlike with viral vector approaches (e.g.,[12]), anatomical specificity is limited. Evidence that the spine-associated neurotrophin Brain-derived Neurotrophic Factor (BDNF) has differential effects on reward sensitivity depending on regional expression patterns reinforces the limitation of whole-brain knockout approaches (reviewed [10]). Moreover, the brief but distinct phases of adolescent prefrontal cortical dendritic spine refinement cannot be selectively manipulated using traditional knockout approaches, thus the relative contributions of dendritic spine proliferation (in early adolescence) and elimination (in mid- and late adolescence) to long-term behavioral outcomes remain unclear.
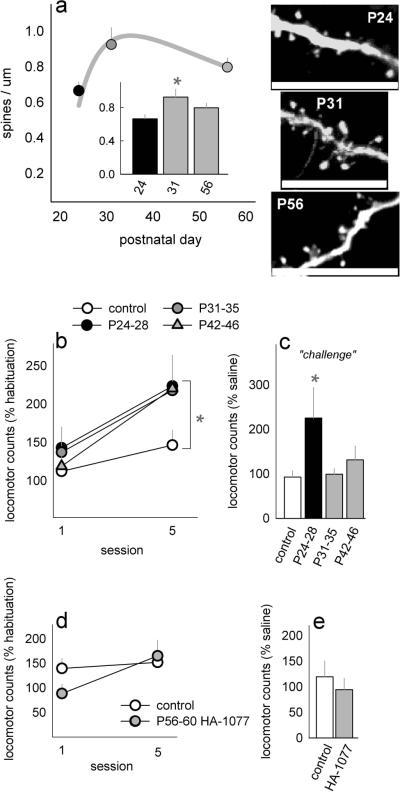
To illustrate these distinct phases here, we enumerated dendritic spines in mice expressing thy1-derived Green Fluorescent Protein (GFP) in deep-layer oPFC [13]. Mounted, 40-μm-thick sections were imaged using a Fluoview confocal microscope, 100× 1.4NA objective, laser excitation at 488 nm, and 0.5 μm step sizes. Collapsed z-stacks collected from the ventral/lateral compartments of the oPFC (50–150 μm from the soma) were enumerated using ImageJ. 18 independent neurons from mice aged 24, 31, and 56 days were scored, corresponding to pre-adolescence, early adolescence, and early adulthood [14]. Spine density was highest at postnatal day (P) 31 [F(2,51)=3.7, p=0.03], and curve fits highlight dendritic spine proliferation between P24–31 and elimination after P31 (fig.1a). These spine counts replicate our previous findings [8] and parallel gross volumetric changes in the rodent oPFC showing that the oPFC is largest at P31 [15].
Figure 1. Rho-kinase (ROCK) inhibition during adolescent dendritic spine maturation confers hyper-sensitivity to cocaine in adulthood.
(a) Deep-layer orbitofrontal cortical spine density increases from P24 to P31 and then declines between P31 and P56. Curve fits are shown for illustrative purposes. Inset: Density measures are also shown in bar graph form. Representative spines are shown at right. Scale bar=5 μm, n=18 neurons (7 mice)/group. (b) Injections of the ROCK inhibitor HA-1077 from P24–28, P31–35, or P42–46 increase psychomotor sensitivity to cocaine in adulthood, n=6–9/group. Control groups did not differ and were collapsed. (c) Despite a 2-week washout period, sensitivity remains exaggerated in adult mice exposed to HA-1077 from P24–28. (d) Psychomotor sensitivity to cocaine does not, however, differ in mice pretreated with HA-1077 in adulthood rather than adolescence, n=5–6/group. (e) We also detected no differences after a 2-week washout period. Bars and symbols represent means+SEMs, *p<0.05 vs. control.
What is the long-term significance of distinct phases of adolescent dendritic spine reorganization?
Next, we inhibited Rho-associated protein kinase II (also called Rho-kinase, or “ROCK”), a substrate of the master cytoskeletal regulator RhoA (Rho). Rho functions as a molecular switch, cycling between an inactive GDP-bound state and an active GTP-bound state in which Rho is targeted to cellular membranes. There, Rho orchestrates the formation of stress fibers and focal adhesions necessary to reorganize cellular membranes through ROCK. Thus, ROCK inhibitors interfere with activity-dependent dendritic spine remodeling (e.g.,[16]).
Here, male C57BL/6 mice bred in-house from Jackson Labs stock were injected with the brain-penetrant ROCK inhibitor HA-1077 (30 mg/kg, i.p., LC Labs) or PBS vehicle from P24–28 or P31–35, corresponding to periods of spine proliferation and elimination during early adolescence. We also injected mice from P42–46, corresponding to the onset of the peri-adolescent period [14]. Mice were left undisturbed until adulthood (P56) when cocaine-induced psychomotor sensitization was tested as an assay of cocaine-induced behavioral plasticity.
We used a within-subjects experimental design described previously [8,9]: Mice were habituated for 1 hour to Med-Associates locomotor monitoring chambers (41×20×20 cm), then injected with cocaine HCl (10 mg/kg, i.p., Sigma) for a total of 5 injections administered every other day. Mice were monitored for 30 min., and time spent repeatedly breaking the same photobeam during the 30 min. after cocaine injection was normalized to time accumulated during the half hour prior to injection; sessions 1 and 5 were compared by 2-factor repeated measures ANOVA with group and session as factors. Fisher's post-hoc comparisons were applied when appropriate.
Repeated cocaine exposure increased locomotor activity, reflecting classical psychomotor sensitization [main effect of session F(1,33)=21.1, p<0.001]. Moreover, all mice with a history of early-life HA-1077 exposure were more active than control mice, indicating heightened sensitivity to cocaine [main effect of HA-1077 F(3,33)=3.0, p=0.04; post-hoc ps≤0.05] (fig.1b).
Following the sensitization procedure, mice were allowed a 2-week washout period, followed by a “challenge” injection. Mice were again habituated to the locomotor chambers for 1 hour, administered PBS, monitored for an additional hour, and then administered a final injection of cocaine (10 mg/kg) and monitored for 30 min. Locomotor time accumulated after the cocaine injection was normalized to locomotor time accumulated in the 30 min. after PBS in order to isolate the locomotor-activating effects of cocaine, as opposed to injection-conditioned locomotion. Remarkably, cocaine-induced locomotor activation remained exaggerated in mice exposed to HA-1077 from P24–28 [F(3,33)=4, p=0.02] (fig.1c), though not in other groups.
Thus, our findings indicate that inhibiting structural plasticity by interfering with early-life Rho signaling confers long-term vulnerability to cocaine. This model predicts that when applied during the relatively static adult period, HA-1077 should have divergent—if any—consequences. We treated adult (P56) mice with HA-1077 for 5 days. Psychomotor sensitivity to cocaine was tested 48 hours later to approximate the age of mice exposed to HA-1077 in adolescence. Sensitivity here was unchanged (F<1) (fig.1d). Sensitivity to a “challenge” injection was also unaffected (t9=−0.7,p=0.5) (fig.1e).
Are there beneficial effects of ROCK inhibition?
Together, our findings provide evidence that disrupting programmed structural plasticity—as during adolescent development—exaggerates subsequent cocaine sensitivity. By contrast, occluding cytoskeletal reorganization that results from pathological stimuli may, however, have distinct—potentially even beneficial—consequences. Indeed, the ROCK inhibitor HA-1077 is in clinical trials to treat ischemic brain damage and other adverse cardiovascular outcomes in adults. HA-1077 also increases hippocampal dendritic branching in a genetic model of Alzheimer's Disease [17], reverses ischemia-related cognitive impairment [18], and facilitates prefrontal cortical-dependent learning and memory in aged rats [19]. These reports raise the possibility that HA-1077, when applied in adulthood, may have beneficial consequences in the context of diverse psychopathologies.
As discussed, psychostimulants are potent regulators of dendritic spine morphology, and exposure during early adolescence results in a net loss of oPFC dendritic spines in adulthood, providing a possible mechanism by which early-life cocaine exposure increases risk of drug dependence across the lifespan [8]. Winstanley and colleagues recently reported that chronic inhibition of the norepinephrine transporter—as occurs with repeated cocaine exposure—during adolescence reduces Bdnf mRNA selectively within the oPFC during adulthood [20]. These findings are provocative because postnatal BDNF expression stabilizes cortical spines in both adolescence and adulthood, thus like early-life cocaine exposure, postnatal BDNF deficiency is associated with cortical dendritic spine loss in adulthood [8,21]. Based on these findings, we hypothesized that: 1) oPFC-selective Bdnf deficiency would confer aberrant reward-seeking, and 2) HA-1077 would normalize behavioral outcomes.
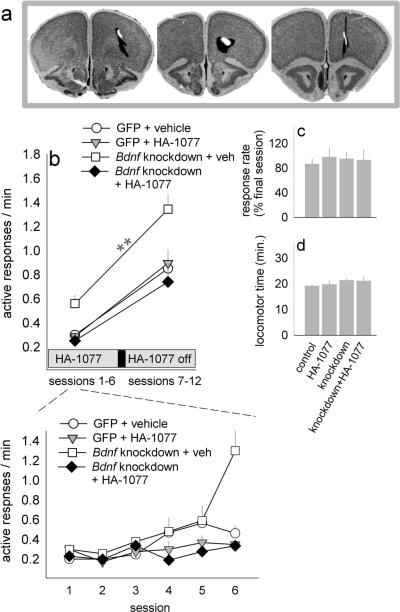
To test these hypotheses, lentiviruses expressing either GFP or Cre Recombinase were infused into the oPFC of adult (P56 or older) mice that were homozygous for a floxed Bdnf gene (exon 5) and maintained on a mixed BALB/C background (Jackson Labs). Females were used. Stereotaxic coordinates were ML+/−1.2, AP+2.6, DV−2.8; the infusion volume was 0.25 μl per hemisphere. Infusion sites were verified by immunostaining for Cre (Sigma; 1:750). Biotinylated Goat Anti-Mouse IgG (Vector Labs, Burlingame, CA; 1:1000) served as a secondary antibody, and signal amplification was achieved using an avidin-biotin complex (ABC) kit in accordance with manufacturer's instructions (Vector Labs, Burlingame, CA). Sections were then stained with Alexa Fluor 488 Streptavidin (Jackson Immuno, West Grove, PA; 1:500) and imaged. Within the oPFC, viruses infected the ventral compartment (fig.2a). Dorsal spread to M2 was noted in some animals, however we do not anticipate this impacted our findings. Mice lacking oPFC infection (n=6) were excluded.
Figure 2. Rho-kinase (ROCK) inhibition in adulthood reverses the behavioral effects of cortical Bdnf knockdown.
(a) Cre expression patterns were transposed onto coronal images from the Mouse Brain Library; black represents the largest infection site, white the smallest. These sections correspond roughly to Bregma +1.98, +2.1, and +2.46, and infusions were bilateral. (b) Bdnf knockdown exaggerates food-reinforced instrumental response rates, and HA-1077 blocks this effect. This pattern is evident both during active HA-1077 treatment (see also inset) and after treatment. n=5–6/group. (c) A single injection of HA-1077 after response acquisition has no additional effects. (d) Locomotor activity counts collected over 30 min. also do not differ. Bars and symbols represent means+SEMs, **p≤0.006 vs. other groups.
One week after surgery, mice were injected daily with HA-1077 (30 mg/kg, i.p.) or vehicle (PBS) in order to coincide with progressive Cre-mediated Bdnf knockdown. Mice were then food-restricted to 93% of their original body weight, and acquisition of a nose poke response to acquire food reinforcers (20 mg grain-based pellets, BioServ) was tested in standard Med-Associates operant conditioning chambers. The start of the session was indicated by illumination of the house light, and mice were reinforced using a continuous reinforcement schedule. Sessions ended when mice acquired 60 pellets or at 135 min., and the house light was extinguished. HA-1077 administration continued during this period, and injections were delayed until at least 2 hours after testing to avoid unforeseen effects on post-session memory consolidation.
Response rates varied, but a clear pattern emerged: Rates were higher in mice with Bdnf knockdown than all other groups, and importantly, HA-1077 blocked this effect [interaction F(1,18)=10.0, p=0.006, Tukey's post-hoc ps<0.001] (fig.2b).
To disambiguate the impact of HA-1077 on instrumental learning vs. performance, we stopped administering HA-1077. Following a 48-hour washout period, mice were tested for an additional 6 sessions. Again, Bdnf knockdown generated higher response rates, and rates normalized in mice with a history of HA-1077 [interaction F(1,18)=4.5, p=0.05, post-hoc ps≤0.006] (fig.2b). When rates were analyzed by 3-factor ANOVA (HA-1077 x knockdown x pre-vs. post-HA-1077 time period), HA-1077 and knockdown were again interacting factors [F(1,18)=9.1, p=0.007]. This pattern indicates that increased response rates in Bdnf-deficient mice reflect an HA-1077-sensitive enhancement of response acquisition rather than response performance since response rates in treated mice remained normalized even after HA-1077 was discontinued. (This experimental approach has been discussed in greater detail by previous investigators [22].) Further supporting this perspective, a single acute injection of HA-1077 prior to a final 13th test session had no additional effects (Fs<1) (fig.2c). We also confirmed the following day that spontaneous locomotor counts (collected as above) did not differ (Fs<1) (fig.2d).
Why might oPFC-selective Bdnf knockdown impact instrumental response acquisition? oPFC lesions that include the ventral compartment infected here impair an animal's ability to learn outcome-predictive contingencies [23,24]. This is likely due to ablation of neurons projecting to the dorsomedial striatum [25], which coordinates decision-making that is based on the association between a response and its outcome [22]. In contrast to the dorsomedial striatum, the dorsolateral striatum is associated with reward-directed responding that is stimulus-dependent [22]. Therefore, disrupting an oPFC-dorsomedial striatal circuit by knocking down oPFC Bdnf might bias decision-making strategies towards those based on dorsolateral striatal-mediated stimulus-response associations. In this case, animals' utilization of reinforcer-related stimuli (the house light, the sound of the pellet delivery, etc.) would invigorate response acquisition and thereby increase response rates, as was observed here.
Future studies will explicitly test the hypothesis that oPFC Bdnf knockdown biases decision-making strategies towards those based on stimulus-response, rather than response-outcome, associations by altering connectivity with the dorsomedial striatum. Also, whether the behavioral effects of cortical Bdnf knockdown can be attributed to dendritic spine reorganization [21] relative to diminished BDNF transport [12] should be explored, in addition to the possible role of sex differences, since our instrumental conditioning studies were conducted with female mice. Identifying interactions between known mechanisms of cortical spine refinement, adolescent stimulant exposure and, for example, estrogen may provide insight into sex-specific drug vulnerabilities.
ROCK inhibition has age-dependent behavioral consequences
A primary finding of the experiments described here is that systemic administration of HA-1077 has developmentally-selective behavioral consequences in the context of psychomotor sensitivity to cocaine. Specifically, adolescent application exaggerates subsequent sensitivity to cocaine in adulthood, while adult application has no obvious effects. Given that psychomotor sensitization is proposed to model drug craving, our findings suggest that exposure to pathological stimuli that interfere with structural refinement during adolescence (such as cocaine [8]) confers lifelong vulnerability to cocaine craving. Other evidence, particularly as it pertains to the orbital subregion of the prefrontal cortex, supports this perspective: First, destabilizing oPFC dendritic spines by infusing inhibitors of F-actin polymerization or the cytoskeletal stabilizer Arg kinase exaggerates cocaine-induced psychomotor sensitization [8,9]. Also, arg−/− mice show oPFC dendritic spine elimination—rather than proliferation—between P24–31, in synchrony with the development of heightened cocaine-induced psychomotor sensitization [8].
Here, timed injections of HA-1077 allowed us to manipulate a primary cytoskeletal regulator in a time-sensitive manner that is not possible using traditional gene knockout strategies. Our experimental design included, for example, mice in which ROCK was inhibited selectively during a discrete developmental period immediately preceding adolescence; in these animals, expression of sensitization was notably pronounced, despite the relatively low dose of cocaine tested. P24–28 corresponds to marked dendritic spine proliferation, thus disrupting proliferation may have distinct adverse consequences relative to disrupting refinement. In this case, isolating the mechanisms of pre-adolescent PFC spine proliferation—e.g., Arg—is critical. These mechanisms may be independent of the mechanisms associated with dendritic spine stabilization. For example, BDNF may be more closely associated with postnatal dendritic spine stabilization rather than postnatal proliferation in the cerebral cortex (e.g.,[21]; discussed also [10]).
Our discussion has focused on dendritic spines since they house the majority of excitatory synapses in the adult brain, and real-time cellular imaging studies emphasize the marked effects of ROCK inhibition on dendritic spine plasticity [16]. HA-1077 also however increases dendritic branching [17], thus it is important to note that gross dendritic remodeling may have contributed to our findings. An additional provision is that only a single dose of HA-1077 was tested. This dose—30 mg/kg—is at the high end of a range shown to be behaviorally active in models of hippocampal-dependent learning and memory (e.g.,[18]). Other doses may have distinct consequences, but this has not, to our knowledge, been tested.
Concluding Remarks
It has become increasingly clear that several highly heritable psychiatric diseases such as drug addiction, autism, and obsessive-compulsive disorder are unlikely to be caused by specific genetic mutations. This recognition stresses the importance of determining common signaling cascades in which so-called “risk genes” function. These cascades—such as those that regulate the actin cytoskeleton through ROCK or other candidates—may serve as promising targets in the development of novel pharmacotherapies. Pharmacotherapies that act on oPFC cellular stability may hold particular promise in the context of cocaine addiction—oPFC gray and white matter volume and integrity are compromised in cocaine dependence, and the oPFC is hypoactive during cocaine withdrawal (reviewed [5]). A major caveat, however, is that the characterization of adolescent vs. adult sensitivities to novel therapeutic-like pharmacological adjuncts is critical—as our findings illustrate, adolescence may confer unique vulnerabilities to the same agents that exert therapeutic-like benefits in adult populations.
Highlights
Orbitofrontal cortical dendritic spines proliferate and refine in adolescence.
Structural instability in adolescence exaggerates adult cocaine sensitivity.
By contrast, destabilizing spines in adulthood can have behavioral benefits.
E.g., Rho-kinase inhibition blocks reward seeking after orbital Bdnf knockdown.
Acknowledgements
This work was supported by Children's Healthcare of Atlanta and the Emory Egleston Children's Research Center. The Yerkes National Primate Research Center has been funded by the National Center for Research Resources P51RR165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. The Emory Viral Vector Core is supported by an NINDS Core Facilities grant, P30NS055077. We thank Dr. Kerry Ressler for generously providing the floxed Bdnf mice used here and Mr. Andrew Swanson for valuable feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: oPFC – orbitofrontal prefrontal cortex; BDNF – Brain-derived Neurotrophic Factor; P – postnatal day; GFP – Green Fluorescent Protein; NA – numerical aperture; ROCK – Rho-kinase; ANOVA – analysis of variance; AP – anterior-posterior; ML – medial-lateral; DV – dorso-ventral; PBS – phosphate-buffered saline
Works Cited
- [1].Lewis DA. Development of the prefrontal cortex during adolescence: Insights into vulnerable neural circuits. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- [2].Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- [4].Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nature Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crombag HS, Gorny G, Li Y, Kolb B, Robinson PV. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- [7].Muhammad A, Kolb B. Mild prenatal stress-modulated behavior and neuronal spine density without affecting amphetamine sensitization. Dev Neurosci. 2011;33:85–98. doi: 10.1159/000324744. [DOI] [PubMed] [Google Scholar]
- [8].Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32:2315–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gourley SL, Koleske AJ, Taylor JR. Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine. Proc Natl Acad Sci U S A. 2009;106:16859–16864. doi: 10.1073/pnas.0902286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gourley SL, Taylor JR, Koleske AJ. Cell adhesion signaling pathways: First responders to cocaine exposure? Comm Integr Biol. 2011;4:30–33. doi: 10.4161/cib.4.1.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Warren MS, Bradley WD, Gourley SL, Lin Y-C, Reichardt LF, Greer CF, Taylor JR, Koleske AJ. Integrin β1 signaling through Arg kinase regulates dendrite arbor stability, synapse density, and behavior. J Neurosci. 2012;32:2824–2834. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR. Prelimbic cortex bdnf knockdown reduces instrumental responding in extinction. Learn Mem. 2009;16:756–760. doi: 10.1101/lm.1547909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- [14].Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. 2000. [DOI] [PubMed] [Google Scholar]
- [15].van Eden CG, Uylings HBM. Postnatal volumetric development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- [16].Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Couch BA, DeMarco GJ, Gourley SL, Koleske AJ. Increased dendrite branching in AβPP/PS1 mice and elongation of dendrite arbors by fasudil administration. J Alzheimers Dis. 2010;20:1003–1008. doi: 10.3233/JAD-2010-091114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang L, He Z, Guo L, Wang H. Improvement of cognitive deficit and neuronal damage in rats with chronic cerebral ischemia via relative long-term inhibition of Rho-kinase. Cell Mol Neurobiol. 2008;28:757–768. doi: 10.1007/s10571-007-9157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huentelman MJ, Stephan DA, Talboom J, Corneveaux JJ, Reiman DM, Gerber JD, Barnes CA, Alexander GE, Reiman EM, Bimonte-Nelson HA. Peripheral delivery of a ROCK inhibitor improves learning and working memory. Behav Neurosci. 2009;123:218–223. doi: 10.1037/a0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- [21].Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. 2012;212:1–18. doi: 10.1016/j.neuroscience.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of the cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of goal-directed action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;423:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]