Abstract
A method is described for simultaneous histological processing of multiple fixed tissue samples. The tissue samples are embedded in a gelatin-albumin protein matrix that is rapidly solidified and bound to the tissue surface using a cross-linking reagent. After freezing and sectioning, the individual sections containing multiple samples can be processed for immunocytochemical and histochemical staining. The method is demonstrated for simultaneous processing of multiple rodent brains, and for reconstruction of fragmented human postmortem brain samples.
Keywords: embedding medium, stereology, cerebral cortex, immunocytochemistry, human, postmortem, brain
Introduction
Microscopic examination of fixed tissue typically requires labor intensive processing of individual samples. Methods that facilitate simultaneous, high-throughput histological processing reduce the labor costs, and at the same time standardize the staining quality across samples. One commonly used method is to create “tissue microarrays” that embed multiple samples in a single paraffin block for simultaneous sectioning (Kononen et al., 1998). Each section contains multiple samples, and can be stained and analyzed on a single microscope slide. A disadvantage is that the tissue is infiltrated with embedding medium (e.g., paraffin), that require immersion in organic solvents, and this is sometimes incompatible with routine histological procedures, such as immunocytochemical staining of sensitive antigens.
An alternative method for embedding tissue is to use gelatin-albumin to infiltrate or encase tissue samples, with subsequent hardening by exposure to formalin (Snodgress and Dorsey, 1963; Crane and Goldman, 1979). A disadvantage of this method is that it requires lengthy exposure to formalin to solidify the embedding material, up to a week for larger tissue samples.
We describe a modification of the gelatin-albumin method that uses a lysine-glutaraldehyde cross-linking reagent to rapidly solidify the embedding material and bind it to the surface of fixed tissue. The method provides a homogeneous embedding matrix that allows simultaneous processing of multiple tissue samples, and is compatible with a variety of histological methods commonly used on frozen sections of fixed tissue. The matrix also provides support for the tissue, reducing cutting artifacts that commonly occur at the tissue edges while cutting frozen tissue. Additionally, optional inclusion of a color reagent creates contrast with the tissue, and simplifies 3-dimensional computer reconstructions from digital images of the block face taken during sectioning, which is especially useful for complex and fragmented samples.
Materials and Methods
Tissue samples were first fixed by exposure to formaldehyde. In the examples shown (Figures 1 and 2), rodent brains were transcardially perfused with 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.2, and human brains were cut into coronal slabs about 3 cm thick, and immersed in phosphate buffered 10% formalin for 5 days. All tissue samples were subsequently cryoprotected by immersion in 20% glycerol plus 0.1 M phosphate, pH 7.2 at 4 °C, until they sank to the bottom of the container. (Alternatively, we used a graded exposure to 10% and then 20% glycerol, without a noticeable effect on tissue preservation). As described below, on the day before sectioning, the samples were embedded in a solid, flexible, protein matrix that is covalently bound to the surface of the tissue samples. After embedding the samples were re-immersed in the 20% glycerol solution overnight at 4 °C, before freezing and sectioning with a sliding microtome.
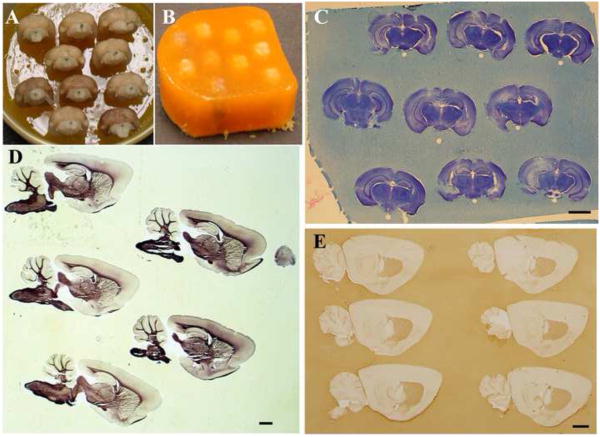
Figure 1.
A. Mouse brains pinned to base of matrix inside a beaker, prior to immersion in embedding matrix. No contrast agent (food coloring) was added to the matrix. B. The same brains after embedding and removal from the beaker, prior to sectioning. C. Nissl stained coronally sectioned mouse brains, embedded in matrix. Uneven staining in the hippocampal region is due to damage caused by microdialyis probes. D. Sagitally sectioned rat brains were mounted on slides and myelin stained (Schmued, 1990). E. Free-floating, sagittally sectioned mouse brains have D1-dopamine receptor immunolabeling in the striatuam (antibody sc-33660, Santa Cruz Biotechnology, Santa Cruz, CA). Scale bars = 2 mm.
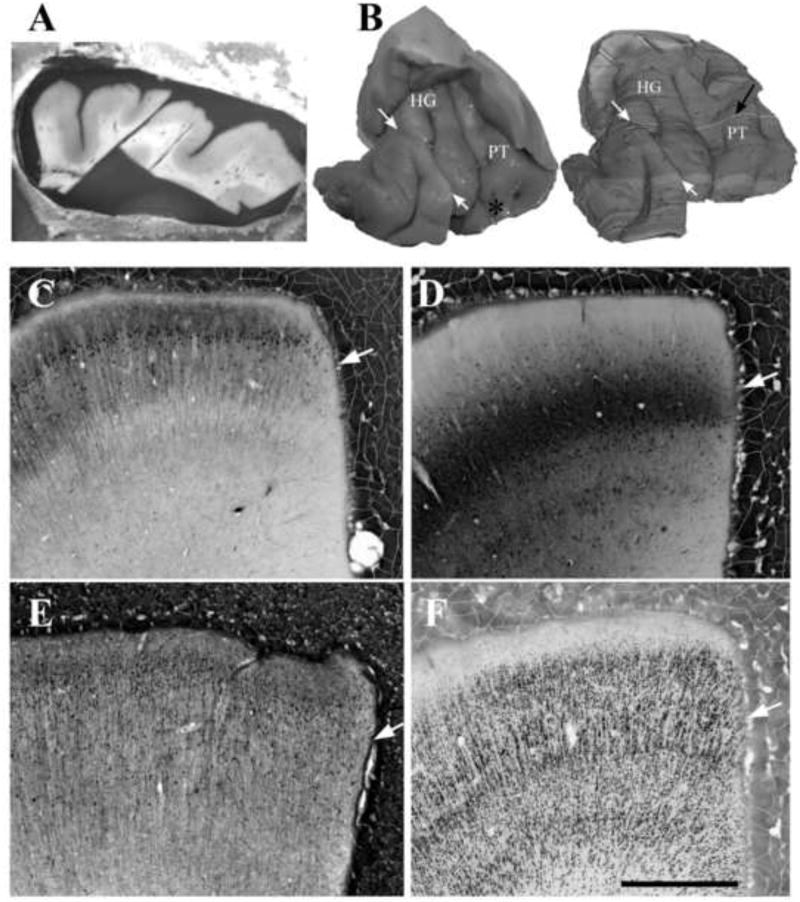
Figure 2.
A.-B. A sample of human postmortem auditory cerebral cortex was embedded in matrix and reconstructed from video images taken from the block face during sectioning. A. Block face images of every section were taken during sectioning, using a digital camera mounted on a tripod above the microtome. B. 3-dimensional reconstructions were made from the block-face digital images. The reconstruction were made using the block face images of all 560 sections through the tissue, in which the tissue image was isolated from the surrounding matrix using image thresholding and manual editing, using ImageJ software [((Abramoff et al., 2004); http://imagej.nih.gov/ij/)]. 3-dimensional reconstructions were then created with the “Volume viewer plugin” in that program. In this example, the original sample had been fragmented at autopsy into 2 coronal pieces, at the location of the white arrows. The highlight section (black arrow) shows the source of the section in A. This example shows Heschl's gyrus (HG) and the adjacent planum temporale (PT), cut perpendicular to the axis of HG. C.-E. Examples of immunolabeling in human postmortem auditory cerebral cortex, using antibodies to the calcium binding proteins calbindin (C), parvablumin (D), calretinin (E), and additionally to neuron nuclear antigen (Neun; F). In some cases, immunolabeling had reduced density at the tissue surface, presumably due to the exposure of the antigens to the glutaraldehyde-lysine cross-linking reagent. In these examples, this effect at the tissue surface was evident with parvalbumin and calbindin labeling (C-D, white arrows), but was difficult to detect with calretinin and Neun labeling (E-F, white arrows). Antibodies shown are mouse anti-Neun (Cat # MAB377, Millipore, Billerica, MA); mouse anti-parvalbumin (Cat. #300, Swant, Switzerland), mouse anti-calbindin (Cat. #235, Swant) and rabbit anti-calretinin (Cat. #7699/3H, Swant). Scale bar = 1 mm, and applies to figures C-F.
The main component of the protein matrix is a “gelatin-egg albumin mixture”, prepared as follows. 3.75 g gelatin (Fisher G7-500) is dissolved into 250 ml of 0.9% NaCl that was first pre-heated to 60-70 °C, and this gelatin solution is left to cool to room temperature. Separately, 225 g egg albumin (Fisher A388-500) is dissolve in 500 ml of 0.9% saline at room temperature. The gelatin and egg albumin solutions are combined, and stirred slowly for 4 hours before filtering through gauze. To each 100 ml of the filtered solution, 50 g sucrose and 1 ml triton X-100 detergent are added. The solution is stored at 4°C, and 0.01% thimerosal is added as an antimicrobial agent for storage of more than a few days.
The following steps are taken immediately prior to pouring the matrix solution over the tissue. With gentle stirring to avoid creating bubbles, 10 ml of 40% formaldehyde (Fisher F77P-4) and 10 ml of household red food coloring (e.g., manufactured by McCormick and Co, Hunt Valley Md.) are added to 150 ml of the above gelatin-egg albumin mixture. The food coloring creates color contrast with the tissue, and is optional. To each 100 ml of this solution is added 7 ml of cross-linking reagent, which is a 1:1 solution of 50% glutaraldehyde (EM grade, Sigma, catalogue number G7651) and 2.5M lysine (= 10 ml dH2O plus 4.57 g of L-lysine monhydrochloride, Sigma-Aldrich L5626), both at room temperature. Upon mixing, the cross-linking reagent turns progressively darker shades of orange. It is gently mixed for 2 minutes, and then added to the gelatin-egg albumin/formaldehyde mixture, while gently stirring with a spatula, and the solution is immediately poured over the tissue samples. The matrix solidifies in 3-4 minutes, and hardens completely after about 15 minutes. The final hardened matrix is flexible, with the consistency of soft rubber.
Tissue samples must be held stationary so that they do not float to the surface of the freshly poured matrix solution. For this purpose, a base of hardened matrix can be formed in the bottom of a beaker (or other suitable mold), and tissue samples affixed to the base with pins that are removed after the tissue is encased in hardened matrix. For rodent brains, we typically pass a fine sewing needle through the ventral brainstem, with the rostral brain pointed downward, so that all brains have the same final coronal orientation during sectioning (Figure 1A). Before pinning the tissue in place, the matrix base and sides of the beaker are coated with glycerol, which acts as a molding release agent and facilitates removal of the hardened matrix from the mold.
After the matrix is hardened and the pins removed, a flat spatula is used to gently pry the matrix from the beaker. The base layer of matrix is peeled off to expose the top cutting surface of the tissue, and excess matrix is trimmed from around the sides of the tissue. It is useful to notch one corner of the matrix as a marker of the original tissue orientation. The embedded tissue is then frozen and sectioned by standard methods. We use a solution of melted 3% agar to glue the tissue to the stage of a sliding microtome, then freeze the tissue and section it at 20-80 um thickness. Each section is a sheet of matrix containing embedded tissue. Sections must be handled gently to avoid separation of the tissue from the matrix, especially with thinner sections. However, we routinely use the sections for free floating immunocytochemistry, or mount them directly on chrome-alum coated slides for on-slide staining.
We have found that matrix embedded tissue cuts more smoothly if left overnight in buffered 20% glycerol, at 4°C. Additionally, the matrix consistency is affected by the quality of the glutaraldehyde. It should be EM-grade, and if it is old or left uncapped the matrix will harden slowly. The gelatin-egg albumin mixture with thimerosol may be used for months after preparation, but lengthy storage can affect the consistency and time of hardening of the matrix. For quantitative measurements of potentially glutaraldehyde-sensitive immunolabels, we make the gelatin-egg albumin mixture within a few days of use.
Results
Figure 1 shows application of this preparation to multiple rodent brains. We have simultaneously processed as many as 20 mouse brains, using 50 × 75 mm microscope slides to accommodate the large sections. Figure 2 shows an application of the method to human postmortem cerebral cortex. The region containing auditory cortex had been fragmented by the pathologist at autopsy. The pieces were pinned together in their original orientation, and then embedded in matrix and sectioned in a parasagittal orientation perpendicular to the axis of Heschl's gyrus. Addition of contrast agent simplified creation of digital 3-dimensional reconstructions of the tissue sample. To create the latter, images of the block face were taken for each section (Figure 2A), using a digital camera mounted directly above the microtome stage. Image thresholding methods separated the tissue image from the much darker surrounding matrix (Figure 2B).
One consideration of the tissue embedding matrix is that it uses glutaraldehyde-lysine as a cross-linking reagent. The tissue is exposed to this cross-linker for a brief period of minutes, before the glutaraldehyde is presumably fully covalently bound to the lysine and to the other available binding sites in the tissue and in the protein-rich matrix. Thus, stains that are highly sensitive to glutaraldehyde may be reduced by this method, especially at the tissue surface that is most exposed to the cross-linker. This effect was subtle or undetectable for most antibody stains that we used. When detectable, it was concentrated at the tissue surface (e.g., Figures 2C-D).
Discussion and Conclusions
Our gelatin-albumin based tissue embedding matrix provides a simple method for simultaneous histological processing of multiple tissue samples. It provides several advantages compared to methods that separately process individual samples. First, the amount of labor involved in sectioning, histological processing and subsequent mounting of the samples is reduced. Second, the matrix surrounding the tissue provides support and protection for the tissue during sectioning, thus reducing damage to the tissue surface that often occurs while cutting frozen sections. Third, by retaining multiple samples in a single section, they can all be treated identically during histological staining, thus reducing variability of staining and tissue thickness between sections. Finally, for anatomically complex and fragmented tissue samples, such as pathological specimens of human brain, the color contrast between the matrix and tissue simplifies 3-dimensional reconstruction of the original tissue, allowing the user to precisely localize the origin of each tissue section in the original sample.
Previous gelatin-ablumen methods used lengthy exposures to formalin to harden the gelatin-albumen embedding medium (Snodgress and Dorsey, 1963; Crane and Goldman, 1979). An alternative approach is to simply use glutaraldehyde in place of formalin as a cross-linking reagent (Levin, 2004). While glutaraldehyde alone also hardens the matrix within minutes, the tissue exposure to glutaraldehyde persists after hardening of the matrix, thus reducing the intensity of labels that are glutaraldehyde sensitive. Our pre-exposure of the glutaraldehyde to equimolar lysine is intended to limit the tissue exposure to glutaraldehyde, by blocking its binding capacity after a brief period of minutes. With the antibodies we tested, the lysine-glutaraldehyde cross-linker caused only minor or undetectable suppression of labeling. We have not systematically tested alternative cross-linking reagents that might have less effect on antibody labeling.
Acknowledgments
We are thankful to Dr. Cecilia Hedin-Pereira (Federal University of Rio de Janeiro) for sharing an earlier gelatin-albumin embedding protocol, to Dr. Andrew J. Dwork (New York Psychiatric Institute) for contributing human cerebral cortex, and to Drs. David Dunlop and Amos Neidle (Nathan Kline Institute, New York) for their helpful discussions and reading the manuscript. Supported by NIH grants MH085208, MH64168 and DC04318.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Crane AM, Goldman PS. An improved method for embedding brain tissue in albumin-gelatin. Stain Technol. 1979;54:71–5. doi: 10.3109/10520297909112637. [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Levin M. A novel immunohistochemical method for evaluation of antibody specificity and detection of labile targets in biological tissue. J Biochem Biophys Methods. 2004;58:85–96. doi: 10.1016/s0165-022x(03)00149-0. [DOI] [PubMed] [Google Scholar]
- Schmued L. A rapid, sensitive histochemical stain for myelin in frozen brain sections. J Histochem Cytochem. 1990;38:717–20. doi: 10.1177/38.5.1692056. [DOI] [PubMed] [Google Scholar]
- Snodgress AB, Dorsey CH. Egg albumin embedding: a procedure compatible with neurological staining techniques. Stain Technol. 1963;38:149–55. doi: 10.3109/10520296309067158. [DOI] [PubMed] [Google Scholar]