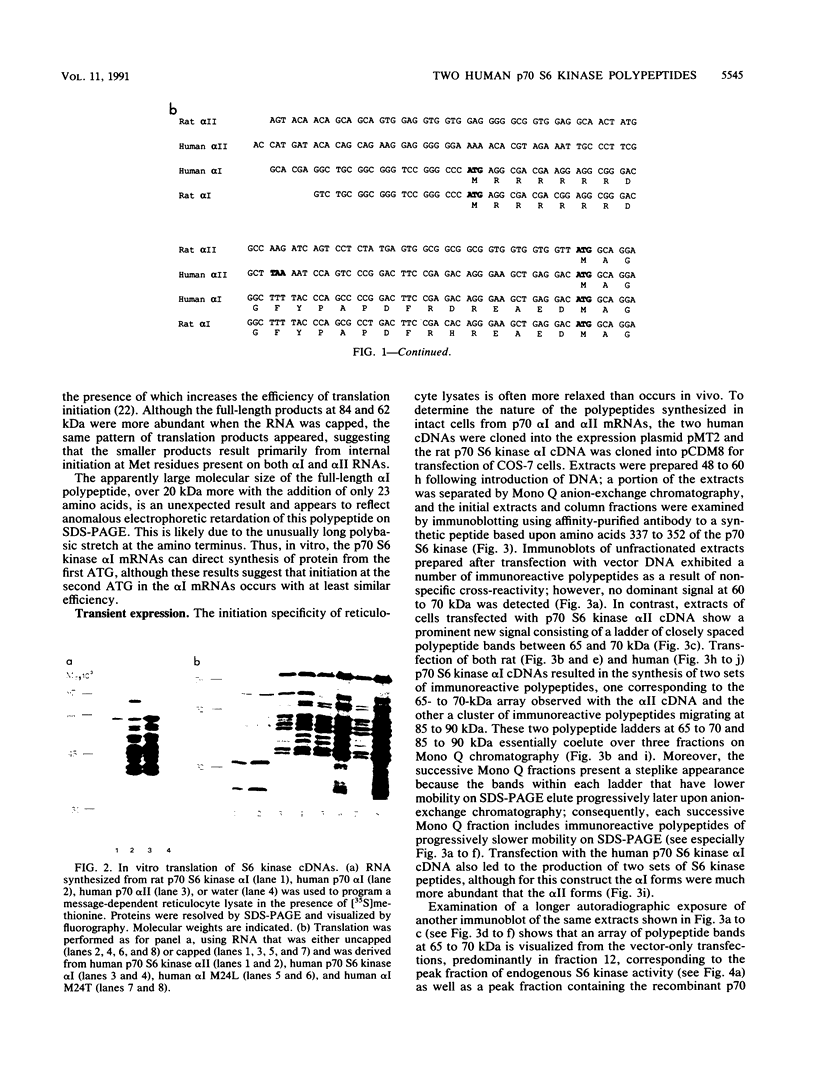
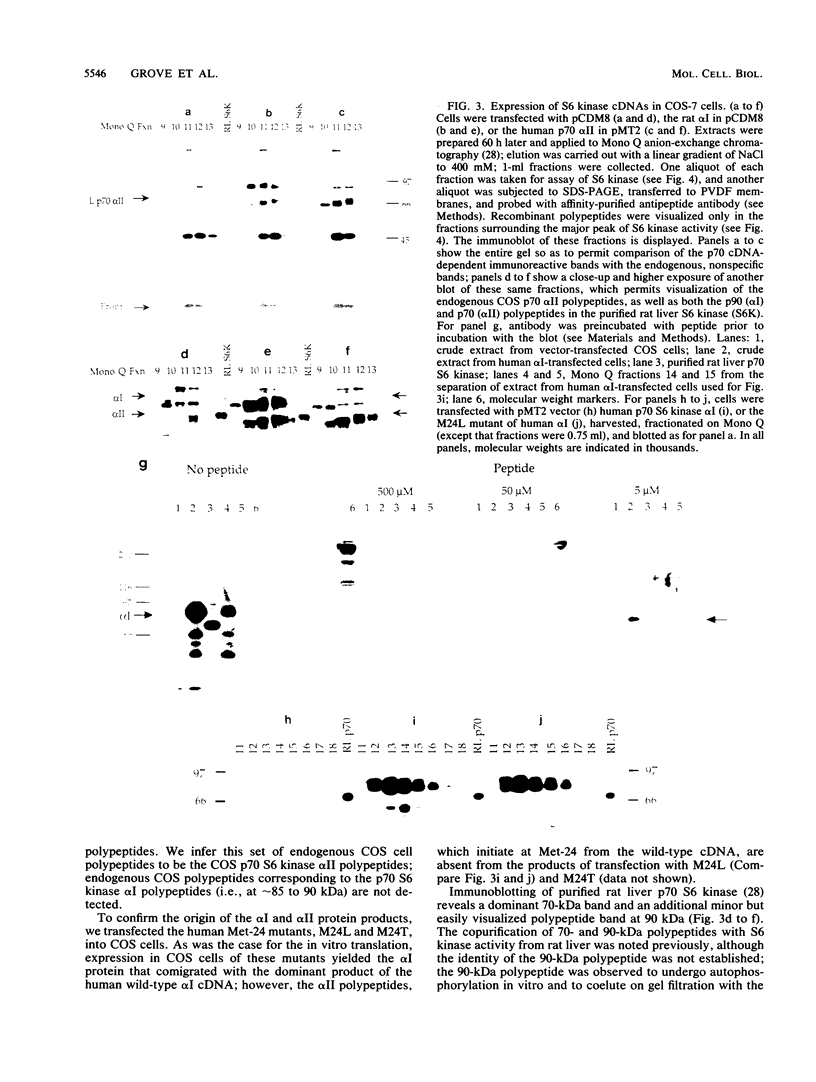
Abstract
Two classes of human cDNA encoding the insulin/mitogen-activated p70 S6 kinase have been isolated; the two classes differ only in the 5' region, such that the longer polypeptide (p70 S6 kinase alpha I; calculated Mr 58,946) consists of 525 amino acids, of which the last 502 residues are identical in sequence to the entire polypeptides encoded by the second cDNA (p70 S6 kinase alpha II; calculated Mr 56,153). Both p70 S6 kinase polypeptides predicted by these cDNAs are present in p70 S6 kinase purified from rat liver, and each is thus expressed in vivo. Moreover, both polypeptides are expressed from a single mRNA transcribed from the (longer) p70 S6 kinase alpha I cDNA through the utilization of different translational start sites. Although the two p70 S6 kinase polypeptides differ by only 23 amino acid residues, the slightly longer alpha I polypeptide exhibits anomalously slow mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), migrating at an apparent Mr of 90,000 probably because of the presence of six consecutive Arg residues immediately following the initiator methionine. Transient expression of p70 alpha I and alpha II S6 kinase cDNA in COS cells results in a 2.5- to 4-fold increase in overall S6 kinase activity. Upon immunoblotting, the recombinant p70 polypeptides appear as a closely spaced ladder of four to five bands between 65 and 70 kDa (alpha II) and 85 and 90 kDa (alpha I). Transfection with the alpha II cDNA yields only the smaller set of bands, while transfection with the alpha I cDNA generates both sets of bands. Mutation of Met-24 in the alpha I cDNA to Leu or Thr suppresses synthesis of the alpha II polypeptides. Only the p70 alpha I and alpha II polypeptides of slowest mobility on SDS-PAGE comigrate with the 70- and 90-kDa proteins observed in purified rat liver S6 kinase. Moreover, it is the recombinant p70 polypeptides of slowest mobility that coelute with S6 kinase activity on anion-exchange chromatography. The slower mobility and higher enzymatic activity of these p70 proteins is due to Ser/Thr phosphorylation, inasmuch as treatment with phosphatase 2A inactivates kinase activity and increases the mobility of the bands on SDS-PAGE in an okadaic acid-sensitive manner. Thus, the recombinant p70 S6 kinase undergoes multiple phosphorylation and partial activation in COS cells. Acquisition of S6 protein kinase catalytic function, however, is apparently restricted to the most extensively phosphorylated recombinant polypeptides.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorta D. A., Crews C. M., Sweet L. J., Bankston L., Jones S. W., Erikson R. L. Sequence and expression of chicken and mouse rsk: homologs of Xenopus laevis ribosomal S6 kinase. Mol Cell Biol. 1989 Sep;9(9):3850–3859. doi: 10.1128/mcb.9.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L. M., Siegmann M., Thomas G. S6 kinase in quiescent Swiss mouse 3T3 cells is activated by phosphorylation in response to serum treatment. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7154–7158. doi: 10.1073/pnas.85.19.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P., Ahmad M. F., Grove J. R., Kozlosky C., Price D. J., Avruch J. Molecular structure of a major insulin/mitogen-activated 70-kDa S6 protein kinase. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8550–8554. doi: 10.1073/pnas.87.21.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Blenis J. Identification of Xenopus S6 protein kinase homologs (pp90rsk) in somatic cells: phosphorylation and activation during initiation of cell proliferation. Mol Cell Biol. 1990 Jun;10(6):3204–3215. doi: 10.1128/mcb.10.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Westermark B., Heldin C. H. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988 Oct;8(10):4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. In vivo phosphorylation and activation of ribosomal protein S6 kinases during Xenopus oocyte maturation. J Biol Chem. 1989 Aug 15;264(23):13711–13717. [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Purification and characterization of a protein kinase from Xenopus eggs highly specific for ribosomal protein S6. J Biol Chem. 1986 Jan 5;261(1):350–355. [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Purification and characterization of ribosomal protein S6 kinase I from Xenopus eggs. J Biol Chem. 1991 Mar 15;266(8):5249–5255. [PubMed] [Google Scholar]
- Erikson E., Maller J. L. Substrate specificity of ribosomal protein S6 kinase II from Xenopus eggs. Second Messengers Phosphoproteins. 1988;12(2-3):135–143. [PubMed] [Google Scholar]
- Franco R., Rosenfeld M. G. Hormonally inducible phosphorylation of a nuclear pool of ribosomal protein S6. J Biol Chem. 1990 Mar 15;265(8):4321–4325. [PubMed] [Google Scholar]
- Gordon J., Nielsen P. J., Manchester K. L., Towbin H., Jimenez de Asua L., Thomas G. Criteria for establishment of the biological significance of ribosomal protein phosphorylation. Curr Top Cell Regul. 1982;21:89–99. doi: 10.1016/b978-0-12-152821-8.50008-6. [DOI] [PubMed] [Google Scholar]
- Harmann B., Kilimann M. W. cDNA encoding a 59 kDa homolog of ribosomal protein S6 kinase from rabbit liver. FEBS Lett. 1990 Oct 29;273(1-2):248–252. doi: 10.1016/0014-5793(90)81096-7. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Protein phosphorylation controls translation rates. J Biol Chem. 1989 Dec 15;264(35):20823–20826. [PubMed] [Google Scholar]
- Jenö P., Ballou L. M., Novak-Hofer I., Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):406–410. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Erikson E., Blenis J., Maller J. L., Erikson R. L. A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc Natl Acad Sci U S A. 1988 May;85(10):3377–3381. doi: 10.1073/pnas.85.10.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma S. C., Ferrari S., Bassand P., Siegmann M., Totty N., Thomas G. Cloning of the mitogen-activated S6 kinase from rat liver reveals an enzyme of the second messenger subfamily. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7365–7369. doi: 10.1073/pnas.87.19.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoinne A., Erikson E., Maller J. L., Price D. J., Avruch J., Cohen P. Purification and characterisation of the insulin-stimulated protein kinase from rabbit skeletal muscle; close similarity to S6 kinase II. Eur J Biochem. 1991 Aug 1;199(3):723–728. doi: 10.1111/j.1432-1033.1991.tb16176.x. [DOI] [PubMed] [Google Scholar]
- Nemenoff R. A., Price D. J., Mendelsohn M. J., Carter E. A., Avruch J. An S6 kinase activated during liver regeneration is related to the insulin-stimulated S6 kinase in H4 hepatoma cells. J Biol Chem. 1988 Dec 25;263(36):19455–19460. [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Price D. J., Gunsalus J. R., Avruch J. Insulin activates a 70-kDa S6 kinase through serine/threonine-specific phosphorylation of the enzyme polypeptide. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7944–7948. doi: 10.1073/pnas.87.20.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. J., Nemenoff R. A., Avruch J. Purification of a hepatic S6 kinase from cycloheximide-treated Rats. J Biol Chem. 1989 Aug 15;264(23):13825–13833. [PubMed] [Google Scholar]
- Rathjen P. D., Toth S., Willis A., Heath J. K., Smith A. G. Differentiation inhibiting activity is produced in matrix-associated and diffusible forms that are generated by alternate promoter usage. Cell. 1990 Sep 21;62(6):1105–1114. doi: 10.1016/0092-8674(90)90387-t. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Weil S., Hunter A. R. Changes in RNA metabolism and accumulation of presumptive messenger RNA during transition from the growing to the quiescent state of cultured mouse fibroblasts. J Mol Biol. 1975 Aug 25;96(4):745–766. doi: 10.1016/0022-2836(75)90150-3. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Sweet L. J., Alcorta D. A., Erikson R. L. Two distinct enzymes contribute to biphasic S6 phosphorylation in serum-stimulated chicken embryo fibroblasts. Mol Cell Biol. 1990 Jun;10(6):2787–2792. doi: 10.1128/mcb.10.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Immunological evidence for two physiological forms of protein kinase C. Mol Cell Biol. 1987 Jan;7(1):85–96. doi: 10.1128/mcb.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]