Abstract
MicroRNA-9 (miR-9) is emerging as a critical regulator of organ development and neurogenesis. It is also deregulated in several types of solid tumors; however, its role in hematopoiesis and leukemogenesis is not yet known. Here we show that miR-9 is detected in hematopoietic stem cells and hematopoietic progenitor cells, and that its expression increases during hematopoietic differentiation. Ectopic expression of miR-9 strongly accelerates terminal myelopoiesis and promotes apoptosis in vitro and in vivo. Conversely, in hematopoietic progenitor cells, the inhibition of miR-9 with a miRNA sponge blocks myelopoiesis. Ecotropic viral integration site 1 (EVI1), required for normal embryogenesis, is considered an oncogene because its inappropriate up-regulation induces malignant transformation in solid and hematopoietic cancers. Here we show that EVI1 binds to the promoter of miR-9-3, leading to DNA hypermethylation of the promoter and repression of miR-9. Moreover, miR-9 expression reverses a myeloid differentiation block that is induced by EVI1. Our findings indicate that EVI1, when inappropriately expressed, delays or blocks myeloid differentiation at least in part by DNA hypermethylation and down-regulation of miR-9. It was reported that Forkhead box class O genes (FoxOs) inhibit myeloid differentiation and prevent differentiation of leukemia-initiating cells. Here we identify both FoxO1 and FoxO3 as direct targets of miR-9 in hematopoietic cells and find that up-regulation of FoxO3 inhibits miR-9–induced myelopoiesis. These results reveal a unique role of miR-9 in myelopoiesis and in the pathogenesis of EVI1-induced myeloid neoplasms and provide insights into the epigenetic regulation of miR9 in tumorigenesis.
MicroRNAs (miRNAs) are emerging regulators of gene expression at the posttranscriptional level; they are short noncoding RNAs of 20–22 nucleotides that destabilize the transcript of target genes or inhibit their translation by interacting with complementary sequences in the 3′ UTR of the target transcripts (1). Growing evidence indicates that miRNAs have an important role in tissue and lineage development and, when deregulated, also in the pathogenesis of cancer. Several miRNAs are expressed in a tissue-specific pattern and have cell context-dependent functions. MicroRNA-9 (miR-9) regulates normal neurogenesis (2–4). In adult neural progenitor cells (NPCs), miR-9 down-regulates cell proliferation and accelerates neural differentiation (5), whereas in early NPCs originating from human ES (hES) cells at the neurosphere stage, miR-9 increases proliferation and suppresses migration of NPCs (6). Taken together, these results indicate that miR-9 has strictly context-dependent functions and can either promote or suppress NPCs proliferation and differentiation at different stages of brain development (7). Less information is available on the role of miR-9 in other tissues, although it was detected in normal hepatocyte differentiation (8) and retina development (9) and has been implicated in the regulation of proinflammatory response (5, 10). To date, there are no published studies on miR-9 function in normal hematopoiesis.
There are many reports of aberrant miR-9 expression in several cancers, suggesting that miR-9 can act as an oncogene or as a tumor suppressor. miR-9 up-regulation was observed in Hodgkin’s lymphoma (11), primary brain tumors (12), caudal-type homeobox protein 2-negative gastric cancer (13), and endometrial cancer (14), whereas miR-9 down-regulation was reported in cervical (15), colorectal (16), non–small-cell lung (17), and ovarian cancer (18), and in hepatocellular (19) and gastric carcinoma (20). miR-9 down-regulation is considered a poor survival marker in cervical cancer (15), lung squamous cell carcinoma (17), and in acute lymphoblastic leukemia (21), and it was associated with development of cancer metastasis (19, 22–24). Silencing of miR-9 by cytosine-phosphate-guanine (CpG) island hypermethylation was found in many tumors (16, 17, 21–23, 25), clearly indicating that miR-9 possesses tumor suppressor features. However, the molecular mechanism leading to miR-9 silencing in cancer has not been defined.
Ecotropic viral integration site 1 (EVI1), first identified as a murine common locus of retroviral integration in myeloid leukemia (26), has been implicated in several human myeloid disorders (27) and more recently in solid tumors (28–30). It is inappropriately activated in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) (31). Forced expression of EVI1 in murine bone marrow (BM) cells leads to a disease with features very similar to those reported in MDS patients (32), including hypercellular marrow, ineffective and dysplastic hematopoiesis, and severe cytopenia. Here, we identify miR-9 as a unique regulator of myeloid differentiation, which can be silenced by EVI1 in lineage-negative (Lin−) BM cells by promoter hypermethylation. Ectopic expression of miR-9 reverses the block of myeloid differentiation induced by EVI1.
The Forkhead box (FOX) transcription factors are characterized by a forkhead DNA-binding domain and are required during embryogenesis and in adult organisms (33). The human FOXO family of transcription factors includes four members, FOXO1, FOXO3, FOXO4, and FOXO6, that regulate the expression of genes associated with cell cycle arrest, DNA repair, oxidative stress resistance, and apoptosis (34). FoxOs are involved in the regulation of myelopoiesis. In mice, the loss of FoxO1/3/4 led to the expansion of myeloid cells (35) and induced the myeloid differentiation of MLL-AF9 leukemia-initiating cells (33). We find that miR-9 binds to the 3′ UTR of FoxO1 and FoxO3, thereby downregulating their expression. Interestingly, up-regulation of FoxO3 suppresses miR-9–induced myelopoiesis, suggesting that in hematopoietic progenitor cells, FoxO1 and FoxO3 are targets of miR-9. Taken together, our data indicate that there is an unexpected link between EVI1, miR-9 repression, and up-regulation of FoxO1 and FoxO3 genes. These findings could help to improve understanding of not only the normal role of miR-9 in hematopoiesis but also the molecular basis of EVI1-positive leukemia.
Results
miR-9 Is Expressed in Mature Hematopoietic Cells.
To determine whether miR-9 is involved in the regulation of hematopoiesis, we examined the expression of this small gene in subpopulations of BM cells. In mouse and human, miR-9 is encoded by three genes, miR-9-1, miR-9-2, and miR-9-3; to determine whether one of them is involved in normal hematopoiesis, we quantified each pre–miR-9 isoform by quantitative RT-PCR (qRT-PCR) in BM cell subpopulations isolated from C57B/L6 mice at 8 wk of age. The results indicate that the miR-9-3 isoform is the most highly expressed, followed by miR9-1, and that miR-9-2 is almost undetectable in the mouse hematopoietic cells (Fig. 1A). A similar trend was observed in human hematopoietic cells (Fig. S1A). Our analysis also indicates that miR-9-3 is more abundant in mature myeloid (Mac-1+, Gr-1+) and erythroid cells (Ter119+) than in the stem cell-enriched population [Lin-c-Kit+Sca-1+ (LSK)] and in subsets of myeloid progenitors, including CMPs (Lin− Sca-1− IL-7R− c-Kit+ FcγRII/IIIlow CD34hi), GMPs (Lin− Sca-1− IL-7R− c-Kit+ FcγRII/IIIhi CD34hi), and megakaryocyte/erythrocyte progenitors (MEPs; Lin− Sca-1− IL-7R− c-Kit+ FcγRII/IIIlow CD34low; Fig. 1B). Mature miR-9 also demonstrated the highest expression in mature myeloid cells (Fig. 1C). The expression of pre–miR-9 was confirmed by gel electrophoresis (Fig. S1B). Together, these data suggest that miR-9-1 and -3 are preferentially expressed during terminal myelopoiesis, and could therefore have a role in myeloid differentiation.
Fig. 1.
Relative expression of pre–miR-9 and miR-9 during myeloid cell development. (A) Relative distribution of pre–miR-9 isoforms in subsets of murine myeloid progenitors and mature myeloid cells. Expression of pre–miR-9-3 (B) and mature miR-9 (C) in subsets of murine BM cells. The expression was determined by qRT-PCR.
In Vitro Ectopic Expression of miR-9 Promotes Myeloid Differentiation, Whereas Inhibition of miR-9 Suppresses Terminal Myelopoiesis.
To determine the function of miR-9 in primary Lin− BM cells, we cloned human miR-9-1 and mouse miR-9-3 in the retroviral vector Migr1 [murine stem cell virus-internal ribosome entry site-EGFP (MSCV-IRES-EGFP)]. The expression of both miRs was confirmed by RT-PCR (Fig. S2A). Serial replating assays in methylcellulose revealed that in the first plating there was a transient increase of clonogenicity and proliferation in the miR-9–positive cells compared with the control cells infected with the empty Migr1 vector. This increase was followed by a significant drop in cell and colony number at the second plating (Fig. 2 A and B). Morphological and FACS analyses (Fig. 2 C and D; Fig. S2B) demonstrated that miR-9 strongly accelerated myeloid differentiation compared with the control cells. In contrast, a miR-9–specific sponge, which competes with the endogenous miR-9 binding sites (24), delayed myelopoiesis (Fig. 2 C and D; Fig. S2B). To further confirm that the results were specific for myelopoiesis, we evaluated the expression of the CCAAT/enhancer-binding protein α (C/ebpα), which is expressed predominantly in granulocytes and monocytes (34). As shown in Fig. 2E, C/ebpα was up-regulated in cells expressing miR-9 in the first plating, suggesting that an increased number of differentiated granulocytes and monocytes are in the culture.
Fig. 2.
miR-9 stimulates myeloid differentiation in vitro. (A and B) Colony formation assay. (C) Representative cytospins of BM cells after 14 d in methylcellulose culture. (D) Analysis of the percentage of myeloid cells by FACS in BM cells after 14 d in methylcellulose culture. (E) Analysis of the C/epbα gene expression in BM cells cultured in methylcellulose by qRT-PCR. *P < 0.05; **P < 0.01; ***P < 0.001.
miR-9 Enhances Myelopoiesis but Inhibits Lymphopoiesis in Vivo.
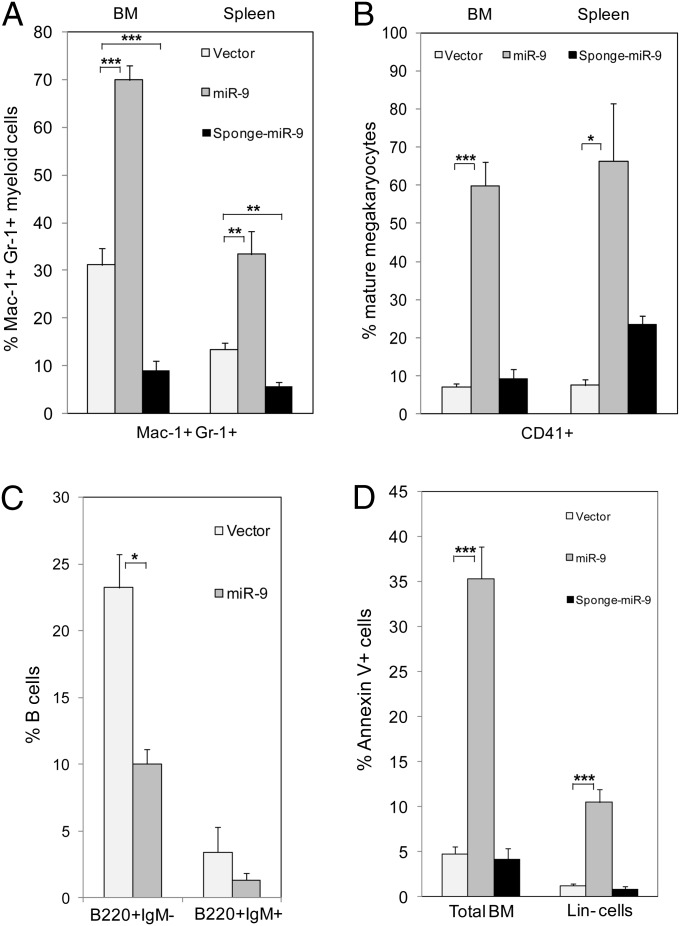
To determine whether forced expression of miR-9 affects myeloid differentiation in vivo, we isolated Lin− BM cells from C57B/L6 mice and infected the cells with the empty Migr1 or miR-9-3 or sponge miR-9 retroviruses, followed by BM transplantation into lethally irradiated recipient mice. At 7 wk after transplantation, EGFP+ BM cells isolated from the reconstituted mice were characterized. As shown in Fig. 3 A and B and Fig. S3, the BM and spleen of miR-9-EGFP+ mice included a significantly higher number of mature myeloid cells (Mac-1+, Gr-1+) and megakaryocytic cells (CD41+) than the BM and spleen of vector EGFP+ control mice, whereas BM and spleen of sponge miR-9-EGFP+ mice contained a lower number of mature myeloid cells than the controls. Sponge miR-9 did not affect megakaryocytic cells (Fig. 3B); the dosage of miR-9 is probably less critical for CD41+ cells than for myeloid cells. In contrast, the frequency of immature B cells (B220+IgM−) and mature B cells (B220+IgM+) in miR-9-EGFP+ was lower than in the controls (Fig. 3C). Taken together, these results indicate that in vivo miR-9 promotes myelopoiesis but inhibits lymphopoiesis.
Fig. 3.
miR-9 stimulates myeloid differentiation, inhibits B-cell differentiation, and stimulates apoptosis in vivo. Flow cytometric analysis of myeloid (A), megakaryocytic (B), B cells (C), cell populations in total BM and apoptotic cells (Annexin V+) in total BM and in Lin− BM cells (D) isolated from mice transplanted with vector (light gray bars), miR-9 (dark gray bars), and sponge miR-9 (black bars). Panels show the relative frequency of cells in each population in EGFP+ BM or spleen cells (mean ± SD; n = 5–8 mice). The mice were analyzed 7 wk post-bone marrow transplantation. *P < 0.05; **P < 0.01; ***P < 0.001.
Ectopic Expression of miR-9 Induces Apoptosis of Lin− BM Cells in Vitro and in Vivo.
Because we observed a significant decrease of the miR-9–positive cells between the first and the second plating (Fig. 2 A and B), we asked whether this drop could correlate with an increase in apoptosis. Therefore, we infected Lin− BM cells with the empty vector, miR-9-3, or sponge miR-9, cultured the cells, and quantified the proapoptotic cells by flow cytometry 4 and 8 d later. We found that the expression of miR-9-3 did not affect apoptosis at day 4 (Fig. S4A) but significantly increased the number of apoptotic cells at day 8 (Fig. S4 B and D), indicating that apoptosis was associated with the late stage of myelopoiesis. Similar results were obtained during the G-CSF–induced differentiation of 32Dcl3 cells (Fig. S4C). To confirm the results in vivo, an aliquot of the infected cells were transplanted in irradiated syngeneic mice. Hematopoietic cells were isolated 7 wk after transplantation and analyzed. The results (Fig. 3D) show that miR-9-EGFP+ Lin− or total BM cells displayed a higher frequency of apoptosis than control EGFP+, whereas sponge miR-9-EGFP+ cells had a slightly decreased apoptosis. These results suggest that miR-9–induced enhanced myeloid differentiation is accompanied by an enhanced apoptosis.
EVI1 Represses miR-9 Expression by Hypermethylation of the Promoter.
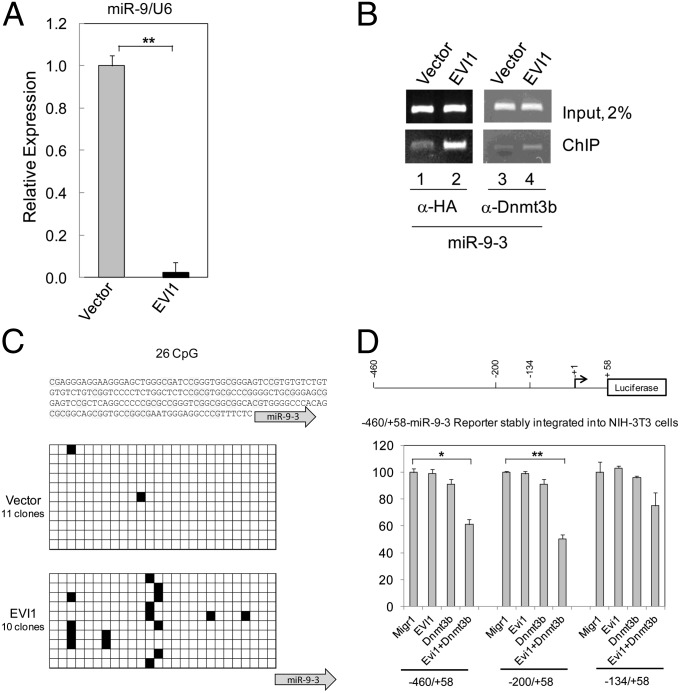
In a previous study, we identified several miRs that are deregulated by EVI1, and found that miR-9 was down-regulated in EVI1+ BM cells by array analysis (36). To confirm this observation, we performed qRT-PCR of miR-9 in EVI1-infected BM cells, and found that miR-9 is indeed suppressed by EVI1 in Lin− BM cells (Fig. 4A). To determine whether EVI1 associates with the regulatory region of miR-9-3, we performed a ChIP assay. 293T cells were transiently transfected with plasmids expressing HA-tagged EVI1 or the empty vector. The nuclear proteins associated with chromatin were immunoprecipitated with anti-HA antibody that recognize the epitope-tagged EVI1 or with anti-DNA (cytosine-5-)-methyltransferase 3-β (Dnmt3b) antibody. As shown in Fig. 4B Lower (lanes 2 and 4), both antibodies efficiently coprecipitate with a DNA fragment located in the regulatory region of miR-9-3, suggesting that EVI1 and Dnmt3b associate with this DNA region.
Fig. 4.
EVI1 down-regulates miR-9 in BM cells through DNA methylation. (A) miR-9 expression in BM cells infected with vector or EVI1 and cultured in vitro for 18 d. The expression of miR-9 was determined by qRT-PCR. Expression of miR-9 is taken arbitrarily as 1 for vector-infected BM cells. (B) ChIP assay with 293T cells transiently transfected with vector or EVI1. (C) miR-9-3 promoter methylation analysis in Lin− BM cells infected with vector or EVI1 and cultured in vitro for 10 d. (Top) The analyzed sequence. (Middle and Bottom) There are 26 CpG dinucleotides within the sequenced stretch of DNA, indicated by black (methylated) or empty (unmethylated) cells. (D) EVI1 synergizes with Dnmt3b to repress the regulatory regions of miR-9-3. (Upper) Diagram of a miR-9-3 luciferase reporter gene. Numbers indicate the nucleotide boundaries of the reporter constructs, and they are numbered with respect to the stem-loop start in the miR-9-3 gene taken as +1 and indicated by the arrow. (Lower) Luciferase reporter assay. NIH 3T3 cells stably transfected with reporter plasmids were transiently cotransfected with effector plasmids as shown.
It was reported that the three miR-9 genes can be aberrantly and preferentially hypermethylated in different malignances leading to their down-regulation (16, 17, 21–23, 25). Because we recently found that EVI1 stimulates de novo DNA methylation in vivo (36, 37), we evaluated the methylation status of the regulatory region of miR-9-3 in BM cells infected with the vector or EVI1. Direct sequencing of bisulfite-converted genomic DNA 10 d after infection clearly showed that EVI1 induces an increase of de novo DNA methylation in this miR-9-3 region. The methylation was significantly higher at the CGGAGTCCG motif (Fig. 4C). The miR-9-3 upstream regulatory region does not contain known EVI1 binding sites close to the CpG-enriched region.
To evaluate the mechanism by which EVI1 induces DNA methylation and the role of the de novo DNA methyltransferase Dnmt3b, we generated several reporter plasmids by inserting fragments of the regulatory region of miR-9-3 (from nucleotides −460, −200, or −134 to nucleotides +58) upstream of the luciferase reporter gene (Fig. 4D, Upper). To avoid artifacts due to transient transfection and to provide a proper chromatin structure for the reporter gene, these plasmids were stably integrated in NIH 3T3 cells. The stably transfected NIH 3T3 cells were used to read the response of the artificial promoter to effector plasmids (empty vector, EVI1, Dnmt3b, and EVI1 + Dnmt3b). The results of the reporter gene assays (Fig. 4D, Lower) show that when EVI1 or Dnmt3b are expressed by themselves, there is no significant repression of the reporter gene. However, when EVI1 is coexpressed with Dnmt3b there is a significant repression of the reporter gene, indicating that EVI1 requires the cooperation of Dnmt3b to down-regulate miR-9-3. This effect is more prominent with the two longer constructs containing the region between nucleotides −460 and −200, and the removal of this region significantly reduces the repression in cells expressing both EVI1 and Dmnt3b. By analogy with results reported for miR-124-3 (37), it is possible that a DNA-methylating complex including EVI1 and DNMT3b could preferentially recognize and bind to this region of miR-9-3 promoter.
miR-9 Reverses the Inhibition of Myeloid Differentiation of EVI1-Positive BM Cells.
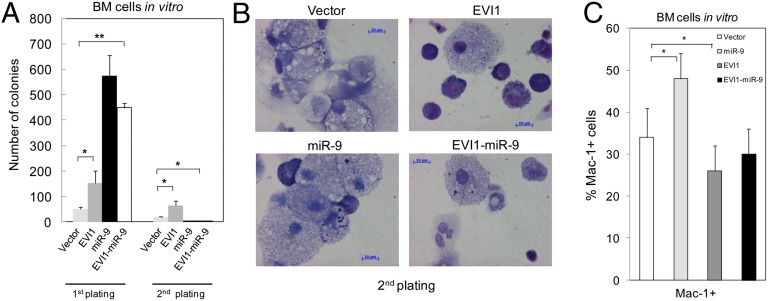
EVI1 delayed or blocked myeloid differentiation in BM primary cells and in a hematopoietic cell line (38, 39). Thus, we hypothesized that miR-9 could partially mediate the inhibition of myeloid cell differentiation induced by EVI1 in BM cells. To confirm this hypothesis, we performed serial replating assays in methylcellulose of Lin− BM cells infected with the empty vector, miR-9-3, EVI1, or EVI1/miR-9-3. In agreement with our previous results, we found that EVI1 delayed myeloid differentiation (Fig. 5 A and B). In contrast, miR-9 accelerated myelopoiesis, indicating that in myelopoiesis, EVI1 and miR-9 have opposite effects. More importantly, we also found that when miR-9 was coexpressed with EVI1, the differentiation of these cells was comparable to the control cells (Fig. 5C). However, miR-9–mediated rescue of myelopoiesis in EVI1-positive cells was not complete, probably because of the deregulation of additional factors by EVI1.
Fig. 5.
miR-9 reverses myeloid cell differentiation inhibited by EVI1. (A) Colony formation assay. (B) Representative cytospins of BM cells after 14 d in methylcellulose culture. (C) Analysis of Mac+ myeloid cells by FACS in BM cells after 7 d in methylcellulose culture. Primary BM cells were infected with vector, miR-9, EVI1, and EVI1/miR-9 retroviruses and cultured in methylcellulose. *P < 0.05.
miR-9 Regulates the Expression of FoxO1 and FoxO3.
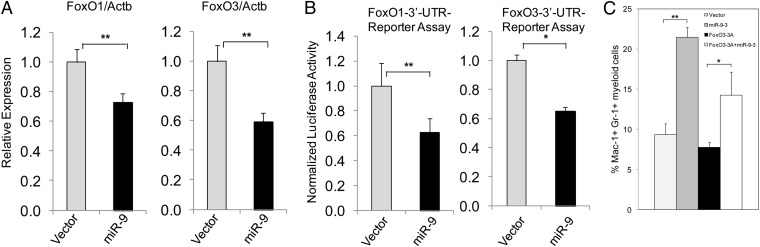
To understand the molecular pathway by which miR-9-3 enhances myelopoiesis, we evaluated the expression of several miR-9 potential targets. This assay was carried out 6 d after infection of Lin− BM cells. There are predicted miR-9 binding sites located on the 3′ UTR region of FOXO1 and FOXO3 genes. In addition, FOXOs are involved in myeloid cell differentiation and leukemogenesis (33, 35). Therefore, we hypothesized that FOXOs may be the critical targets of miR-9 in hematopoietic cells. We determined the expression of FoxO1 and FoxO3 in miR-9–expressing cells by qRT-PCR, and found that these cells had a reduced expression of FoxO1 and FoxO3 compared with control cells (Fig. 6A). To validate these genes as true miR-9 targets, we cloned 583-bp or 477-bp DNA fragments containing the predicted miR-9 binding sites from the 3′ UTR of the murine FoxO1 or FoxO3 into the vector promoter Mir-Report (pMir-Report) and performed reporter gene assays. As shown in Fig. 6B, miR-9 repressed the luciferase activity of the reporter. To determine whether FoxO3 mediates the function of miR-9 in myelopoiesis, we coexpressed miR-9 with a constitutively active form of FoxO3 (FoxO3-3A) in Lin− BM cells in vitro. The percentage of myeloid cells was analyzed by flow cytometry 3 d after infection. The mature myeloid cells (Mac-1+ Gr-1+) were increased about threefold in miR-9–positive BM cells compared with control BM cells; however, these differentiated cells were significantly reduced when miR-9 and FoxO3-3A were coexpressed in BM cells (Fig. 6C; Fig. S5). We found that expression of FoxO3-3A alone did affect the myeloid cell differentiation of BM cells in vitro. These data suggest that the expression of FoxO3-3A suppressed the myeloid cell differentiation induced by miR-9 (Fig. 6C; Fig. S5). By analysis of a published dataset of 82 AML samples (USA-Set-III; ref. 40), after excluding the five MLL leukemia samples, we found a significant positive correlation between EVI1 and FOXO3 expression (r = 0.45, P < 0.0001; Fig. S6).
Fig. 6.
miR-9 down-regulates FoxO1 and FoxO3 genes, and overexpression of FoxO3-3A inhibits myeloid cell differentiation induced by miR-9. (A) qRT-PCR analysis of the FoxO1 and FoxO3 genes’ expression in BM cells infected with vector or miR-9 retroviruses and cultured in methylcellulose for 6 d. (B) miR-9 binds to and represses pMir-Report reporters for both FoxO1 and FoxO3 3′ UTR regions. (C) Diagrams show the summary of FACS analysis of BM cells in vitro. The BM cells were infected with vector, FoxO3-3A, miR-9-3, and FoxO3-3A + miR-9-3 retrovirus. Three days after infection, Lin− cells were isolated, and differentiation of Lin− cells were analyzed 3 d later. The experiments were repeated three times. *P < 0.05; **; P < 0.01.
Discussion
During the past few years, it has become clear that miRNAs have crucial roles in the regulation and maintenance of normal and malignant cells. Several miRNAs have been associated with hematopoietic differentiation, and their deregulation was reported in leukemia (41). Our findings identify miR-9 as a unique player in normal and malignant myelopoiesis. In human, miR-9 is processed from three precursors (miR-9-1, -2, and -3) located on chromosomes 1, 5, and 15, respectively. miR-9-1 and miR-9-2, but not miR-9-3, are expressed in vertebrate brain (42). Here, we report that in hematopoietic cells miR-9-3 is the most abundant isoform, whereas miR-9-2 is virtually undetected. A recent study showed that miR-9-2, miR-9-3, and miR-9-2/3 double-knockout mice displayed different phenotypes (4). Together, these data indicate that the three miR-9 isoforms are activated in a tissue-specific pattern and may have alternative and nonoverlapping functions in different cells. Interestingly, we found that ectopic expression of miR-9 enhances myelopoiesis but inhibits B-cell lymphopoiesis, suggesting that miR-9 may act as a critical regulator in cell fate determination in the development of hematopoietic cells.
How miRNAs are regulated in normal cells and are deregulated in transformed cells is not clear. Whereas it is generally accepted that miRNA’s down-regulation in cancer cells is often a consequence of inappropriate DNA hypermethylation, the molecular pathways leading to DNA hypermethylation are not known. Here we show that EVI1 inhibits miR-9 transcription by hypermethylation of the miR’s promoter. This result suggests that a classic oncoprotein, such as EVI1, in addition to inappropriate interactions with specific transcription factors, can also be involved in DNA methylation, leading to the perturbation of chromatin structure and to the inappropriate deregulation of genes. Our finding also suggests that to maintain normal myelopoiesis, miR-9 expression must be strictly controlled.
A question that arises from our results is which genes are regulated by miR-9 and have a role in myelopoiesis. Each miRNA can target multiple transcripts. The different down-stream targets of miR-9 may contribute to its cell type- or tissue-specific functions. Several potential target genes of miR-9 have been identified in different cell types (7). Here, we demonstrate that miR-9 inhibits the expression of FoxO1 and FoxO3 genes by binding to conserved sites in the 3′ UTR of both genes, indicating that miR-9 is indeed an upstream regulator of FoxO1 and FoxO3. The miR-9 binding sites are conserved in vertebrates, suggesting that the regulatory function of miR-9 on FoxO1 and FoxO3 is not species specific. Previous in vivo studies showed that loss of FoxOs resulted in an expansion of myeloid cells (33). In this study, we showed that overexpression of FoxO3 suppresses myeloid cell differentiation induced by miR-9. Taken together, the data indicate that FoxO3/FoxO1 are critical targets of miR-9 in hematopoietic cells and mediate the function of miR-9 in regulation of myeloid lineage commitment and differentiation.
It was reported that miR-9 could act as an oncogene or tumor suppressor in different contexts. We find that in BM cells miR-9 is down-regulated by EVI1 and that it reduces the EVI1-induced suppression of myeloid cell differentiation, suggesting that miR-9 may act as a tumor suppressor in a subset of myeloid neoplasms expressing EVI1. However, it is possible that miR-9 is also suppressed in some EVI1-negative hematopoietic malignancies through some other factors facilitating de novo DNA methylation.
Materials and Methods
DNA Plasmids.
The EVI1 plasmids used in this study have been described (36). To express murine miR-9-3 and human miR-9-1, 111-bp (miR-9-3) and 441-bp (miR-9-1) PCR fragments, including the stem loops, were obtained by genomic PCR and cloned into XhoI and EcoRI sites of the Migr1 vector. The murine miR-9-3 regulatory region between nucleotides −460 and +58 (where +1 corresponds to the stem-loop start site of the miR) was amplified by genomic PCR and cloned in the promoterless luciferase reporter plasmid pGL4.20 (Promega). The EVI1/miR-9/Migr1 construct was obtained by insertion of a 111-bp PCR fragment containing murine miR-9-3 into the XhoI site of the EVI1/Migr1 plasmid. To obtain the 3′ UTR FoxO1/pMir-Report or 3′ UTR FoxO3/pMir-Report constructs, a 583-bp or a 447-bp PCR fragment containing conserved binding sites for miR-9 was amplified from murine cDNA and cloned in the SacI and HindIII restriction sites of the pMir-Report vector (Applied Biosystems). Sponge miR-9 plasmid was obtained from Addgene (catalog no. 25040). Dnmt3b/Myc plasmid was kindly provided by Chih-Lin Hsien (University of Southern California, Los Angeles). HA-tagged FoxO3-3A/pBabe mutant plasmid was a generous gift from Paul J. Coffer (University Medical Center, Lundlaan, The Netherlands). Whole FoxO3-3A ORF was subcloned into EcoRI/XhoI sites of pMSCVneo vector (Clontech) and a 111-bp miR-9-3 PCR fragment was added into the XhoI site of FoxO3-3A/pMSCV plasmid. All PCR-amplified DNA fragments were verified by DNA sequencing.
Cell Infection and Transfection.
To generate infectious retrovirus particles, we transfected 10–20 µg of plasmid/10-cm plate in the packaging GP2-293T cells (Clontech) with MegaTrans 1.0 reagent (OriGene). DNA transfection of adherent cells was performed by the calcium phosphate precipitation method.
Cell Culture.
The 293T and NIH 3T3 cell lines were maintained as described (37). 32Dcl3 culture and differentiation were described (36).
BM Transplantation.
The isolation and infection of Lin− cells and the reconstitution of irradiated syngeneic recipient mice have been described (43). All of the animal studies were performed in accordance with the guidelines of the Animal Care Committee of the University of Illinois at Chicago.
Colony Formation Assay.
Lin− BM cells were infected with the retroviruses. The GFP-positive cells were sorted by flow cytometry and plated in duplicate in methylcellulose medium (MethoCult M3234; StemCell Technologies) supplemented with murine IL-3, IL-6, GM-CSF, and stem cell factor. The colonies were cultured and counted as described (43).
Flow Cytometry Assays.
Single-cell suspensions from BM were stained with the indicated fluorochrome-conjugated antibodies (BD Bioscience). Flow cytometry was performed at the University of Illinois at Chicago facility using CyAn flow cytometer. For the detection of apoptosis, BM or 32Dcl3 cells were stained with Annexin V (BD Bioscience) and DAPI following the manufacturer’s instructions. All data were analyzed by FlowJo software (TreeStar). For isolation of lineage-specific cell populations by FACS, we used total BM cells for the mature populations or pooled Lin− BM cells from five wild-type mice and proceeded. The subpopulation of hematopoietic stem/progenitor cells, including LSK, CMP, GMP, and MEP, were immunostained and sorted as described previously (44).
qRT-PCR.
Total RNA extracted from BM cells was transcribed into cDNA and analyzed with an ABI 7000 Real-Time PCR System (Applied Biosystems). The primers used include C/ebpα, forward: 5′-gaggattcctgcttcctctc-3′, reverse: 5′-cagactcaaatccccaacac-3′; FoxO1, forward: 5′-tcgtacgccgacctcatca-3′, reverse: 5′-tccttgaagtagggcacgctc-3′, FoxO3, forward: 5′-acttcaaggataagggcgacagca-3′, reverse: 5′-cttcattctgaacgcgcatgaagc-3′; Actb, forward: 5′-gtgacgaggcccagagcaaga-3′, reverse: 5′-ggctggggtgttgaaggtctc-3′; premiR-9-1, forward: cggggttggttgttatctttgg, reverse: gctttatgaagactccacaccac; premiR-9-2, forward: gttgttatctttggttatctagc, reverse: cggttatctagctttatgaagac; premiR-9-3, forward: ggaggcccgtttctctctttg, reverse: gctttatgacggctctgtggc. Mature miR-9 was detected by using TaqMan miR-9 assay and U6 assay as normalization control (Applied Biosystems).
DNA Bisulfite Sequencing.
Lin− BM cells infected with EVI1 or the empty vector were cultured in vitro for 10 d. Genomic DNA was converted with EpiTect Bisulfite Kit (Qiagen). An aliquot of bisulfite-converted DNA was amplified with methylation-insensitive primers, forward: 5′-ygttygaggttgtttaagygtagag-3′; reverse: 5′-aaatcraatacctaatcccracrta-3′, cloned into Pcr4-topo vector (Invitrogen), and sequenced.
Reporter Gene Studies.
The reporter gene assays were performed with wild-type 293T cells (pMir-Report assay) or with NIH 3T3 cells stably transfected with a plasmid expressing the reporter gene under the control of regulatory regions of miR-9-3. The regulatory regions used are nucleotides −460 to +58, −200 to +58, or −134 to +58, where +1 corresponds to the stem-loop start site of miR-9-3. NIH 3T3 cells were transiently transfected with effector plasmids. After 48 h, equal numbers of cells were lysed and analyzed. For the pMir-Report assay, we used Renilla control for reporter readings normalization. All measurements were done in triplicate.
ChIP Assay.
ChIP assays were performed with transiently transfected 293T cells using a SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology) according to the manufacturer’s instructions. The DNA fragments were analyzed by PCR using two primers (5′-aaaccgattgtgccaagca-3′ and 5′-tgattccgggagacccaa-3′) designed to amplify 222 bp of the miR-9-3 regulatory region. We used commercially available monoclonal rat antibody to the HA epitope (Roche) and anti-Dnmt3b monoclonal antibody (Imgenex).
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 CA140979 (to Z.Q.), R01 CA9644 (to G.N.), and HL79580 (to G.N.), and American Cancer Society (Illinois Division) Grant 229105 (to V.S.).
Footnotes
The authors declare no conflict of interest.
The article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302645110/-/DCSupplemental.
References
- 1.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24(4):857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leucht C, et al. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci. 2008;11(6):641–648. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- 4.Shibata M, Nakao H, Kiyonari H, Abe T, Aizawa S. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci. 2011;31(9):3407–3422. doi: 10.1523/JNEUROSCI.5085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16(4):365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaloy C, et al. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6(4):323–335. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuva-Aydemir Y, Simkin A, Gascon E, Gao FB. MicroRNA-9: Functional evolution of a conserved small regulatory RNA. RNA Biol. 2011;8(4):557–564. doi: 10.4161/rna.8.4.16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim N, et al. Expression profiles of miRNAs in human embryonic stem cells during hepatocyte differentiation. Hepatol Res. 2011;41(2):170–183. doi: 10.1111/j.1872-034X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 9.Arora A, et al. Prediction of microRNAs affecting mRNA expression during retinal development. BMC Dev Biol. 2010;10:1. doi: 10.1186/1471-213X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazzoni F, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA. 2009;106(13):5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie K, et al. MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in Hodgkin/Reed-Sternberg cells: A potential pathogenetic lesion in Hodgkin lymphomas. Am J Pathol. 2008;173(1):242–252. doi: 10.2353/ajpath.2008.080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nass D, et al. miR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol. 2009;19(3):375–383. doi: 10.1111/j.1750-3639.2008.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotkrua P, Akiyama Y, Hashimoto Y, Otsubo T, Yuasa Y. MiR-9 downregulates CDX2 expression in gastric cancer cells. Int J Cancer. 2011;129(11):2611–2620. doi: 10.1002/ijc.25923. [DOI] [PubMed] [Google Scholar]
- 14.Myatt SS, et al. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010;70(1):367–377. doi: 10.1158/0008-5472.CAN-09-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, et al. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70(4):1441–1448. doi: 10.1158/0008-5472.CAN-09-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandres E, et al. Epigenetic regulation of microRNA expression in colorectal cancer. Int J Cancer. 2009;125(11):2737–2743. doi: 10.1002/ijc.24638. [DOI] [PubMed] [Google Scholar]
- 17.Heller G, et al. Genome-wide miRNA expression profiling identifies miR-9-3 and miR-193a as targets for DNA methylation in non-small cell lung cancers. Clin Cancer Res. 2012;18(6):1619–1629. doi: 10.1158/1078-0432.CCR-11-2450. [DOI] [PubMed] [Google Scholar]
- 18.Guo LM, et al. MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-kappaB1. FEBS J. 2009;276(19):5537–5546. doi: 10.1111/j.1742-4658.2009.07237.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan HX, et al. MicroRNA-9 reduces cell invasion and E-cadherin secretion in SK-Hep-1 cell. Med Oncol. 2010;27(3):654–660. doi: 10.1007/s12032-009-9264-2. [DOI] [PubMed] [Google Scholar]
- 20.Luo H, et al. Down-regulated miR-9 and miR-433 in human gastric carcinoma. J Exp Clin Cancer Res. 2009;28:82. doi: 10.1186/1756-9966-28-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Otero P, et al. Deregulation of FGFR1 and CDK6 oncogenic pathways in acute lymphoblastic leukaemia harbouring epigenetic modifications of the MIR9 family. Br J Haematol. 2011;155(1):73–83. doi: 10.1111/j.1365-2141.2011.08812.x. [DOI] [PubMed] [Google Scholar]
- 22.Lujambio A, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA. 2008;105(36):13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrandt MA, et al. Hsa-miR-9 methylation status is associated with cancer development and metastatic recurrence in patients with clear cell renal cell carcinoma. Oncogene. 2010;29(42):5724–5728. doi: 10.1038/onc.2010.305. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minor J, et al. Methylation of microRNA-9 is a specific and sensitive biomarker for oral and oropharyngeal squamous cell carcinomas. Oral Oncol. 2012;48(1):73–78. doi: 10.1016/j.oraloncology.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucenski ML, Taylor BA, Copeland NG, Jenkins NA. Chromosomal location of Evi-1, a common site of ecotropic viral integration in AKXD murine myeloid tumors. Oncogene Res. 1988;2(3):219–233. [PubMed] [Google Scholar]
- 27.Testoni N, et al. 3q21 and 3q26 cytogenetic abnormalities in acute myeloblastic leukemia: biological and clinical features. Haematologica. 1999;84(8):690–694. [PubMed] [Google Scholar]
- 28.Brooks DJ, et al. Expression of the zinc finger gene EVI-1 in ovarian and other cancers. Br J Cancer. 1996;74(10):1518–1525. doi: 10.1038/bjc.1996.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Chen L, Ko TC, Fields AP, Thompson EA. Evi1 is a survival factor which conveys resistance to both TGFbeta- and taxol-mediated cell death via PI3K/AKT. Oncogene. 2006;25(25):3565–3575. doi: 10.1038/sj.onc.1209403. [DOI] [PubMed] [Google Scholar]
- 30.Choi YW, et al. Comparative genomic hybridization array analysis and real time PCR reveals genomic alterations in squamous cell carcinomas of the lung. Lung Cancer. 2007;55(1):43–51. doi: 10.1016/j.lungcan.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Haase D, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: Evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–4395. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 32.Buonamici S, et al. EVI1 induces myelodysplastic syndrome in mice. J Clin Invest. 2004;114(5):713–719. doi: 10.1172/JCI21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26(47):6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 35.Sykes SM, et al. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias. Cell. 2011;146(5):697–708. doi: 10.1016/j.cell.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickstein J, et al. Methylation and silencing of miRNA-124 by EVI1 and self-renewal exhaustion of hematopoietic stem cells in murine myelodysplastic syndrome. Proc Natl Acad Sci USA. 2010;107(21):9783–9788. doi: 10.1073/pnas.1004297107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senyuk V, Premanand K, Xu P, Qian Z, Nucifora G. The oncoprotein EVI1 and the DNA methyltransferase Dnmt3 co-operate in binding and de novo methylation of target DNA. PLoS ONE. 2011;6(6):e20793. doi: 10.1371/journal.pone.0020793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morishita K, Parganas E, Matsugi T, Ihle JN. Expression of the Evi-1 zinc finger gene in 32Dc13 myeloid cells blocks granulocytic differentiation in response to granulocyte colony-stimulating factor. Mol Cell Biol. 1992;12(1):183–189. doi: 10.1128/mcb.12.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sitailo S, Sood R, Barton K, Nucifora G. Forced expression of the leukemia-associated gene EVI1 in ES cells: A model for myeloid leukemia with 3q26 rearrangements. Leukemia. 1999;13(11):1639–1645. doi: 10.1038/sj.leu.2401585. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, et al. Up-regulation of a HOXA-PBX3 homeobox-gene signature following down-regulation of miR-181 is associated with adverse prognosis in patients with cytogenetically abnormal AML. Blood. 2012;119(10):2314–2324. doi: 10.1182/blood-2011-10-386235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bissels U, Bosio A, Wagner W. MicroRNAs are shaping the hematopoietic landscape. Haematologica. 2012;97(2):160–167. doi: 10.3324/haematol.2011.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J, et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian Z, et al. Enhanced expression of FHL2 leads to abnormal myelopoiesis in vivo. Leukemia. 2009;23(9):1650–1657. doi: 10.1038/leu.2009.78. [DOI] [PubMed] [Google Scholar]
- 44.Qian Z, Chen L, Fernald AA, Williams BO, Le Beau MM. A critical role for Apc in hematopoietic stem and progenitor cell survival. J Exp Med. 2008;205(9):2163–2175. doi: 10.1084/jem.20080578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.