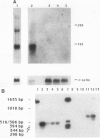
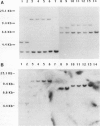
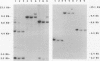
Abstract
A variety of experimental approaches have been devised recently to mutate mammalian genes by homologous recombination. In this report, we describe the disruption of the Hox1.3 locus by using two of these approaches, namely, positive-negative selection and activation of a promoterless gene. Interestingly, we observe similarly high frequencies of targeted disruption with both procedures. The high frequency of targeted disruption with a promoterless vector was unexpected given the extremely low level of Hox1.3 expression in the embryonic stem cell line used for these studies. These data indicate that minimal expression of the target gene is required to enrich for homologous recombination events with promoterless vectors and thus enhance the promoterless gene approach as a general strategy to mutate mammalian genes by homologous recombination.
Full text
PDF


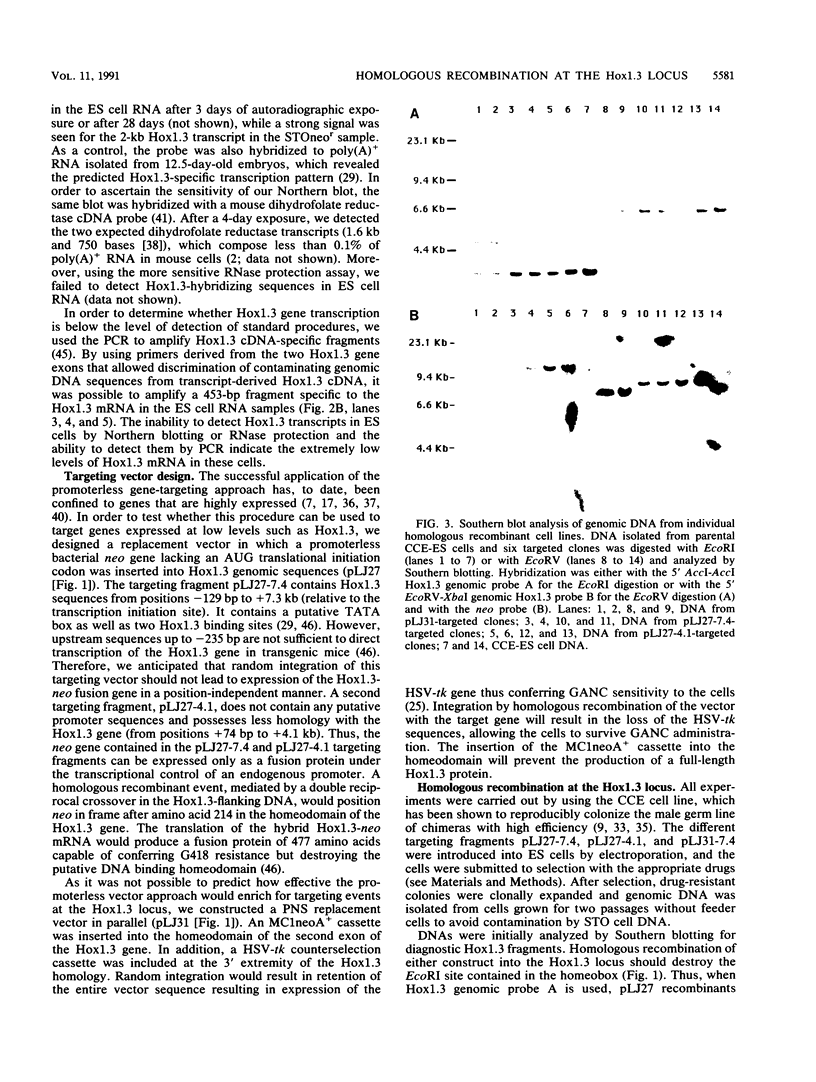



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Nairn R. S., Wilson J. H., Seidman M. M., Brotherman K. A., MacKinnon C., Scheerer J. B. Targeted homologous recombination at the endogenous adenine phosphoribosyltransferase locus in Chinese hamster cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4574–4578. doi: 10.1073/pnas.86.12.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddington R. S., Robertson E. J. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989 Apr;105(4):733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Charron J., Malynn B. A., Robertson E. J., Goff S. P., Alt F. W. High-frequency disruption of the N-myc gene in embryonic stem and pre-B cell lines by homologous recombination. Mol Cell Biol. 1990 Apr;10(4):1799–1804. doi: 10.1128/mcb.10.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Dawid I. B. How to Prepare a Manuscript for PNAS. Proc Natl Acad Sci U S A. 1989 Jan;86(1):1–1. doi: 10.1073/pnas.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara T. M., Efstratiadis A., Robertson E. J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990 May 3;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Doetschman T., Maeda N., Smithies O. Targeted mutation of the Hprt gene in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8583–8587. doi: 10.1073/pnas.85.22.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dony C., Gruss P. Specific expression of the Hox 1.3 homeo box gene in murine embryonic structures originating from or induced by the mesoderm. EMBO J. 1987 Oct;6(10):2965–2975. doi: 10.1002/j.1460-2075.1987.tb02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Jasin M., Berg P. Homologous integration in mammalian cells without target gene selection. Genes Dev. 1988 Nov;2(11):1353–1363. doi: 10.1101/gad.2.11.1353. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Sheng M., Greenberg M. E., Kolodner R. D., Papaioannou V. E., Spiegelman B. M. Targeting of nonexpressed genes in embryonic stem cells via homologous recombination. Science. 1989 Sep 15;245(4923):1234–1236. doi: 10.1126/science.2506639. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Herrup K., Auerbach B. A., Davis C. A., Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991 Mar 8;251(4998):1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R. H., Evans M. J. Changes in protein synthesis during differentiation of embryonal carcinoma cells, and a comparison with embryo cells. J Embryol Exp Morphol. 1980 Oct;59:187–206. [PubMed] [Google Scholar]
- Malicki J., Schughart K., McGinnis W. Mouse Hox-2.2 specifies thoracic segmental identity in Drosophila embryos and larvae. Cell. 1990 Nov 30;63(5):961–967. doi: 10.1016/0092-8674(90)90499-5. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McGinnis N., Kuziora M. A., McGinnis W. Human Hox-4.2 and Drosophila deformed encode similar regulatory specificities in Drosophila embryos and larvae. Cell. 1990 Nov 30;63(5):969–976. doi: 10.1016/0092-8674(90)90500-e. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Odenwald W. F., Taylor C. F., Palmer-Hill F. J., Friedrich V., Jr, Tani M., Lazzarini R. A. Expression of a homeo domain protein in noncontact-inhibited cultured cells and postmitotic neurons. Genes Dev. 1987 Jul;1(5):482–496. doi: 10.1101/gad.1.5.482. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Robertson E. J. Using embryonic stem cells to introduce mutations into the mouse germ line. Biol Reprod. 1991 Feb;44(2):238–245. doi: 10.1095/biolreprod44.2.238. [DOI] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Goff S. P., Robertson E. J. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989 Nov 10;246(4931):799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Robertson E. J., Goff S. P. Targeted gene disruption of the endogenous c-abl locus by homologous recombination with DNA encoding a selectable fusion protein. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3210–3214. doi: 10.1073/pnas.87.8.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedivy J. M., Sharp P. A. Positive genetic selection for gene disruption in mammalian cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Jan;86(1):227–231. doi: 10.1073/pnas.86.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Stanton B. R., Reid S. W., Parada L. F. Germ line transmission of an inactive N-myc allele generated by homologous recombination in mouse embryonic stem cells. Mol Cell Biol. 1990 Dec;10(12):6755–6758. doi: 10.1128/mcb.10.12.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990 Aug 30;346(6287):847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Thompson S., Clarke A. R., Pow A. M., Hooper M. L., Melton D. W. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell. 1989 Jan 27;56(2):313–321. doi: 10.1016/0092-8674(89)90905-7. [DOI] [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Zakany J., Tuggle C. K., Patel M. D., Nguyen-Huu M. C. Spatial regulation of homeobox gene fusions in the embryonic central nervous system of transgenic mice. Neuron. 1988 Oct;1(8):679–691. doi: 10.1016/0896-6273(88)90167-5. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989 Nov 23;342(6248):435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]