Abstract
Pentatransmembrane glycoprotein prominin-1 (CD133) is expressed at the cell surface of multiple somatic stem cells, and it is widely used as a cell surface marker for the isolation and characterization of human hematopoietic stem cells (HSCs) and cancer stem cells. CD133 has been linked on a cell biological basis to stem cell-fate decisions in human HSCs and emerges as an important physiological regulator of stem cell maintenance and expansion. Its expression and physiological relevance in the murine hematopoietic system is nevertheless elusive. We show here that CD133 is expressed by bone marrow-resident murine HSCs and myeloid precursor cells with the developmental propensity to give rise to granulocytes and monocytes. However, CD133 is dispensable for the pool size and function of HSCs during steady-state hematopoiesis and after transplantation, demonstrating a substantial species difference between mouse and man. Blood cell numbers in the periphery are normal; however, CD133 appears to be a modifier for the development of growth-factor responsive myeloerythroid precursor cells in the bone marrow under steady state and mature red blood cells after hematopoietic stress. Taken together, these studies show that CD133 is not a critical regulator of hematopoietic stem cell function in mouse but that it modifies frequencies of growth-factor responsive hematopoietic progenitor cells during steady state and after myelotoxic stress in vivo.
Keywords: 5-fluorouracil, hematopoietic recovery, IL-3 complex, radiosensitivity, CFU-S
Hematopoietic stem cells (HSCs) continuously provide supply of newly generated mature blood cells by asymmetric cell division through a series of cellular intermediates (reviewed in ref. 1). On a cell biological basis, loss of proliferation/differentiation options in one daughter cell is the functional hallmark of asymmetric division, and it was suggested to be associated with nonhomogeneous distribution of proteins during cell division, for instance, in mammalian neural stem cells (2, 3), male germ-line stem cells of the fruit fly Drosophila melanogaster (4), and human HSCs (5).
Prominin-1 (CD133) is a five-transmembrane–spanning cholesterol-binding protein expressed on numerous somatic stem cells notably human HSCs and hematopoietic progenitor cells (HPCs) (6–10) (reviewed in refs. 11, 12). Indeed, CD133 is widely used as a cell surface antigen to prospectively isolate human HSCs that can reconstitute hematopoiesis upon transplantation into mice (13, 14), sheep (9), and humans (15). Besides HSCs derived from cord blood, bone marrow, and apheresis products (13, 14, 16), CD133 is detected on cancer cells from various malignant hematopoietic diseases, including acute and chronic myeloid and lymphoblastic leukemias (reviewed in ref. 17) and solid cancers (18). From a cell biological point of view, CD133 is a unique marker of both plasma membrane protrusions (6, 8) and cholesterol-based membrane microdomains (19, 20) and could be differentially inherited to daughter cells upon cell division as demonstrated in murine neural stem cells (2), human HSCs (11, 12), and human lung and brain cancer cells (21, 22). Furthermore, a link between the asymmetric cell distribution of CD133 and the cellular fate has been elegantly demonstrated in neural stem cells (2). The level of complexity to understand the biological role of CD133 in stem cells has recently increased by the finding that small CD133-containing membrane vesicles can be released from human HSCs and neural stem cells during the differentiation process (23). Irrespective of the cellular mechanisms underlying the decrease or loss of CD133 (24), it has been proposed that CD133-containing membrane microdomains might act as stem cell-specific signal transduction platforms, and their reduction will somehow lead to cellular differentiation (23, 25). In these contexts, whether CD133 itself is important for HSC fate decisions and/or for hematopoiesis in the mouse remains however unknown.
In the present study, we have investigated the influence of CD133 in HSC maintenance and hematopoiesis using wild-type and CD133 knockout (KO) mice (26). The latter animals are viable and fertile but are affected with a retinal degeneration leading to blindness (26). No obvious hematopoietic defects were reported in CD133 KO mice, although this issue was not investigated vigorously (26). Here, we demonstrated that CD133 is indeed expressed by mouse HPCs but that HSC purification based on CD133 protein is not possible, suggesting a substantial species difference for the role of CD133 on HSCs. Further, HSC function under steady state and after transplantation is independent of CD133 expression. Nevertheless, CD133 is a modifier for the proper development of growth factor-responsive myeloid progenitor cells during steady state and of mature red blood cells after myelotoxic stress in vivo.
Results
CD133 Is Expressed by Murine HSCs and Granulocyte Monocyte Progenitor Cells.
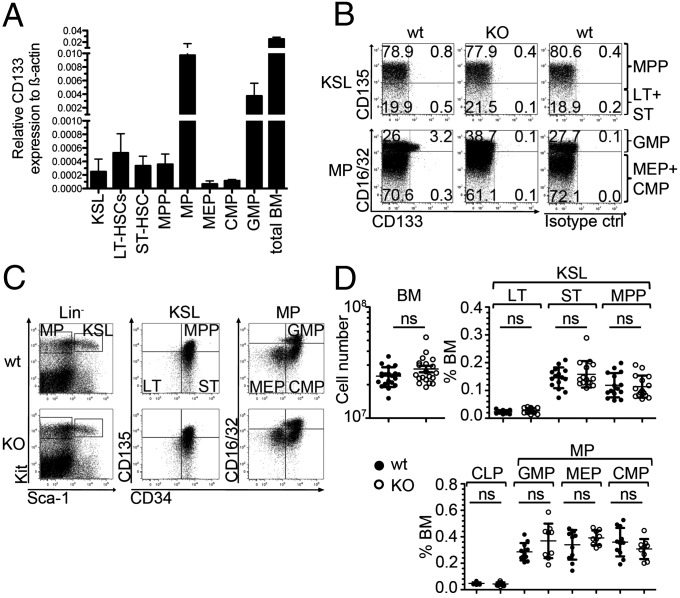
To decipher the role of CD133 in mouse HSC biology and hematopoiesis we first documented its gene expression by quantitative PCR in progenitor cells. CD133 transcripts were strongly expressed in total bone marrow cells and, to a lower level, in HSC-containing Kit+Sca-1+Lineage– (KSL) cells (Fig. 1A). The fractionation of KSL cells into long-term (LT) and short-term (ST) HSCs and multipotent progenitor cells (MPPs) (Fig. S1) (27–29) revealed that all subsets contained low levels of CD133 transcripts (Fig. 1A). Likewise, precursor cells restricted to differentiate into myeloid cells, myeloid progenitors (MPs), were separated into megakaryocyte erythroid progenitors (MEPs), common MPs (CMPs), and granulocyte monocyte progenitors (GMPs) (30), out of which CD133 was mainly expressed in GMPs and at a low level in CMPs and MEPs. In agreement with the mRNA expression levels, we detected the cell surface expression of CD133 protein mainly on GMP cells (Fig. 1B). To investigate a potential role of CD133 in the function of hematopoietic stem and progenitor cells, we analyzed frequencies and numbers of HSCs/HPCs in mice deficient for CD133 (26) and wild-type controls (Fig. 1 C and D). Frequencies of KSL cells and further subpopulations, and myeloid or lymphoid progenitor cells were identical in wild-type and CD133 KO mice. In conclusion, cell surface expression of CD133 was exclusively detected on GMPs but seemed not to be required for the development of their normal cell numbers.
Fig. 1.
Expression of CD133 on mouse HSCs/HPCs and its effects on stem or progenitor cell numbers. (A) Quantitative expression analysis of CD133 by indicated purified cell populations (KSL, LT-HSC, ST-HSC, MPP, MP, MEP, CMP, GMP) or total bone marrow (BM) cells from wild-type mice. Results show mean ± SD of CD133 transcript expression relative to transcripts of β-actin (Actb) (n = 2). (B) Dot plots show the expression of CD133 on KSL (Upper) or MP (Lower) of wild-type (wt) (Left) and CD133 KO (KO) (Center) mice. Staining of the isotype control antibody on wild-type cells is depicted (Right). KSL population is further resolved discriminating between MPPs and ST-HSCs (ST) plus LT-HSCs (LT) (Upper), and the MP population is further resolved discriminating between GMP and MEP plus CMP (Lower). Data are representative for eight wild-type and eight CD133 KO mice analyzed in two independent experiments. In all wild-type mice, CD133 cell surface expression was only detected in a fraction of GMPs (2.7 ± 0.9%, n = 8). (C) Dot plots show the expression of Kit versus Sca-1 on lineage negative cells (Lin−) (Left), CD135 versus CD34 on KSL cells (Center), and CD16/32 versus CD34 on MPs from wild-type (wt) (Upper) and CD133 KO (KO) (Lower) mice. Data are representative for 15 mice per genotype analyzed in three independent experiments for KSL, and 11 wild-type and 9 CD133 KO mice analyzed in two independent experiments for MP analysis. Data are quantified in D. (D) Plots show the numbers of BM cells and frequencies of LT-HSCs (LT), ST-HSCs (ST), and MPPs or common lymphoid progenitors (CLPs) and MPs separated into MEPs, CMPs, and GMPs in wild-type mice (wt) (closed circles) and CD133 KO mice (KO) (open circles) as shown in C. Data are pooled from three independent experiments using 20 mice per genotype for BM cell numbers, 15 mice per genotype for KSL analysis, and 11 wild-type and 9 CD133 KO mice for CLP and MP analysis. Results represent means ± SD. ns, not significant.
Functionality of LT-HSCs Is Independent of CD133 Expression.
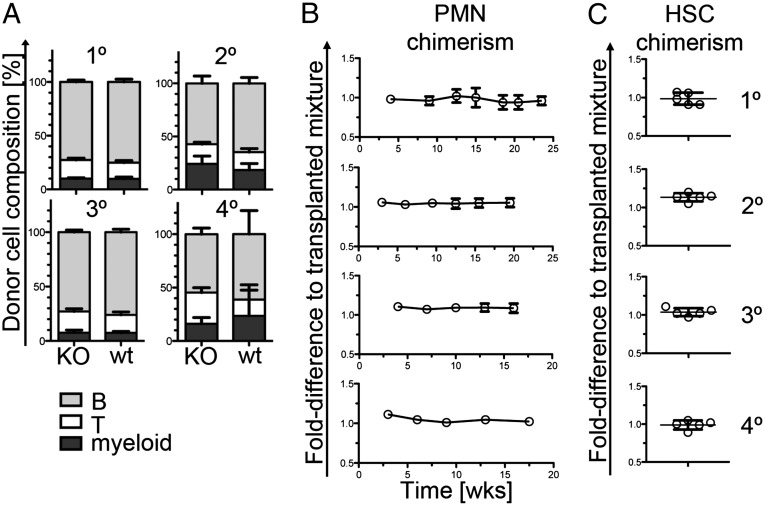
To determine whether lack of CD133 protein has functional consequences in hematopoietic compartments, we performed a series of transplantation experiments. First, CD133 KO or wild-type lineage-depleted (Lin−) bone marrow cells were transplanted together with competitor wild-type Lin− bone marrow cells into five consecutive recipient mice, and their engraftment was monitored over time (Fig. 2). Donor-cell composition at late time points (15–18 wk) after transplantation was the same from CD133 KO or wild-type HSCs throughout the engraftment in primary, secondary, tertiary, and quaternary recipient mice (Fig. 2A). Also, contribution of CD133 KO donor cells to blood neutrophils, a compartment with a high turnover rate that closely reflects the cellular composition of the HSC compartment (31) stayed constant over the series of the four successive transplantations (Fig. 2B). Analysis of the HSC compartment in recipients at the time point of analysis confirmed constant ratio of wild-type and CD133 KO cells (Fig. 2C), indicating that lack of CD133 is dispensable for normal HSC function and expansion after transplantation.
Fig. 2.
CD133-deficient HSCs can competitively and serially reconstitute immune cells and the HSC compartment of irradiated recipient mice. (A) Bars show the composition of graft-derived leukocytes (CD3+ T cells, B220+ B cells, and CD11b+ myeloid cells) in the blood of primary (1°), secondary (2°), tertiary (3°), and quaternary (4°) recipient mice 15, 15.5, 16, and 17.5 wk after transplantation, respectively. Lin– bone marrow cells of CD133 KO or wild-type mice were mixed with Lin– wild-type competitor cells and transplanted into irradiated wild-type recipient mice. All genotypes were identified using antibodies specific for different CD45 isotypes. Five replicate recipient mice for either condition were analyzed. Results represent means ± SD. A significant difference was found between T-cell frequencies in quaternary recipients (P = 0.014). (B) Plots show the fold difference of the ratio of the relative contribution of CD133 KO and wild-type cells to blood neutrophils (PMN). Data are presented as fold difference to the initially transplanted mix of wild-type and CD133 KO HSCs over time. Results show means ± SD of five replicate mice. No statistically significant differences were obtained. (C) Plots show the fold-difference of the ratio of the relative contribution of CD133 KO or wild-type competitor cells to the HSC compartment (KSL) in the bone marrow at the time point of analysis. Data from all replicate mice are shown. Time points of analysis after transplantation were as follows: primary recipients, 24 wk; secondary recipients, 20 wk; tertiary recipients, 16 wk; quaternary recipients, 17.5 wk.
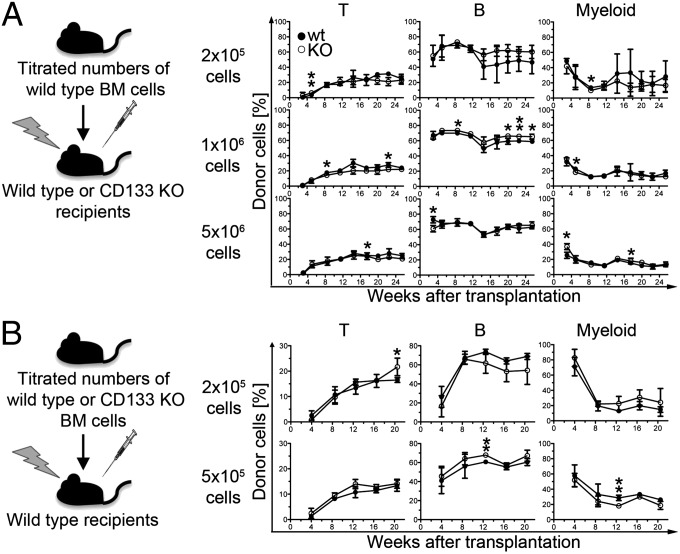
Second, we aimed at determining whether lack of CD133 affects the functionality of the stem cell niche because human CD133-positive stromal cells were suggested to support human HSC maintenance (32). To this end, we transplanted wild-type bone marrow cells into lethally irradiated CD133 KO or wild-type mice and analyzed quality and quantity of hematopoietic reconstitution (outlined in Fig. 3A, Left). Donor-cell composition was not affected by lack of CD133 in the recipient mice (Fig. 3A, Right), and the engraftment of wild-type HSCs was comparable in wild-type or CD133 recipient mice (Fig. S2 A and B). Consistently, donor-cell contribution after competitive transplantation into secondary recipients revealed no negative effect by the passage of wild-type cells through a CD133-deficient environment in the primary recipient mice (Fig. S2C).
Fig. 3.
Graft composition is independent of CD133 on donor or recipient cells. (A) Outline of the experiment (Left): titrated numbers of wild-type bone marrow cells were transplanted into irradiated wild-type or CD133 KO mice and the composition of donor leukocytes monitored over time (Right). Percentages of wild-type–derived (closed circles) or CD133 KO-derived (open circles) T cells (left plot), B cells (center plot), and myeloid cells (right plot) are depicted over time for each donor cell number. At each time point data from two (donor cell number: 2 × 105) or three (donor cell number: 1 × 106 and 5 × 106) recipient mice was pooled. Significant differences were indicated. *P = 0.05–0.01; **P = 0.01–0.001. (B) Titrated numbers of wild-type or CD133 KO bone marrow cells were transplanted into irradiated wild-type recipients. Composition of donor cells in recipient mice that had received 2 × 105 (Upper) or 5 × 105 (Lower) bone marrow cells is depicted as described in A. At each time point, data from four recipients of wild-type cells and two recipients of CD133 KO cells (2 × 105 donor cells) or data from three recipients of wild-type cells and four recipients of CD133 KO cells (5 × 105 donor cells) are shown. Significant differences indicated as described in A.
Third, to evaluate a potential role of CD133 in HSC regeneration, we transplanted titrated numbers of CD133 KO or wild-type cells into lethally irradiated wild-type mice and followed the reconstitution of T, B, and myeloid cell types over time (Fig. 3B). Our analysis revealed no evidence for any functional defect in CD133-deficient HSCs even when a lower number of donor cells was used (Fig. 3B). Taken together, we conclude that CD133 expression is dispensable for normal LT-HSC function.
In agreement with the proper homing of transplanted CD133 KO cells into the bone marrow niche, knocked-down CD133 in primary human HSCs/HPCs revealed no impairment in their migrational behavior. In agreement with previous analysis (33), the RNA interference-mediated knockdown of CD133 in human (h) HSCs showed that CD133 is dispensable for both the morphological polarization and migration of these cells (Fig. S3). Upon CD133 knockdown, hHSCs still exhibited various types of plasma membrane protrusions, including a uropod and leading edge, two typical structures observed in migrating HSCs (Fig. S3) (34). Functionally, their migration is indistinguishable to the control samples, as monitored by time-lapse video microscopy. Thus, in human, just like in the mouse, the migration of HSCs did not rely on CD133.
Lack of CD133 Results in Reduced Frequencies of Growth Factor-Responsive Myeloerythroid Precursor Cells in Vitro.
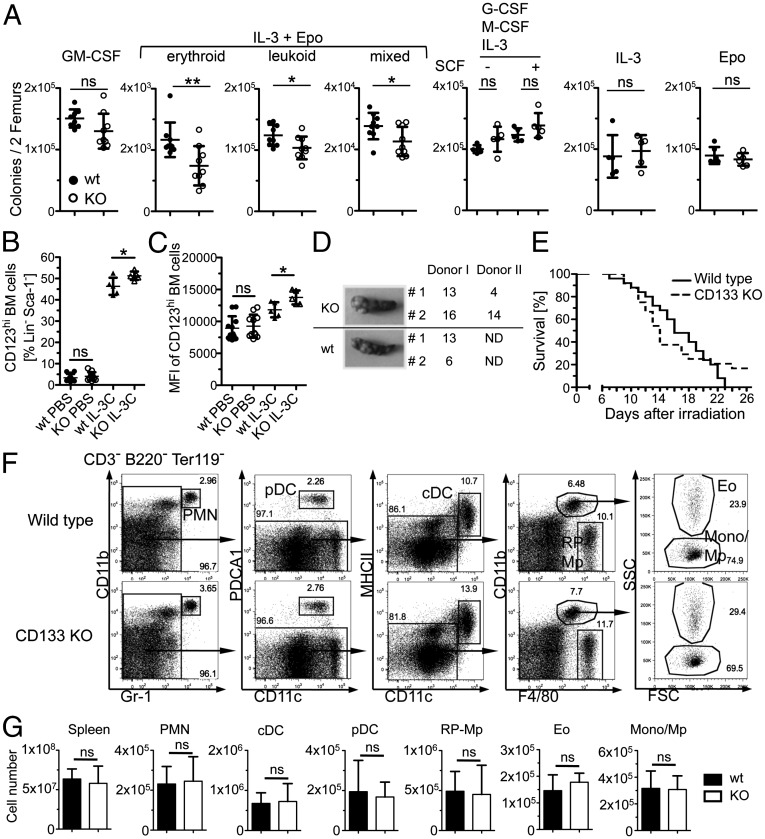
To evaluate whether lack of CD133 expression on GMPs affects frequencies of functional myeloid progenitor cells in the bone marrow, we analyzed in vitro colony formation in response to GM-CSF, IL-3 plus erythropoietin (Epo), G-CSF plus M-CSF plus IL-3 with and without stem cell factor (SCF), and IL-3 or Epo alone (Fig. 4A). We found decreased progenitor frequencies in the bone marrow of CD133 KO mice in response to IL-3 plus Epo but not after stimulation with other cytokines or combinations thereof (Fig. 4A), suggesting that CD133 is a modifier of the function of early myeloerythroid progenitors. Lack of CD133 had no effect on frequency and density of IL-3 or Epo receptor expression on bone marrow cells under steady-state conditions or on the signaling pathways or the induction of target genes after in vitro stimulation with Epo or IL-3 (Fig. S4). To address whether lack of CD133 has effects on in vivo responses to cytokine stimulation, we injected IL-3 complexes and analyzed hematopoietic progenitor frequencies in the bone marrow. Increased numbers of mature basophils and basophil progenitors, GMPs, and CMPs confirmed the effectiveness of IL-3 complex injections (Fig. S5). However, the response of these cell types was identical in wild-type and CD133 KO mice. In contrast, we found an increase in the frequency of bone marrow progenitors that expressed high levels of the IL-3 receptor (Fig. 4B and Fig. S4C) and, additionally, an increased density of IL-3 receptors on a per cell basis on cells of CD133 KO mice (Fig. 4C). These findings suggest that malfunctioning synergism between IL-3 and Epo receptor causes reduced colony formation in vitro and, correspondingly, an accumulation of IL-3 receptor-expressing precursor cells in the bone marrow.
Fig. 4.
Changes in in vitro colony formation and IL-3 receptor expression but no effect on CFU-S formation, sensitivity to γ irradiation, and the pool size of mature myeloid populations. (A) Plots show in vitro colony formation after culture of wild-type or CD133 KO bone marrow cells with GM-CSF, IL-3 plus erythropoietin (Epo), G-CSF plus M-CSF plus IL-3 with and without SCF, IL-3, or Epo (from left to right). Plots show means ± SD of nine mice per genotype (GM-CSF, IL-3+Epo), six mice per genotype (Epo), and five mice per genotype (G-CSF+M-CSF+IL-3± SCF, IL-3). Significant differences were indicated. *P = 0.05–0.01; **P = 0.01–0.001. (B) Plot shows frequency of CD123hi cells in Lin− Sca-1− bone marrow cells with or without in vivo stimulation with IL-3 complexes (IL-3C). Results show means ± SD of 11 or 5 mice per genotype for PBS control or IL-3 complex injections, respectively. (C) Plot shows the mean fluorescence intensity (MFI) of CD123 expression on CD123hi cells. Data presentation as described in B. (D) Macroscopically visible colonies in the spleen of recipient mice were counted 8 d [(CFU-S day 8 (CFU-Sd8)] after transplantation of CD133 KO or wild-type bone marrow (BM) cells. Photographs show one representative recipient spleen from each donor. (E) Survival curve of wild-type (solid line) and CD133 KO (broken line) mice after lethal irradiation. Data are pooled from three independent experiments using a total of 25 wild-type or CD133 KO mice. (F) Dot plots show the expression of CD11b vs. Gr-1, PDCA1 vs. CD11c, MHCII vs. CD11c, CD11b vs. F4/80, and side scatter (SSC) vs. forward scatter (FCS) (from left to right) on spleen cells from wild-type (Upper) and CD133 KO (Lower) mice on CD3-, B220-, and Ter119-negative cells. Arrows indicate the population that was further resolved in the following dot plot. cDC, conventional dendritic cells; Eo, eosinophils; Mono/Mp, monocytes/macrophages; pDC, plasmacytoid dendritic cells; PMN, neutrophils; RP-Mp, red pulp macrophages. Data are summarized in E. (G) Graphs show the cell number of spleen cells, PMNs, cDCs, pDCs, RP-Mps, eosinophils, and monocytes/macrophages (from left to right) in wild-type (closed bar) and CD133 KO (open bar) mice identified as shown in C. Means ± SD are given (n = 6 mice per genotype).
LT-HSC function was independent from prominin-1 (Prom1, CD133) gene expression, and we, therefore, aimed to analyze the functionality of early hematopoietic precursor cells beyond the HSC stage preceding the GMP stage. The capacity to form macroscopically visible spleen colonies after injection into irradiated wild-type mice [colony-forming unit-spleen (CFU-S)] has been mainly assigned to these developmental stages (28, 35, 36), and, therefore, we compared CFU-S formation from CD133 KO and wild-type bone marrow cells (Fig. 4D). We found no differences in colony formation between the bone marrow of test and control mice and conclude that CD133 expression is dispensable for the function of early hematopoietic progenitor cells. Consistently, CD133 KO and wild-type mice showed no differences in the rate and kinetic of death after lethal irradiation (radiosensitivity; Fig. 4E).
To analyze whether adaptive mechanisms compensate for the loss of CD133 in the adult mouse, we analyzed fetal hematopoiesis at embryonic day 14.5 (Fig. S6). We analyzed fetal liver cellularity, frequencies of hematopoietic stem and progenitor cells, in vitro colony formation, and the expression of key genes in red blood cell development but found no evidence for an effect of CD133 deficiency in fetal hematopoiesis.
To further analyze whether the constitutive knockout suppresses the biological relevance of CD133, we knocked down CD133 in primary murine HSCs/HPCs. Surprisingly, using three of four shRNAs, the knockdown of CD133 in murine HSCs/HPCs revealed an increase in colony-forming frequencies (Fig. S7), suggesting that an acute manipulation of CD133 expression interferes with the responsiveness of precursor cells. In a full knockout, a mechanism of adaption may operate as also suggested for similar experimental discrepancies found for the role of N-cadherin in HSC regulation (37, 38).
We conclude that CD133 expression modifies normal function of erythromyeloid precursor cells in the adult murine bone marrow in the steady state. However, CD133 is dispensable for fetal hematopoiesis and for numbers and function of adult progenitor cells preceding the GMP stage.
Normal Numbers of Mature Myeloid Cells in the Spleen of CD133 KO Mice.
Next, we analyzed whether the reduced frequencies of IL-3-plus-Epo–responsive progenitor cells results in a decreased production of mature myeloid and erythroid cells under steady-state conditions. Frequencies and absolute numbers of mature myeloid populations (neutrophils, eosinophils, conventional dendritic cells, plasmacytoid dendritic cells, red pulp macrophages, and monocytes) were normal in the spleen (Fig. 4 F and G) of CD133 KO mice compared with wild-type mice. Furthermore, leukocytes and red blood cell parameters were comparable between wild-type and CD133 KO mice (Table S1). We deduce that functional differences between GMP progenitor cells of wild-type and CD133 KO mice are compensated in vivo and that CD133 has redundant functions during steady-state hematopoiesis.
Compromised Recovery from Hematopoietic Insult in the Absence of CD133.
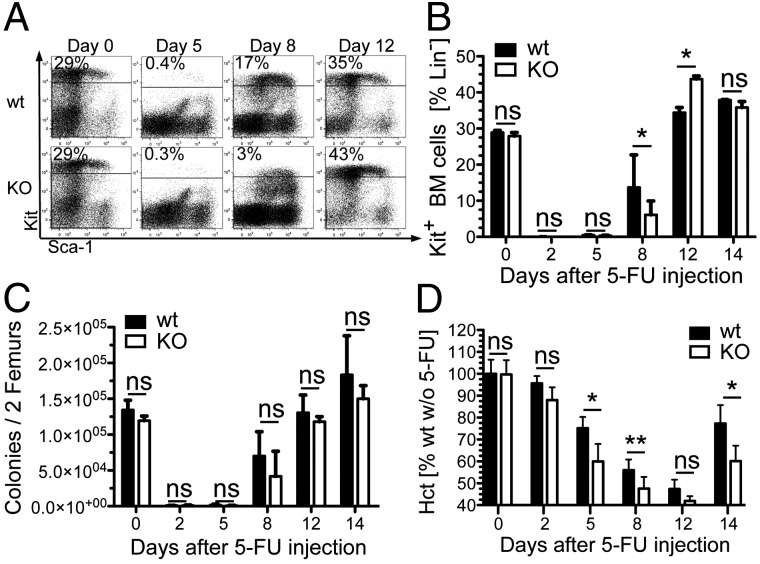
Reduced frequencies of growth factor-responsive myeloid precursor cells suggest that CD133 may play a role during stress hematopoiesis and, thus, in situations where a large number of hematopoietic cells are generated in response to a massive depletion of progenitors or mature cells (39). To analyze for a potential role of CD133 in hematopoietic stress response we injected 5-fluorouracil (5-FU) and analyzed the recovery kinetic (Fig. 5). Cycling progenitor cells are depleted by 5-FU treatment and hematopoiesis is restored from noncycling LT-HSCs (39) that transiently lose the expression of the Kit receptor on the cell surface (40). Recovery of Kit-positive bone marrow cells was mildly affected in the absence of CD133 compared with wild-type mice (Fig. 5A). Eight days after 5-FU injection, ∼17% of all immature bone marrow cells expressed the Kit receptor in wild-type mice, whereas only ∼3% of the lineage-negative compartment in CD133 KO mice expressed Kit, suggesting that the recovery of precursor cells was delayed (Fig. 5B). To assess whether the frequencies of growth-factor responsive progenitors was also altered under stress conditions, we analyzed colony growth in response to IL-3 plus Epo on different days after 5-FU injection and found that paucity of Lin−Kit+ cells at day 8 was not accompanied by reduced frequencies of myeloid progenitor cells (Fig. 5C). However, reduced frequencies of immature bone marrow cells had a functional consequence on the recovery kinetic of red blood cells shown by the delayed recovery of the hematocrit in CD133 KO mice compared with controls (Fig. 5D). The recovery kinetic of mature myeloid cells was not affected (Fig. S8). We conclude that CD133 delays the normal recovery of hematopoietic insult after myeloablation.
Fig. 5.
CD133 KO mice have a compromised recovery after myelotoxic stress in vivo. (A) Dot plots show the frequency of Kit and Sca-1 cell surface expression on Lin– bone marrow cells from wild-type (Upper) and CD133 KO mice (Lower) at the indicated time point after injection of 5-FU. Data are representative for 2 (day 0, 5, and 12) and 13 (day 8) mice per genotype. Three independent experiments were performed, and the data from all mice are summarized in B. (B) Plot shows the frequency of Kit+ bone marrow cells in the Lin– compartment of wild-type (solid bars) and CD133 KO (open bars) mice at the indicated time points after injection of 5-FU. Mean and SD are given [n = 2 (day 0, 2, 5, 12, and 14) or n = 13 (day 8) mice per genotype]. *P = 0.05–0.01; **P = 0.01–0.001. Data are pooled from three independent experiments as outlined in A. (C) Colony numbers per two femurs from wild-type (closed bars) or CD133 KO (open bars) mice in methylcellulose-containing medium supplemented with IL-3 and Epo at the indicated time points after 5-FU injection are shown. Data presentation and mice analyzed are as described in B. (D) Plot depicts the hematocrit (Hct) calculated as percentage from the average Hct of wild-type mice without 5-FU at the indicated time points after 5-FU injection. Data of wild-type (closed bars) or CD133 KO (open bars) mice from two independent experiments were pooled. Means ± SD are given [n = 9 (day 0 and 8), n = 4 (day 2 and 5), and n = 5 wild-type and n = 4 CD133 KO (day 12 and 14) mice per genotype].
Discussion
The molecular and cellular characterizations of the murine CD133 antigen in the hematopoietic bone marrow compartment highlight four findings. First, the expression of CD133 in murine hematopoietic progenitor cell types is detectable at low levels but apparently more highly expressed in GMPs. Second, CD133 expression is dispensable for HSC function during steady state and during stress hematopoiesis. Third, CD133 is required for the generation of normal frequencies of growth factor-responsive cells under steady-state conditions. Fourth, the lack of CD133 on hematopoietic cells results in a delayed recovery of precursor cells and, as a functional consequence, also of mature red blood cells in response to myelotoxic stress.
Although the expression of CD133 by human HSCs is recognized including the therapeutic relevance of bone marrow-derived CD133 positive cells, very little is known about its counterpart in the murine system (8, 41). We showed here that CD133 is expressed by mouse HSCs and HPCs. However, HSC functions, including engraftment to bone marrow niche and fate choices between self-renewing and differentiation divisions, seem to be independent of CD133. Indeed, to increase the selection and proliferation pressure for a rigorous evaluation of HSC potential, we have performed (i) serial competitive transplantation experiments into four consecutive recipient mice and (ii) transplantations with titrated numbers of CD133 KO and wild-type donor cells; to assay for a role of CD133 in HSC microenvironment, we have used CD133-deficient mice as recipients for wild-type cells that were subsequently transplanted into secondary recipient mice. Surprisingly, CD133-deficient HSCs showed the same reconstitution capacity compared with wild-type ones, and the passage through a CD133-deficient microenvironment had no effect on the functionality of HSCs, suggesting that maintenance and function of LT-HSCs is independent of CD133. Such a conclusion is in line with the fact that no obvious hematopoietic defect is observed in individuals affected with recessive or dominant mutations in PROM1 gene (42–44) and also with the results obtained by knockdown approaches of CD133 in human HPCs indicating that CD133 is not fully required for their migration, hence their homing and colony formation [our data (33)].
The normal hematopoiesis in CD133 KO mice might simply be explained by the lack of CD133 protein expression at the cell surface of normal HSCs. However, such conclusion needs to be interpreted with caution. Although the expression of Prom1 gene is observed at a transcriptional level by a sensitive method such as PCR, the absence of CD133 protein as monitored by flow cytometry might reflect numerous phenomena that are not mutually exclusive. In addition to a low level of expression and/or even the lack of translation, we could not exclude, for instance, an intracellular localization (e.g., endosomal compartment) of CD133 as recently reported in human HSCs and HPCs (23). Although the 13A4 epitope of mouse CD133 is independent of N-linked sugar moieties (6), in contrast to the AC133 epitope of human CD133 (45), it could not be ruled out that it became embedded in cholesterol-based membrane microdomains impeding its immunodetection (reviewed in ref. 46). The direct interaction of mouse and human CD133 with membrane cholesterol is consistent with such scenario (19, 47). Similarly, a fast turnover of CD133 at the cell surface might also lead to false negative, or its translocation to an internal pool and/or release by means of small membrane vesicles might account for such situation (23). Irrespective of its biological reasons, the lack of CD133 protein on the cell surface of murine HSCs, and lack of functional consequences on murine hematopoiesis in its absence, marks a substantial species difference between mouse and human and adds CD133 to the list of cell surface markers and cell-fate regulators that are not conserved across species (reviewed in ref. 48).
Myelotoxic stress induced, for instance, by the injection of 5-FU increases the rate and frequency of dividing HSCs/HPCs, resulting in an excessive rebound reaction (39). The 5-FU injection into CD133 KO mice resulted after 8 d in a significant reduction of phenotypic HPCs in the bone marrow by comparison with the control wild-type animals. As a consequence, recovery of mature red blood cells was delayed in CD133 KO mice. Such information highlights the possibility that CD133 is indeed a discrete modulator of HSCs/HPCs, which is revealed under the provoked hematopoiesis where dividing stem and progenitor cells became suddenly active. Furthermore, and in line with this interpretation we find apparent differences in proliferative responses between adult wild-type cells where CD133 was knocked down and in the same cells from a constitutive CD133-deficient animal. Discrepancies between the phenotypes of knockdown and constitutive knockout approaches have been reported before (37, 38) and can be explained by compensatory other molecules that may have masked the effects of CD133 deficiency in vivo. In our case, the finding also suggests that a threshold of CD133 expression levels might influence the balance of cell division as it has been reported previously for ES cells (49). A certain link between the expression of CD133 and status of cellular proliferation seems to exist and may explain the general expression of CD133 in numerous cancer stem cells originating from various organ systems.
In conclusion, mouse CD133 specifically modifies the red blood cell recovery kinetic after hematopoietic insults. Despite reduced precursor frequencies in the bone marrow, frequencies and absolute numbers of mature myeloid cell types in the spleen were normal during steady state, suggesting that the deficit in generating progenitor cell numbers can be overcome at later time points during differentiation and that other pathways regulating later stages of mature myeloid cell formation can compensate for the lack of CD133. Thus, CD133 plays a redundant role in the differentiation of mature myeloid cell compartments during steady state mouse hematopoiesis but is important for the normal recovery of red blood cells under hematopoietic stress.
Materials and Methods
C57BL/6 (B6), and B6.SJL-PtprcaPep3b/BoyJ (B6.SJL) mice were purchased (The Jackson Laboratory) and CD133 KO mice were generated and made congenic on C57BL/6JOlaHsd background (N11) as described (26). Mice were kept under specific pathogen-free conditions in the animal facility at the Medical Theoretical Center of the University of Technology Dresden. Experiments were performed in accordance with German animal welfare legislation and were approved by the relevant authorities, the Landesdirektion Dresden. Details on transplantation procedures, 5-FU treatment, colony assays and flow cytometry, expression analysis, and statistical analysis are given in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Piontek and S. Böhme for expert technical assistance. We thank W. B. Huttner and A.-M. Marzesco for supplying animals. We thank M. Bornhäuser for blood samples for HSC isolation and primary mesenchymal stromal cells, and A. Muench-Wuttke for automated determination of mouse blood parameters. We thank F. Buchholz for providing shRNA-containing transfer vectors directed against mouse CD133. C.W. is supported by the Center for Regenerative Therapies Dresden and Deutsche Forschungsgemeinschaft (DFG) Grant Sonderforschungsbereich (SFB) 655 (B9). D.C. is supported by DFG Grants SFB 655 (B3), Transregio 83 (6), and CO298/5-1. The project was further supported by an intramural CRTD seed grant. The work of P.C. is supported by long-term structural funding: Methusalem funding from the Flemish Government and by Grant G.0595.12N, G.0209.07 from the Fund for Scientific Research of the Flemish Government (FWO).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215438110/-/DCSupplemental.
References
- 1.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosodo Y, et al. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23(11):2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461(7266):947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng J, et al. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456(7222):599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: Identification of asymmetrically segregating proteins. Blood. 2007;109(12):5494–5501. doi: 10.1182/blood-2006-11-055921. [DOI] [PubMed] [Google Scholar]
- 6.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94(23):12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freund D, et al. Comparative analysis of proliferative potential and clonogenicity of MACS-immunomagnetic isolated CD34+ and CD133+ blood stem cells derived from a single donor. Cell Prolif. 2006;39(4):325–332. doi: 10.1111/j.1365-2184.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbeil D, et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275(8):5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 9.Yin AH, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 10.Miraglia S, et al. A novel five-transmembrane hematopoietic stem cell antigen: Isolation, characterization, and molecular cloning. Blood. 1997;90(12):5013–5021. [PubMed] [Google Scholar]
- 11.Fargeas CA, Fonseca AV, Huttner WB, Corbeil D. Prominin-1 (CD133): From progenitor cells to human diseases. Future Lipidol. 2006;1(2):213–225. [Google Scholar]
- 12.Bauer N, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133) Cells Tissues Organs. 2008;188(1-2):127–138. doi: 10.1159/000112847. [DOI] [PubMed] [Google Scholar]
- 13.de Wynter EA, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16(6):387–396. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]
- 14.Hess DA, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107(5):2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bornhäuser M, et al. Rapid reconstitution of dendritic cells after allogeneic transplantation of CD133+ selected hematopoietic stem cells. Leukemia. 2005;19(1):161–165. doi: 10.1038/sj.leu.2403563. [DOI] [PubMed] [Google Scholar]
- 16.Ito CY, et al. The AC133+CD38-, but not the rhodamine-low, phenotype tracks LTC-IC and SRC function in human cord blood ex vivo expansion cultures. Blood. 2010;115(2):257–260. doi: 10.1182/blood-2009-07-228106. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia M. AC133 expression in human stem cells. Leukemia. 2001;15(11):1685–1688. doi: 10.1038/sj.leu.2402255. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 19.Röper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2(9):582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 20.Giebel B, et al. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104(8):2332–2338. doi: 10.1182/blood-2004-02-0511. [DOI] [PubMed] [Google Scholar]
- 21.Pine SR, Ryan BM, Varticovski L, Robles AI, Harris CC. Microenvironmental modulation of asymmetric cell division in human lung cancer cells. Proc Natl Acad Sci USA. 2010;107(5):2195–2200. doi: 10.1073/pnas.0909390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lathia JD, et al. Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis. 2011;2:e200. doi: 10.1038/cddis.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer N, et al. Haematopoietic stem cell differentiation promotes the release of prominin-1/CD133-containing membrane vesicles—a role of the endocytic-exocytic pathway. EMBO Mol Med. 2011;3(7):398–409. doi: 10.1002/emmm.201100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbeil D, Marzesco AM, Wilsch-Bräuninger M, Huttner WB. The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro)epithelial cell differentiation. FEBS Lett. 2010;584(9):1659–1664. doi: 10.1016/j.febslet.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 25.Marzesco AM, et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118(Pt 13):2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 26.Zacchigna S, et al. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29(7):2297–2308. doi: 10.1523/JNEUROSCI.2034-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, et al. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105(7):2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 29.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 31.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakondi B, Spees JL. Human CD133-derived bone marrow stromal cells establish ectopic hematopoietic microenvironments in immunodeficient mice. Biochem Biophys Res Commun. 2010;400(2):212–218. doi: 10.1016/j.bbrc.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majka M, et al. Expression, regulation and function of AC133, a putative cell surface marker of primitive human haematopoietic cells. Folia Histochem Cytobiol. 2000;38(2):53–63. [PubMed] [Google Scholar]
- 34.Fonseca AV, Freund D, Bornhäuser M, Corbeil D. Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network. J Biol Chem. 2010;285(41):31661–31671. doi: 10.1074/jbc.M110.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Till JE, McCULLOCH EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 36.Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347(6289):188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 37.Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1(2):204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Hosokawa K, et al. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116(4):554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 39.Lerner C, Harrison DE. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990;18(2):114–118. [PubMed] [Google Scholar]
- 40.Randall TD, Weissman IL. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89(10):3596–3606. [PubMed] [Google Scholar]
- 41.Zhang CC, Lodish HF. Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion. Blood. 2005;105(11):4314–4320. doi: 10.1182/blood-2004-11-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maw MA, et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9(1):27–34. doi: 10.1093/hmg/9.1.27. [DOI] [PubMed] [Google Scholar]
- 43.Yang Z, et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest. 2008;118(8):2908–2916. doi: 10.1172/JCI35891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arrigoni FI, et al. Extended extraocular phenotype of PROM1 mutation in kindreds with known autosomal dominant macular dystrophy. Eur J Hum Genet. 2011;19(2):131–137. doi: 10.1038/ejhg.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med (Berl) 2008;86(9):1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fargeas CA, Karbanová J, Jászai J, Corbeil D. CD133 and membrane microdomains: Old facets for future hypotheses. World J Gastroenterol. 2011;17(36):4149–4152. doi: 10.3748/wjg.v17.i36.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzesco AM, et al. Release of extracellular membrane vesicles from microvilli of epithelial cells is enhanced by depleting membrane cholesterol. FEBS Lett. 2009;583(5):897–902. doi: 10.1016/j.febslet.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 48.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10(2):120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Jaksch M, Múnera J, Bajpai R, Terskikh A, Oshima RG. Cell cycle-dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res. 2008;68(19):7882–7886. doi: 10.1158/0008-5472.CAN-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.