Abstract
Pulsatile release of hypothalamic gonadotropin-releasing hormone (GnRH) is essential for pituitary gonadotrope function. Although the importance of pulsatile GnRH secretion has been recognized for several decades, the mechanisms underlying GnRH pulse generation in hypothalamic neural networks remain elusive. Here, we demonstrate the ultradian rhythm of GnRH gene transcription in single GnRH neurons using cultured hypothalamic slices prepared from transgenic mice expressing a GnRH promoter-driven destabilized luciferase reporter. Although GnRH promoter activity in each GnRH neuron exhibited an ultradian pattern of oscillations with a period of ∼10 h, GnRH neuronal cultures exhibited partially synchronized bursts of GnRH transcriptional activity at ∼2-h intervals. Surprisingly, pulsatile administration of kisspeptin, a potent GnRH secretagogue, evoked dramatic synchronous activation of GnRH gene transcription with robust stimulation of pulsatile GnRH secretion. We also addressed the issue of hierarchical interaction between the circadian and ultradian rhythms by using Bmal1-deficient mice with defective circadian clocks. The circadian molecular oscillator barely affected basal ultradian oscillation of GnRH transcription but was heavily involved in kisspeptin-evoked responses of GnRH neurons. In conclusion, we have clearly shown synchronous bursts of GnRH gene transcription in the hypothalamic GnRH neuronal population in association with episodic neurohormone secretion, thereby providing insight into GnRH pulse generation.
Keywords: biological rhythm, GnRH pulse generator, circadian rhythm, dynamic transcription
Diverse forms of biological oscillation are found in biochemical reactions, cellular events, and physiological processes (1). Intrinsic daily rhythms are generated by molecular circadian clockwork, which is based on transcription–translation feedback loops composed of transcriptional activators such as the CLOCK:BMAL1 heterodimer and inhibitory factors including PERIODs and CRYPTOCHROMEs (2, 3). Dynamic regulation of the neuroendocrine system occurs due to the integrative actions of multiple types of biological oscillators. For example, corticosteroids in circulation exhibit robust circadian oscillation and hourly pulsatility (4).
The pulsatile release of hypothalamic neurohormones into the hypothalamic–pituitary portal vessels is a classic example of ultradian oscillation. Gonadotropin-releasing hormone (GnRH) is the most extensively studied neurohormone. Knobil (5) elegantly demonstrated that the pulsatile neurosecretion of GnRH is crucial for normal reproductive function. Although the physiological importance of pulsatile GnRH secretion is recognized, the cellular mechanisms underlying GnRH pulse generation remain unclear. An intrinsic mechanism for generating pulsatile secretion within individual GnRH neurons may exist. Pulsatile GnRH release has been demonstrated in immortalized GnRH-producing GT1 cells and in embryonic GnRH neuronal cultures (6, 7). Intracellular calcium oscillation (8), episodic gene expression coupled with exocytic activity (9, 10), voltage-dependent ion channel-mediated synchronization (11), and cellular circadian oscillators (12) have been implicated in pulsatile secretion of GnRH. Electrophysiological studies have suggested that a synchronization mechanism underlies the autonomous pulse generation (13, 14). However, most GnRH neuronal cell bodies are located in the preoptic area (POA) of the hypothalamus and direct contact between GnRH neuronal cell bodies is only occasionally found despite considerable dendro–dendritic interactions among them (14). Indeed, these anatomical features make it possible that scattered GnRH neurons form networks to coordinate their function along with neighboring non-GnRH neurons.
Kisspeptin, a neuropeptide encoded by the Kiss1 gene, is one of the strongest secretagogues of GnRH and luteinizing hormone (LH) (15, 16). Mutations in the kisspeptin receptor (GPR54) gene are associated with hypogonadotropic hypogonadism (17). Kisspeptin regulates GnRH neurons during multiple processes including pubertal maturation and mammalian reproduction (15, 17–19). Kisspeptin-producing cells reside in two distinct hypothalamic regions, the anteroventral periventricular nucleus and the arcuate nucleus, and extend their neurites adjacent to axon terminals and cell bodies of GnRH neurons (15, 18). Notably, kisspeptin release in the stalk-median eminence is pulsatile and exhibits a strong correlation with GnRH pulses (20). Furthermore, pulsatile kisspeptin administration drives gonadotropin secretion in juvenile male monkeys primed with GnRH. However, continuous administration of kisspeptin abolishes gonadotropin secretion after an acute stimulatory effect, presumably owing to receptor desensitization (21–23). Thus, kisspeptin may participate in GnRH pulse generation, although the underlying mechanisms remain elusive (24, 25).
We used organotypic cultures of hypothalamic GnRH neurons to elucidate the episodic pattern of GnRH gene expression and secretion in response to kisspeptin stimulation. Further, we examined the role of the molecular circadian clock in the ultradian rhythmicity of GnRH neurons.
Results
Ultradian Rhythm of GnRH Promoter Activity in Single GnRH Neurons.
We generated transgenic mice bearing a destabilized luciferase reporter under the control of the 3.0-kb rat GnRH promoter (GnRHp-dsLuc) (Fig. S1A). We compared expression of the luciferase reporter with endogenous GnRH to validate the model. In the POA, most luciferase-immunoreactive neurons coexpressed the GnRH decapeptide (Fig. S1B). The relative luciferase activities correlated well with GnRH content. The strongest transgene expression was found in the POA. Considerable expression was also found in the olfactory bulb and hippocampus (Fig. S1 C and D). Moreover, GnRH promoter-driven luciferase activity exhibited cyclical changes similar to endogenous GnRH during the estrous cycle (Fig. S1 E and F). These observations clearly indicate that luciferase expression in GnRHp-dsLuc transgenic mice closely parallels the spatiotemporal regulation of endogenous GnRH biosynthesis.
We prepared coronal slice cultures using the POA of transgenic animals on postnatal days 5–7. These cultures were analyzed by real-time bioluminescence imaging after 2–4 wk of cultivation (Fig. S1 G and H). The GnRH promoter activity in an individual neuron exhibited irregular but distinct ultradian oscillations (Fig. 1 A and B and Fig. S2). The mean interpulse interval was ∼10 h (594.51 ± 13.49 min, n = 124 from five slices) and the amplitude was 105.12 ± 4.66% of the average bioluminescence (n = 124 from five slices). Similar ultradian profiles were observed in GnRH neurons in cultures of the diagonal band of Broca and sagittally cut hypothalamic slices (Fig. S3). The POA cultures, which were prepared from adult transgenic mice, also exhibited an ultradian pattern of GnRH gene transcription. These data suggest that a neonatal culture model accompanied by maturation ex vivo may mimic the dynamic GnRH gene expression profiles in the adult tissues (Fig. S4). Furthermore, the episodic GnRH promoter activity was maintained in the presence of tetrodotoxin, a sodium channel blocker, and nimodipine, an L-type calcium channel blocker (Fig. S5). These results collectively indicate that the ultradian rhythm of spontaneous GnRH gene transcription with a period of ∼10 h is an intrinsic and cell-autonomous feature of postmitotic GnRH neurons.
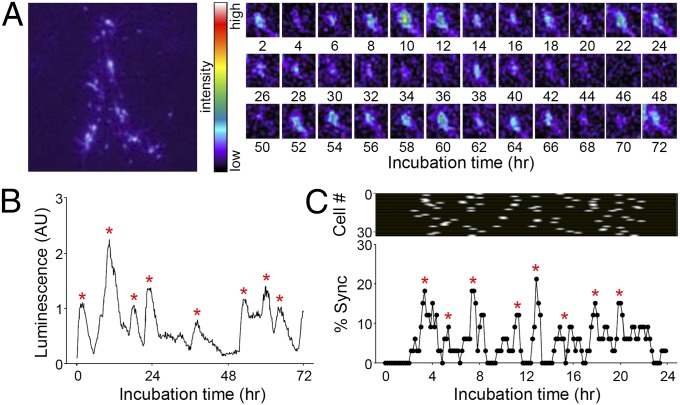
Fig. 1.
Ultradian rhythm of GnRHp-dsLuc under basal conditions. (A) Real-time bioluminescence in GnRHp-dsLuc mice. Representative time-lapse images of a GnRH neuron are shown on the right. (B) Quantitative luminescence profile of a GnRH neuron shown in A. (C) Spontaneous synchronization of a GnRH neuronal population. Pulsatile peaks of 33 individual luciferase-positive cells in a POA culture are plotted. Each row represents an individual neuron (white dot, peak). Synchronization index (% Sync) is the percentage of luciferase-positive cells simultaneously reaching their peaks. Asterisks represent peaks identified by Cluster-8.
The 10-h interpulse interval for the GnRH transcriptional activity of a single cell is longer than endogenous episodic neurohormone secretion, which is known to be ∼30 min in rodents (13). Because pulsatile GnRH release from the hypothalamus is a result of coordinated discharge of the decapeptide from hypophysiotropic GnRH neurons, synchronization of individual rhythms in a given cultured brain slice (typically 20–40 luciferase-expressing cells) is worth examining. We found that a small but significant subset of GnRH neurons (13.44 ± 0.80% of luciferase-positive cells from six independent experiments) formed synchronized peaks with ∼2-h intervals (118 ± 21 min) under basal conditions (Fig. 1C). This finding suggests that in vivo hypothalamic GnRH gene expression results from coordinated activity of a subset of GnRH neurons and follows an oscillation pattern with a shorter period.
Synchronous Activation of GnRH Transcription and Secretion by Intermittent Kisspeptin Administration.
We analyzed the kinetics of GnRH gene transcription in association with secretion of the neurohormone in response to the secretagogue kisspeptin in hypothalamic GnRH neurons. Although inhibition of kisspeptin signaling modulates pulsatile GnRH secretion in several species (24, 25), the role of kisspeptin in episodic GnRH gene expression remains elusive. We examined the effects of kisspeptin pulses on ultradian GnRH gene transcription in individual GnRH neurons and examined neuronal synchronization and secretion of the decapeptide. We treated POA cultures with kisspeptin-10, the physiologically active form (26). Cultures received either a single bolus of 10 nM kisspeptin for 15 min, six doses of 10 nM kisspeptin given intermittently (15 min on, 45 min off), or chronic infusion of 2.5 nM kisspeptin for 6 h. GnRH promoter activity and secretion into the perifused media were simultaneously measured from the same explants.
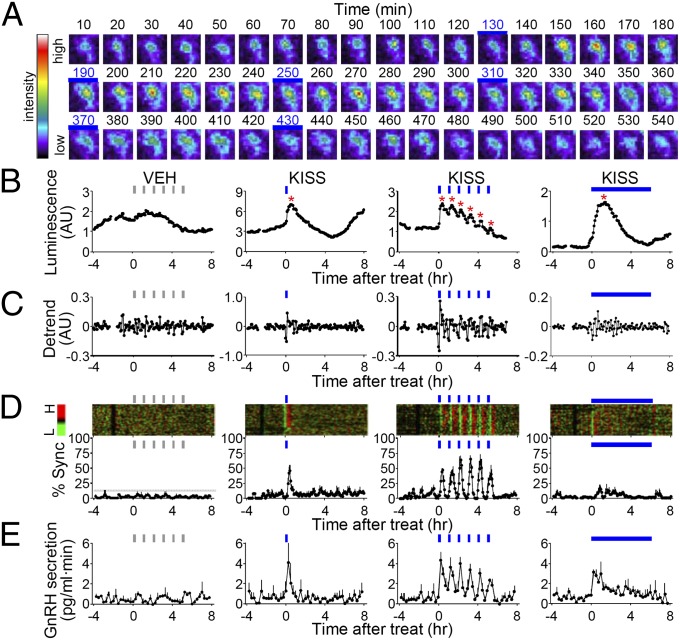
More than 40% of luciferase-positive cells showed an immediate spike-like increase in GnRH promoter activity 10–15 min after the end of the initial kisspeptin pulse (Fig. 2 A–C and Fig. S6). Another 30–40% of cells exhibited an increment of luminescence during the first one or two boluses of episodic stimulation followed by pulsatile responses later. Thus, kisspeptin-evoked synchronous bursts of GnRH transcription in a slice culture were reinforced in up to 80% of luciferase-positive cells (Fig. 2D). The remaining GnRH neurons were unresponsive even after six pulses of kisspeptin. These results demonstrate the heterogeneous response of GnRH neurons to kisspeptin. GnRH secretion was sharply induced to form distinct peaks, which seemed to coincide or precede transcriptional activation of GnRH in single cells (Fig. 2E). Continuous infusion of kisspeptin was less effective at inducing episodic bursts of GnRH secretion and synchronous transcription in comparison with a brief administration of kisspeptin owing to sustained and variable responses among GnRH neurons (Fig. 2 B–E, last panels).
Fig. 2.
Synchronous activation of GnRH promoter activity and secretion by intermittent kisspeptin administration. (A) Time-lapse luminescence images of an individual GnRH neuron stimulated with intermittent kisspeptin pulses (10 nM, 15 min on, 45 min off for 6 h). Blue bar, kisspeptin administration. (B and C) Representative profiles of an individual GnRH neuron stimulated with vehicle (VEH, gray bar) or kisspeptin (KISS, blue bar) (continuous KISS, 2.5 nM; single or intermittent KISS, 10 nM; VEH, 0.1% distilled water). Data are shown as raw (B) or detrended profile (C). (D) Synchronization of GnRH neuronal population. Raster plot of normalized detrended luminescence of a representative batch is color-coded according to scale shown at left (H, high; L, low). Each row represents an individual GnRH neuron. % Sync represents the percentage of luciferase-positive cells simultaneously reaching their peaks. Gray line, typical range of synchronization under basal conditions. (E) GnRH secretion in perifused media (gray bar, VEH; blue bar, kisspeptin). For D–E, data are shown as the mean ± SEM (n = 61–92 cells from three to four batches per treatment). Asterisks represent peaks identified by Cluster-8.
Recently, a subpopulation of kisspeptin neurons in the arcuate nucleus that coexpress neurokinin B and dynorphin A has been found to form an interconnective network with reciprocal cooperation between positive and negative regulators (27). This network may be capable of producing episodic transmission of kisspeptinergic signaling, which may modulate pulsatile GnRH secretion (19, 27, 28). In contrast to kisspeptin, neither dynorphin A nor senktide, a neurokinin B receptor agonist, had any influence on GnRH transcription or secretion (Fig. S7), supporting the idea that kisspeptin directly regulates triphenotypic neurons to control GnRH neuronal pulsatility. The effect of kisspeptin was distinguished from effects induced by nonpeptidergic neurotransmitters that influence GnRH secretion, including norepinephrine, glutamate, and γ-aminobutyric acid (Fig. S8). Our findings strongly suggest that kisspeptin contributes to GnRH pulse generation as a prominent and selective activator of GnRH transcription and secretion.
G Protein-Coupled Receptor 54 (GPR54) and Protein Kinase C (PKC) Mediate the Responses to Kisspeptin Stimulation.
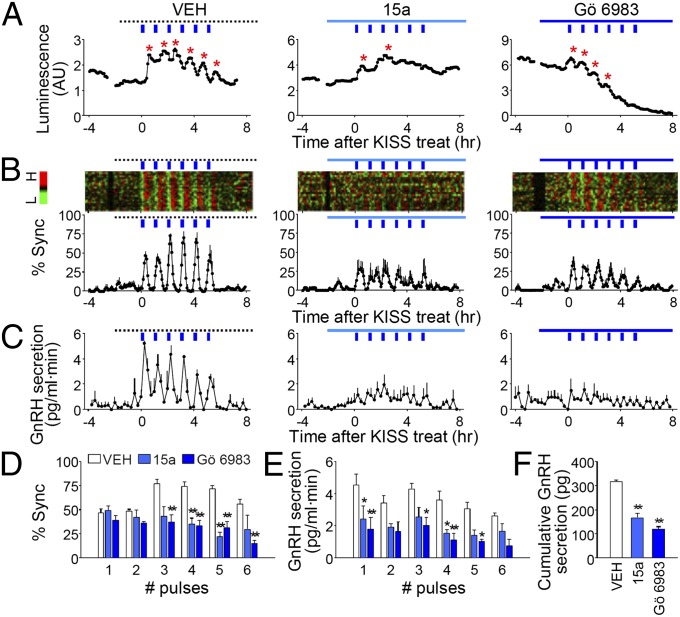
Kisspeptin activates G protein-coupled receptor 54, which is expressed in GnRH neurons (16) and works primarily through the Gq protein-initiated pathway (26). PKC is a major mediator of Gq protein-initiated signaling. We pretreated GnRH neurons for 2 h with a recently developed GPR54 antagonist, 2-acylamino-4,6-diphenylpyridine derivative (15a) (29), or with a PKC inhibitor, Gö 6983, before stimulating with kisspeptin. Kisspeptin-induced luciferase expression was significantly attenuated in the presence of these inhibitors, thereby suppressing the synchronous transcriptional response of GnRH neurons (Fig. 3 A and B; Fig. S9 shows effects on unstimulated slices). Inhibition of GPR54 or PKC also significantly impaired kisspeptin-evoked GnRH secretion (Fig. 3C). Transient transcriptional induction and secretion of GnRH during consecutive kisspeptin applications were significantly reduced to approximately half of the control level (Fig. 3 D–F). Although pharmacological manipulation did not completely abolish the effects of kisspeptin, these results suggest that activation of kisspeptin–GPR54 may mediate episodic GnRH gene transcription and secretion in response to intermittent kisspeptin stimulation.
Fig. 3.
GPR54–PKC pathway in kisspeptin-induced synchronous activation of GnRH transcription and secretion. (A) Representative profiles of an individual GnRH neuron stimulated with kisspeptin with vehicle or indicated antagonist (15a, 30 μM GPR54 antagonist; Gö 6983, 10 μM PKC inhibitor; VEH, 0.1% dimethyl sulfoxide). Asterisks represent peaks identified by Cluster-8. (B) Raster plot of GnRH promoter activity for a single POA culture. Each row represents an individual GnRH neuron. Normalized detrended values are color-coded according to the scale on left (H, high; L, low). % Sync is the percentage of luciferase-positive cells simultaneously reaching their peaks. (C) GnRH secretion in perifused media (blue bar, kisspeptin pulse; line, vehicle or indicated drug). (D) Peak value of synchronization. Two-way repeated measures analysis of variance (RM ANOVA), P < 0.01 (pharmacological agents), P < 0.01 (number of kisspeptin pulses), P < 0.01 (interaction). (E) Peak value of GnRH secretion. Two-way RM ANOVA, P < 0.01 (pharmacological agents), P < 0.01 (number of kisspeptin pulses), P = 0.9710 (interaction). In D and E, *P < 0.05; **P < 0.01 vs. VEH, Bonferroni posttest. (F) Cumulative GnRH secretion during 6 h after initiation of kisspeptin, **P < 0.01 vs. VEH, t test. In B–F, data are shown as mean ± SEM (n = 52–72 cells from three to four batches per treatment).
Involvement of de Novo Protein Synthesis and Secretory Pathway.
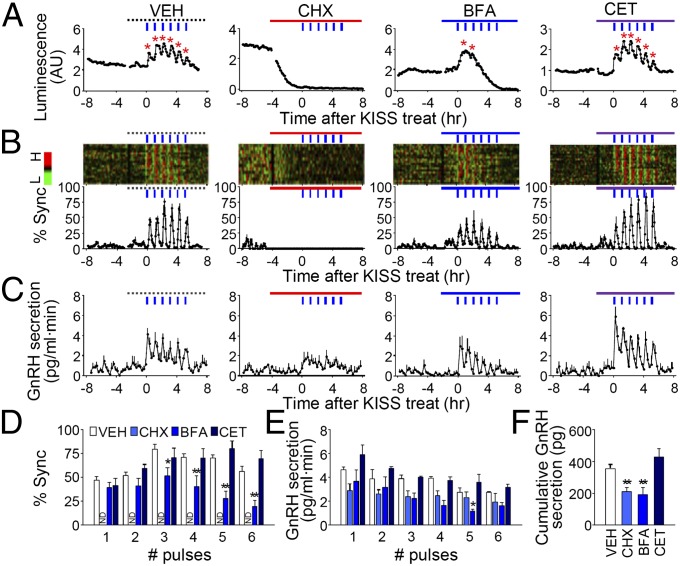
Concomitant activation of episodic GnRH gene transcription and secretion suggests that these processes may be directly related. To address this issue, we inhibited de novo protein synthesis or secretory pathway during pulsatile applications of kisspeptin. Although previous reports claimed that spontaneous episodic GnRH secretion or exocytic activity in GT1 cells require neither transcription nor translation (10, 30), pretreatment with cycloheximide for 4 h completely abolished reporter expression (Fig. 4 A, B, and D; Fig. S9 shows effects on unstimulated slices). Episodic release of neurohormones in response to kisspeptin was significantly impaired by cycloheximide (Fig. 4 C and E) in addition to a significant reduction in cumulative GnRH secretion (Fig. 4F). Thus, GnRH biosynthesis may be required to maintain kisspeptin-evoked pulsatile release, which may be related to a robust discharge effect on GnRH neurons (16).
Fig. 4.
Effect of de novo protein synthesis and secretory pathway. (A) Representative profiles of an individual GnRH neuron with pulsatile kisspeptin stimulation with vehicle or indicated drug (BFA, brefeldin A, 10 μg/mL; CET, cetrorelix, 10 μM; CHX, cycloheximide, 100 μM; VEH, 0.1% dimethyl sulfoxide). Asterisks represent peaks identified by Cluster-8. (B) Synchronization of GnRH neuronal population. Raster plot of normalized detrended luminescence of a single POA culture is shown as a pseudocolor scale (H, high; L, low). Each row represents an individual GnRH neuron. % Sync is the percentage of luciferase-positive cells simultaneously reaching their peaks. (C) GnRH secretion in perifused media (blue bar, kisspeptin; line, vehicle or indicated drugs). (D) Peak value of synchronization. Two-way RM ANOVA, P < 0.01 (inhibitors), P < 0.01 (number of kisspeptin pulses), P < 0.01 (interaction). (E) Peak value of GnRH secretion. Two-way RM ANOVA, P < 0.05 (inhibitors), P < 0.01 (number of kisspeptin pulses), P = 0.1977 (interaction). In D and E, *P < 0.05, **P < 0.01 vs. VEH, Bonferroni posttest. (F) Cumulative GnRH secretion during 6 h after initiation of kisspeptin. **P < 0.01 vs. VEH, t test. In B–F, data are shown as mean ± SEM (n = 65–89 cells from three to five batches per treatment).
However, secretion may be important for maintaining synchronized GnRH gene transcription after intermittent kisspeptin stimulation. Pretreatment of brefeldin A (BFA), an inhibitor of protein trafficking and secretory pathway, led to a gradual attenuation in kisspeptin-induced episodic GnRH transcription in single GnRH neurons (Fig. 4A). As a result, synchronized bursts of GnRH gene transcription were reduced (Fig. 4 B and D). Neurohormone release was significantly impaired in the presence of BFA (Fig. 4 C, E, and F). Consistent with a report that blockade of exocytosis impairs spontaneous GnRH transcription pulses in GT1 cells (10), our results suggest that a secreted factor mediates the harmonized transcriptional response of GnRH neurons in response to kisspeptin pulses. Because autocrine feedback has been demonstrated for GnRH (31–33), we examined whether GnRH receptor inhibition elicits effects similar to BFA. However, pretreatment with cetrorelix (CET), a GnRH antagonist, barely altered kisspeptin-mediated effects (Fig. 4). Therefore, mechanisms associated with secretory processes other than autocrine signaling may play an important role in coupling GnRH transcription and secretion and in maintaining responsiveness to kisspeptin.
Hierarchical Interaction Between Circadian and Ultradian Rhythms.
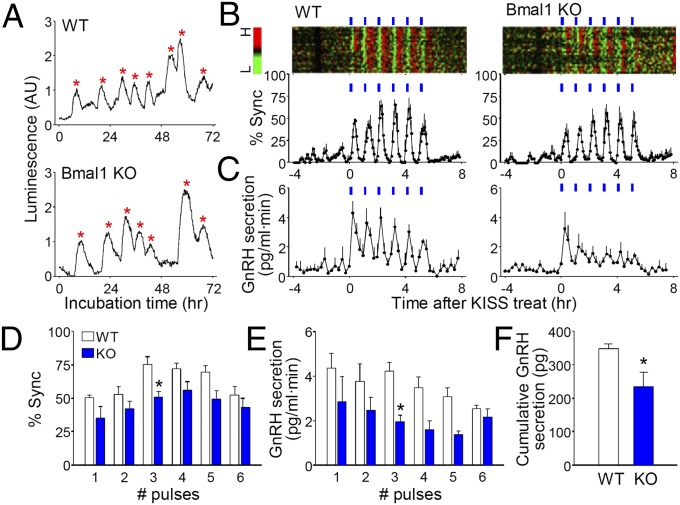
Mutant mice bearing a defective allele of the Clock gene are subfertile and exhibit abnormal estrous cycles as a result of hypothalamic defects (12, 34). Cellular circadian clock machinery may be required for spontaneous GnRH pulsatility in the GT1 cell line (12). The present study tried to elucidate the role of the molecular clockwork in spontaneous and kisspeptin-evoked GnRH transcription and secretion in cultured hypothalamic slices. We monitored GnRH promoter activity and secretion in POA slice cultures obtained from GnRHp-dsLuc transgenic mice lacking functional BMAL1 (GnRHp-dsLuc;Bmal1−/−), a key transcriptional regulator of the circadian molecular clock (35). Under basal conditions, ultradian oscillations of GnRH promoter activity in Bmal1−/− slices were similar to those in wild-type controls in terms of period (606.29 ± 15.31 min for Bmal1+/+ and 573.14 ± 18.16 min for Bmal1−/−, P > 0.05; n = 77 and 58 cells with each genotype, respectively) and amplitude (100.57 ± 4.94% for wild-type and 95.51 ± 5.75% for Bmal1−/−, P > 0.05; Fig. 5A). This result strongly suggests that intrinsic ultradian GnRH gene expression is driven by an unidentified oscillator distinct from the circadian clockwork. However, Bmal1-deficient cultures exhibited impaired responses to pulsatile kisspeptin administration with slight but significant reductions in synchronous bursts of GnRH gene transcription (Fig. 5 B and D). Episodic GnRH secretion in response to kisspeptin was far more impaired in Bmal1−/− cultures (Fig. 5 C and E), leading to a significant reduction in cumulative secretion during kisspeptin administration (Fig. 5F).
Fig. 5.
Ultradian rhythm of GnRHp-dsLuc and kisspeptin-induced synchronization in Bmal1 knock-out mice. (A) Representative luminescence profiles under basal conditions in POA cultures obtained from GnRHp-dsLuc in WT or Bmal1−/− (Bmal1 KO) background. Asterisks represent peaks identified by Cluster-8. (B) Synchronization of GnRH neuronal population derived from WT or Bmal1 KO mouse stimulated with kisspeptin. Raster plot of normalized detrended luminescence for a representative POA culture is shown as a pseudocolor scale (H, high; L, low). Each row represents an individual GnRH neuron. % Sync is the percentage of luciferase-positive cells simultaneously reaching their peaks. (C) GnRH secretion in perifused media (blue bar, kisspeptin pulse). (D) Peak value of synchronization. Two-way RM ANOVA, P < 0.01 (genotype), P < 0.01 (number of kisspeptin pulses), P = 0.7842 (interaction). (E) Peak value of GnRH secretion. Two-way RM ANOVA, P < 0.01 (genotype), P = 0.1245 (number of kisspeptin pulses), P = 0.6422 (interaction). In D and E, *P < 0.05 vs. VEH, Bonferroni posttest. (F) Cumulative GnRH secretion during 6 h after initiation of kisspeptin. *P < 0.05 vs. VEH, t test. In B–F, data are presented as mean ± SEM (n = 81–93 cells from four batches per genotype).
Discussion
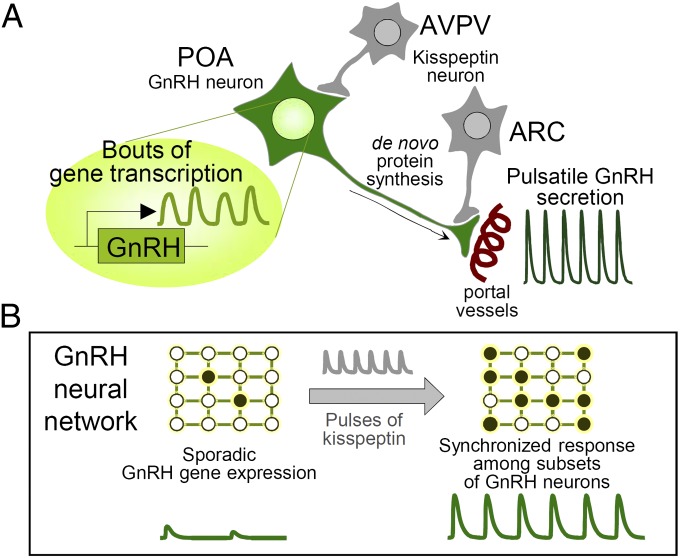
This study examined GnRH pulse generation, which is a classic example of an ultradian biological rhythm. We characterized spontaneous and kisspeptin-evoked ultradian GnRH gene transcription ex vivo in cultured hypothalamic slices derived from GnRHp-dsLuc transgenic mice. We showed that pulsatile GnRH promoter activity occurs in single GnRH neurons residing in hypothalamic neural networks, demonstrating their populational properties relative to GnRH secretion (Fig. 6).
Fig. 6.
A hypothetical model for GnRH pulse generator synchronized by intermittent kisspeptin stimulation. (A) GnRH neuron, which secretes GnRH neurohormone into the hypothalamic–pituitary portal vessels, is innervated by kisspeptinergic neurons; cell bodies are located in the anteroventral periventricular nucleus (AVPV) or arcuate nucleus (ARC) of the hypothalamus. (B) Under basal conditions, GnRH gene expression is sporadic (Left). Pulsatile stimulation of kisspeptin dramatically recruits a subset of GnRH neurons to synchronize GnRH transcription with episodic GnRH secretion (Right). Each circle represents a single GnRH neuron residing in the hypothalamic neural network. Closed circles, GnRH neurons in sync; open circles, GnRH neurons out of sync. De novo protein synthesis and secretory pathway may mediate coupled bursting of GnRH transcription and secretion.
GnRH neurons may harbor intrinsic oscillatory mechanisms that drive pulsatile GnRH secretion (13). We showed that GnRH promoter activity exhibited spontaneous oscillations in single neurons. Despite stochastic synchronization at ∼2-h intervals in ∼10% of GnRH-expressing cells, the ultradian rhythm of GnRH gene transcription was not directly linked with episodic GnRH secretion because of differences in their periods. Moreover, the transcriptional rhythm of single GnRH neurons was intrinsic and did not require voltage-gated ion channel activity, which is important for pulsatile GnRH release (32, 36). Thus, voltage-gated ion channels and intracellular calcium oscillation may regulate neurohormone secretion by acting on axon terminals in the median eminence. However, the intrinsic rhythmicity of GnRH neurons is still important, because ultradian gene transcription in each cell and stochastic synchrony may contribute to GnRH pulse generation as demonstrated by repetitive kisspeptin stimulation.
Hypothalamic GnRH neurons receive various inputs in vivo by interacting with multiple cell types. Accumulating evidence suggests that kisspeptin–GPR54 signaling plays a key role in GnRH pulse generation. Inhibition of hypothalamic kisspeptin signaling suppresses pulsatile GnRH/LH secretion (24, 25). Kisspeptin levels were episodic and temporally coincided with or preceded GnRH pulses (20, 37, 38). However, the mechanism by which synchronous gene expression occurs in kisspeptin-stimulated GnRH neurons remains elusive. The most important finding of this study is transient coupling of GnRH transcription and secretion in response to kisspeptin. This is important to highlight, because it suggests that direct or indirect pulsatile kisspeptinergic inputs to GnRH neurons may drive GnRH pulse generation. Considering the response kinetics and the effects of cycloheximide and BFA, concurrent activation does not mean that transcriptional activation simply leads to episodic GnRH secretion. Instead, a robust discharge of secreted GnRH may affect transcriptional activation. Inhibition of secretory processes accelerated a desensitization-like decline in transcription during consecutive treatments with kisspeptin, supporting this hypothesis. Thus, concomitant activation of GnRH transcription and secretion may be required for optimal response of GnRH neurons to kisspeptin pulses.
Kisspeptin–GPR54 signaling is important for the onset of puberty (15, 17–19). Prepubertal vertebrates exhibit a quiescent period of GnRH secretion, which becomes activated with the onset of puberty (39). GPR54-mediated signaling increases during sexual maturation and is required for pubertal onset and reproduction in many mammalian species (15, 17–19). Expression of kisspeptin and GPR54, and particularly the kisspeptin immunoreactivities juxtaposed to the GnRH fibers, are dramatically induced upon pubertal onset, suggesting that kisspeptin stimulation may activate puberty and maintain GnRH pulse generation in adulthood (40). We used organotypic cultures from sexually immature neonatal mice, which subsequently matured ex vivo. The spontaneous ultradian rhythmicity of GnRH transcription in acutely prepared neonatal slices was similar to that in adult slices (Fig. S4). Thus, prepubertal GnRH neurons may possess intrinsic mechanisms for pulse generation and spontaneous ultradian rhythmicity, which become activated by puberty and enhanced kisspeptinergic inputs.
Hierarchical interaction between different biological rhythms is an important topic. Previous work supports functional cross-talk between circadian and ultradian rhythms. For example, circadian rhythms in the hypothalamic–pituitary–adrenal axis are interlocked with ultradian oscillations (4). Both circadian clockwork and hormonal pulsatility are required for normal reproductive neuroendocrine axis function (35, 36). Bmal1-deficient cultures had reduced synchrony between transcription and secretion during kisspeptin pulses despite similarities in spontaneous GnRH gene transcription (Fig. 5). Thus, our results implicate the circadian clockwork in the processing, storage, or secretion of GnRH and synchronization of the GnRH neuronal population. However, it is unlikely to directly control ultradian oscillations of GnRH gene transcription. The response of GnRH neurons to kisspeptin may be controlled by the molecular circadian clock, because daily variations in kisspeptin sensitivity and cyclic accumulation of GPR54 mRNA were recently found in GT1 cells (41, 42).
In conclusion, we show that intermittent kisspeptin stimulation evokes pulsatile GnRH gene transcription and release in GnRH neurons. Kisspeptin–GPR54 signaling may be a pivotal regulator of GnRH pulse generation. Synchrony in hypothalamic neural networks may be important for GnRH pulse generation. Episodic GnRH gene expression and secretion provides valuable insight into ultradian rhythms, which are widely found in the neuroendocrine system.
Materials and Methods
GnRHp-dsLuc Transgenic Mice and Organotypic Slice Culture.
The rat GnRH promoter (3.0 kb, −3,002 to +88 from the transcription start site) was fused upstream to the destabilized luciferase-coding sequence to construct GnRHp-dsLuc. Transgenic mice expressing GnRHp-dsLuc were generated by microinjection of purified DNA into the pronuclei of fertilized eggs of C57BL/6J mice. For slice cultures, mice at the age of postnatal days 5–7 were anesthetized with ether, and brains were immediately transferred to ice-cold Gey’s balanced salt solution (with 10 mM Hepes and 30 mM glucose) bubbled with 5% CO2 and 95% O2. Coronal slices (400 μm thick) were made with a vibratome (Campden Instruments). Brain slices were carefully dissected under a stereomicroscope to minimize the outside regions of the POA. One POA explant (∼1 mm long and 1 mm wide) was obtained per mouse and four explants were maintained on a membrane (Millicell-CM; Millipore), which was dipped into culture medium (50% minimum essential medium, 25% HBSS, 25% horse serum, 36 mM glucose, and 100 U/mL penicillin–streptomycin) at 36 °C.
Real-Time Bioluminescence Monitoring and Measurement of GnRH Secretion.
POA slice cultures were maintained in a customized chamber with two input ports and one output port made of stainless steel (Live Cell Instruments) and perifused with the recording medium (DMEM:Ham’s F12 supplemented with 1× N2 supplement, 36 mM glucose, and 100 U/mL penicillin–streptomycin) at a flow rate of 2.4 mL/h. Kisspeptin was administered by replacing the normal media input to a kisspeptin-containing input. For pulsatile kisspeptin administration, six consecutive kisspeptin pulses (15min of kisspeptin at 10 nM followed by 45 min of media washout) were administered to the slice culture. For continuous treatment, cultures were perifused with kisspeptin-containing media (2.5 nM) for 6 h. The total amount of administered kisspeptin is the same between intermittent (10 nM for 15 min per cycle, six cycles) and continuous (2.5 nM for 6 h) mode. Bioluminescence images were acquired at 5-min intervals using the Cellgraph (ATTO) in the presence of 1 mM D-luciferin (Promega). Average luminescence intensities within the region of interest were presented as arbitrary units after background correction. The detrended value was obtained by successively subtracting average luminescence intensities of a neighboring 25-min window. Peak and nadir of the pulses were identified using the Cluster-8 program (43). Synchronization of a GnRH neuronal population was calculated as previously described (44) with minor modifications. For the raster plots, the detrended value was normalized by SD and color-coded. GnRH concentrations in the media collected at 15-min intervals were determined by RIA as described previously (45). Cumulative GnRH secretion was calculated by summation of the amount collected for 6 h after the intermittent kisspeptin stimulation was started. Detailed experimental procedures can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
Bmal1-mutant mice were generously provided by Drs. Marina Antoch (Roswell Park Cancer Institute) and Karyn Esser (University of Kentucky). BioScience Writers provided English editing services for the manuscript. This work was supported by grants from the Korea Ministry of Education, Science, and Technology (MEST) through the Brain Research Center for the 21st Century Frontier Research and Development Program in Neuroscience. H.K.C. and H.K. were supported by Brain Korea 21 Research Fellowships from MEST.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213594110/-/DCSupplemental.
References
- 1.Goldbeter A. Biological rhythms: Clocks for all times. Curr Biol. 2008;18(17):R751–R753. doi: 10.1016/j.cub.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 4.Lightman SL, Conway-Campbell BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci. 2010;11(10):710–718. doi: 10.1038/nrn2914. [DOI] [PubMed] [Google Scholar]
- 5.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- 6.Martínez de la Escalera G, Choi AL, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: Intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci USA. 1992;89(5):1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140(3):1432–1441. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- 8.Jasoni CL, Romanò N, Constantin S, Lee K, Herbison AE. Calcium dynamics in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2010;31(3):259–269. doi: 10.1016/j.yfrne.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Nuñez L, Faught WJ, Frawley LS. Episodic gonadotropin-releasing hormone gene expression revealed by dynamic monitoring of luciferase reporter activity in single, living neurons. Proc Natl Acad Sci USA. 1998;95(16):9648–9653. doi: 10.1073/pnas.95.16.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazquez-Martinez R, et al. Pulsatile exocytosis is functionally associated with GnRH gene expression in immortalized GnRH-expressing cells. Endocrinology. 2001;142(12):5364–5370. doi: 10.1210/endo.142.12.8551. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez-Martinez R, Shorte SL, Boockfor FR, Frawley LS. Synchronized exocytotic bursts from gonadotropin-releasing hormone-expressing cells: Dual control by intrinsic cellular pulsatility and gap junctional communication. Endocrinology. 2001;142(5):2095–2101. doi: 10.1210/endo.142.5.8123. [DOI] [PubMed] [Google Scholar]
- 12.Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci. 2003;23(35):11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24(2):79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA. 2009;106(26):10835–10840. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 18.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29(1):48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Navarro VM, Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol. 2012;8(1):40–53. doi: 10.1038/nrendo.2011.147. [DOI] [PubMed] [Google Scholar]
- 20.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tovar S, et al. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology. 2006;147(6):2696–2704. doi: 10.1210/en.2005-1397. [DOI] [PubMed] [Google Scholar]
- 22.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147(2):1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 23.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): A finding with therapeutic implications. Endocrinology. 2006;147(5):2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 24.Li XF, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE. 2009;4(12):e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roseweir AK, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtaki T, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 27.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro VM, et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi T, et al. 2-acylamino-4,6-diphenylpyridine derivatives as novel GPR54 antagonists with good brain exposure and in vivo efficacy for plasma LH level in male rats. Bioorg Med Chem. 2010;18(14):5157–5171. doi: 10.1016/j.bmc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 30.Pitts GR, Nunemaker CS, Moenter SM. Cycles of transcription and translation do not comprise the gonadotropin-releasing hormone pulse generator in GT1 cells. Endocrinology. 2001;142(5):1858–1864. doi: 10.1210/endo.142.5.8137. [DOI] [PubMed] [Google Scholar]
- 31.Krsmanović LZ, Stojilković SS, Mertz LM, Tomić M, Catt KJ. Expression of gonadotropin-releasing hormone receptors and autocrine regulation of neuropeptide release in immortalized hypothalamic neurons. Proc Natl Acad Sci USA. 1993;90(9):3908–3912. doi: 10.1073/pnas.90.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stojilkovic SS, Krsmanovic LZ, Spergel DJ, Catt KJ. Gonadotropin-releasing hormone neurons: Intrinsic pulsatility and receptor-mediated regulation. Trends Endocrinol Metab. 1994;5(5):201–209. doi: 10.1016/1043-2760(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 33.Xu C, Xu XZ, Nunemaker CS, Moenter SM. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology. 2004;145(2):728–735. doi: 10.1210/en.2003-0562. [DOI] [PubMed] [Google Scholar]
- 34.Miller BH, et al. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krsmanović LZ, et al. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA. 1992;89(18):8462–8466. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta) Endocrinology. 2012;153(4):1887–1897. doi: 10.1210/en.2011-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic control of kisspeptin release in prepubertal monkeys: implications to the mechanism of puberty onset. Endocrinology. 2012;153(7):3331–3336. doi: 10.1210/en.2012-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 40.Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324(1-2):45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 41.Zhao S, Kriegsfeld LJ. Daily changes in GT1-7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89(4):448–457. doi: 10.1159/000192370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonsfeldt KJ, Goodall CP, Latham KL, Chappell PE. Oestrogen induces rhythmic expression of the Kisspeptin-1 receptor GPR54 in hypothalamic gonadotrophin-releasing hormone-secreting GT1-7 cells. J Neuroendocrinol. 2011;23(9):823–830. doi: 10.1111/j.1365-2826.2011.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veldhuis JD, Johnson ML. Cluster analysis: A simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250(4 Pt 1):E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 44.Moore JP, Jr, Shang E, Wray S. In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci. 2002;22(20):8932–8941. doi: 10.1523/JNEUROSCI.22-20-08932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho S, Cho H, Geum D, Kim K. Retinoic acid regulates gonadotropin-releasing hormone (GnRH) release and gene expression in the rat hypothalamic fragments and GT1-1 neuronal cells in vitro. Brain Res Mol Brain Res. 1998;54(1):74–84. doi: 10.1016/s0169-328x(97)00325-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.