Abstract
Fragile X syndrome (FXS) is the most common inherited form of autism and intellectual disability and is caused by the silencing of a single gene, fragile X mental retardation 1 (Fmr1). The Fmr1 KO mouse displays phenotypes similar to symptoms in the human condition—including hyperactivity, repetitive behaviors, and seizures—as well as analogous abnormalities in the density of dendritic spines. Here we take a hypothesis-driven, mechanism-based approach to the search for an effective therapy for FXS. We hypothesize that a treatment that rescues the dendritic spine defect in Fmr1 KO mice may also ameliorate autism-like behavioral symptoms. Thus, we targeted a protein that regulates spines through modulation of actin cytoskeleton dynamics: p21-activated kinase (PAK). Our results demonstrate that a potent small molecule inhibitor of group I PAKs reverses dendritic spine phenotypes in Fmr1 KO mice. Moreover, this PAK inhibitor—which we call FRAX486—also rescues seizures and behavioral abnormalities such as hyperactivity and repetitive movements, thereby supporting the hypothesis that a drug treatment that reverses the spine abnormalities can also treat neurological and behavioral symptoms. Finally, a single administration of FRAX486 is sufficient to rescue all of these phenotypes in adult Fmr1 KO mice, demonstrating the potential for rapid, postdiagnostic therapy in adults with FXS.
Keywords: drug discovery, neurodevelopmental disorder
Autism is a diverse and complex family of disorders, and its prevalence is on the rise: one in 110 children have autism today, an increase of nearly 300% in just 12 y (1). Although there are two drugs available to treat repetitive behaviors and irritability, associated adverse effects limit their use (2). Furthermore, there are no effective treatments for the core symptoms, which often include language and communication deficits, intellectual disability, epilepsy, attention deficits, and hyperactivity (3). The quest for a cure is challenging because of the heterogeneity of the disorder, but also because more than 90% of cases of autism are idiopathic (4). Fortunately, one cause has been discovered: silencing of the single-gene fragile X mental retardation 1 (Fmr1) causes an autism-like disorder called fragile X syndrome (FXS) (5). The mouse model of FXS, the Fmr1 KO mouse, displays phenotypes similar to symptoms in the human condition—including hyperactivity, repetitive behaviors, and seizures (6–12).
Fundamental research on the Fmr1 KO mouse has provided promising insights into the cellular and molecular underpinnings of the disease, and suggested two pathways to target for pharmacological therapies. Numerous animal studies have shown that treatments aimed at reversing a protein synthesis phenotype, through inhibition of metabotropic glutamate receptors and downstream signaling kinases such as ERK1/2, ameliorate mutant phenotypes (13, reviewed in ref. 14, 15). Another therapeutic strategy is built on the observation that the density and morphology of dendritic spines are abnormal in humans with FXS and Fmr1 KO mice (8, 16–20). Because dendritic spines are the sites of connections between neurons and critical substrates for learning, neuroanatomical abnormalities in dendritic spines may contribute to disease symptoms and severity.
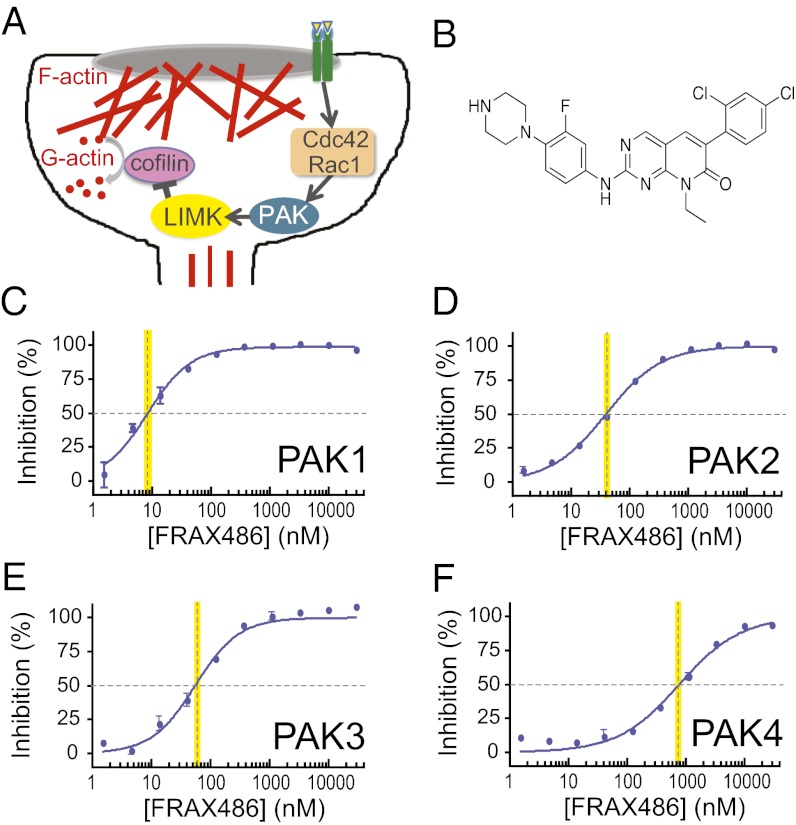
The structural integrity of dendritic spines and the functional efficacy of their synapses depend on modulation of the actin cytoskeleton and therefore the regulation of proteins in the actin remodeling pathway (21, 22). Actin dynamics are mediated by actin-binding proteins and the signaling pathways upstream of them, including p21-activated kinase (PAK)—which has been linked to intellectual disability and Alzheimer’s disease—and the upstream small guanosine triphosphatases cell division cycle 42 (Cdc42) and ras-related C3 botulinum toxin substrate 1 (Rac1) (Fig. 1A; 23–26). Accumulating evidence suggests a link between the Rac/PAK pathway and Fmr1. In Drosophila, there is evidence for a genetic interaction between the fly orthologs of Fmr1 and Rac1 (27). In murine fibroblasts, the protein product of Fmr1 (FMRP) binds to Rac1 mRNA and interferes with Rac1-induced actin remodeling (28). In the hippocampus of Fmr1 KO mice, physiological activation of the Rac/PAK pathway is defective (29). Finally, partial inhibition of PAK activity in vivo through the expression of a dominant negative PAK transgene (dnPAK) results in dendritic spine abnormalities opposite to those reported in Fmr1 KO mice and FXS humans (30). These observations suggest that PAK and FMRP antagonize one another to regulate spine number and shape, and led us to hypothesize that inhibition of PAK may reverse phenotypes in Fmr1 KO mice. Indeed, expression of dnPAK in the forebrain of adult Fmr1 KO mice on the C57/BL6 (B6) background strain was shown to be sufficient to reverse the morphological, physiological, and behavioral abnormalities in the mouse model of FXS (8).
Fig. 1.
FRAX486 is an inhibitor of group I PAKs. (A) Schematic of the actin cytoskeleton remodeling pathway in a dendritic spine including PAK activators Cdc42 and Rac1, PAK substrate LIMK, downstream target cofilin, globular G-actin, and filamentous F-actin. (B) Chemical structure of FRAX486. (C–F) In vitro kinase assay tested the ability of full-length PAK isoforms to phosphorylate an optimized peptide substrate in the presence of the putative kinase inhibitor, FRAX486. The IC50 kinase concentration is indicated by a yellow vertical line. Values are mean ± SEM (n = 2). Dose–response curves demonstrate that FRAX486 inhibited PAK1, PAK2, and PAK3—group I PAKs—with nanomolar potency (IC50 = 8.25, 39.5, and 55.3 nM, respectively), but is a poor inhibitor of PAK4—a group II PAK (IC50 = 779 nM). LIMK, LIM domain kinase 1.
Although a genetic rescue strategy is an excellent proof of concept, a pharmacological approach would be needed for human therapies. Until this point, there has been no potent small molecule PAK inhibitor for in vivo studies (31). In this work, we present a small molecule that we call FRAX486 and test whether it can rescue behavioral and spine density phenotypes when administered to adult Fmr1 KO mice. Our results demonstrate that seizures and behavioral abnormalities such as hyperactivity and repetitive movements are rescued by both acute and prolonged treatment with FRAX486. Moreover, because FRAX486 reverses spine phenotypes in adult Fmr1 KO mice, our data support the hypothesis that a drug treatment that reverses spine abnormalities, even later in life, could also treat neurological and behavioral symptoms.
Results
Discovery of an Inhibitor of Group I PAKs.
The discovery that genetic inhibition of PAK reverses abnormalities in Fmr1 KO mice on the B6 background strain highlighted the PAK pathway as a potential target for therapeutics for FXS and related neurodevelopmental disorders (8). More specifically, dnPAK targets the autoinhibitory domain of group I PAKs, but does not inhibit group II PAKs. The former group consists of three isoforms—PAK1, PAK2, and PAK3—which are enriched in the brain and critical for brain growth and proper cognitive function (32–34).
To find a potent inhibitor of group I PAKs, we performed a high-throughput screen of a 12,000 kinase–focused small molecule library. In addition, we counterscreened for one group II PAK—PAK4—to evaluate group II selectivity. The high-throughput screen identified a promising scaffold that was further refined to identify FRAX486 [6-(2,4-dichlorophenyl)-8-ethyl-2-(3-fluoro-4-(piperazin-1-yl)phenylamino)pyrido[2,3-d] pyrimidin-7(8H)-one; structure shown in Fig. 1B]. Using an in vitro kinase assay, we found that FRAX486 is an inhibitor of PAK, with excellent selectivity for group I over group II PAKs (Fig. 1 C–F).
FRAX486 Is Bioavailable and Penetrates the Brain.
A significant challenge in drug discovery research and the treatment of central nervous system diseases is effective blood–brain barrier (BBB) penetration. Because only ∼2% of small molecule drugs can cross the BBB (35), it was important to determine whether FRAX486 achieves optimum in vivo exposure in the brain before assaying impacts on neuroanatomy and behavior.
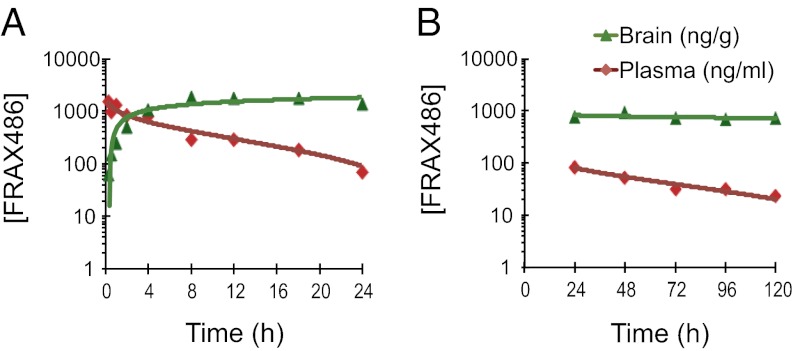
In the initial experiment, WT mice of the FVB.129P2 background strain were administered a single s.c. injection of 20 mg/kg FRAX486, and blood and brain samples were collected at various time points. Plasma levels of FRAX486—analyzed and quantified by liquid chromatography with tandem MS—were highest at the initial time point (15 min) and remained >100 ng/mL (∼200 nM) for up to 18 h (Fig. 2A). One hour after drug administration, brain levels of FRAX486 reached 155 ± 25.5 ng/g, equivalent to 301 ± 50.0 nM and therefore ∼36 times the IC50 for PAK1, 7.6 times the IC50 for PAK2, and 5.4 times the IC50 for PAK3.
Fig. 2.
Pharmacokinetics of FRAX486. Pharmacokinetic profiles of plasma (ng/mL) and brain concentrations (ng/g) of FRAX486 at different time points following (A) a single s.c. injection (n = 2–3) or (B) daily administration of 20 mg/kg FRAX486 (n = 3–4). Values are mean ± SEM, plotted on a logarithmic scale.
Not only did FRAX486 cross the BBB at levels that will inhibit group I PAKs, FRAX486 levels remained high in the brain for many hours. FRAX486 levels rose steadily in the brain until peaking at 8 h, maintained near 951 ± 27.0 ng/g (1.84 ± 63.5 µM) from 8 h to 18 h (Fig. 2A), and began to decrease at 24 h. These data demonstrate that FRAX486 crosses the BBB and that therapeutically useful concentrations of FRAX486 are in the brain as early as 1 h and remain as long as 24 h after administration, with the maximum concentration in the target tissue at 8 h.
To determine whether daily dosing results in steady-state levels of drug or whether drug accumulates in the body, samples were taken from mice that received daily injections of 20 mg/kg FRAX486 for up to 5 d. Each blood and brain sample was taken 24 h following the ultimate injection (Fig. 2B). Plasma levels of FRAX486 remained consistent from day to day (plasma, P = 0.1503; brain, P = 0.7928). In this way, once-a-day dosing of 20 mg/kg FRAX486 via s.c. injection results in steady-state levels of FRAX486 in the brain.
FRAX486 Rescues the Dendritic Spine Abnormality in Fmr1 KO Mice.
Dendritic spine density is increased on cortical neurons in FXS humans and Fmr1 KO mice compared with neurotypical humans and WT mice (18, 19). Expression of dnPAK in the forebrain of adult Fmr1 KO mice on the B6 background rescues the increase in spine density, a phenotype observed on the apical but not basal dendrites in Fmr1 KO mice (8). To determine whether this genetic strategy could be translated into an effective pharmacological strategy, we assessed the impact of PAK inhibitor FRAX486 on spine density of the same population of neurons—pyramidal cells in cortical layers II/III of the temporal lobe—in WT and Fmr1 KO mice on the FVB background strain.
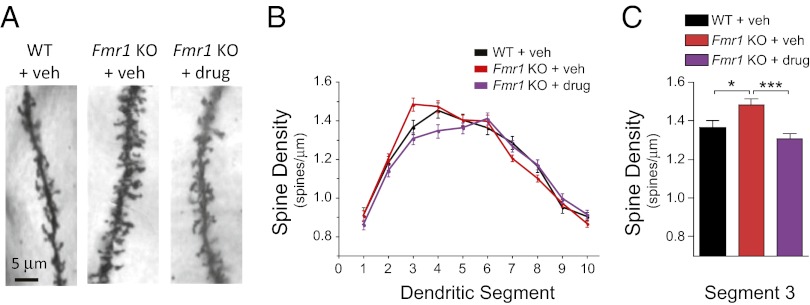
Mice were administered FRAX486 at a concentration of 20 mg/kg, and brain tissue was collected 8 h after drug treatment. Golgi staining showed that in cortical neurons of Fmr1 KO mice, the mean density of apical dendritic spines was greater than in WT neurons in segment 3 (WT + vehicle vs. KO + vehicle: P < 0.05; Fig. 3). Therefore, we tested whether our small-molecule PAK inhibitor was sufficient to reverse the segment 3 abnormality. Indeed, the phenotype was rescued by treatment with FRAX486 8 h after a single treatment because spine density was decreased to levels similar to WT mice but significantly lower than untreated Fmr1 KO mice (WT + vehicle vs. KO + drug: P > 0.05; KO + vehicle vs. KO + drug: P < 0.001; Fig. 3C). FRAX486 also decreased spine density in Fmr1 KO mice in the neighboring segment 4 (KO + vehicle vs. KO + drug: P < 0.05). Importantly, FRAX486 does not have an effect in segments 1–2 and 5–10, in which there is no phenotype in the mutant mice.
Fig. 3.
FRAX486 rescued increased density of apical dendritic spines in Fmr1 KO mice. (A) Representative apical dendritic segments of layer II/III pyramidal neurons from WT + vehicle (veh), Fmr1 KO + veh, and Fmr1 KO + drug (20 mg/kg FRAX486). (B) Dendrites were divided into 10 segments of 10 µm each based on distance from the soma (proximal to distal, left to right). (C) Spine density was increased in Fmr1 KO + veh compared with WT + veh in segment 3. This phenotype was rescued by FRAX486. Values are mean ± SEM (WT + veh, n = 40 neurons, 4 mice; Fmr1 KO + veh, n = 60 neurons, 6 mice; Fmr1 KO + drug, n = 60 neurons, 6 mice). One-way ANOVA comparing all groups for segment 3, P = 0.0004. Results of Tukey multiple comparison post hoc test comparing two genotypes/treatment groups at each segment are on graph C: *P < 0.05; ***P < 0.001.
Furthermore, FRAX486 did not impact spine density in the basal dendrites in Fmr1 KO cortical neurons nor in the apical or basal dendrites in neurons in WT mice (KO + vehicle vs. KO + drug: P > 0.05; WT + vehicle vs. WT + drug: P > 0.05; Figs. S1 and S2). These findings support the notion that FRAX486 is specifically rescuing the Fmr1 KO abnormality in which the spine phenotype is present in apical neurons and not simply decreasing spine density irrespective of genotype or existence of a phenotype.
FRAX486 Rescues Audiogenic Seizures in Fmr1 KO Mice.
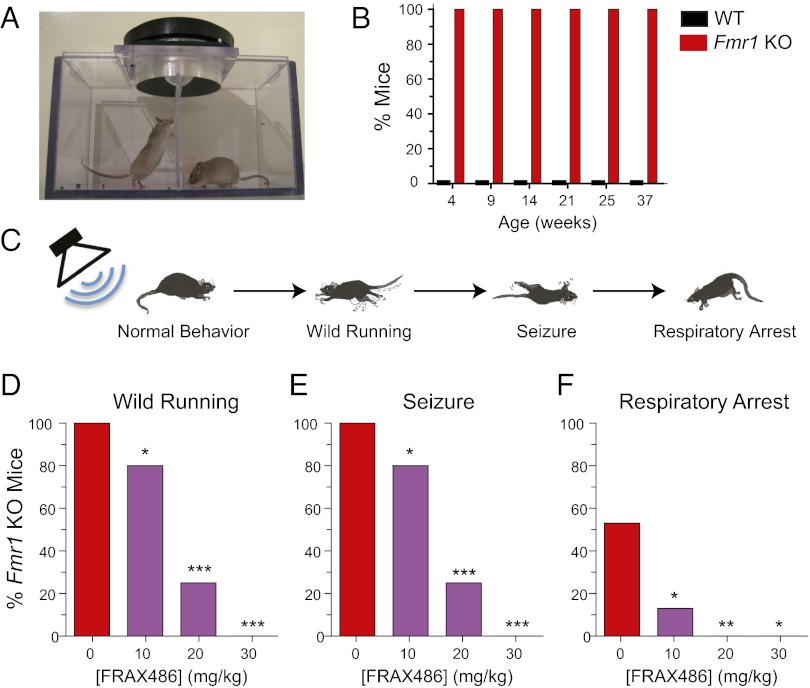
Seizures occur in conjunction with FXS and autism in up to one-quarter of children with these disorders (36, 37). Increased susceptibility to sound-induced seizures, called audiogenic seizures (AGS), is a robust and reliable phenotype in the Fmr1 KO mice that does not occur in WT mice (Fig. 4 A and B). In response to a loud siren-like noise, Fmr1 KO mice exhibited a sequential seizure response of wild running, tonic-clonic seizures, and often respiratory arrest, leading to death.
Fig. 4.
Audiogenic seizure susceptibility was reduced in Fmr1 KO mice by FRAX486. (A) AGS apparatus included a speaker that delivered an auditory stimulus of 124 dB. (B) Fmr1 KO mice of all ages were susceptible to AGS (n = 7). In contrast, WT mice never displayed a seizure phenotype (n = 5–9). (C) Fmr1 KO mice had a sequential seizure response to a loud auditory stimulus. First, there was a phase of wild running and jumping. Then seizures were composed of clonic convulsions and tonic hind limb tensing and extension. Finally, although some Fmr1 KO mice recovered, for many, respiratory arrest led to death (schematic modified from ref. 13). (D–F) FRAX486 reduced incidence of sound-induced wild running (D), seizure (E), and respiratory arrest (F) in Fmr1 KO mice. Percentage of Fmr1 KO animals exhibiting each phase of the sequential seizure response is plotted. Single injections of 0, 10, 20, or 30 mg/kg FRAX486 were administered 8 h before the assay. Values are mean ± SEM (Fmr1 KO + veh, n = 15; Fmr1 KO + 10 mg/kg, n = 15; Fmr1 KO + 20 mg/kg, n =12; Fmr1 KO + 30 mg/kg, n = 5). χ2 test was used to compare incidence in drug treated mice to that of vehicle control: *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether pharmacological inhibition of PAK can rescue seizures, we treated Fmr1 KO mice with vehicle or 10, 20, or 30 mg/kg FRAX486 and induced AGS 8 h later. The incidences of wild running and seizure were reduced from 100% to 80% by 10 mg/kg, to 25% by 20 mg/kg, and completely abolished by 30 mg/kg (P = 0.0339; P < 0.0001; P < 0.0001, respectively; Fig. 4). A dose-dependent decrease in the percentage of Fmr1 KO mice that died as a result of respiratory arrest was also observed (10 mg/kg, P = 0.0101; 20 mg/kg, P = 0.0013; 30 mg/kg, P = 0.0175; the higher dose of 30 mg/kg made some mice sluggish and appear sick; therefore, it was not used in subsequent experiments). As a control, we also treated WT mice with vehicle or 20 mg/kg FRAX486 and, as expected, neither WT group displayed AGS phenotypes (WT + vehicle, n = 9; WT + 20 mg/kg, n = 19). In summary, a single dose of FRAX486 is sufficient to rescue the AGS phenotype in Fmr1 KO mice.
To determine whether daily administration of FRAX486 also rescues the seizure phenotype, we delivered 10 or 20 mg/kg FRAX486 for 5 d and conducted the AGS assay 8 h after the final injection. Daily administration of FRAX486 also reduced seizure susceptibility: The incidence of wild running was reduced from 100% to 75% by 10 mg/kg and to 54% by 20 mg/kg, and the incidence of seizure was reduced from 96% to 50% by 10 mg/kg and to 42% by 20 mg/kg (P = 0.0041; P < 0.0001; P = 0.002; P < 0.0001, respectively; Fig. S3).
To test whether a single dose of FRAX486 has prolonged effects on seizure susceptibility, we administered vehicle or one dose of 20 mg/kg FRAX486 and waited 5 d before conducting the AGS assay. This single dose of FRAX486 was not able to prevent seizures 5 d later (incidence of seizure = 100% and 100%; Fmr1 KO + vehicle, n = 11, Fmr1 KO + 20 mg/kg, n = 10). That is, once the drug was cleared from the body, no lasting effect on seizure susceptibility was observed.
FRAX486 Reduces Hyperactivity and Stereotypical Behaviors.
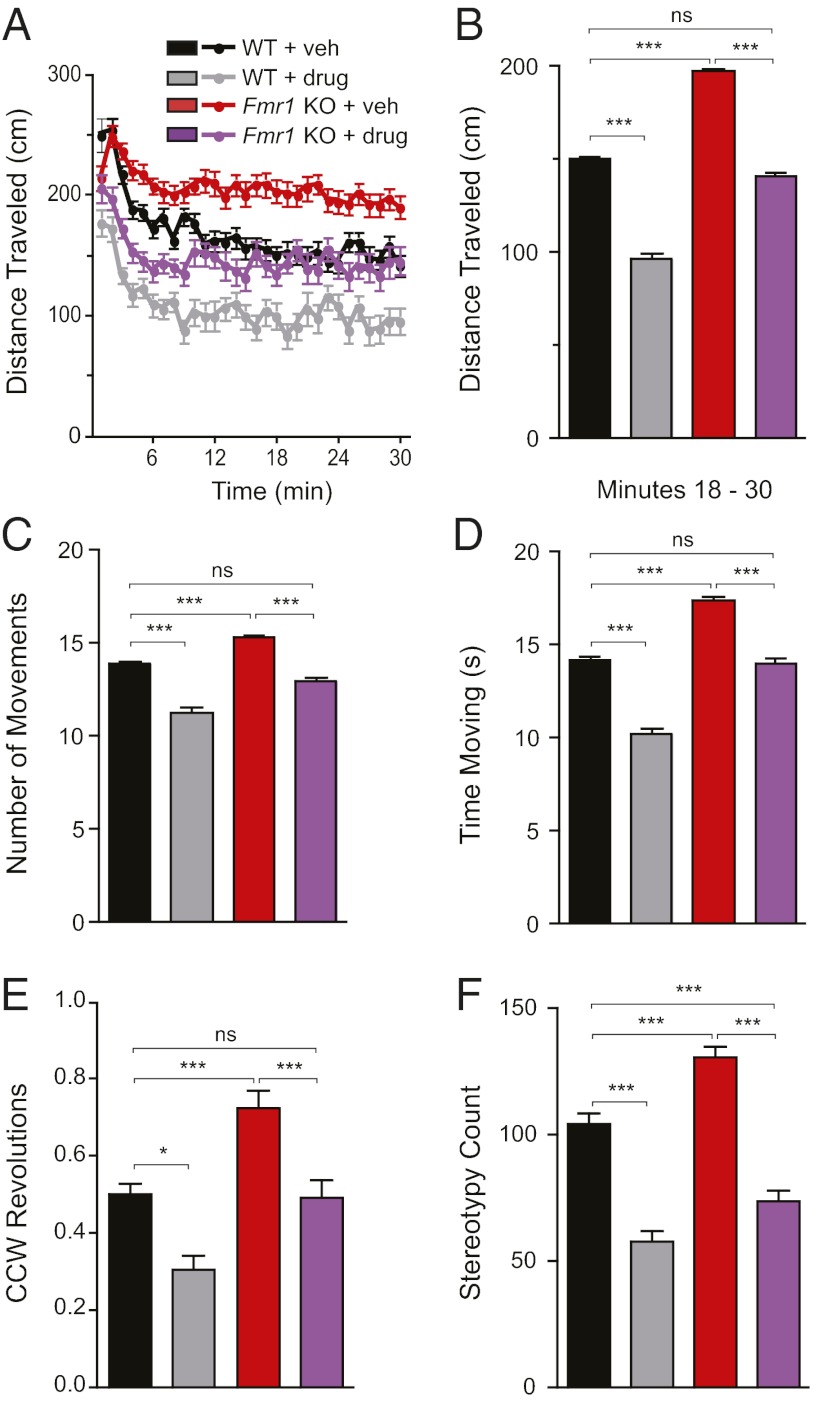
Fmr1 KO mice display the autism-like phenotypes of hyperactivity and restrictive or repetitive behaviors (6, 8). Inhibition of PAK through expression of dnPAK is sufficient to rescue these phenotypes (8). To test whether a pharmacological version of this strategy is also effective, we treated mice with FRAX486 or vehicle alone and conducted an open-field assay. In this assay, a single mouse is introduced to a novel, spacious environment, and its movements are tracked for 30 min. In the later part of the 30-min exploration period, WT mice tend to habituate to the novel environment, but Fmr1 KO mice continue to be active (38). Therefore, although data are presented for each 1-min bin for the initial graph of distance traveled over time (Fig. 5A), all subsequent bar graphs present averages for minutes 18–30 (Fig. 5 B–F).
Fig. 5.
FRAX486 reversed hyperactivity and repetitive behaviors in Fmr1 KO mice. Fmr1 KO mice were more active in the open-field test than were WT littermates. The mutant phenotype manifested itself toward the latter half of the 30-min exploration session, as shown in the bar graphs that average values from minutes 18–30 (B–F). This hyperactivity was rescued by administration of FRAX486 for 5 d. (A and B) Distance traveled. Fmr1 KO + veh traveled a greater distance than WT + veh. However, this increase in motor activity was reversed by treatment with FRAX486; these values were not significantly different than WT + veh, although they were significantly less than values for Fmr1 KO + veh. (C and D) Movement. Fmr1 KO + veh made more horizontal movements and spent more time moving than WT + veh and drug-treated Fmr1 KO. Fmr1 KO + drug did not differ from WT + veh. (E and F) Repetitive behaviors. Fmr1 KO + veh made more counterclockwise revolutions and stereotypical movements than WT + veh. Repetitive behaviors were decreased by drug treatment in both genotypes. Values are mean ± SEM (WT + veh, n = 61; WT + drug, n = 29; Fmr1 KO + veh, n = 62; Fmr1 KO + drug, n = 50). (A) One-minute bins. (B–F) One-way ANOVA with post hoc Tukey-Kramer multiple comparison test for minutes 18–30: *P < 0.05; **P < 0.01; ***P < 0.001, ns = not significant.
As shown in Fig. 5A, the patterns of distance traveled differ for Fmr1 KO mice and their WT littermate controls. First, Fmr1 KO + vehicle traveled a greater distance in minutes 18–30 compared with WT + vehicle (P < 0.01; Fig. 5B). This phenotype was rescued by treatment with FRAX486 for 5 d (P < 0.01), because drug-treated Fmr1 KO mice traveled a distance indistinguishable from WT controls (P > 0.05). Similarly, Fmr1 KO + vehicle made more distinct horizontal movements and spent more time moving than did WT + vehicle (P < 0.05); this hyperactivity was successfully reversed by treatment with FRAX486 for 5 d (P < 0.05; Fig. 5 C and D).
In humans, repetitive behaviors—which include motor stereotypies, repetitive use of objects, and excessive adherence to ritualized patterns of behavior—are one of three criteria for a diagnosis of autism (39). To demonstrate that Fmr1 KO mice exhibit these abnormal behaviors and to assess the ability of the PAK inhibitor FRAX486 to treat these autism-like symptoms, we counted revolutions in small circles and stereotypies in the open field. Stereotypy count is quantified as the number of times a mouse interferes with the same infrared beam (or a set of beams) in a bout of stereotypic activity that typically involves behaviors such as grooming and head bobbing. Fmr1 KO + vehicle revolved in the counterclockwise (CCW) direction significantly more than WT + vehicle (P < 0.05; Fig. 5E). This phenotype was rescued by FRAX486, because CCW revolutions were significantly lower than in Fmr1 KO + vehicle (P < 0.05) but similar to CCW revolutions in WT + vehicle. Fmr1 KO + vehicle exhibited higher levels of stereotypy counts compared with WT + veh, and this phenotype was reverse by FRAX486 treatment (Fig. 5F). In summary, the mouse model of FXS exhibited restrictive and repetitive behaviors, and FRAX486 reversed this phenotype.
Interestingly, FRAX486 also reduced activity—as measured by distance traveled, time spent moving, and number of distinct movements including CCW revolutions and stereotopies—in WT mice. This decrease was not due to sedation because there was no difference between vehicle and FRAX486-treated WT and Fmr1 KO mice on the rotarod test for motor coordination and balance (Fig. S4). In conclusion, the PAK inhibitor FRAX486 reduces hyperactivity and stereotypical movements, both of which are phenotypes that characterize the mouse model of FXS.
Discussion
Here we present evidence that treatment of adult Fmr1 KO mice with the PAK inhibitor FRAX486 is sufficient to reverse the spine density phenotype and to ameliorate autism-like symptoms. We chose an animal model with robust phenotypes that mimic the human FXS symptoms because our primary aim was to develop a therapy with human relevance. The Fmr1 KO mouse on the FVB background exhibits hyperactivity, repetitive behaviors, and seizures into adulthood. All experiments were conducted on the same background strain so that we could correlate the results of neuroanatomical, neurological, and behavioral assays.
Strikingly, FRAX486 rescued every one of the human-like, brain-related phenotypes observed in Fmr1 KO mice. First, humans with FXS and autism have abnormally high densities of dendritic spines (18, 19, 40). Similarly, the cortical neurons of Fmr1 KO mice have increased spine density in a portion of their dendritic tree, which FRAX486 reversed. Second, many FXS and autism patients suffer from seizures (36, 37, 41). Analogously, Fmr1 KO mice have audiogenic seizures that often lead to death (10–12, 42). FRAX486 had a remarkable effect, decreasing seizure susceptibility from 100% to 25% in Fmr1 KO mice. Next, three-quarters of people with FXS also have attention deficit and hyperactivity disorder (43, 44). This symptom, modeled in mice by hyperactivity in the open field, was also reversed by FRAX486. Finally, restrictive behaviors—adherence to routines or repetitive hand flapping—are core criteria for the diagnosis of autism (39, 45). Similarly, Fmr1 KO mice show increased circling behavior and stereotypical movements, both of which were decreased by FRAX486. Interestingly, FRAX486 did not impact body weight or the macroorchidism phenotype (Figs. S5 and S6).
FRAX486 rescues all of these brain-related phenotypes in a manner that is compatible with its potential for human therapeutics. First, rescue was observed in adult mice, suggesting that FRAX486 can reverse symptoms after their onset. Second, FRAX486 has excellent pharmacokinetic properties, crossing the BBB and remaining in the brain for at least 24 h at levels well above the IC50 for group I PAKs. Third, FRAX486 reversed phenotypes rapidly, rescuing seizure and spine phenotypes 8 h after a single drug treatment. This cellular change, which parallels the behavioral improvements we observed, is consistent with the growing literature describing rapid spine elimination or formation in vivo, including spine elimination following fear conditioning and spine formation following ketamine treatment in a stress model of depression (46, 47). Fourth, FRAX486 selectively acted in the same region of dendrite where the Fmr1 mutation increased spine density (segment 3 and neighboring segment 4), but had no effect on other regions of the dendrite. We hypothesize that FRAX486 does not decrease spine density nondiscriminately, but may interact with the FMRP pathway—as PAK and FMRP physically interact—or at least in the same dendritic segments where FMRP is normally active (8). Importantly, this selectivity is likely to reduce the possibility that FRAX486 will have side effects in humans. The localization of the spine phenotype to segment 3 is one of a number of differences between the Fmr1 KO mouse on the FVB and B6 backgrounds, which, in the latter, we previously showed that density increases span segments 1–6 (8). Background strain also influences seizure susceptibility and behavior. Seizure susceptibility was not age-dependent on the FVB background—which allowed us to test the drug in adults—whereas in B6 mice, it is limited to a 3-d window during the juvenile stage (12). In addition, activity levels are higher in both WT and Fmr1 KO mice with genes from the FVB strain (F1 cross of FVB/NJ and B6) than mice of at least six other genetic backgrounds (7). This gives insight into our open-field results, in which FRAX486 may be correcting abnormally high levels of activity in all FVB mice. This reduction in activity regardless of genotype was not mirrored in our spine analysis. This may be related to possible impacts of FRAX486 on spines in other regions of the brain. In this study, we focused on pyramidal neurons of the temporal cortex, a part of the brain implicated in autism in humans, specifically sensitive to PAK inhibition, and previously studied in the Fmr1 KO mouse (8, 30, 40). It remains possible that FRAX486 broadly decreases activity in mice through mechanisms unrelated to spine density; however, the rotarod results demonstrate that the decrease in activity is not due to sedation.
In summary, these discoveries—that a small-molecule PAK inhibitor rescued all of the Fmr1 KO mouse behavioral phenotypes we studied—represent a breakthrough in research for a treatment of FXS. Given that spine phenotypes have also been observed in other forms of autism and intellectual disability, FRAX486 and PAK pathway inhibitors more generally may have therapeutic potential for multiple brain disorders.
Materials and Methods
Adult male Fmr1 KO mice (FVB.129P2-Fmr1tm1Cgr/J) and age-matched WT mice [FVB.129P2-Pde6b+ Tyrc−ch/AntJ (48)], of the genetic background that includes the WT Pde6b allele so that the mice do not suffer from blindness resulting from retinal degeneration, 10–17 wk old were used (Jackson Labs). Animals were housed two to five per cage, with food and water freely available. Mice were kept on a 12-h light/dark cycle and cared for in accordance with the standards of the Massachusetts Institute of Technology Committee on Animal Care and in compliance with National Institutes of Health guidelines.
The small-molecule inhibitor FRAX486 was discovered through a traditional, two-part structure-activity relationship approach. A distinct chemical series was identified in a high-throughput screen. Using IMAP technology from Molecular Devices, 12,000 compounds in a kinase-focused small-molecule library were screened using a FRET-based assay for their ability to inhibit group I PAKs. The 218 small molecules that met the criterion threshold of 50% inhibition at 10 mM were considered hits. These hits, confirmed with a seven-point dose–response assay, constituted a class of unsubstituted pyridopyrimidinones that demonstrated moderate group I inhibition. Although these initial compounds were indeed group I inhibitors, they demonstrated similar inhibition of group II PAKs. A total of 143 of the compounds were handpicked for further profiling and grouped into chemical series. Through a scaffold optimization strategy, we identified FRAX486, a pyridopyrimidinone with a substituted aryl group, which provided good group I potency and selectivity over group II PAKs. Efficacy of this putative inhibitor was tested again with Invitrogen in vitro kinase assays (SelectScreen Biochemical Kinase Profiling Service using a Z′-LYTE functional biochemical assay for 10 titrations of FRAX486). The values (mean ± SEM, n = 2) were graphed as semilog plots, and sigmoidal dose–response curves with variable slope were fit to the data and used to calculate IC50 values.
For in vivo experiments, FRAX486 was dissolved in 20% (wt/vol) hydroxypropyl-β-cyclodextrin vehicle (Sigma-Aldrich). A 2-mg/mL solution of drug or vehicle alone control was administered via s.c. injection at 20 mg/kg in a volume proportional to the animals weight. For pharmacokinetics, forebrain tissue was weighed, fast frozen, and homogenized in 2× volumes of cold PBS. Levels of FRAX486 were determined via liquid chromatography/MS/MS (Apredica). Open-field and rotarod experiments were conducted 4–5.5 h after FRAX486 administration. Audiogenic seizure and dendritic spine analyses were conducted 8 h after drug treatment.
The brains of drug treated 11-wk-old littermates were prepared and analyzed via the Golgi-Cox technique (49, 50). Dendrites of layer II/III pyramidal neurons of the temporal cortex were analyzed in 10 consecutive 10-µm segments to quantify spine density. Primary apical dendrites observed in this study originate 50–100 µm from the cell body.
For the audiogenic seizure assay, a clear acrylic box with two identical square chambers (13 × 13 × 16 cm) divided by a clear partition was designed and built (Fig. 4A). A mouse was placed on either side of the partition and was acclimated to the apparatus for 2 min. Then a speaker in the top of the box presented an auditory stimulus (124-dB siren) until seizures occurred but not longer than 5 min. Mouse behavior was observed for wild running and jumping, seizure, and cardiac arrest leading to death.
The open-field assay was performed in a VersaMax activity monitor chamber with the associated VersaDat software (Accuscan Instruments). Infrared sensors tracked the animal’s horizontal and vertical movements, and the data were combined into 1-min bins. Criteria were established to remove outlier mice based on extreme hyperactivity or adverse effects of FRAX486: mice that scored more than 2 SDs from the mean in total distance traveled over the 30-min period or did not travel horizontally for 5 consecutive minutes were discarded from further analysis.
The rotarod treadmill consisted of a motor-driven, computer-controlled, rotating cylinder divided up into five test zones. One mouse was placed in each test zone on the stationary cylinder such that it was facing away from the eventual direction of the rotation. When all mice were in position, rotation of the cylinder was initiated starting at one revolution per 12 s and accelerating every 40 s. The latency to fall or jump off the rotating cylinder was measured for each mouse (maximum of 3 min). Each mouse was given three trials that are separated by 30–40 min, and the average latency for each mouse is used for comparison.
Supplementary Material
Acknowledgments
We thank Alexander J. Rivest, Junghyup Suh, and Jennie Young for their insightful comments. This work was supported by National Institutes of Health Grants R01-MH078821 and P50-MH58880 (to S.T.), the RIKEN Brain Science Institute, and the Simons Center for the Social Brain at the Massachusetts Institute of Technology.
Footnotes
Conflict of interest statement: S.T., B.M.D., S.G.D., D.A.C., and B.V. have financial interests in Afraxis, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219383110/-/DCSupplemental.
References
- 1.Boyle CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127(6):1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 2.McPheeters ML, et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127(5):e1312–e1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- 3.Hagerman RJ, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123(1):378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verkerk AJ, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 6.The Dutch-Belgian Fragile X Consortium Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78(1):23–33. [PubMed] [Google Scholar]
- 7.Spencer CM, et al. Modifying behavioral phenotypes in Fmr1KO mice: Genetic background differences reveal autistic-like responses. Autism Res. 2011;4(1):40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi ML, et al. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci USA. 2007;104(27):11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176(1):66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103(4):1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 11.Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004;3(6):337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 12.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49(7):1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Dölen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhakar AL, Dölen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses) Annu Rev Neurosci. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalon A, et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudelli RD, et al. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol. 1985;67(3-4):289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- 17.Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41(3):289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 18.Irwin SA, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet. 2001;98(2):161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Comery TA, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94(10):5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin SA, et al. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet. 2002;111(2):140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- 21.Fukazawa Y, et al. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38(3):447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 22.Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18(5):524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16(1):95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 25.Allen KM, et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20(1):25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9(2):234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 27.Schenck A, et al. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38(6):887–898. doi: 10.1016/s0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- 28.Castets M, et al. FMRP interferes with the Rac1 pathway and controls actin cytoskeleton dynamics in murine fibroblasts. Hum Mol Genet. 2005;14(6):835–844. doi: 10.1093/hmg/ddi077. [DOI] [PubMed] [Google Scholar]
- 29.Chen LY, et al. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010;30(33):10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi ML, et al. Altered cortical synaptic morphology and impaired memory consolidation in forebrain-specific dominant-negative PAK transgenic mice. Neuron. 2004;42(5):773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Deacon SW, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15(4):322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boda B, et al. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J Neurosci. 2004;24(48):10816–10825. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asrar S, et al. Regulation of hippocampal long-term potentiation by p21-activated protein kinase 1 (PAK1) Neuropharmacology. 2009;56(1):73–80. doi: 10.1016/j.neuropharm.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, et al. p21-Activated kinases 1 and 3 control brain size through coordinating neuronal complexity and synaptic properties. Mol Cell Biol. 2011;31(3):388–403. doi: 10.1128/MCB.00969-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardridge WM. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry-Kravis E. Epilepsy in fragile X syndrome. Dev Med Child Neurol. 2002;44(11):724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- 37.Hara H. Autism and epilepsy: A retrospective follow-up study. Brain Dev. 2007;29(8):486–490. doi: 10.1016/j.braindev.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: Effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005;135(3):999–1009. doi: 10.1016/j.neuroscience.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 39.American Psychiatric Association . Diagnostic Criteria from DSM-IV. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- 40.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 41.Musumeci SA, et al. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40(8):1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 42.Musumeci SA, et al. Audiogenic seizure susceptibility is reduced in fragile X knockout mice after introduction of FMR1 transgenes. Exp Neurol. 2007;203(1):233–240. doi: 10.1016/j.expneurol.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Simonoff E, et al. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 44.Backes M, et al. Cognitive and behavioral profile of fragile X boys: Correlations to molecular data. Am J Med Genet. 2000;95(2):150–156. [PubMed] [Google Scholar]
- 45.Fryns JP. The fragile X syndrome. A study of 83 families. Clin Genet. 1984;26(6):497–528. doi: 10.1111/j.1399-0004.1984.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 46.Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483(7387):87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 47.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Errijgers V, et al. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6(6):552–557. doi: 10.1111/j.1601-183X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 49.Ramon-Moliner E. The Golgi-Cox Technique in Contemporary Research Methods in Neuroanatomy. New York: Springer; 1970. [Google Scholar]
- 50.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.