Abstract
Decreased cerebral blood flow (CBF) may contribute to the pathology of multiple sclerosis (MS), but the underlying mechanism is unknown. We investigated whether the potent vasoconstrictor endothelin-1 (ET-1) is involved. We found that, compared with controls, plasma ET-1 levels in patients with MS were significantly elevated in blood drawn from the internal jugular vein and a peripheral vein. The jugular vein/peripheral vein ratio was 1.4 in patients with MS vs. 1.1 in control subjects, suggesting that, in MS, ET-1 is released from the brain to the cerebral circulation. Next, we performed ET-1 immunohistochemistry on postmortem white matter brain samples and found that the likely source of ET-1 release are reactive astrocytes in MS plaques. We then used arterial spin-labeling MRI to noninvasively measure CBF and assess the effect of the administration of the ET-1 antagonist bosentan. CBF was significantly lower in patients with MS than in control subjects and increased to control values after bosentan administration. These data demonstrate that reduced CBF in MS is mediated by ET-1, which is likely released in the cerebral circulation from reactive astrocytes in plaques. Restoring CBF by interfering with the ET-1 system warrants further investigation as a potential new therapeutic target for MS.
Multiple sclerosis (MS) is a poorly understood chronic disorder of the CNS, characterized by focal inflammatory demyelinating lesions and degenerative processes (1). Immune responses play a crucial role in the pathogenesis of focal lesions that constitute the pathological substrate for relapses. However, the underlying mechanism of the progressive degeneration of axons, which is the primary determinant of long-term disability in MS, is not clear (2), and treatment is lacking.
A number of studies found that cerebral blood flow (CBF) is globally impaired in early diagnosed relapsing-remitting MS and primary progressive MS, indicating that it is an integral part of the disease that is already present at the time of diagnosis (3–5). Animal studies have shown that chronic hypoperfusion of the brain can lead to neurodegenerative changes, including axonal degeneration (6).
The underlying mechanism of reduced CBF in MS is unknown. Plasma levels of the potent vasoconstrictor endothelin-1 (ET-1) (7) were found to be elevated in patients with MS (8), and this was associated with alterations of extraocular blood flow (9). The reason for the increase in ET-1 levels in MS was unclear, and the findings have not received much attention. We hypothesized that ET-1 might play a role in reducing CBF in MS.
Results
Internal Jugular Vein ET-1 Levels.
Individuals with critical illness and systemic conditions known to be associated with increased levels of ET-1 were excluded (10). Ten subjects with MS according to the revised McDonald criteria (11) and 10 matched control subjects were included. Patients with MS were autonomously seeking treatment at the department of cardiovascular and thoracic surgery of Onze-Lieve-Vrouw Ziekenhuis Aalst for so-called chronic cerebrospinal venous insufficiency syndrome, which is presumed to be caused by stenoses or obstructions of the internal jugular and/or azygos veins (12). However, jugular vein stenoses can also be found in healthy individuals (13). Patients with MS with internal jugular vein stenosis detected by CT venography were scheduled at their own explicit request for angioplasty. The median Expanded Disability Status Scale score of the patients with MS was 4.5 (range, 2–7), and median disease duration was 16 y (range, 2–28 y). Only two patients with MS received immunomodulatory treatment (IFN-β-1a and natalizumab). All patients with MS were in clinically stable condition without evidence of an exacerbation within 3 mo before inclusion. Control subjects were patients without neurological disease who underwent central venous catheter placement before elective cardiac surgery or pacemaker placement at UZ Brussel. There was no significant difference in age and sex between patients with MS and control subjects (56 ± 7 y vs. 59 ± 13 y; five men and five women in both groups). Demographics and clinical characteristics of patients with MS and control subjects are provided in Table S1.
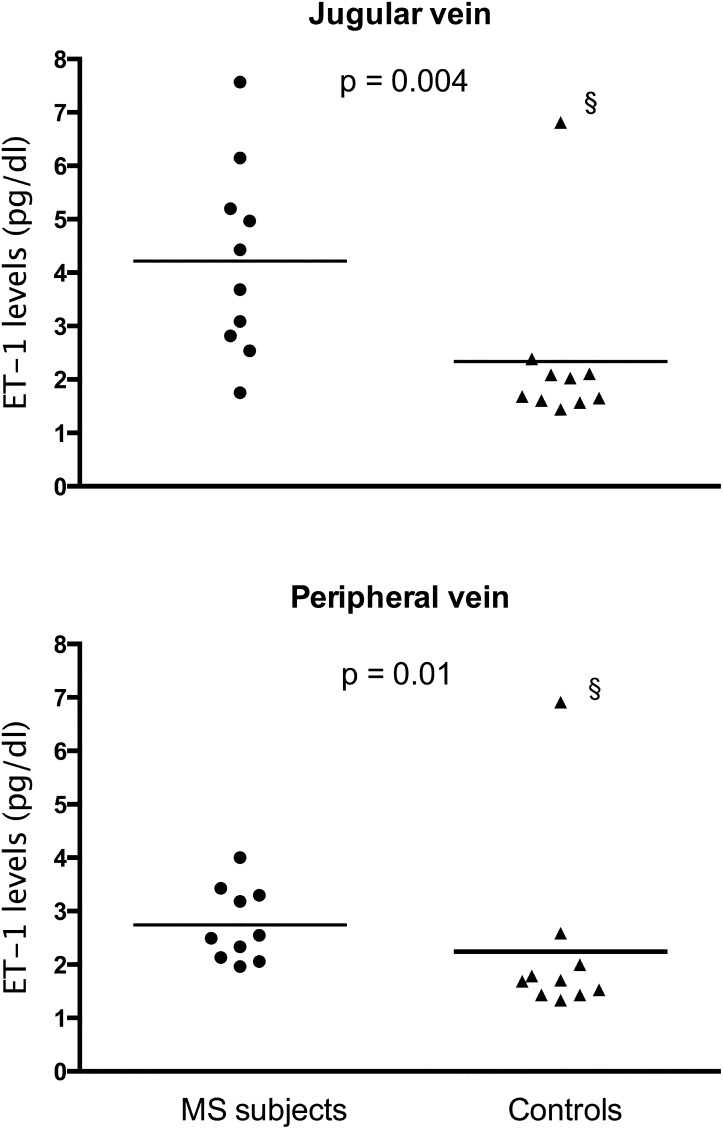
Compared with controls, patients with MS had increased ET-1 levels in plasma derived from the internal jugular vein (mean ± SD, 3.92 ± 1.68 vs. 2.34 ± 1.6 pg/dL; P = 0.004) and peripheral vein (2.74 ± 0.69 vs. 2.24 ± 1.68 pg/dL; P = 0.01; Fig. 1). In patients with MS, but not in control subjects, internal jugular ET-1 plasma levels were significantly higher than those in peripheral vein plasma (P = 0.008). Internal jugular vein/peripheral vein ET-1 ratios were 1.40 ± 0.38 in patients with MS and 1.10 ± 0.25 in controls (P = 0.03).
Fig. 1.
ET-1 plasma levels from the internal jugular vein and a peripheral vein in subjects with MS and control subjects (bar indicates the mean). (§In the control group, there was an outlier with high ET-1 levels, but his internal jugular vein/peripheral vein ET-1 ratio was 0.98, indicating that this was not caused by ET-1 coming from the brain.)
ET-1 Immunohistochemistry.
Characteristics of five patients with MS and five control subjects are shown in Table 1. Standard immunohistochemical techniques of white matter sections of the MS cases showed normal-appearing white matter (NAWM) and six chronic active plaques characterized by demyelination, central gliosis surrounded by phagocytic macrophages, and reactive astrocytes. Reactive astrocytes displayed hypertrophy and increased staining for the intermediate filament GFAP. Blood vessels stained positive for ET-1, but there were no visual differences in intensity of staining between blood vessels in MS plaques, MS NAWM, and white matter of control subjects (Fig. 2). In NAWM of MS cases and control white matter, we found no ET-1 immunostaining of glial cells. In all six plaques, double-labeling immunohistochemistry showed reactive astrocytes that were strongly immunoreactive for ET-1 (Fig. 3).
Table 1.
Characteristics of patients with MS and control subjects on postmortem studies
| Patient no. | Age, y/sex | Condition | Postmortem time, h |
| 1 | 60/M | SP MS | 9 |
| 2 | 43/F | SP MS | 6.5 |
| 3 | 47/F | SP MS | 11 |
| 4 | 51/F | SP MS | 6 |
| 5 | 48/M | SP MS | 7 |
| 6 | 76/M | Control | 12 |
| 7 | 68/F | Control | 10.5 |
| 8 | 69/M | Control | 8 |
| 9 | 54/F | Control | 11 |
| 10 | 72/F | Control | 14 |
SP MS, secondary progressive MS.
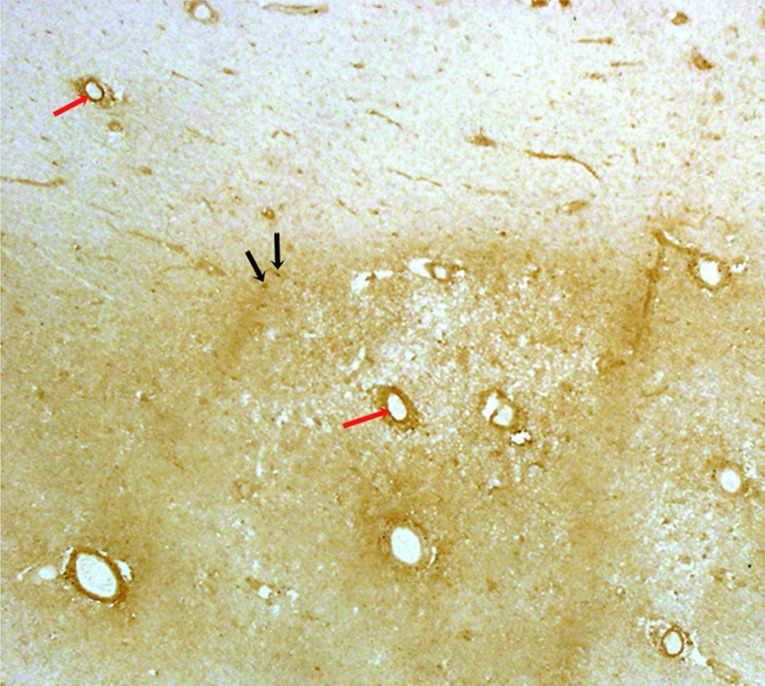
Fig. 2.
Immunostaining for ET-1. Representative photograph a chronic active MS plaque (darker area) and surrounding NAWM (lighter area) immunostained for ET-1 by using the 3′3-diaminobenzidine tetrahydrochloride method. Blood vessel walls in the plaque and NAWM (red arrows) were positive for ET-1. Reactive astrocytes in the plaque (black arrows) were ET-1–positive, whereas astrocytes in NAWM were ET-1–negative (magnification of 2×).
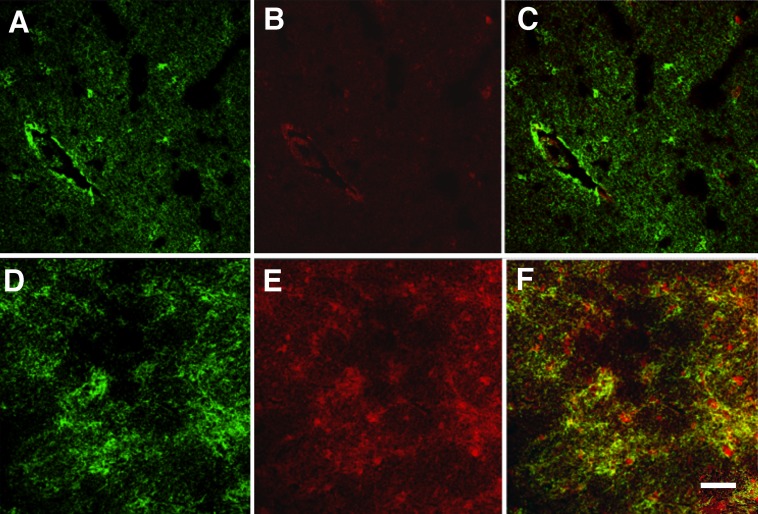
Fig. 3.
Double immunostainings for ET-1 and GFAP. Representative photographs of tissue sections double-immunostained for GFAP (green, A and D) and ET-1 (red, B and E), visualized by confocal laser microscopy. (A–C) Cerebral white matter of a control. There was no colocalization between GFAP and ET-1 (C), indicating that normal astrocytes do not express ET-1. Blood vessels were ET-1–positive. (D–F) Chronic active MS plaque. ET-1 is present in reactive astrocytes (colocalization shown in yellow, F). (Scale bar: 50 µm.)
CBF.
In 15 patients with MS according to the revised McDonald criteria (11) and 15 matched healthy control subjects, CBF was measured noninvasively by using continuous arterial spin labeling (ASL). Patients with MS and control subjects were studied contemporaneously. Regions of interest were NAWM of the centrum semiovale, frontoparietal cortex, thalamus, and cerebellar hemispheres. The median Expanded Disability Status Scale score of the patients with MS was 2 (range, 0–8), and the median disease duration was 7 y (range, 1–22 y). All patients with MS were in clinically stable condition without evidence of an exacerbation within 3 mo before inclusion. Nine patients with MS received immunomodulatory drugs (six were receiving IFN-β-1a, two IFN-β-1b, and one glatiramer acetate). Characteristics of the participants are shown in Table 2. There were no significant differences in age or sex between patients with MS and healthy control subjects. A blinded physicist and radiologist analyzed CBF measurements.
Table 2.
Demographics and baseline CBF in patients with MS and control subjects
| Characteristic | MS (n = 15) | Control (n = 15) | P value |
| Age, y | 0.96 | ||
| Median | 48 | 52 | |
| Range | 27–64 | 25–60 | |
| Female sex | 6 | 6 | — |
| Mean CBF (SD), mL/100 g/min | |||
| NAWM centrum semiovale | 39.96 (17.29) | 47.72 (14.86) | 0.02 |
| Frontoparietal cortex | 54.62 (20.17) | 61.65 (18.61) | 0.10 |
| Thalamus | 49.59 (21.05) | 59.34 (18.7) | 0.01 |
| Cerebellum | 42.4 (17.89) | 53.41 (16.31) | 0.003 |
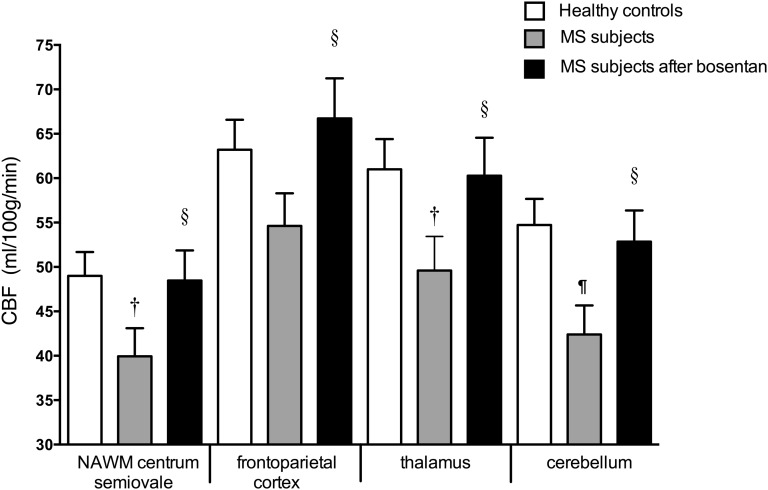
CBF was approximately 20% lower in subjects with MS than in control subjects (Fig. 4 and Table 2). In the patients with MS, CBF measurements were repeated 4 h (peak plasma concentrations) after oral intake of 62.5 mg of the ET-1 receptor antagonist bosentan. After bosentan, CBF in the subjects with MS significantly increased by 20% to values obtained in the control subjects (Fig. 4).
Fig. 4.
CBF in control subjects and in patients with MS before and 4 h after bosentan (†P = 0.02 and ¶P = 0.003, patients with MS at baseline vs. controls; §P < 0.001, patients with MS after bosentan vs. baseline).
Discussion
Our study shows that ET-1 plays a prominent role in the cerebral hypoperfusion in patients with MS. Compared with control subjecs, the internal jugular vein/peripheral vein ET-1 ratio was significantly elevated in patients with MS. Because the jugular venous system is the main route of venous efflux from the brain, this indicates that ET-1 is released form the brain to the circulation. Cerebral blood vessel walls were ET-1–positive, but without differences in intensity of ET-1 immunoreactivity between MS cases and controls. Similar to previous studies, we found that normal astrocytes did not show ET-1 immunoreactivity (14). However, reactive astrocytes in MS plaques stained strongly positive for ET-1. ET-1 produced and released from reactive astrocytes may reach intracerebral arterioles and induce long-lasting vasoconstriction (14). The mechanism responsible for ET-1 up-regulation in reactive astrocytes is speculative, but cytokines that are elevated in MS plaques, including TNF-α and IL-1β (15), can enhance the transcription of ET-1 (16, 17). ET-1 levels were also found to be increased in cerebrospinal fluid of patients with MS (18).
Chronic hypoperfusion of the CNS in animal models induces mitochondrial energy failure and oxidative stress leading to neuronal death (19). White matter is more susceptible to chronic hypoperfusion than gray matter, with an involvement of axonal and myelin components (6). Evidence is evolving that mitochondrial failure and oxidative stress play a crucial role in the axonal degeneration in MS (20, 21). In animals, chronic cerebral hypoperfusion also generates white matter lesions with apoptosis of oligodendrocytes, myelin breakdown, inflammatory reactions, and gliosis, which are all pathological features of MS (22–24). Some actively demyelinating lesions in MS show histological characteristics that are similar to acute white matter ischemic lesions, with a preferential loss of myelin-associated glycoprotein and apoptotic-like oligodendrocyte destruction (25, 26). Epidemiological studies found that patients with MS also have a higher risk for ischemic stroke (27, 28).
Chronic cerebral hypoperfusion in rats is associated with cognitive impairment and neuronal loss in the hippocampal CA1 region (24). MRI studies in patients with MS revealed selective and progressive hippocampal atrophy localized initially to the CA1 subregion (29), which may contribute, among other factors, to cognitive impairment that occurs in many people with MS (30). Axonal degeneration, apoptosis of oligodendrocytes, myelin loss, and hippocampal atrophy are also described in chronic ischemic leukoencephalopathy and Alzheimer’s disease (31–33), which are disorders that are also associated with reactive astrocytes expressing ET-1 immunoreactivity (14) and a global reduced CBF.
The most important limitation of our study is the small number of subjects. This was mainly based on ethical considerations, such as sampling from the jugular vein in patients with MS during an intervention that neurologists do not support because of lack of evidence, and possible hepatotoxicity of bosentan. We did not assess the effect of bosentan in the healthy control subjects. However, this was not the aim of this study, and it would not change our conclusion that reduced CBF in MS improved with bosentan to levels measured in healthy individuals, which supports the role of ET-1. MS groups in the different studies consisted of a mix of patients with relapsing and progressive MS. However, reduced CBF appears an integral part of the disease, which is independent of the course (relapsing onset or progressive onset). It is already present in patients who present with the first clinical manifestations of MS (3–5). Our finding that reduced CBF in subjects with MS is reversible with an ET-1 antagonist opens the door for the exploration of new therapeutic approaches for the degenerative changes MS.
Materials and Methods
Endovascular Procedures.
The ethics committees of Universitair Ziekenhuis (UZ) Brussel and Onze-Lieve-Vrouw Ziekenhuis Aalst approved the study, and written informed consent was obtained from all patients. Endovascular procedures in the patients with MS were always performed by the same experienced cardiovascular surgeon, and in the controls by the same anesthesiologist. Blood samples were collected from the right internal jugular vein in both groups, and from the right femoral vein in patients with MS or antecubital vein in control subjects. Correct localization in the internal jugular vein was radiographically controlled in all subjects. All samples were taken in the morning between 7:00 AM and 12:00 PM. Samples in subjects with MS were obtained before venous dilations, and in controls before any other intervention. Patients with MS and control subjects were studied contemporaneously.
ET-1 Levels.
Blood samples were immediately centrifuged, and plasma was stored at −80 °C. Plasma ET-1 levels were coded and assayed with a commercially available ELISA kit in blinded fashion by a laboratory assistant (detection range, 0–25 ng/mL; Phoenix Pharmaceuticals).
ET-1 Immunohistochemistry.
We obtained approval from the institutional review board for the study of postmortem brain specimens. We studied snap-frozen postmortem obtained brain samples from five patients with pathologically confirmed MS and five control subjects without neurological disease. The samples consisting of white matter and chronic active plaques were stored at stored at −80 °C. They were sectioned (20 μm thick) on a freezing-sliding microtome, placed on polylysine-coated glass slides, fixed for 30 min in buffered paraformaldehyde (pH 7), and treated with 10 µg/mL proteinase-K solution containing 0.1% triton during 10 min at 37 °C to preserve antigen presentation. Tissue sections were analyzed by routine H&E staining for histopathologic changes, by Luxol fast blue staining for evidence of myelin loss, and by immunohistochemistry with CD68 and GFAP for the detection of macrophagic infiltrates and astrocytes, respectively.
We performed single immunostainings on serial sections with mouse anti–human ET-1 (1/100; Abcam) and rabbit anti-human GFAP (1/100; Lifespan Biosciences). Sections were incubated for 48 h at 4 °C, after which they were incubated with secondary antibody, either HRP-conjugated sheep anti-mouse or HRP-conjugated goat anti-rabbit (1/200; Jackson ImmunoResearch), for 2 h at room temperature. Before the addition of the secondary antibodies, sections were treated with 0.1% hydrogen peroxide solution for 20 min to suppress endogenous peroxidase activity. Between all steps, the sections were rinsed thoroughly with PBS solution. Sections were rinsed in 50 mM Tris buffer (pH 7.6), and immunocomplexes were visualized with 3′3-diaminobenzidine tetrahydrochloride (30 mg/100 mL) and 0.01% hydrogen peroxide. After staining, sections were dehydrated in ethanol, cleared in xylol, and embedded in DePeX mounting medium. Sections were examined by light microscopy (BX-50; Olympus). Specificity of the secondary antibody immunoreactivity was assessed by the incubation of tissue sections in 5/100 donkey serum instead of primary antibodies.
Next, we performed double immunostainings with mouse anti–human ET-1 (1/100) and rabbit anti-human GFAP (1/100) by incubating sections in PBS solution overnight at 4 °C. Thereafter, sections were incubated with secondary antibodies: Alexa Fluor-488 goat anti-mouse IgG (FITC-conjugated; 1/100) and Alexa Fluor-568 goat anti-rabbit igG [tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC)-conjugated; 1/100; Molecular Probes] in PBS solution for 2 h at room temperature. Between all steps, the sections were rinsed thoroughly with PBS solution. After the incubation with the antibodies, sections were treated with a solution of 5% Sudan black B (Sigma-Aldrich) in 70% ethanol for 5 min, to block lipofuscin-like fluorescence. Excess of Sudan black B was removed by rinsing the sections quickly in 70% ethanol and distilled water. Sections were embedded in Prolong Gold antifading fluorescent mounting medium (Invitrogen Life Technologies). Fluorescence serial images were taken by using confocal scanning laser microscopy (TCS SP2/AOBS; Leica).
ASL.
The ethics committees of UZ Brussel approved this part of the study. Written informed consent was obtained from all patients. MRI of the brain was performed in supine position by using a 3-T machine (Achieva; Philips). The protocol contained a magnetization prepared 3D TurboFLASH (repetition time, 7.8 ms; echo time, 3.8 ms; inversion time, 970 ms; flip angle, 8°) for anatomical reference. Field of view was 240 × 240 × 150 mm; voxel size was 1 × 1 × 2 mm. Acquisition time was 4.47 min. Cerebral and cerebellar blood flow was assessed with the ASL technique. We used an echo-planar imaging and signal targeting with alternating radio-frequency (EPISTAR) sequence with following parameters: repetition time/echo time of 4,000 ms/20 ms, 11 slices, slice thickness of 8 mm, field of view of 280 × 280 mm, pixel size of 2.88 × 3.71 mm, and labeling delay of 1,200 ms. To increase SNR, we averaged over 40 measurements, for a total acquisition time of 5.28 min. The imaging volume was centered on the centrum semiovale, and slice orientation was transversal. To measure equilibrium magnetization, an EPI sequence with the same parameters and orientation was used, without the labeling pulses.
Postprocessing of the ASL data was performed according to the method described by Golay et al. (34). CBF was calculated in the NAWM of the left and right centrum semiovale (using a T2-weighted scan to avoid placement in plaques), frontoparietal cerebral cortex, thalamus, and cerebellar hemispheres by means of manual region of interest placement. Longitudinal relaxation rates for tissue and blood were 0.91 s−1 and 0.59 s−1, respectively. For determination of the equilibrium magnetization of blood M0b, we started from the equilibrium magnetization of white matter M0wm (35). T2 relaxation times for blood and white matter were 100 ms and 55 ms, respectively. This yielded following simple relationship: M0b = 1.28 M0wm. The final CBF results in each region of interest represent the average value from both hemispheres.
Statistical Analysis.
All statistical analyses were performed with SPSS software (version 17.0; SPSS). A Fisher exact test was used for the analysis of categorical data. Mann–Whitney U or Wilcoxon signed-rank tests were used for the analysis of continuous data where appropriate. All reported P values are two-tailed and were declared statistically significant at the 0.05 level.
Supplementary Material
Acknowledgments
We thank Nadine Wilczak and Anke Desmet for technical support and the Human Brain and Spinal Fluid Resource Center of Los Angeles for providing brain samples. This work was supported by the Research Foundation Flanders (FWO), the Belgian Charcot Foundation, and the Willy Gepts Fund UZ Brussel. M.C. is an FWO fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222560110/-/DCSupplemental.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins A, Scolding N. Protecting axons in multiple sclerosis. Mult Scler. 2008;14(8):1013–1025. doi: 10.1177/1352458508091370. [DOI] [PubMed] [Google Scholar]
- 3.Law M, et al. Microvascular abnormality in relapsing-remitting multiple sclerosis: Perfusion MR imaging findings in normal-appearing white matter. Radiology. 2004;231(3):645–652. doi: 10.1148/radiol.2313030996. [DOI] [PubMed] [Google Scholar]
- 4.D’haeseleer M, Cambron M, Vanopdenbosch L, De Keyser J. Vascular aspects of multiple sclerosis. Lancet Neurol. 2011;10(7):657–666. doi: 10.1016/S1474-4422(11)70105-3. [DOI] [PubMed] [Google Scholar]
- 5.Adhya S, et al. Pattern of hemodynamic impairment in multiple sclerosis: Dynamic susceptibility contrast perfusion MR imaging at 3.0 T. Neuroimage. 2006;33(4):1029–1035. doi: 10.1016/j.neuroimage.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakita H, et al. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924(1):63–70. doi: 10.1016/s0006-8993(01)03223-1. [DOI] [PubMed] [Google Scholar]
- 7.Yanagisawa M, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 8.Haufschild T, Shaw SG, Kesselring J, Flammer J. Increased endothelin-1 plasma levels in patients with multiple sclerosis. J Neuroophthalmol. 2001;21(1):37–38. doi: 10.1097/00041327-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Pache M, et al. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur Neurol. 2003;49(3):164–168. doi: 10.1159/000069085. [DOI] [PubMed] [Google Scholar]
- 10.Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res. 2001;20(3):319–349. doi: 10.1016/s1350-9462(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 11.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 12.Zamboni P, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(4):392–399. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wattjes MP, et al. No association of abnormal cranial venous drainage with multiple sclerosis: A magnetic resonance venography and flow-quantification study. J Neurol Neurosurg Psychiatry. 2011;82(4):429–435. doi: 10.1136/jnnp.2010.223479. [DOI] [PubMed] [Google Scholar]
- 14.Zhang WW, et al. Structural and vasoactive factors influencing intracerebral arterioles in cases of vascular dementia and other cerebrovascular disease: a review. Immunohistochemical studies on expression of collagens, basal lamina components and endothelin-1. Dementia. 1994;5(3-4):153–162. doi: 10.1159/000106714. [DOI] [PubMed] [Google Scholar]
- 15.Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konsman JP, Drukarch B, Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond) 2007;112(1):1–25. doi: 10.1042/CS20060043. [DOI] [PubMed] [Google Scholar]
- 17.Rubanyi GM, Polokoff MA. Endothelins: Molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46(3):325–415. [PubMed] [Google Scholar]
- 18.Speciale L, et al. Endothelin and nitric oxide levels in cerebrospinal fluid of patients with multiple sclerosis. J Neurovirol. 2000;6(suppl 2):S62–S66. [PubMed] [Google Scholar]
- 19.Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox Res. 2003;5(7):491–504. doi: 10.1007/BF03033159. [DOI] [PubMed] [Google Scholar]
- 20.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8(3):280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- 21.Cambron M, et al. White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J Cereb Blood Flow Metab. 2012;32(3):413–424. doi: 10.1038/jcbfm.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomimoto H, et al. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003;106(6):527–534. doi: 10.1007/s00401-003-0749-3. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40(3) suppl:S20–S23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohta H, Nishikawa H, Kimura H, Anayama H, Miyamoto M. Chronic cerebral hypoperfusion by permanent internal carotid ligation produces learning impairment without brain damage in rats. Neuroscience. 1997;79(4):1039–1050. doi: 10.1016/s0306-4522(97)00037-7. [DOI] [PubMed] [Google Scholar]
- 25.Aboul-Enein F, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62(1):25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Lassmann H. Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci. 2003;206(2):187–191. doi: 10.1016/S0022-510X(02)00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch-Henriksen N, Brønnum-Hansen H, Stenager E. Underlying cause of death in Danish patients with multiple sclerosis: Results from the Danish Multiple Sclerosis Registry. J Neurol Neurosurg Psychiatry. 1998;65(1):56–59. doi: 10.1136/jnnp.65.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain. 2004;127(Pt 4):844–850. doi: 10.1093/brain/awh104. [DOI] [PubMed] [Google Scholar]
- 29.Sicotte NL, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131(pt 4):1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 30.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 31.Shiino A, et al. Morphometric characterization of Binswanger’s disease: comparison with Alzheimer’s disease. Eur J Radiol. 2012;81(9):2375–2379. doi: 10.1016/j.ejrad.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Alterations in glia and axons in the brains of Binswanger’s disease patients. Stroke. 1997;28(7):1423–1429. doi: 10.1161/01.str.28.7.1423. [DOI] [PubMed] [Google Scholar]
- 33.Sjöbeck M, Haglund M, Englund E. Decreasing myelin density reflected increasing white matter pathology in Alzheimer’s disease—a neuropathological study. Int J Geriatr Psychiatry. 2005;20(10):919–926. doi: 10.1002/gps.1384. [DOI] [PubMed] [Google Scholar]
- 34.Golay X, Petersen ET, Hui F. Pulsed star labeling of arterial regions (PULSAR): A robust regional perfusion technique for high field imaging. Magn Reson Med. 2005;53(1):15–21. doi: 10.1002/mrm.20338. [DOI] [PubMed] [Google Scholar]
- 35.Wong EC, Buxton RB, Frank LR. A theoretical and experimental comparison of continuous and pulsed arterial spin labeling techniques for quantitative perfusion imaging. Magn Reson Med. 1998;40(3):348–355. doi: 10.1002/mrm.1910400303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.