Abstract
The ability of the brain to adapt to environmental demands implies that neurons can change throughout life. The extent to which single neurons actually change remains largely unstudied, however. To evaluate how functional properties of single neurons change over time, we devised a way to perform in vivo time-lapse electrophysiological recordings from the exact same neuron. We monitored the contralateral and ipsilateral sensory-evoked spiking activity of individual L2/3 neurons from the somatosensory cortex of mice. At the end of the first recording session, we electroporated the neuron with a DNA plasmid to drive GFP expression. Then, 2 wk later, we visually guided a recording electrode in vivo to the GFP-expressing neuron for the second time. We found that contralateral and ipsilateral evoked responses (i.e., probability to respond, latency, and preference), and spontaneous activity of individual L2/3 pyramidal neurons are stable under control conditions, but that this stability could be rapidly disrupted. Contralateral whisker deprivation induced robust changes in sensory-evoked response profiles of single neurons. Our experiments provide a framework for studying the stability and plasticity of single neurons over long time scales using electrophysiology.
Keywords: electroporation, two-photon imaging
It is well established that the morphology and response properties of neurons are highly dynamic during development. Immature cortical neurons are particularly amenable to change during specific stages of development, when spontaneous activity and sensory experience shape their morphology and physiology (1–4). Once formed and stabilized, neurons are thought to maintain some aspects of this plasticity during adulthood, albeit at a lower level (5–7); however, the extent to which single-neuron physiology changes or remains stable in the adult mammalian brain is poorly documented.
Limitations to the study of single-neuron plasticity stem from technical difficulties as well as from the inherent heterogeneity of single neurons in a circuit. Fine changes, like those that underlie functional plasticity, can go undetected due to data averaging and homeostatic events that may cancel out each other. As a result, plasticity is made experimentally more tractable by studying populations after gross manipulations like sensory deprivation or injury. For example, receptive fields of neurons across adult rodent cortices are known to shift in response to different paradigms of sensory input manipulation (8–14). Whether and how other forms of plasticity, such as learning and memory, mark their signature on single neurons is more difficult to deduce from gross manipulation studies. One way to overcome some of these difficulties is through experiments based on time-lapse imaging and electrophysiology (14–18). However, the ability to measure fine temporal physiological events of identifiable single neurons over long time scales remains limited, especially with electrophysiology.
To establish a method to follow the electrophysiology of single neurons over time, we focused on the rodent vibrissa system, an accessible and well-characterized circuit. Ascending inputs from the whiskers on one side of the facial pad innervate layer 4 of the contralateral somatosensory cortex barrel field (S1BF), and the information is then transmitted across other layers (8, 19–21). The ipsilateral whiskers also send information to the same circuits, but these inputs originate from interhemispheric connections (8, 20, 22). We targeted neurons in layer (L) L2/3 and recorded their spiking response preference to ipsilateral and contralateral whisker deflections. These responses provided information about the relative strength and timing of the net contralateral (intrahemispheric) and ipsilateral (interhemispheric) inputs to these cells.
To study long-term plasticity of these neurons, we targeted the same cells twice. We first made blind juxtasomal recordings and then electroporated plasmid DNA into the cells to drive GFP expression. Then, 2 wk later, we used two-photon targeted patching (TPTP) (23, 24) to record from the exact same neuron a second time. During the second recording session, we imaged the dendritic and axonal ramifications of the neuron, enabling further classification based on morphology. Our experiments reveal that single L2/3 pyramidal neurons are remarkably stable under control conditions, but that this stability is readily disrupted by experience-dependent manipulations.
Results
Heterogeneity of SIBF L2/3 Neuron Response Properties.
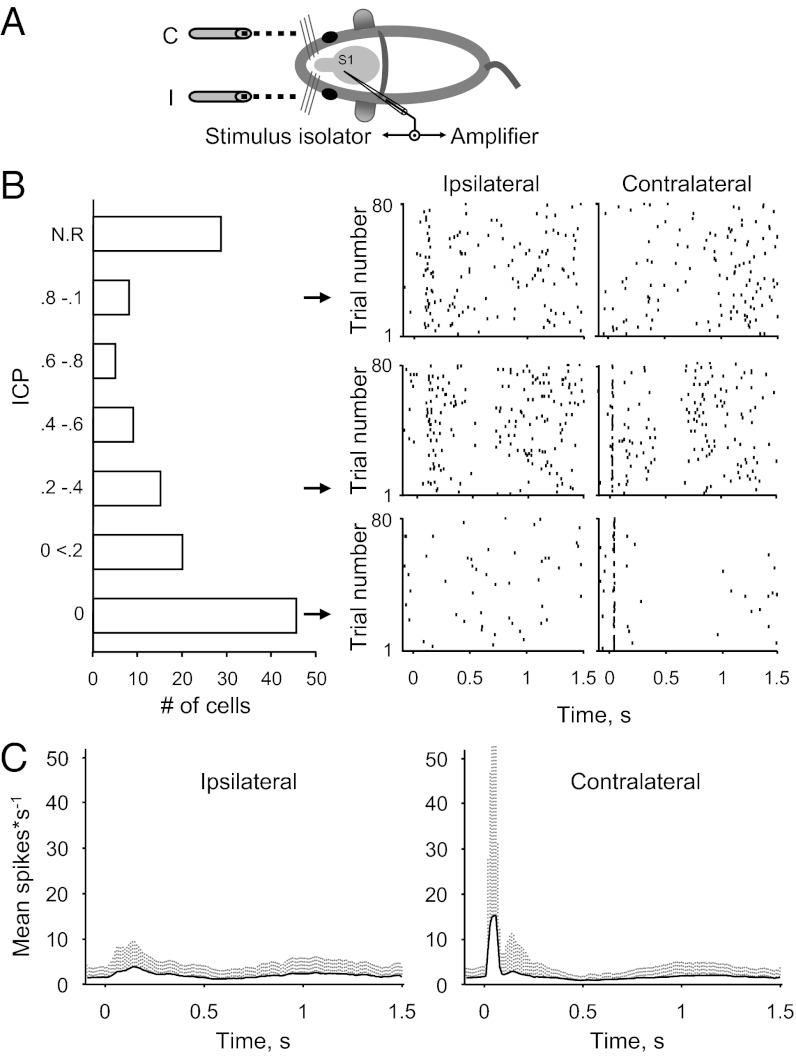
To assess the contralateral and ipsilateral evoked responses in S1BF, we blindly recorded single-unit spike output while presenting alternating air puffs to the contralateral and ipsilateral whiskers (Fig. 1A). Spike output was recorded in cell-attached configurations at 250–450 μm subpial depth (i.e., targeting putative L2/3 neurons). We characterized a response signature of each neuron by calculating an index of ipsilateral-to-contralateral preference [ICP = ipsilateral/(ipsilateral + contralateral)]. An ICP of 0 indicates exclusive dominance of the contralateral responses, and an ICP of 1 indicates an exclusive ipsilateral dominance. We recorded a total of 147 neurons from 38 mice. Using spike waveform analysis, we identified 13 of these 147 cells as fast-spiking neurons. Considering the unique properties of fast-spiking neurons, we analyzed these neurons separately (Fig. S1). Most of the regular spiking neurons (106 of 134) responded to stimuli within a wide range of ICP values (Fig. 1B). As expected from previous anatomic explorations as well as other studies, the majority of neurons responded preferentially to stimulation of the contralateral whiskers (Fig. 1B; mean ICP, 0.21 ± 0.3). On average, the response to contralateral stimuli was greater, more synchronized, and had a shorter latency (Fig. 1C); however, there was a high degree of variability among neurons at the single-cell level (Fig. 1 B and C; note the high SD).
Fig. 1.
Single-cell responses in S1BF are heterogeneous. (A) Experimental scheme. An electrode connected via an electrical relay to an amplifier was used to record from single- neurons in the left hemisphere of S1BF in response to ipilateral (I) and contralateral (C) air puffs. (B) Neuronal responses of single neurons in the barrel field have heterogeneous profiles, as measured here by their ICP. (Left) ICP distribution histogram showing the contralateral preference for L2/3 neurons (mean ICP, 0.21 ± 0.3; n = 106). (Right) Raster plot examples from individual neurons with a range of ICPs. Arrows indicate the pool of distribution from which the cell was taken. NR, not responsive. (C) Population post stimulus time histograms (PSTHs) to ipsilateral and contralateral stimuli (mean ± SD; 10-ms time bins). (Left) Population PSTHs to ipsilateral stimuli (n = 60 neurons). (Right) Population PSTHs to contralateral stimuli (n = 99 neurons).
These data highlight the fact that L2/3 neurons have a rich array of response properties, even when challenged with a simple set of stimuli and even among seemingly similar neuronal subtypes. This heterogeneity poses major challenges to the study of neural coding in general and neural plasticity in particular. In the context of plasticity, one way to deal with such heterogeneity is to study the same neurons repeatedly, an approach that has proven highly useful in anatomical studies (8, 10, 25–31). Thus, we devised a way to study the physiology of single cells over long time scales. We focused on testing the stability of single-neuron ICPs, spontaneous firing rates, probability of response, and latency of response.
Response Profiles of L2/3 Pyramidal Neurons Are Highly Stable.
To study the long-term functional properties of individual pyramidal neurons, we labeled the neurons after the first recording session (designated day 0), thereby enabling future retargeting of the same cell 2 wk later (designated day 14). We used the same electrode to perform juxtasomal recordings from a single neuron and then electroporated it with a DNA plasmid for GFP expression. Details of the design and examples of assessing successes and failures are provided in Figs. S2 and S3. Of note, no neurons that discharged a train of high-frequency spikes after electroporation expressed GFP in the second recording session (Fig. S3). We recorded and electroporated several cells in each animal (range, 1–5 neurons) until we had one neuron with stable activity after electroporation.
Only 50% of the cells with stable activity after electroporation expressed GFP in the second recording session (Fig. S3B). GFP expression was bright enough to allow identification of the same cell again at the second time point by in vivo imaging, in most cases even by epifluorescence (Fig. S4A). Of note, in most (although not all) neurons, both the dendritic and axonal ramifications of single cells were brightly labeled (Fig. 2A and Fig. S4B). Thus, this approach can be used to record from, as well as reveal the local morphology of, cortical neurons in vivo (Fig. 2A).
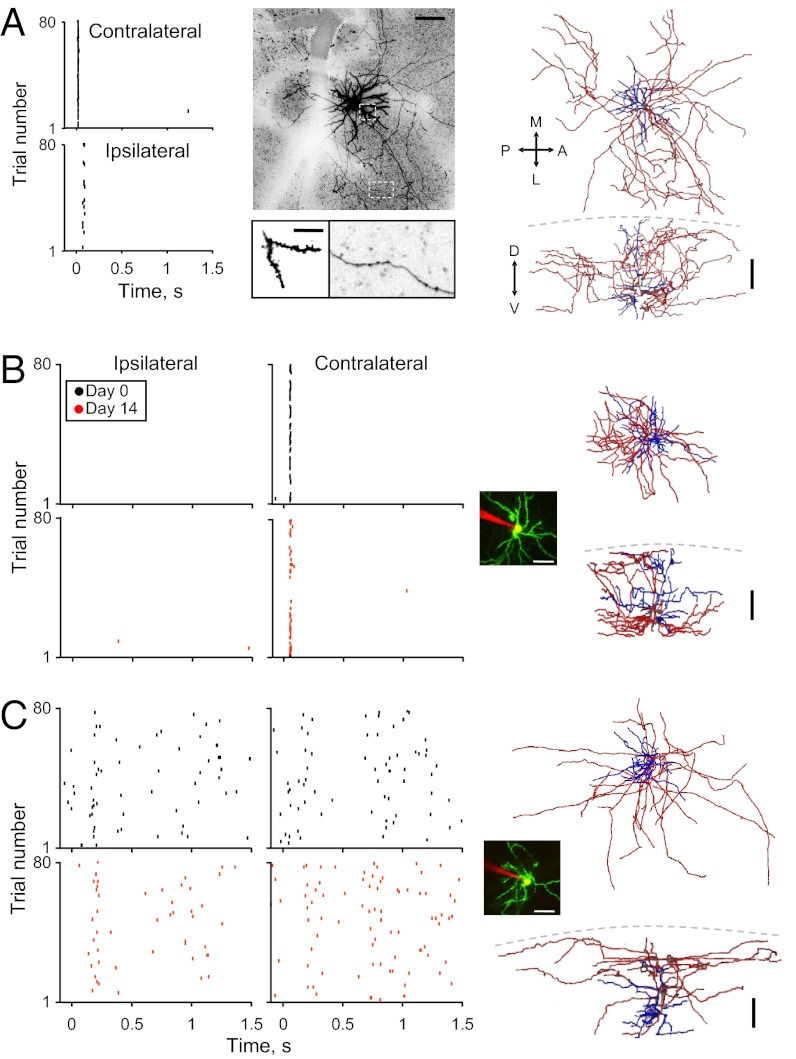
Fig. 2.
Single-neuron responses are highly stable over long time scales. (A) An example of a neuron that was recorded, labeled, and consequently reconstructed. (Left) Raster plots (ICP = 0.3). (Middle, Upper) In vivo micrograph of the same neuron (maximum projection of 400 µm from the surface) at 5 wk after its recording and electroporation. (Scale bar: 100 µm.) (Middle, Lower) In vivo micrographs of a short section of a dendritic (Left) and an axonal (Right) process. (Scale bar: 20 µm.) (Right) Top and side views of a 3D morphological reconstruction of the neuron shown on the left (blue, dendrite; red, axon). A, anterior; D, dorsal; L, lateral; M, medial; P, posterior; V, ventral. The image of GFP fluorescence has been gray-scaled and inverted for clarity. (B and C) Two representative examples from the time-lapse recording experiment. (Left) Raster plots for ipsilateral (Left) and contralateral (Right) air puffs (black top, day 0; red bottom, day 14). (Inset) Maximum projection image of the neuron and recording electrode as imaged in vivo. (Scale bar: 50 µm.) (Right) Three-dimensional reconstructions of the neurons for which responses are shown on the left. Orientation is as in A. (Scale bar: 100 µm.)
We designed our experimental protocol to record from the same neurons twice. To this end, we patched neurons 14 d apart. On both day 0 and day 14, animals were anesthetized with ketamine/medetomidine (Methods). We recorded the responses of L2/3 neurons to contralateral and ipsilateral air puffs and electroporated them with DNA to express GFP (Fig. 2 B and C; black raster plots, day 0). This method allowed us to preselect the neurons for electroporation by screening their functional properties online. We screened for neurons with similar evoked activity as published for pyramidal neurons in S1BF L2/3 (32). Specifically, we electroporated only neurons with low spontaneous firing rates (< 0.5 Hz) that responded to sensory stimuli with a low number of spikes per trial (≤1 evoked spike/stimulus). In addition, we preselected neurons for electroporation according to ICP value. Specifically, we recorded and electropotated L2/3 neurons such that our sample covered the entire ICP range (five neurons from five mice with ICP ranging from 0 to 0.76).
To ensure that we returned to the exact same neuron, we labeled only one neuron per animal (i.e., the first neuron the we encountered with a suitable response profile and with stable activity after electroporation). Then, 2 wk later, we used TPTP to once again record the spontaneous and evoked spiking output of the same neurons (Fig. 2 B and C; red raster plots, day 14). The five neurons that we recorded were verified as being from L2/3, with cell bodies located at a mean 300 ± 60 µm subpial depth. Moreover, each neuron was anatomically imaged and fully reconstructed for anatomical identification. All five neurons were L2/3 spiny pyramidal neurons with an apical dendrite normally branching in layer 1 and multiple branched axonal arbor extending vertically and laterally to varying extents (Fig. 2 A–C). Such a heterogeneous morphology was previously described for neurons located at the supragranular layers of S1 (33).
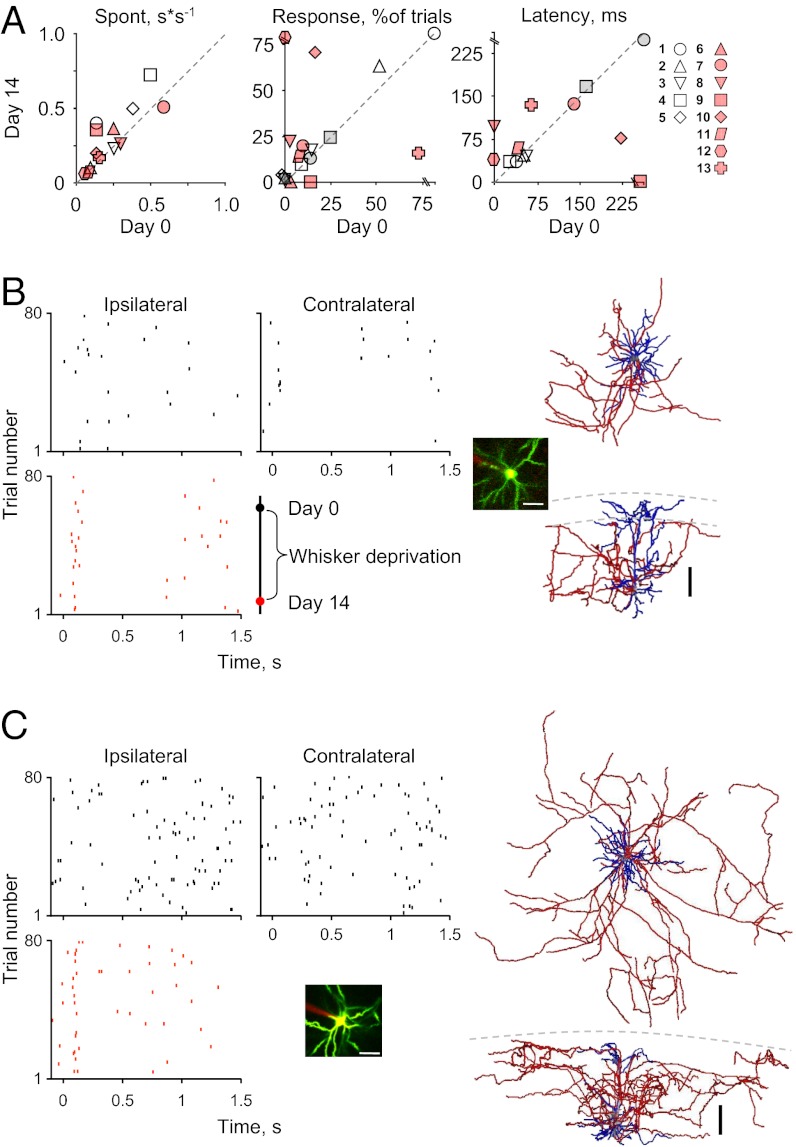
Electrophysiological recordings from the same neurons twice allowed us to evaluate whether spike response patterns of neocortical neurons are stable or dynamic over relatively long time scales. Remarkably, all parameters of all neurons remained stable, and a single neuron could be uniquely identified based on its sensory-evoked physiological fingerprint. For example, ipsilateral and contralateral evoked responses remained similar, as indicated by the stable ICP values over the 2-wk period (Table 1, cells 1–5). High stability was also observed for probability of response, spike latencies, and spontaneous firing rates of individual neurons (Table 1, cells 1–5 and Fig. 3A; note that all gray markers fall close to the diagonal). Notably, in control neurons, none of the physiological parameters differed significantly after 2 wk.
Table 1.
Functional properties of individual L2/3 neurons 14 d apart (day 0 vs. day 14)
| Cell | Spontaneous, spikes⋅s−1 |
Probability, % of trials |
Latency, ms |
ICP |
||||||||
| Contralateral |
Ipsilateral |
Contralateral |
Ipsilateral |
|||||||||
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | |
| 1 | 0.12 ± 0.10 | 0.40 ± 0.20 | 100 | 100 | 13 | 13 | 41 ± 3 | 38 ± 4 | 308 ± 20 | 282 ± 51 | 0.11 | 0.11 |
| 2 | <0.10 | <0.10 | 53 | 62 | 0 | 0 | 50 ± 3 | 48 ± 3 | — | — | 0 | 0 |
| 3 | 0.25 ± 0.12 | 0.25 ± 0.12 | 13 | 16 | 0 | 0 | 49 ± 8 | 44 ± 7 | — | — | 0 | 0 |
| 4 | 0.50 ± 0.16 | 0.75 ± 0.23 | 8 | 10 | 24 | 24 | 28 ± 31 | 43 ± 51 | 165 ± 10 | 177 ± 19 | 0.76 | 0.71 |
| 5 | 0.37 ± 0.16 | 0.50 ± 0.18 | 0 | 0 | 0 | 0 | — | — | — | — | — | — |
| 6 | 0.25 ± 0.14 | 0.37 ± 0.18 | 0 | NA | 0 | 0 | — | — | — | — | — | — |
| 7 | 0.57 ± 0.23 | 0.50 ± 0.20 | 8 | NA | 6 | 22* | 60 ± 7 | — | 142 ± 19 | 134 ± 71 | 0.43 | — |
| 8 | 0.50 ± 0.20 | 0.25 ± 0.12 | 0 | NA | 0 | 21* | — | — | — | 94 ± 8* | — | — |
| 9 | 0.12 ± 0.10 | 0.37 ± 0.60 | 18 | NA | 8 | 0* | 38 ± 11 | — | 288 ± 11 | —* | 0.31 | — |
| 10 | 0.12 ± 0.10 | 0.20 ± 0.12 | 45 | NA | 14 | 73 | 40 ± 2 | — | 219 ± 27 | 73 ± 26* | 0.24 | — |
| 11 | <0.10 | <0.10 | 85 | NA | 8 | 12 | 38 ± 6 | — | 40 ± 16 | 54 ± 28 | 0.07 | — |
| 12 | <0.10 | <0.10 | 92 | NA | 0 | 82* | 44 ± 3 | — | — | 32 ± 4* | 0 | — |
| 13 | 0.14 ± 0.14 | 0.15 ± 0.12 | 52 | NA | 72 | 16* | 150 ± 42 | — | 63 ± 7 | 122 ± 16* | 0.63 | — |
For each neuron, the following parameters are shown: spontaneous spike rate (mean ± SD), probability of response (% of trials for each stimulus), latency (mean ± SD), and ICP at the two time points (day 0 and day 14). Cells 1–5, control experiments; cells 6–13, sensory-deprived experiments. NA, not applicable. Significant changes in probability of response (χ2 test, df = 1), and latency (t test) are indicated by asterisks (*P < 0.05). Note that none of the neurons in the control condition changed significantly.
Fig. 3.
Single-cell plasticity after deprivation is heterogeneous. (A) Stability/changes in response properties for all neurons that we recorded twice at 14 d apart. The five control neurons are indicated by open (ipsilateral) and gray (contralateral) symbols. The eight neurons of the deprivation experiment are shown in red symbols (ispilateral only). (Note that cell 13 is a fast-spiking neuron.) (B and C) Two representative examples of single-cell time-lapse recording. Electrophysiology measurements were performed before and 2 wk after sensory deprivation. (Left) Raster plots in response to ipsilateral air puffs (black top, day 0; red bottom, day 14). Contralateral responses are shown only for day 0, when whiskers were intact. (Inset) Maximum projection image of the neuron and recording electrode. (Scale bar: 50 µm.) (Right) Three-dimensional reconstructions of the neurons for which responses are shown on the left. Orientation is as in Fig. 2A. (Scale bar: 100 µm.)
Neurons maintained stable response statistics regardless of their initial response profile or morphology. For example, the neuron shown in Fig. 2B (Table 1, cell 2) hardly fired spontaneously in either session, but maintained its contralateral-specific response latency (50 ± 3 ms vs. 48 ± 3 ms) and probability of response (53% vs. 62%). The neuron shown in Fig. 2C (Table 1, cell 4) displayed ipsilateral selectivity in both sessions (ICP, 0.76 on day 0, 0.71 on day 14), along with stable ipsilateral and contralateral response reliability, latency, and spontaneous spike rate (Table 1). Taken together, these data show that the spiking output of individual L2/3 neurons changed little over the 2-wk period.
Experience-Dependent Plasticity of Single Neurons.
Given our finding that the physiology of L2/3 pyramidal neurons remains stable over long time scales, we next investigated the extent to which this stability endures when challenged with a manipulation of sensory experience. We determined the response properties of individual neurons and then partially deprived the mice of sensory input by removing all contralateral whiskers relative to our recording site (eight neurons from eight mice with ICP ranging from 0 to 0.63). We chose this manipulation because previous whisker deprivation experiments have shown that neuronal responses can be modulated in adult rodents (8, 34).
We found that 2 wk of contralateral whisker trimming induced marked changes in single-neuron responses to stimulation of the intact ipsilateral whiskers (Table 1, cells 6–13 and Fig. 3). Deprivation affected the neuronal probability of response and timing of response, but not spontaneous activity (Fig. 3A, red markers). Two neurons with different initial response profiles are shown in Fig. 3 B and C. The neuron in Fig. 3B (Table 1, cell 7) had an initial ICP of 0.43, with a low probability to respond to ipsilateral and contralateral stimuli at day 0. After 2 wk of contralateral whisker removal, the same cell displayed a fourfold increase in its probability to respond to the ipsilateral stimulus (from 6% to 22%), with no apparent effect on response latency (Fig. 3A, red circle). The neuron shown in Fig. 3C (Table 1, cell 8) did not respond to either ipsilateral or contralateral stimuli at day 0. By day 14 of whisker deprivation, the neuron displayed a marked ispilateral response with a latency of 94 ± 8 ms, generating a spike every fifth trial on average (i.e., probability of response to 21% of trials; Fig. 3A, red triangle).
Of note, not all neurons responded to contralateral whisker removal in the same manner. For example, one of the neurons (Table 1, cell 9 and Fig. 3A, red square) that responded to ipsilateral and contralateral stimuli at day 0 (ICP = 0.31) did not respond to sensory stimuli by day 14, and two neurons exhibited no significant change (Table 1, cells 6 and 11 and Fig. 3A). Thus, sensory experience affects the evoked response of individual cortical neurons in different ways and to varying degrees.
Discussion
In this work, we combined juxtasomal electrical recording with single-cell electroporation and TPTP to patch single neurons twice, in vivo. We found that response profiles of individual neocortical neurons remained highly stable under control conditions (Table 1 and Figs. 2 and 3A), but individual neuron response profiles readily changed in a heterogeneous manner when mice were deprived of sensory input.
A central motivation for our study was the fact that cortical neurons are often highly heterogeneous (35–37). This variability poses challenges to the study of functional plasticity using “snapshot” measurements. In particular, studies of plasticity using snapshot measurements can be missing information, because biologically significant changes in opposite directions may be canceled out when averaged across populations. Of note, recent improvements in genetically encoded calcium indicators allow measurements of physiological activity (by genetically encoded calcium-binding proteins) of the same individual neurons over time (14, 16, 18, 38, 39). In addition, single-cell electroporation has been used to load neurons with dyes and indicators. Loading can be done within minutes, and neurons can be studied for a few hours after electroporation (40–42). What then are the advantages of our technique, which is based on a very low sampling density?
Alrhough each method has its strengths and weaknesses, the advantages of our method over calcium imaging include high unit isolation, high temporal resolution for determining precise spike timing at ms resolution, and the ability to reveal near-complete neuronal morphology. These advantages can be instrumental not only in complementing the limited temporal information of calcium imaging studies, but also in their own right. For example, our method can be used to study cell-autonomous molecular mechanisms of long-term plasticity by introducing genetic material (e.g., shRNA) to single neurons in an otherwise unperturbed environment. Our technique is still limited in terms of sampling density and success rate (Fig. S3). Future studies may improve this rate by, for example, providing visual online feedback during the experiment and optimizing electrode parameters for electroporation.
Our time-lapse electrical recordings of individual L2/3 pyramidal neurons revealed highly stable timing and probability of response to contralateral and ipsilateral whisker deflection in S1BF L2/3 pyramidal neurons (Figs. 2 and 3A and Table 1). Although response properties and morphologies varied considerably among neurons, long-term stability did not. This result suggests that the intrahemispheric and interhemispheric synaptic input to individual L2/3 neurons in the adult neocortex remain in a stable steady state. The well-established phenomena of presynaptic and postsynaptic terminal replacement (10, 25, 28) do not seem to significantly alter the spiking output of a neuron, at least not for simple stimuli. Taken together with earlier imaging experiments, our data show that physiological stability is a general feature of pyramidal neurons in the somatosensory cortex (14, 16). In control mice, the mature cortical network is under homeostatic control, which keeps sensory information processing continuously in tune (8, 43).
Considering the sensitivity of L2/3 neurons to manipulations of sensory experience, the strongly modulated neuronal response properties that we measured were not surprising (Fig. 3). Our data, however, highlight that even massive changes in sensory input induce heterogeneous effects at the single-cell level (Table 1 and Fig. 3). Such heterogeneous forms of functional plasticity could be important for teasing apart cellular mechanisms of cortical plasticity. Future analyses of morphological and functional dynamics from other neuronal cell types or across layers should provide more insight into how experience adjusts cortical circuit function over time. Our work provides a proof of principle that time-lapse electrophysiological recordings of single neurons is a feasible experimental tool for studying the dynamic relationship between neuronal structure and function.
Methods
Surgical Procedures.
All experimental procedures used in this study were approved by Hebrew University’s Animal Care and Use Committee. Male NMRI or CD1 mice (n = 60, 6 wk or 8 wk old) were anesthetized in both recording sessions using ketamine and medetomidine (100 and 0.83 mg/kg i.p., respectively). Rectal temperature was maintained at 36 ± 1 °C and monitored continuously. A metal pin was glued to the skull using dental cement and then connected to a custom stage, to allow precise positioning and repositioning of the head during the time-lapse recording sessions.
In the first recording session, a small opening (<0.2 mm2) was made with a drill over the left hemisphere barrel field to accommodate insertion of the glass pipette through one or two separate penetration points at a shallow angle (2.5 to −3 mm lateral to the midline, 1–1.5 mm posterior to bregma, −0.25 to −0.45 mm from the brain surface). In the second session, a larger craniotomy (>4 mm2) was performed over the same area.
Whisker Deprivation.
Once the mice recovered from the first recording session, all contralateral whiskers were removed every other day for 2 wk.
Electrophysiology and Electroporation.
Cell-attached recordings were obtained using blind/targeted patch-clamp recordings (23, 24, 44). Electrodes (∼7 MΩ) were pulled from filamented, thin-walled borosilicate glass (outer diameter, 1.5 mm; inner diameter, 1.0 mm; Hilgenberg). The internal solution contained 140 mM K-gluconate, 10 mM KCl, 10 mM Hepes, 10 mM Na2-phosphocreatine, 4 mM MgATP, 0.4 mM Na2GTP, and 0.5 mM EGTA, and was adjusted to pH 7.25 with KOH. In the first recording session, the internal solution also contained 0.2 µg/µL plasmid DNA (driving GFP expression under a CAG promoter), which was replaced in the second session by 20 µM Alex Fluor 568. After amplification, the plasmid was extracted, purified, concentrated to ∼5 µg/µL (in double-distilled water), and then diluted to final concentration in the internal pipette solution. Recordings were acquired at 10 kHz with an intracellular amplifier in current clamp mode (Multiclamp 700B; Molecular Devices) and filtered using a 50-Hz high-pass filter (Digidata 1440A; Molecular Devices).
A custom-made relay configuration allowed us to toggle between electrical recording and electroporation using the same electrode. The double-pole, double-throw relay (DPDT basic relay; Teledyne Relays) served two functions. First, it allowed us to switch between an amplifier configuration for recording the small currents generated by spikes and a high-voltage source configuration for DNA electroporation using large currents (Fig. S2). Second, by grounding the head-stage amplifier during electroporation, it protected the amplifier’s input and minimized the period needed to reestablish the recording session (Figs. S2C and S3). In addition, grounding the current injection system during recording minimized line noise. The large current required for electroporation was generated by a stimulus isolator (ISO-Flex; AMPI) driven by a computer-controlled programmable pulse stimulator (Master-8; AMPI).
Electroporation Parameters.
Between 50 and 80 square 1–2 µA (10–20 V) pulses of 0.5-ms duration at a frequency of 50 Hz were effective for electroporation. The electroporation parameters were optimized for each cell by changing the injected current amplitude, following a calculation of the effective negative voltage in the range of 20–40 V [(pipette resistance + membrane seal resistance) × injected current = effective voltage].
Neurons firing at a high frequency (>20 Hz) after electroporation could not be reidentified in S1BF at the second recording session 2 wk later, suggesting that these cells did not recover from the electroporation (Fig. S3). Therefore, we recorded and electroporated several neurons in each animal (range, 1–5) until we had at least one neuron with stable activity after electroporation.
Imaging.
In vivo imaging was performed using an Ultima two-photon microscope (Prairie Technologies). Two-photon excitation (900 nm) was delivered with a DeepSee femtosecond laser (Spectraphysics). The laser beam was expanded to fill the large back aperture of the 16× objective (0.8 NA, Nikon CF175). Z-stack images of the cell (512 × 512 or 1,024 × 1,024 pixels) were acquired at 1.32- or 0.66-μm/pixel resolution in the XY dimension and 2–4 μm between frames in the Z dimension. Care was taken to adjust fluorescence levels to partial saturation of the central region of the cell bodies. For several neurons, we acquired four partially overlapping Z-stacks, to obtain a larger portion of the neuron. Z-stack 3D stitching was performed with the Fiji 3-D Stitching plug-in. Anatomical 3D reconstruction was performed with the Fiji Simple Neurite Tracer plug-in.
Somatosensory Stimuli.
Eighty air puffs (10 psi, 100 ms) were delivered at 0.625 Hz, alternating between contralateral and ipsilateral whiskers (a total of 160 trials). Stimuli were controlled by an electrical valve triggered by a programmable stimulator (Master-8; AMPI). The air puff delivery system had a constant 14-ms delay from the time of triggering to whisker deflection. Latency values were measured from the time of triggering.
Data Analysis.
Analysis was performed using custom-written Matlab code (MathWorks). Spikes recorded in cell-attached mode were extracted from raw voltage traces by thresholding. Spike times were then assigned to the local peaks of suprathreshold segments and rounded to the nearest millisecond.
For spontaneous firing rate, all trials were assigned to a raster plot based on chronological order. A neuron's spontaneous firing rate was calculated based on the 200 ms preceding each stimulus presentation (for a total 32 s per cell). Responsiveness to air puffs was assessed by calculating the firing rates over all trials for ipsilateral and contralateral stimuli (i.e., the PSTH, using a 10-ms bin size) within a response window. The response window was identified for each neuron separately as follows. The PSTH vector mean was subtracted from the PSTH to locate the onset and offset of the evoked response with the shortest latency (i.e., around the first peak in the PSTH after onset of the stimulus). The size of the response window varied between ∼20 and ∼100 ms depending on response jitter. The spike rate in this window was compared with the spike rate before stimulus onset; if there was significant increase in spike rate in the response window, then the neuron was considered responsive to air puffs.
Each neuron’s ICP value was calculated for the spike rates in the response window. The probability of response to a stimulus was calculated as the percentage of trials in which a spike was generated within the response window. Latency was calculated as the timing (mean ± SD) of the appearance of spikes within the response window for ipsilateral and contralateral stimuli.
Supplementary Material
Acknowledgments
We thank the members of the A.M. laboratory for comments and discussions. This work was supported by the European Research Council [Grant 203994 (to A.M.)], the Israeli Science Foundation [Grant 1338/10 (to Y.Y.)], and by the Gatsby Charitable Foundation. L.C. is supported by a fellowship from the Edmond and Lily Safra Center for Brain Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214434110/-/DCSupplemental.
References
- 1.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 4.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 5.de Villers-Sidani E, Merzenich MM. Lifelong plasticity in the rat auditory cortex: Basic mechanisms and role of sensory experience. Prog Brain Res. 2011;191:119–131. doi: 10.1016/B978-0-444-53752-2.00009-6. [DOI] [PubMed] [Google Scholar]
- 6.Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82(3):109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Sun QQ. The balance between excitation and inhibition and functional sensory processing in the somatosensory cortex. Int Rev Neurobiol. 2011;97:305–333. doi: 10.1016/B978-0-12-385198-7.00012-6. [DOI] [PubMed] [Google Scholar]
- 9.Jacob V, Petreanu L, Wright N, Svoboda K, Fox K. Regular spiking and intrinsic bursting pyramidal cells show orthogonal forms of experience-dependent plasticity in layer V of barrel cortex. Neuron. 2012;73(2):391–404. doi: 10.1016/j.neuron.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 11.De Paola V, et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49(6):861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436(7048):261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 13.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 14.Margolis DJ, et al. Reorganization of cortical population activity imaged throughout long-term sensory deprivation. Nat Neurosci. 2012;15(11):1539–1546. doi: 10.1038/nn.3240. [DOI] [PubMed] [Google Scholar]
- 15.Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009;7(7):e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber D, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484(7395):473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mank M, et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5(9):805–811. doi: 10.1038/nmeth.1243. [DOI] [PubMed] [Google Scholar]
- 18.Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front Cell Neurosci. 2010;4:3. doi: 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56(2):339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Douglas RJ, Martin KA. Mapping the matrix: The ways of neocortex. Neuron. 2007;56(2):226–238. doi: 10.1016/j.neuron.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 2006;4(12):e382. doi: 10.1371/journal.pbio.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer LM, et al. The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science. 2012;335(6071):989–993. doi: 10.1126/science.1217276. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura K, Judkewitz B, Kano M, Denk W, Häusser M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat Methods. 2008;5(1):61–67. doi: 10.1038/nmeth1150. [DOI] [PubMed] [Google Scholar]
- 24.Margrie TW, et al. Targeted whole-cell recordings in the mammalian brain in vivo. Neuron. 2003;39(6):911–918. doi: 10.1016/j.neuron.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 26.Chen JL, et al. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14(5):587–594. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JL, et al. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74(2):361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441(7096):979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 29.Leslie JH, Nedivi E. Activity-regulated genes as mediators of neural circuit plasticity. Prog Neurobiol. 2011;94(3):223–237. doi: 10.1016/j.pneurobio.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livneh Y, Mizrahi A. Experience-dependent plasticity of mature adult-born neurons. Nat Neurosci. 2012;15(1):26–28. doi: 10.1038/nn.2980. [DOI] [PubMed] [Google Scholar]
- 31.Mizrahi A. Dendritic development and plasticity of adult-born neurons in the mouse olfactory bulb. Nat Neurosci. 2007;10(4):444–452. doi: 10.1038/nn1875. [DOI] [PubMed] [Google Scholar]
- 32.de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol. 2007;581(Pt 1):139–154. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruno RM, Hahn TT, Wallace DJ, de Kock CP, Sakmann B. Sensory experience alters specific branches of individual corticocortical axons during development. J Neurosci. 2009;29(10):3172–3181. doi: 10.1523/JNEUROSCI.5911-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glazewski S, Benedetti BL, Barth AL. Ipsilateral whiskers suppress experience-dependent plasticity in the barrel cortex. J Neurosci. 2007;27(14):3910–3920. doi: 10.1523/JNEUROSCI.0181-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat Neurosci. 2010;13(3):353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- 36.Ohki K, Reid RC. Specificity and randomness in the visual cortex. Curr Opin Neurobiol. 2007;17(4):401–407. doi: 10.1016/j.conb.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11(7):749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- 38.Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484(7392):62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6(12):875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nevian T, Helmchen F. Calcium indicator loading of neurons using single-cell electroporation. Pflugers Arch. 2007;454(4):675–688. doi: 10.1007/s00424-007-0234-2. [DOI] [PubMed] [Google Scholar]
- 41.Hovis KR, Padmanabhan K, Urban NN. A simple method of in vitro electroporation allows visualization, recording, and calcium imaging of local neuronal circuits. J Neurosci Methods. 2010;191(1):1–10. doi: 10.1016/j.jneumeth.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagayama S, et al. In vivo simultaneous tracing and Ca(2+) imaging of local neuronal circuits. Neuron. 2007;53(6):789–803. doi: 10.1016/j.neuron.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10(3):358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 44.Judkewitz B, Rizzi M, Kitamura K, Häusser M. Targeted single-cell electroporation of mammalian neurons in vivo. Nat Protoc. 2009;4(6):862–869. doi: 10.1038/nprot.2009.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.