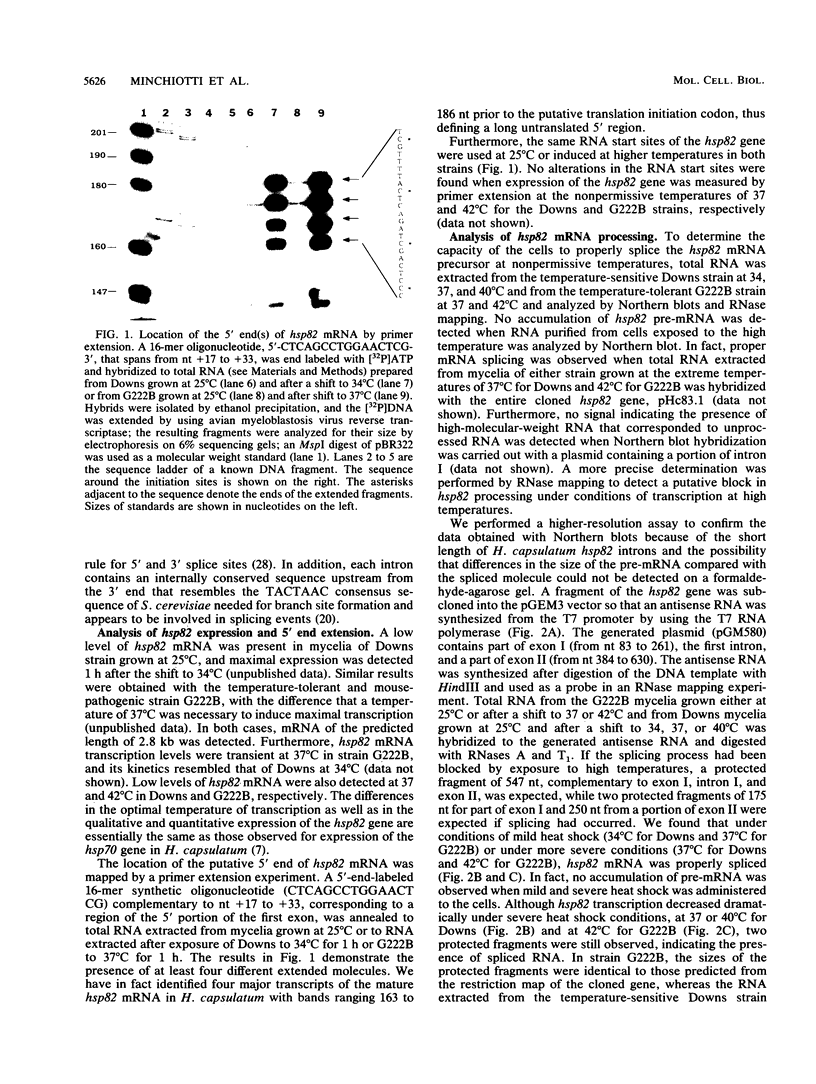
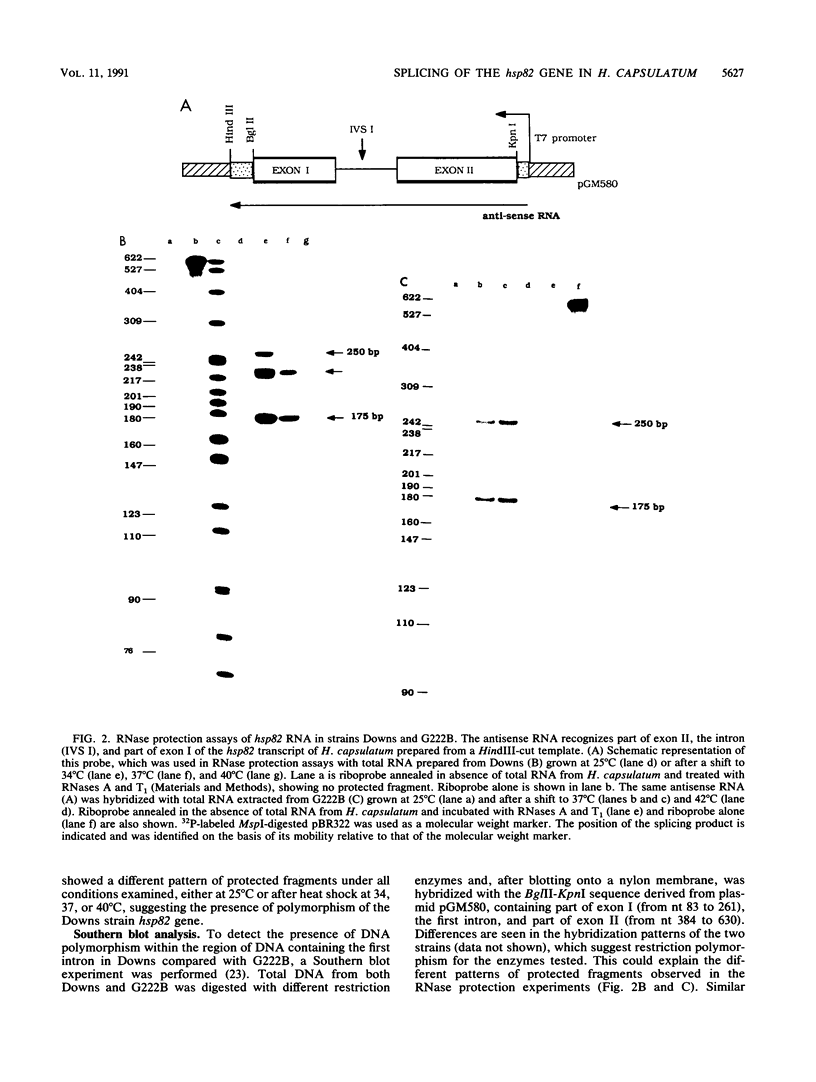
Abstract
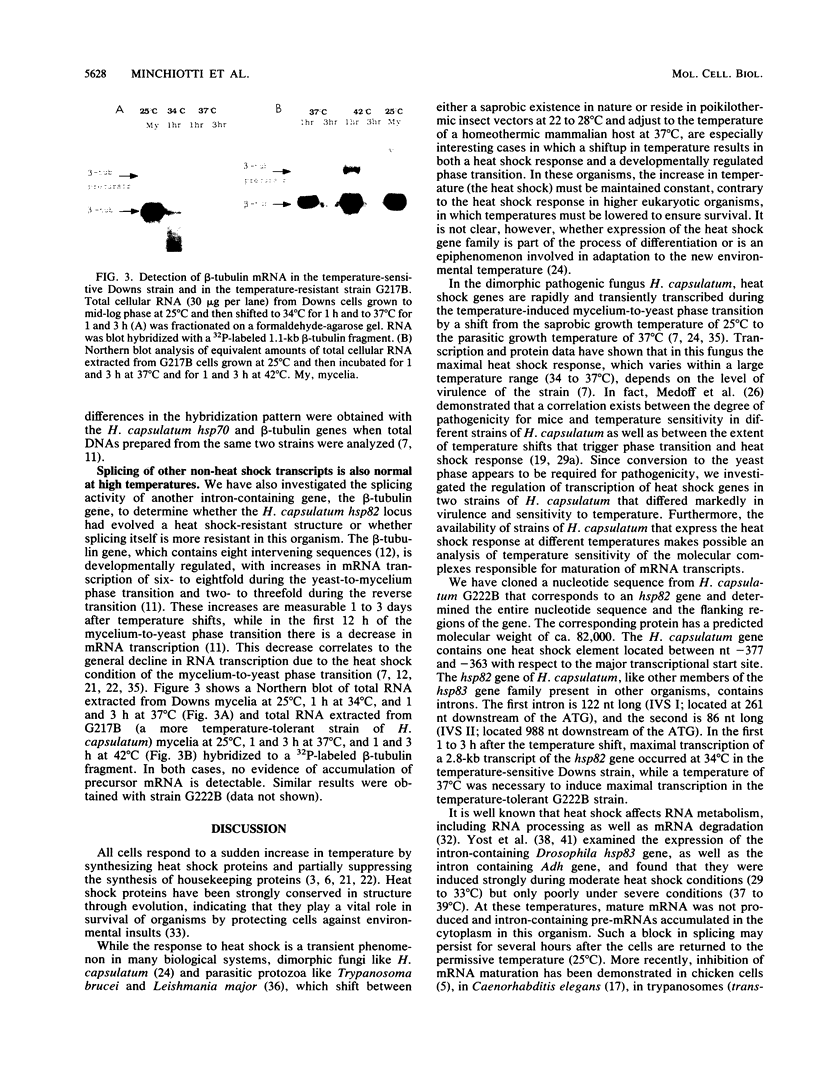
We have isolated and characterized a heat-inducible gene, hsp82, from the dimorphic pathogenic fungus Histoplasma capsulatum, which is a filamentous mold at 25 degrees C and a unicellular yeast at 37 degrees C. This gene, which has a high degree of homology with other members of the hsp82 gene family, is split into three exons and two introns of 122 and 86 nucleotides, respectively. Contrary to what has been demonstrated in Drosophila melanogaster, Saccharomyces cerevisiae, and other organisms, hsp82 mRNA in H. capsulatum is properly spliced during the severe heat conditions of 37 to 40 degrees C in the temperature-sensitive Downs strain. Splicing accuracy was also observed at 42 degrees C in the temperature-tolerant G222B strain, which showed no evidence of accumulation of primary transcripts. Furthermore, the intron containing the beta-tubulin gene is also properly spliced at the upper temperature range, suggesting that the lack of a block in splicing may be a general phenomenon in this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajello L. Coccidioidomycosis and histoplasmosis. A review of their epidemiology and geographical distribution. Mycopathol Mycol Appl. 1971 Dec 6;45(3):221–230. doi: 10.1007/BF02051969. [DOI] [PubMed] [Google Scholar]
- Amin J., Ananthan J., Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988 Sep;8(9):3761–3769. doi: 10.1128/mcb.8.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bond U. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 1988 Nov;7(11):3509–3518. doi: 10.1002/j.1460-2075.1988.tb03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Heat-shock proteins and development. Adv Genet. 1987;24:1–29. doi: 10.1016/s0065-2660(08)60005-x. [DOI] [PubMed] [Google Scholar]
- Caruso M., Sacco M., Medoff G., Maresca B. Heat shock 70 gene is differentially expressed in Histoplasma capsulatum strains with different levels of thermotolerance and pathogenicity. Mol Microbiol. 1987 Sep;1(2):151–158. doi: 10.1111/j.1365-2958.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Feramisco J. R., Helfman D. M. Cloning of the chick hsp 90 cDNA in expression vector. Nucleic Acids Res. 1985 Sep 11;13(17):6035–6047. doi: 10.1093/nar/13.17.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S. S., Kobayashi G. S., Schlessinger D., Medoff G. RNA metabolism during morphogenesis in Histoplasma capsulatum. J Gen Microbiol. 1974 Jun;82(2):301–307. doi: 10.1099/00221287-82-2-301. [DOI] [PubMed] [Google Scholar]
- Farrelly F. W., Finkelstein D. B. Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem. 1984 May 10;259(9):5745–5751. [PubMed] [Google Scholar]
- Harris G. S., Keath E. J., Medoff J. Characterization of alpha and beta tubulin genes in the dimorphic fungus Histoplasma capsulatum. J Gen Microbiol. 1989 Jul;135(7):1817–1832. doi: 10.1099/00221287-135-7-1817. [DOI] [PubMed] [Google Scholar]
- Harris G. S., Keath E. J., Medoff J. Expression of alpha- and beta-tubulin genes during dimorphic-phase transitions of Histoplasma capsulatum. Mol Cell Biol. 1989 May;9(5):2042–2049. doi: 10.1128/mcb.9.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Herrick D., Parker R., Jacobson A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Smale G., Lloyd D., Weber L. A. Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol Cell Biol. 1989 Jun;9(6):2615–2626. doi: 10.1128/mcb.9.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R. J., Russnak R. H., Jones D., Mathias C., Candido E. P. Expression of intron-containing C. elegans heat shock genes in mouse cells demonstrates divergence of 3' splice site recognition sequences between nematodes and vertebrates, and an inhibitory effect of heat shock on the mammalian splicing apparatus. Nucleic Acids Res. 1987 May 11;15(9):3723–3741. doi: 10.1093/nar/15.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M., Kobayashi G. S., Painter A., Medoff G. Possible relationship of morphogenesis in pathogenic fungus, Histoplasma capsulatum, to heat shock response. Nature. 1983 Jun 30;303(5920):806–808. doi: 10.1038/303806a0. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Klinz F. J., Donath C., Gallwitz D. Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell. 1984 Mar;36(3):645–653. doi: 10.1016/0092-8674(84)90344-1. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Maresca B., Kobayashi G. S. Dimorphism in Histoplasma capsulatum: a model for the study of cell differentiation in pathogenic fungi. Microbiol Rev. 1989 Jun;53(2):186–209. doi: 10.1128/mr.53.2.186-209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S., Painter A., Travis S. Morphogenesis and pathogenicity of Histoplasma capsulatum. Infect Immun. 1987 Jun;55(6):1355–1358. doi: 10.1128/iai.55.6.1355-1358.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Maresca B., Lambowitz A. M., Kobayashi G., Painter A., Sacco M., Carratu L. Correlation between pathogenicity and temperature sensitivity in different strains of Histoplasma capsulatum. J Clin Invest. 1986 Dec;78(6):1638–1647. doi: 10.1172/JCI112757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. K., Kozak C., Robinson E. A., Ullrich S. J., Appella E. Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem. 1989 Apr 5;264(10):5343–5351. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Boothroyd J. C. Synthesis of trypanosome hsp70 mRNA is resistant to disruption of trans-splicing by heat shock. J Biol Chem. 1989 May 5;264(13):7107–7110. [PubMed] [Google Scholar]
- Rebbe N. F., Ware J., Bertina R. M., Modrich P., Stafford D. W. Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene. 1987;53(2-3):235–245. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Sadis S., Hickey E., Weber L. A. Effect of heat shock on RNA metabolism in HeLa cells. J Cell Physiol. 1988 Jun;135(3):377–386. doi: 10.1002/jcp.1041350304. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Shearer G., Jr, Birge C. H., Yuckenberg P. D., Kobayashi G. S., Medoff G. Heat-shock proteins induced during the mycelial-to-yeast transitions of strains of Histoplasma capsulatum. J Gen Microbiol. 1987 Dec;133(12):3375–3382. doi: 10.1099/00221287-133-12-3375. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Giannini S. H., Cantor C. R. Heat shock genes: regulatory role for differentiation in parasitic protozoa. Science. 1985 Jun 21;228(4706):1443–1446. doi: 10.1126/science.4012301. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. Heat shock proteins affect RNA processing during the heat shock response of Saccharomyces cerevisiae. Mol Cell Biol. 1991 Feb;11(2):1062–1068. doi: 10.1128/mcb.11.2.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. Translation of unspliced transcripts after heat shock. Science. 1988 Dec 16;242(4885):1544–1548. doi: 10.1126/science.3201243. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Petersen R. B., Lindquist S. RNA metabolism: strategies for regulation in the heat shock response. Trends Genet. 1990 Jul;6(7):223–227. doi: 10.1016/0168-9525(90)90183-7. [DOI] [PubMed] [Google Scholar]