Abstract
Hyaluronan (HA) has an extraordinarily high turnover in physiological tissues, and HA degradation is accelerated in inflammatory and neoplastic diseases. CD44 (a cell surface receptor) and two hyaluronidases (HYAL1 and HYAL2) are thought to be responsible for HA binding and degradation; however, the role of these molecules in HA catabolism remains controversial. Here we show that KIAA1199, a deafness gene of unknown function, plays a central role in HA binding and depolymerization that is independent of CD44 and HYAL enzymes. The specific binding of KIAA1199 to HA was demonstrated in glycosaminoglycan-binding assays. We found that knockdown of KIAA1199 abolished HA degradation by human skin fibroblasts and that transfection of KIAA1199 cDNA into cells conferred the ability to catabolize HA in an endo-β-N-acetylglucosaminidase–dependent manner via the clathrin-coated pit pathway. Enhanced degradation of HA in synovial fibroblasts from patients with osteoarthritis or rheumatoid arthritis was correlated with increased levels of KIAA1199 expression and was abrogated by knockdown of KIAA1199. The level of KIAA1199 expression in uninflamed synovium was less than in osteoarthritic or rheumatoid synovium. These data suggest that KIAA1199 is a unique hyaladherin with a key role in HA catabolism in the dermis of the skin and arthritic synovium.
Keywords: extracellular matrix, hyaluronic acid, hyaluronate, hearing loss
Hyaluronan (HA) is a high molecular weight, linear glycosaminoglycan (GAG) composed of only two sugars: β-(1,3)-linked-d-glucuronic acid and β-(1,4)-linked-N-acetyl-d-glucosamine. HA is ubiquitously present as a major constituent of the extracellular matrix (ECM) in vertebrate tissues, providing structural and functional integrity to cells and organs. Although many organs maintain high concentrations of HA, skin contains approximately half the total body HA (1). HA is rapidly depolymerized within tissues, from extralarge native molecules of 1,000–10,000 kDa, to intermediate-size fragments of 10–100 kDa present in the extracellular milieu (2). Approximately one-third of total body HA is replaced daily, and the skin is a major determinant organ for HA turnover, with a metabolic half-life of 1–1.5 d (2). HA degradation is enhanced under certain pathological conditions and its lower molecular weight products are commonly detected in diseases, such as arthritis and cancers (3–5). The reduced average molecular weight of HA (as low as 200 kDa) in synovial fluids from patients with osteoarthritis (OA) or rheumatoid arthritis (RA) leads to decreased synovial viscosity and is associated with synovial inflammation (6). In addition, much lower molecular weight HA fragments (∼20 kDa) are known to stimulate neovascularization and facilitate tumor cell motility and invasion (5, 7, 8).
There are six human hyaluronidase-related genes clustered on two chromosomal loci, 3p21.3 (HYAL1, HYAL2, and HYAL3) and 7q31.3 (HYAL4, HYALP1, and SPAM1) (9). However, because HYALP1 is a pseudogene (9), and HYAL4 and SPAM1 have restricted expression patterns, HYALP1, HYAL4, and SPAM1 are unlikely to have major roles in constitutive HA degradation in vivo. HYAL3 has a restricted expression pattern (9) and its ability to degrade HA is questionable (10). Therefore, HYAL1 and HYAL2 are most likely to have key roles in degrading HA.
One current model suggests that high molecular weight HA is tethered to cell surfaces by CD44 (a HA receptor) concentrated in caveolin-rich lipid rafts, and then cleaved by HYAL2 into intermediate-size fragments in acidic microenvironments created by the Na+-H+ exchanger (11). The intermediate-size fragments are degraded to oligosaccharides within cells by lysosomal HYAL1 in coordination with lysosomal β-glucuronidase and β-N-acetyl-glucurosaminidase (12). However, this model is insufficient to explain the rapid catabolism of HA in vivo. First, neither HYAL1 nor HYAL2 are expressed in the brain (9, 13), a major organ containing large amounts of HA. This finding suggests that other molecules degrade HA in the brain. Second, HYAL2 has little or no hyaluronidase activity (14), and the finding that HYAL1 degrades HA into oligosaccharides of ∼0.8 kDa intracellularly (10, 12) is inconsistent with the presence of HA catabolites of 10–100 kDa in tissues (2). Third, mice deficient in the HYAL1 or HYAL2 gene do not show significant accumulation of HA within tissues (15, 16). Finally, the evidence for HA degradation by HYAL1 and HYAL2 was obtained in a breast carcinoma MDA-MB231 cell line (11) and in cells stably transfected with these genes (10); thus, only limited evidence for the direct involvement of HYAL1 and HYAL2 in HA degradation in cells such as fibroblasts is available. Collectively, these lines of evidence reveal that HA degradation by the HYAL enzymes remains elusive, and that new HA-degradation pathways independent of the HYAL1, HYAL2, and CD44 system may exist.
In the present study, we first tested the involvement of HYAL1, HYAL2, and CD44 in HA depolymerization in normal human skin fibroblasts, and found that knockdown of these genes with siRNAs did not abrogate HA depolymerization. This result prompted us to investigate new mechanisms for HA degradation. Using microarray analysis, we screened genes whose expression levels paralleled the extent of HA depolymerization in cultured skin fibroblasts under stimulated conditions. Intriguingly, our data provided unique evidence that a deafness gene of unknown function, known as KIAA1199 (17), has a key role in the binding and depolymerization of HA, and that this activity is independent of CD44 and HYAL enzymes. We also demonstrate that KIAA1199 is expressed predominantly by dermal fibroblasts in normal skin and is overexpressed by synovial fibroblasts and tissues from arthritic joints.
Results
CD44 and HYAL Enzymes Are Dispensable for HA Degradation in Cultured Skin Fibroblasts.
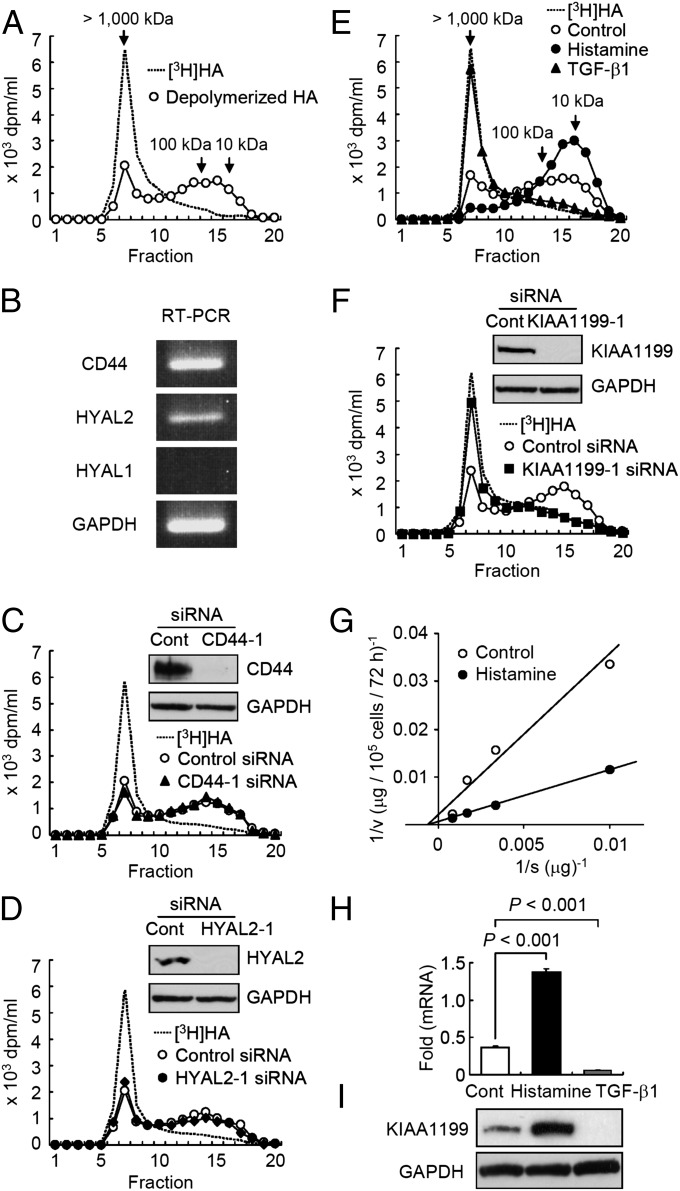
Human embryonic skin fibroblasts (Detroit 551 cells) degraded exogenous high molecular weight [3H]-labeled HA of >1,000 kDa ([3H]HA) to intermediate size fragments with molecular weights ranging from 10 kDa to 100 kDa, and accumulated the catabolites extracellularly (Fig. 1A). The fibroblasts selectively digested fluoresceinamine-labeled HA of 1,760 kDa (FA-HA H1), but not other FA-labeled GAGs, such as chondroitin sulfate A, C, and D (FA-CSA, FA-CDC, and FA-CSD), dermatan sulfate (FA-DS), heparin (FA-Hep), or heparan sulfate (FA-HS) (Fig. S1), suggesting that the depolymerizing machinery of the fibroblasts is specific for HA. To study the involvement of CD44 and the enzymes HYAL1 and HYAL2 in HA depolymerization, we examined expression of these molecules and found that skin fibroblasts express CD44 and HYAL2 but not HYAL1 (Fig. 1B). Interestingly, knockdown of CD44 and HYAL2 using two different siRNAs specific to these molecules showed no effect on HA depolymerization (Fig. 1 C and D). These data suggest that the HA-degrading machinery present in Detroit 551 cells is independent of CD44 and HYAL2 or HYAL1.
Fig. 1.
HA degradation via KIAA1199 and regulation of KIAA1199 expression by histamine and TGF-β1 in human skin fibroblasts. (A) Detroit 551 skin fibroblasts were cultured with [3H]HA for 48 h and HA fragments in the culture medium were examined by size-exclusion chromatography. (B) Expression of CD44, HYAL2, and HYAL1 by RT-PCR. GAPDH, a loading control. (C and D) CD44 and HYAL2 were knocked down by treating cells with siRNAs to CD44 or HYAL2. For controls, the cells were transfected with control nonsilencing siRNA (Control siRNA). Cells with siRNA were cultured with [3H]HA for 48 h, and degraded HA was examined by size-exclusion chromatography. Knockdown efficiency for CD44 and HYAL2 was evaluated by immunoblotting (Insets). GAPDH, a loading control. Representative data for two siRNAs are shown. (E) Effect of histamine and TGF-β1 on HA depolymerization. Cells were treated with or without histamine or TGF-β1 and cultured with [3H]HA for 48 h. The HA-degrading activity was analyzed by chromatography. Control, untreated cells. (F) Abrogation of HA-degrading activity by knockdown of KIAA1199 with two different siRNAs specific for KIAA1199. The cells were cultured with [3H]HA for 48 h, and HA degradation was determined. (Inset) Immunoblotting for KIAA1199 and GAPDH (a loading control). (G) Kinetic study of HA degradation by cells treated with or without histamine for 72 h. Control and Histamine, cells treated with vehicle alone and histamine, respectively. (H and I) The expression levels of KIAA1199 mRNA and protein in cells treated with histamine, TGF-β1 or vehicle alone (Cont) for 24 h. Levels of mRNA and protein expression were measured by real-time PCR and immunoblotting. Values (relative mRNA expression, fold KIAA1199 to GAPDH) represent mean ± SD (n = 3). The Dunnett test was used for statistical analysis. The protein expression levels (ratio of KIAA1199 to GAPDH) were estimated by using densitometric scanning Multi Gauge v.2.1 (Fuji Film). A representative finding of three different experiments is shown.
KIAA1199 Is Required for HA Depolymerization and Expressed by Dermal Fibroblasts in Normal Skin.
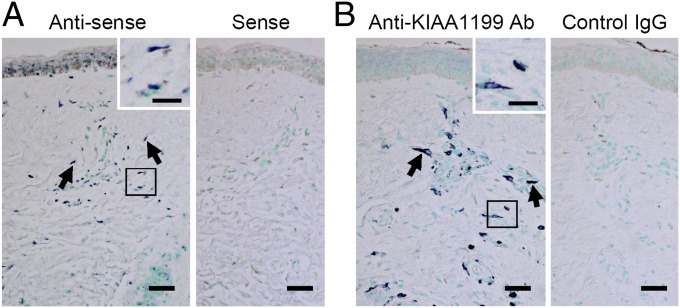
To identify molecules involved in the HA depolymerization, we monitored changes in depolymerization in Detroit 551 skin fibroblasts following stimulation with IL-1α, IL-1β, TGF-α, TGF-β1, epidermal growth factor, hepatocyte growth factor, IFN-γ, TNF-α, or histamine, and found that HA depolymerization is strikingly up-regulated and down-regulated only by treatment with histamine and TGF-β1, respectively (Fig. 1E). Thus, we searched for genes associated with HA depolymerization using microarray analysis and identified 25 genes that were up-regulated by histamine and down-regulated by TGF-β1 (Table S1). We then used siRNA to knock down the 25 genes and discovered that transfection of two different siRNAs targeting the KIAA1199 gene abolished HA depolymerization in Detroit 551 cells (Fig. 1F and Fig. S2A). Similar results were obtained with other human skin fibroblast cell lines, including HS27 (neonatal skin fibroblasts) and NHDF-Ad (adult skin fibroblasts) (Fig. S2 B and C). The HA depolymerizing activity in Detroit 551 cells showed an apparent Vmax of 370 μg/105 cells/72 h and Km of 1,480 μg/mL. Histamine treatment showed a 3.8-fold increase in Vmax (1,370 μg/105 cells/72 h) without affecting Km (1,500 μg/mL) (Fig. 1G). Under stimulation with histamine, the mRNA and protein expression of KIAA1199 was significantly increased by 3.7-fold (0.37 ± 0.02 vs.1.37 ± 0.04, control vs. histamine; n = 3; P < 0.001) (Fig. 1H) and 4.2-fold (0.31 ± 0.10 vs. 1.31 ± 0.10; n = 3; P < 0.001) respectively (Fig. 1I), whereas TGF-β1 reduced the expression to a negligible level (Fig. 1 H and I). These results show that KIAA1199 expression is essential for HA depolymerization in skin fibroblasts and the amount of KIAA1199 protein directly determines the velocity of HA depolymerization. In situ hybridization (Fig. 2A) and immunohistochemistry (Fig. 2B) showed that KIAA1199 is expressed predominantly by dermal fibroblasts in normal human skin.
Fig. 2.
Expression of KIAA1199 by dermal fibroblasts in normal human skin. (A) The mRNA expression of KIAA1199 was examined by in situ hybridization using antisense (Left) and sense (Right) RNA probes. (B) KIAA1199 protein expression was analyzed by immunohistochemistry with anti-KIAA1199 Ab (Left) and control rat IgG2aκ (Right). (Insets) High-power views of the boxed areas. Arrows, KIAA1199+ cells. (Scale bars, 50 μm; Insets, 20 μm.) Representative data from three subjects are shown.
Cells Transfected with KIAA1199 cDNA Acquire HA-Degrading Capability.
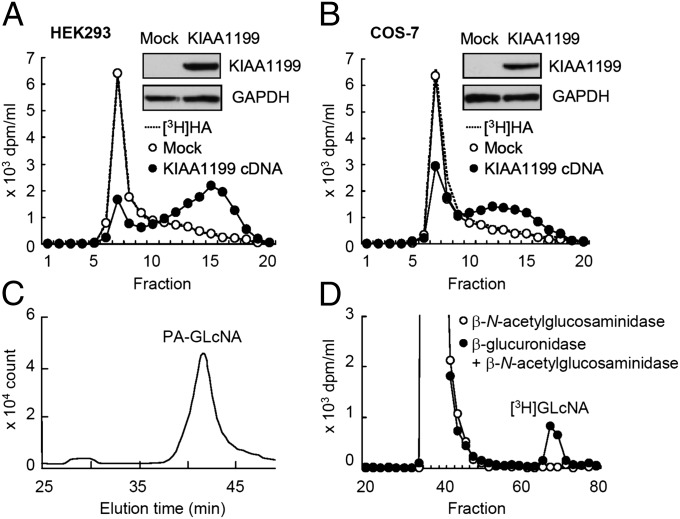
To further examine the activity of KIAA1199 in HA depolymerization, we transfected HEK293 (human embryonic kidney) and COS-7 (monkey kidney fibroblast) cell lines—neither of which have HA-degrading activity—with KIAA1199 cDNA. KIAA1199 transfectants reduced [3H]HA to intermediate size fragments of 10–100 kDa, releasing the catabolites into the medium as seen with skin fibroblasts, whereas mock transfectants showed negligible activity (Fig. 3 A and B). We then prepared stable transfectants of KIAA1199 in HEK293 cells (KIAA1199/HEK293 cells) and showed that KIAA1199/HEK293 cells selectively digest FA-HA H1, but not other FA-GAGs (FA-CSA, FA-CDC, FA-CSD, FA-DS, FA-Hep, and FA-HS) (Fig. S3). In addition, KIAA1199/HEK293 cells degraded FA-HA species with different average molecular weights (FA-M1, 907 kDa; FA-L1, 197 kDa; FA-S1, 56 kDa; FA-T1, 28 kDa; FA-U1, 9.8 kDa) (Fig. S3). Determination of the terminal sugars on the fragments showed that the reducing and nonreducing terminal sugars were N-acetylglucosamine and glucuronic acid, respectively (Fig. 3 C and D), indicating that cleavage occurs at the β-endo-N-acetylglucosamine bonds. Importantly, we found that additional, transient expression of HYAL1 or HYAL2 in KIAA1199/HEK293 cells did not enhance HA degradation (Fig. S4).
Fig. 3.
HA depolymerization by KIAA1199 transfectants and determination of HA cleavage sites. (A and B) HEK293 and COS-7 cells were transiently transfected with empty vector (Mock) or vector containing KIAA1199 cDNA, and then incubated with [3H]HA for 24 h. HA depolymerization was examined by size-exclusion chromatography. Expression of KIAA1199 protein in Mock and KIAA1199 transfectants was assessed by immunoblotting (Insets). (C and D) Determination of the reducing and nonreducing terminal sugars of depolymerized HA. HPLC pattern of pyridylaminated N-acetylglucosamine (PA-GLcNA) obtained from HA depolymerized by KIAA1199/HEK293 cells (C). Sephadex G-25 column chromatogram of depolymerized [3H]HA after incubation with β-N-acetylglucosaminidase (○) or β-glucuronidase followed by incubation with β-N-acetylglucosaminidase (●) (D).
HA Is Catabolized by KIAA1199 via the Clathrin-Coated Pit Pathway.
Accumulated lines of evidence have shown that HA degradation occurs extracellularly and via receptor-mediated endocytosis (2, 10–12, 18–20). HARE (HA receptor for endocytosis; also called Stabilin-2) and CD44 are thought to be associated with clathrin-coated pits and caveolae, respectively (2, 11). Because KIAA1199 appeared to be located in the cytoplasm and on the cell membrane, as shown in Fig. 2B, we assessed the possible involvement of clathrin-coated pits and caveolae for their roles in HA degradation. When expression of the clathrin heavy chain (CHC) and α-adaptin subunit of AP-2, an adaptor protein complex functioning as a major organizer of clathrin coats, was knocked down by siRNAs in KIAA1199/HEK293 cells, HA degradation was reduced (Fig. 4 A and B). In contrast, knockdown of caveolin-1 caused no changes in HA degradation (Fig. 4C). Immunoprecipitation with anti-KIAA1199 antibody showed that CHC coprecipitates with KIAA1199 (Fig. 4D). Because these results suggest that the clathrin-coated pit pathway is most likely involved in KIAA1199-mediated HA depolymerization, we further studied the effects of inhibitors on HA degradation. These effects included inhibitors for receptor recycling (monensin), endosome-lysosome system acidification (NH4Cl), a vacuolar (H+)-ATPase (bafilomycin A1), dynamin, a GTPase implicated in endocytosis and scission of newly formed vesicles (dynasore), and polymerization of microtubules and trafficking from early to late endosome (nocodazole) (19). As shown in Fig. 4E, treatment of KIAA1199/HEK293 cells with these chemicals, except for nocodazole, inhibited HA depolymerization, suggesting that HA is depolymerized in acidic compartments before endosome-lysosome fusion (e.g., clathrin-coated vesicles or early endosomes). The idea that HA depolymerization is unlikely to occur in lysosomes is supported by the observations that additional expression of lysosomal HYAL1 or HYAL2 in KIAA1199/HEK293 cells did not affect HA depolymerization (Fig. S4). By immunohistochemistry, KIAA1199 was localized mainly to the vesicles in the periphery of Detroit 551 skin fibroblasts (Fig. S5 A and B). Double immunostaining of KIAA1199 and CHC in KIAA1199/HEK293 cells showed that signals of both molecules are detected in a vesicular pattern, and KIAA1199 is localized closely to CHC in some vesicles (Fig. S5 C and D). When localization of high molecular weight HA added to skin fibroblasts and KIAA1199/HEK293 cells was examined by confocal microscopy, HA was observed in the periphery of these cells, showing a vesicular pattern, but no fluorescence was shown by incubation with Streptomyces hyaluronidase-digested HA (Fig. S5 E–G). Importantly, [3H]HA was almost completely recovered in the medium after addition to KIAA1199/HEK293 cells, and intracellular HA accumulation was not observed (Table S2). In addition, [3H]HA was not depolymerized when it was incubated with conditioned media from KIAA1199/HEK293 cells. All these results suggest that KIAA1199-mediated HA depolymerization may occur through rapid vesicle endocytosis and recycling without intracytoplasmic accumulation or digestion in lysosomes.
Fig. 4.
Clathrin-specific HA depolymerization and HA-specific binding of KIAA1199 in KIAA1199/HEK293 cells. (A–C) CHC (A), α-adaptin (B), and caveolin-1 (C) were knocked down by siRNAs to each gene in KIAA1199/HEK293 cells. For controls, cells were transfected with control nonsilencing siRNA. The cells were incubated with [3H]HA for 6 h, and HA fragments in the media were analyzed by size-exclusion chromatography. Efficiency of the knockdown was evaluated by immunoblotting (Insets). Representative data from two siRNAs are shown. Note that knockdown of CHC and α-adaptin but not caveolin-1 decreased HA depolymerization. (D) Coimmunoprecipitation of CHC with KIAA1199. Cell lysates were immunoprecipitated with control IgG2aκ or anti-KIAA1199 antibody, followed by immunoblotting (IMB) for KIAA1199 and CHC. Input is shown in the Upper panel. IP, immunoprecipitation. (E) Effects of inhibitors on HA depolymerization. KIAA1199/HEK293 cells were incubated for 3 h in the absence or presence of monensin, NH4Cl, bafilomycin A1, dynasore, or nocodazol, followed by additional incubation for 6 h with [3H]HA. HA depolymerization was determined by chromatography. (F) GAG-binding assay for KIAA1199 protein. Cell lysates of KIAA1199/HEK293 cells were incubated with H2O (negative control) or unlabeled HA (HA-H2), chondroitin sulfate A, C, and D (CSA, CSC, and CSD), dermatan sulfate (DS), heparin (Hep), and heparan sulfate (HS) (Upper), or HA-H2, HA-M2, HA-L2, HA-S2 or HA-T2 (Lower). The samples were precipitated with cetylpyridium chloride and analyzed by NuPAGE and immunoblotting with anti-KIAA1199 antibody. (G) KIAA1199 binding to HA-Sepharose. Cell lysates were incubated with control or HA-coupled Sepharose 4B beads (Upper), and were preincubated with H2O (control) or GAGs before application to HA-Sepharose (Lower). Bound materials to the beads were eluted with NuPAGE LDS sample-loading buffer, and analyzed by immunoblotting with anti-KIAA1199 antibody.
KIAA1199 Has HA-Specific Binding Capability.
The interaction between KIAA1199 protein and GAGs was examined by immunoblotting for KIAA1199 in GAG precipitates from the KIAA1199/HEK293 cell lysates after incubation with GAGs. As shown in Fig. 4F, KIAA1199 was selectively coprecipitated with HA (Fig. 4F, Upper), and also coprecipitated with HA species of varying average molecular weights including HA-H2 (1,452 kDa), HA-M2 (1,039 kDa), HA-L2 (219 kDa), HA-S2 (52 kDa), and HA-T2 (28 kDa) (Fig. 4F, Lower). In addition, KIAA1199 in KIAA1199/HEK293 cell lysates could bind to HA-Sepharose beads (Fig. 4G, Upper), and this binding was competitively blocked by preincubation of KIAA1199/HEK293 cell lysates with soluble HA-H2, but not other GAGs (Fig. 4G, Lower). Note, however that HA-degrading activity was not detected in cell lysates from KIAA1199/HEK293 cells. Recombinant KIAA1199 protein expressed in a wheat-germ cell-free expression system showed no definitive HA degrading activity at a pH range between 4.0 and 7.0 (Fig. S6).
KIAA1199 Is Overexpressed by Synovial Fibroblasts and Synovial Tissues from OA and RA Patients.
To assess the possible role of KIAA1199 in human disease, we next examined HA-degrading activity in cultured synovial fibroblasts. The activity appeared higher in OA and RA synovial fibroblasts compared with normal synovial fibroblasts (Fig. 5A and Fig. S7) and closely correlated with the expression levels of KIAA1199 mRNA and protein (Fig. 5B). Importantly, knockdown of KIAA1199 by siRNA reduced the HA-degrading activity to almost negligible levels (Fig. 5A and Fig. S7). These data suggest that KIAA1199 is essential for HA degradation in synovial fibroblasts and that overexpression of KIAA1199 elicits enhanced HA degradation in OA and RA synovial cells. Real-time PCR analysis showed that KIAA1199 gene expression tended to be higher in OA synovial tissues (P = 0.088) and was significantly higher in RA synovial tissues (P < 0.05) compared with noninflammatory synovial tissue (Fig. 5C). In situ hybridization and immunohistochemistry showed that KIAA1199 is expressed mainly by synovial lining and some sublining cells of RA synovial tissues, whereas only background signal was observed with a control sense probe and nonimmune IgG (Fig. 5 D and E).
Fig. 5.
KIAA1199-mediated HA degradation in OA and RA synovial fibroblasts, and expression of KIAA1199 by synovial lining and sublining cells in the RA patients. (A) Synovial fibroblasts from a normal subject (n = 1), OA, and RA patients (n = 3 each) were treated with control nonsilencing siRNA or siRNAs for KIAA1199, and incubated with [3H]HA for 48 h. HA depolymerization was analyzed by size-exclusion chromatography. Protein expression of KIAA1199 was assessed by immunoblotting (Insets). Representative data of three OA (OA-2) and RA synovial fibroblasts (RA-3) are shown, and data of other patients are presented in Fig. S7. Representative data from two siRNAs are shown. (B) The expression of KIAA1199 mRNA (Upper) and protein (Lower) in cultured normal (n = 1), OA (n = 3), and RA (n = 3) synovial fibroblasts was analyzed by real-time PCR and immunoblotting. GAPDH, loading control. (C) The expression levels of KIAA1199 in synovial tissues from the patients with noninflammatory joint disease (n = 3), OA (n = 10) or RA (n = 8) were determined by real-time PCR. Data are presented as a scatter blot with mean ± SD. Statistical analysis was done by the Dunnett test. (D and E) Identification of cells expressing KIAA1199 in RA synovial tissue. The expression of KIAA1199 mRNA and protein was determined by in situ hybridization using antisense (Left) and sense (Right) RNA probes (D) and by immunohistochemistry with anti-KIAA1199 Ab (Left) and control rat IgG2aκ (Right) (E). Arrows, KIAA1199-expressing cells. (Scale bars, 50 μm.) Representative data of eight subjects are shown.
Missense Mutations of KIAA1199 in Hearing-Loss Patients Reduce HA Degradation.
Because four missense mutations in KIAA1199 have been associated with hearing loss (17), the effect of KIAA1199 mutations on HA-degrading activity was examined. As shown in Fig. S7, four mutant KIAA1199 proteins (R187C, R187H, H783R, and V1109I) and wild-type protein were expressed at similar levels by transient transfection in HEK293 cells. Cells expressing R187C and R187H mutants showed marked reductions in HA degrading activity compared with the H783R and V11091 mutants and wild-type proteins.
Discussion
Two hyaluronidases (HYAL1 and HYAL2) and CD44 are thought to have key roles in HA degradation. However, our present experiments on expression of these molecules and KIAA1199, modulation of their expression in normal skin and arthritic synovial fibroblasts, and transfection of KIAA1199 cDNA failed to confirm that notion, and instead provide unique data showing that HA depolymerization mediated by KIAA1199 occurs independently of CD44/HYALs. We found that: (i) normal human skin and arthritic synovial fibroblasts degrade HA into intermediate-sized fragments, dependent on the expression of KIAA1199; (ii) transfection of KIAA1199 cDNA confers the ability to specifically bind to and degrade HA; (iii) overexpression of HYAL1 or HYAL2 in KIAA1199 transfectants does not increase HA depolymerization; and (iv) HA depolymerization by KIAA1199 involves the clathrin-coated pit pathway, rather than the caveolae pathway via CD44 and HYAL2.
Extralarge (1,000–10,000 kDa) native HA molecules within tissues are initially depolymerized into intermediate fragments of 10–100 kDa (2). Most fragments are then released from the ECM, drained into lymphatic vessels, and catabolized within the lymph nodes (2). The remaining HA fragments reach the circulation and are fully degraded by an acid-active hyaluronidase in lysosomes following HARE-mediated clathrin-coated pit endocytosis, predominantly in the liver, kidney, and spleen (2), where both HYAL1 and HYAL2 are highly expressed (9, 13). Patients with mucopolysaccharidosis (MPS) IX caused by HYAL1 deficiency in humans, and HYAL2 knockout mice, show elevated levels of HA in the plasma (13, 16). Lysosomal accumulation of HA is also known in MPS IX patients within macrophages and fibroblasts (13) and HYAL1 knockout mice within chondrocytes (15). These data suggest that HYAL1 and HYAL2 contribute to HA degradation via HARE- and CD44-mediated endocytosis in these organs. However, these HYAL enzymes are not expressed in the brain (9, 13), indicating a requirement for an alternative pathway in the brain. KIAA1199 is expressed in a wide range of normal human tissues, including brain, lung, pancreas, testis, and ovary, but its expression is notably absent in the liver, kidney, and spleen (21). Therefore, it seems likely that KIAA1199 may have a more ubiquitous role in HA catabolism in tissues that do not express HYAL1 or HYAL2, such as the brain. Moreover, our data show that KIAA1199, but not the HYAL enzymes, is involved in HA degradation in normal skin fibroblasts and arthritic synovial fibroblasts. The differential roles of KIAA1199 and the CD44/HYALs system in degrading HA in other tissues remain to be clarified in future work.
The KIAA1199 protein sequence shows no substantial homology to other molecules, including HYAL enzymes, HA-binding proteins, and bacterial hyaluronidases (9, 12), and lacks HA-link modules (22), B(X7)B HA-binding motifs (23), and the transmembrane domain (21). However, the protein has two GG domains, consisting of two well-conserved Gly residues, one G8 domain that contains eight conserved Gly residues in five β-strand pairs (24, 25), four PbH1 domains (26), and seven predicted N-glycosylation sites (26). In the present study, we have shown that mutations of the ARG187 residue (R187C and R187H) located in the GG domain eliminate HA-degrading activity. No information is available for the function of the GG domain at present, but the G8 domain has predicted roles in extracellular ligand binding (25). In addition, the cellular role of PbH1 domain is supposed to be polysaccharide hydrolysis according to the InterPro member database SMART (www.ebi.ac.uk/interpro). Thus, it is possible to speculate that the GG and G8 domains and PbH1 domains are responsible for KIAA1199-mediated binding and depolymerization of HA. Like HYAL2 (14), cell lysates of KIAA1199 stable transfectants or recombinant KIAA1199 protein lacked HA depolymerizing activity. This lack could reflect the need for a specific conformational change in KIAA1199 that is conferred by its microenvironment or interactions with other molecules. Because our study showed that KIAA1199 is located near the CHC+ small vesicles, and KIAA1199-mediated HA degradation is dependent on vacuolar (H+)-ATPase and dynamin, the acidic, dynamic and energy-coupling microenvironment may be critical for KIAA1199 activity. Alternatively, we cannot exclude the possibility that KIAA1199 may function as an adaptor molecule for an unknown hyaluronidase. It is well known that free radicals randomly attack HA chains, generating HA fragments of various sizes (27). However, our finding that KIAA1199 can mediate HA cleavage at β-endo-N-acetylglucosamine bonds shows that HA degradation by KIAA1199 is independent of free-radical activity.
The study presented here shows that KIAA1199 gene expression is up-regulated and down-regulated by histamine and TGF-β1, respectively. A reduction in molecular weight and mass of HA because of increased HA degradation is commonly observed in arthritic synovial fluids (3, 4) and in the dermis of the UV-damaged skin (28). Under these pathological conditions, locally, overproduced histamine is released from mast cells into the extracellular environment (29, 30). It is therefore plausible that up-regulation of KIAA1199 expression by histamine enhances HA degradation in these conditions. In contrast, TGF-β1 plays an essential role in the overproduction of ECM molecules, including HA, under pathophysiological conditions such as wound healing (31). Although accumulation of HA within wounded tissues is explained mainly by enhanced HA synthesis following TGF-β–induced expression of HA synthases 1 and 2 (32), our data suggest that TGF-β1 might simultaneously down-regulate HA degrading activity by reducing KIAA1199 gene expression, which contributes further to the accumulation of HA.
We have shown that the R187C and R187H mutants of KIAA1199 substantially reduce HA depolymerization. HA is present in the inner ear as a molecular filter against intercellular ionic leakage (33) and KIAA1199 is highly expressed in the spiral ligament of the cochlea (17), which has an important role in the homeostasis of inner ear fluids (33). Thus, a decline in HA catabolism might affect the static equilibrium of electrolytes and water in the inner ear fluids, causing hearing impairment. Another possible link between KIAA1199 and genetic disorders is Werner syndrome. Werner syndrome is an adult-onset progeroid disease caused by mutations in the WRN gene encoding a protein with helicase and exonuclease activities (34). Interestingly, Werner patients show hyperhyaluronic aciduria together with elevated serum HA concentration (35) and scleroderma-like skin with an aged appearance, characterized by reduced amounts of HA (36). Skin fibroblasts isolated from a patient with Werner syndrome overexpress KIAA1199 (21) and generate small molecular weight HA (37). Although further studies are needed to obtain direct evidence, it is tempting to speculate that enhanced degradation and release of HA from the skin by fibroblasts overexpressing KIAA1199 may contribute to the clinical characteristic of Werners syndrome. Previous studies have shown that KIAA1199 is up-regulated in gastric and colorectal cancers and immortalized renal cell carcinomas, and a splice variant is detected in primary colon adenocarcinomas, although the functional significance of these findings is not clear (21, 26, 38). In addition, enhanced expression and activity of HYAL1 and HYAL2/CD44 are reportedly related to growth and malignancy of tumor cells (11, 39). Because HA fragments of <20 kDa can induce inflammation by promoting angiogenesis (7, 8) and cell migration (5), one can postulate that HA fragments generated by KIAA1199 or HYAL enzymes might contribute to enhanced neovascularization and tumor cell invasion in cancer tissues. However, further detailed studies are necessary to better understand the roles of KIAA1199 and HYAL enzymes in cancer.
In summary, the present study provides evidence that KIAA1199 is a unique HA-binding protein, with a role in HA catabolism, that is independent of CD44 and HYAL enzymes. The expression of KIAA1199 by dermal fibroblasts in normal skin and by synovial cells in RA synovial tissue suggests that this molecule has key roles in catabolizing HA in the dermis of healthy skin and the synovium of arthritis patients. These findings provide fresh insight into our understanding of physiological and pathological HA catabolism, and suggest that therapeutic interventions targeting KIAA1199 may be of clinical value.
Materials and Methods
[3H]HA of >1,000 kDa was prepared as described in SI Materials and Methods. [3H]HA depolymerization after incubation with cultured cells was determined by application of the media to a Sepharose CL-2B (GE Healthcare) column. The cDNA of human KIAA1199 was chemically synthesized. A rat monoclonal antibody against human KIAA1199 was raised against the amino acid sequence CA762RYSPHQDADPLKPRE777, corresponding to amino acids Ala762 to Glu777 of KIAA1199 (GenBank accession no. NM_018689). Immunohistochemistry and in situ hybridization studies were done on paraffin sections of formalin-fixed human normal skin and rheumatoid synovium. More detailed information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Y. Endo and Dr. T. Sawasaki of Ehime University for their generous support with a wheat-germ cell-free expression system; Dr. S. Higashiyama of Ehime University and Dr. M. Ito of the New York University School of Medicine for discussions; and Dr. A. Fosang of the University of Melbourne, Dr. S. Nakanishi of Osaka Bioscience Institute, and Dr. Y. Yamaguchi of Sanford-Burnham Medical Research Institute for reviewing the manuscript. This work was partially supported by a Grant-in-Aid for Scientific Research (A) 24249022 from the Japan Society for the Promotion of Science (to Y.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215432110/-/DCSupplemental.
References
- 1.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: Its nature, distribution, functions, and turnover. J Intern Med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 2.Pandey MS, Harris EN, Weigel JA, Weigel PH. The cytoplasmic domain of the hyaluronan receptor for endocytosis (HARE) contains multiple endocytic motifs targeting coated pit-mediated internalization. J Biol Chem. 2008;283(31):21453–21461. doi: 10.1074/jbc.M800886200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh P. The role of hyaluronic acid (hyaluronan) in health and disease: Interactions with cells, cartilage and components of synovial fluid. Clin Exp Rheumatol. 1994;12(1):75–82. [PubMed] [Google Scholar]
- 4.Yoshida M, et al. Expression analysis of three isoforms of hyaluronan synthase and hyaluronidase in the synovium of knees in osteoarthritis and rheumatoid arthritis by quantitative real-time reverse transcriptase polymerase chain reaction. Arthritis Res Ther. 2004;6(6):R514–R520. doi: 10.1186/ar1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugahara KN, et al. Hyaluronan oligosaccharides induce CD44 cleavage and promote cell migration in CD44-expressing tumor cells. J Biol Chem. 2003;278(34):32259–32265. doi: 10.1074/jbc.M300347200. [DOI] [PubMed] [Google Scholar]
- 6.Vuorio E, Einola S, Hakkarainen S, Penttinen R. Synthesis of underpolymerized hyaluronic acid by fibroblasts cultured from rheumatoid and non-rheumatoid synovitis. Rheumatol Int. 1982;2(3):97–102. doi: 10.1007/BF00541160. [DOI] [PubMed] [Google Scholar]
- 7.Rooney P, Kumar S, Ponting J, Wang M. The role of hyaluronan in tumour neovascularization (review) Int J Cancer. 1995;60(5):632–636. doi: 10.1002/ijc.2910600511. [DOI] [PubMed] [Google Scholar]
- 8.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 9.Csóka AB, Scherer SW, Stern R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics. 1999;60(3):356–361. doi: 10.1006/geno.1999.5876. [DOI] [PubMed] [Google Scholar]
- 10.Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem. 2007;282(8):5597–5607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- 11.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279(26):26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 12.Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20(8):499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- 13.Triggs-Raine B, Salo TJ, Zhang H, Wicklow BA, Natowicz MR. Mutations in HYAL1, a member of a tandemly distributed multigene family encoding disparate hyaluronidase activities, cause a newly described lysosomal disorder, mucopolysaccharidosis IX. Proc Natl Acad Sci USA. 1999;96(11):6296–6300. doi: 10.1073/pnas.96.11.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai SK, et al. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98(8):4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin DC, et al. A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum Mol Genet. 2008;17(13):1904–1915. doi: 10.1093/hmg/ddn088. [DOI] [PubMed] [Google Scholar]
- 16.Jadin L, et al. Skeletal and hematological anomalies in HYAL2-deficient mice: A second type of mucopolysaccharidosis IX? FASEB J. 2008;22(12):4316–4326. doi: 10.1096/fj.08-111997. [DOI] [PubMed] [Google Scholar]
- 17.Abe S, Usami S, Nakamura Y. Mutations in the gene encoding KIAA1199 protein, an inner-ear protein expressed in Deiters’ cells and the fibrocytes, as the cause of nonsyndromic hearing loss. J Hum Genet. 2003;48(11):564–570. doi: 10.1007/s10038-003-0079-2. [DOI] [PubMed] [Google Scholar]
- 18.Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002;21(1):15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 19.Tammi R, et al. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem. 2001;276(37):35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, et al. Extracellular depolymerization of hyaluronic acid in cultured human skin fibroblasts. Biochem Biophys Res Commun. 1990;172(1):70–76. doi: 10.1016/s0006-291x(05)80174-3. [DOI] [PubMed] [Google Scholar]
- 21.Michishita E, Garcés G, Barrett JC, Horikawa I. Upregulation of the KIAA1199 gene is associated with cellular mortality. Cancer Lett. 2006;239(1):71–77. doi: 10.1016/j.canlet.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Kohda D, et al. Solution structure of the link module: A hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86(5):767–775. doi: 10.1016/s0092-8674(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13(2):286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, Cheng H, Zhao S, Yu L. GG: A domain involved in phage LTF apparatus and implicated in human MEB and non-syndromic hearing loss diseases. FEBS Lett. 2006;580(2):581–584. doi: 10.1016/j.febslet.2005.12.076. [DOI] [PubMed] [Google Scholar]
- 25.He QY, et al. G8: A novel domain associated with polycystic kidney disease and non-syndromic hearing loss. Bioinformatics. 2006;22(18):2189–2191. doi: 10.1093/bioinformatics/btl123. [DOI] [PubMed] [Google Scholar]
- 26.Birkenkamp-Demtroder K, et al. Repression of KIAA1199 attenuates Wnt-signalling and decreases the proliferation of colon cancer cells. Br J Cancer. 2011;105(4):552–561. doi: 10.1038/bjc.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern R, Kogan G, Jedrzejas MJ, Soltés L. The many ways to cleave hyaluronan. Biotechnol Adv. 2007;25(6):537–557. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Averbeck M, et al. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 2007;127(3):687–697. doi: 10.1038/sj.jid.5700614. [DOI] [PubMed] [Google Scholar]
- 29.Frewin DB, Cleland LG, Jonsson JR, Robertson PW. Histamine levels in human synovial fluid. J Rheumatol. 1986;13(1):13–14. [PubMed] [Google Scholar]
- 30.Gilchrest BA, Soter NA, Stoff JS, Mihm MC., Jr The human sunburn reaction: Histologic and biochemical studies. J Am Acad Dermatol. 1981;5(4):411–422. doi: 10.1016/s0190-9622(81)70103-8. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Asteriou T, Bernert B, Heldin CH, Heldin P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: Importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem J. 2007;404(2):327–336. doi: 10.1042/BJ20061757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiyama Y, Shimada A, Sayo T, Sakai S, Inoue S. Putative hyaluronan synthase mRNA are expressed in mouse skin and TGF-β upregulates their expression in cultured human skin cells. J Invest Dermatol. 1998;110(2):116–121. doi: 10.1046/j.1523-1747.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 33.Anniko M, Arnold W. Hyaluronic acid as a molecular filter and friction-reducing lubricant in the human inner ear. ORL J Otorhinolaryngol Relat Spec. 1995;57(2):82–86. doi: 10.1159/000276716. [DOI] [PubMed] [Google Scholar]
- 34.Kudlow BA, Kennedy BK, Monnat RJ., Jr Werner and Hutchinson-Gilford progeria syndromes: Mechanistic basis of human progeroid diseases. Nat Rev Mol Cell Biol. 2007;8(5):394–404. doi: 10.1038/nrm2161. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe M, Goto M. Elevation of serum hyaluronan level in Werner’s syndrome. Gerontology. 2001;47(2):77–81. doi: 10.1159/000052777. [DOI] [PubMed] [Google Scholar]
- 36.Higuchi T, Ishikawa O, Hayashi H, Ohnishi K, Miyachi Y. Disaccharide analysis of the skin glycosaminoglycans in patients with Werner’s syndrome. Clin Exp Dermatol. 1994;19(6):487–491. doi: 10.1111/j.1365-2230.1994.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, et al. Hyaluronate synthesized by cultured skin fibroblasts derived from patients with Werner’s syndrome. Biochim Biophys Acta. 1992;1139(1–2):84–90. doi: 10.1016/0925-4439(92)90086-3. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzaki S, et al. Clinicopathologic significance of KIAA1199 overexpression in human gastric cancer. Ann Surg Oncol. 2009;16(7):2042–2051. doi: 10.1245/s10434-009-0469-6. [DOI] [PubMed] [Google Scholar]
- 39.Chao KL, Muthukumar L, Herzberg O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry. 2007;46(23):6911–6920. doi: 10.1021/bi700382g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.