Abstract
Malaria parasites use hemoglobin (Hb) as a major nutrient source in the intraerythrocytic stage, during which heme is converted to hemozoin (Hz). The formation of Hz is essential for parasite survival, but to date, the underlying mechanisms of Hb degradation and Hz formation are poorly understood. We report the presence of a ∼200-kDa protein complex in the food vacuole that is required for Hb degradation and Hz formation. This complex contains several parasite proteins, including falcipain 2/2′, plasmepsin II, plasmepsin IV, histo aspartic protease, and heme detoxification protein. The association of these proteins is evident from coimmunoprecipitation followed by mass spectrometry, coelution from a gel filtration column, cosedimentation on a glycerol gradient, and in vitro protein interaction analyses. To functionally characterize this complex, we developed an in vitro assay using two of the proteins present in the complex. Our results show that falcipain 2 and heme detoxification protein associate with each other to efficiently convert Hb to Hz. We also used this in vitro assay to elucidate the modes of action of chloroquine and artemisinin. Our results reveal that both chloroquine and artemisinin act during the heme polymerization step, and chloroquine also acts at the Hb degradation step. These results may have important implications in the development of previously undefined antimalarials.
Malaria remains a major infectious disease in part because of the lack of an effective vaccine and widespread drug resistance (1). The human malaria parasite, Plasmodium falciparum, digests ∼60%–80% of the Hb present in red blood cells during the intraerythrocytic stage of its life cycle (2). The degradation of Hb occurs in a specialized acidic organelle within the parasite called the food vacuole. A number of studies have suggested that Hb degradation is a cooperative process that involves proteases of multiple catalytic classes, including cysteine, aspartic, and metallo proteases (3). These proteases produce short peptides that are further degraded to amino acids, probably by aminopeptidases (4). During the process of Hb degradation, heme released in the food vacuole is toxic to Plasmodium, as it induces oxygen-derived free radical formation, lipid peroxidation (5), and protein and DNA oxidation (6, 7). Organisms such as Plasmodium, Schistosoma, and Rhodnius, which use Hb as a nutrient source, have evolved different strategies to detoxify this free heme. Plasmodium spp. convert heme to β-hematin, which is a dark brown pigment also known as hemozoin (Hz), through a process that is essential for the life cycle of these organisms (8, 9). Hz is a cyclic dimer of ferriprotoporphyrin IX [Fe(III)PPIX] in which the propionate group of each Fe(III)PPIX molecule coordinates the Fe(III) center of its partner (9). However, the exact mechanism of Hz formation and the factor or factors required for it are not clear (10). Some groups have shown that Hz synthesis is a protein/enzyme- or lipid-driven process (11, 12). Egan has reported the process of Hz formation to be nonenzymatic and autocatalytic, with preformed Hz supporting its further formation (9). It was also suggested that neutral lipids promote Hz formation (13). Recently, Jani et al. identified and characterized a novel Plasmodium heme detoxification protein (HDP) that was extremely potent in converting heme into Hz (14).
Although our understanding of parasite biology has advanced considerably with the sequencing of the Plasmodium genome (15), no new class of antimalarials has been introduced into clinical practice since 1996. The processes of Hb degradation and Hz formation are potential targets of some of the current antimalarials. Quinolines, aryl alcohol, artemisinin, and other peroxides get concentrated in the food vacuole, where they have been suggested to affect these processes (16–19). However, the specific mechanism of Hz formation and the modes of action of these antimalarial agents are not yet clear. We report the existence of a multiprotein Hb degradation/Hz formation complex in the food vacuole of P. falciparum. This complex contains falcipain 2 and falcipain 2′, which are almost identical copies (20), and other parasite proteases including plasmepsin II, plasmepsin IV, and histo aspartic protease (HAP) in association with the HDP. We also provide evidence for the direct association of falcipain 2 (a major hemoglobinase) with HDP (a major heme polymerization protein). In addition, we developed an in vitro system that efficiently converts Hb to Hz and used this assay to investigate the modes of action of two important antimalarials: chloroquine and artemisinin. Insights from our findings may help guide the development of previously undefined antimalarial drugs.
Results
Falcipain 2 Coexists with HDP and Forms a Hz Formation Complex in the Plasmodium Food Vacuole.
The possibility that multiple proteins involved in the process of Hb degradation and Hz formation are physically associated was assessed. Food vacuoles were isolated from the trophozoite stages of P. falciparum (21) and the lysates immunoprecipitated with anti-falcipain 2 (or preimmune) sera bound to Protein A/G Sepharose beads (Pierce). The bound proteins were identified by MALDI-TOF/TOF analysis of peptides from the eluted proteins after resolution by SDS/PAGE and digestion with trypsin (Fig. S1A). This identified parasite proteins including hemoglobinases and HDP, which has been shown to be involved in conversion of heme to Hz (14) (Table S1). The sequences of the peptides from the identified proteins are presented in Table S2.
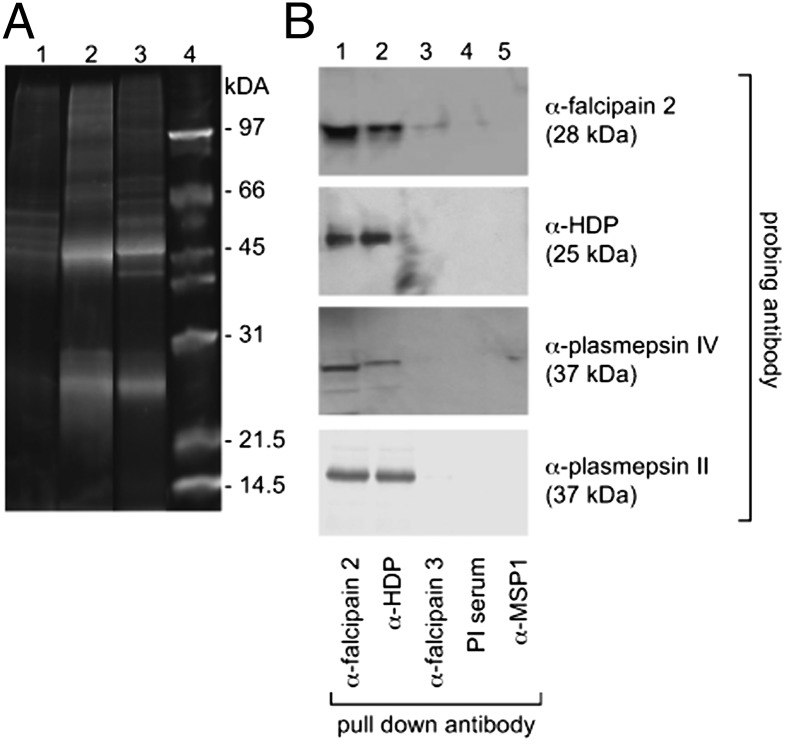
The association between HDP and falcipain 2 was analyzed further using antibody against recombinant HDP protein that had been expressed in Escherichia coli and purified by nickel affinity chromatography (Fig. S1 B and C). Sypro Ruby (Invitrogen)–stained SDS/PAGE of immunoprecipitates from food vacuole lysates using anti-HDP antibody revealed protein bands with similar mobilities to those of immunoprecipitates that were generated using anti-falcipain 2 antibody (Fig. 1A). These banding patterns were distinct from those seen when food vacuole extract was immunoprecipitated with the preimmune serum. Liquid chromatography mass spectrometry(LC-MS/MS) analysis of the whole fractionated gel lanes that had been digested with trypsin identified peptides from several proteins including falcipain 2/2′ and plasmepsin II, plasmepsin IV, HAP, falcilysin, and HDP. Notably, peptides from the same proteins were identified in LC-MS/MS analysis of both anti-falcipain 2 and anti-HDP pull downs (Table 1). The number of unique peptides identified for each protein and their sequences, along with the sequence coverage, are given in Fig. S1 D and E, and Table S3. The sequence coverage of peptides corresponding to falcilysin was low considering its size of 125 kDa. Peptides that correspond to a few abundant parasite proteins such as erythrocyte membrane protein, histone proteins, and chaperones were also identified in the immunoprecipitated lanes, including the preimmune serum-precipitated lane.
Fig. 1.
Association of HDP with hemoglobinases. (A) Sypro Ruby–stained SDS/PAGE of proteins immunoprecipitated from purified food vacuole lysate, using preimmune sera control (lane 1), rabbit anti-falcipain 2 (lane 2), mouse anti-HDP (lane 3), and molecular mass marker (lane 4). (B) Immunoblot analysis of anti-falcipain 2, anti-HDP, anti-falcipain 3, anti-merozoite surface protein 1(MSP1), and preimmune antiserum immunoprecipitates from purified food vacuole lysates probed with anti-falcipain 2, anti-HDP, anti-plasmepsin II, and anti-plasmepsin IV antibodies.
Table 1.
Identification of malarial proteins immunoprecipitated using anti-falcipain 2 and anti-HDP antibody by LC-MS/MS
| Sample no. | Protein | Plasmo DB accession no. | Molecular mass (kDa) |
Sequence coverage |
No. unique peptides |
|||
| Proenzyme | Active enzyme | Anti-fal2 | Anti-HDP | Anti-fal2 | Anti-HDP | |||
| 1 | Falcipain 2/2′ | PF11_0165/PF11_0161 | 56.4 | 28 | 26.7 | 13.4 | 28 | 7 |
| 2 | Plasmepsin II | PF14_0077 | 41.9 | 37 | 16.6 | 7.1 | 4 | 3 |
| 3 | HAP | PF14_0078 | 51.7 | 37 | 21.7 | 8.9 | 10 | 4 |
| 4 | Plasmepsin IV | PF14_0075 | 51.2 | 37 | 15.8 | 23.8 | 5 | 9 |
| 5 | Falcilysin | PF13_0322 | 138.9 | 125 | 8.0 | 2.0 | 8 | 2 |
| 6 | HDP | PF14_0446 | 24.5 | 25 | 12.7 | 28.3 | 2 | 5 |
Western blot analysis confirmed the presence of falcipain 2, plasmepsin II, plasmepsin IV, and HDP in the immunoprecipitates of food vacuole lysates using both the antibodies (anti-falcipain 2 and anti-HDP). However, neither HDP nor plasmepsins II and IV were observed when antibodies to falcipain 3 or merozoite surface protein 1 (MSP1) or preimmune sera were used to immunoprecipitate food vacuole lysates (Fig. 1B). Taken together, these results suggest the presence of a complex containing proteases and HDP in the food vacuole of P. falciparum, which we will for convenience refer to here as Hz formation complex HFC. The coexistence of cysteine and aspartic proteases in this complex may help explain the synergistic action of the inhibitors of these proteases on parasite culture (22). As falcipain 3 was not identified in any immunoprecipitation of the food vacuole extract, this suggests that falcipain 2/2′ may be the major hemoglobinase present in this parasite organelle (23).
HFC in P. falciparum.
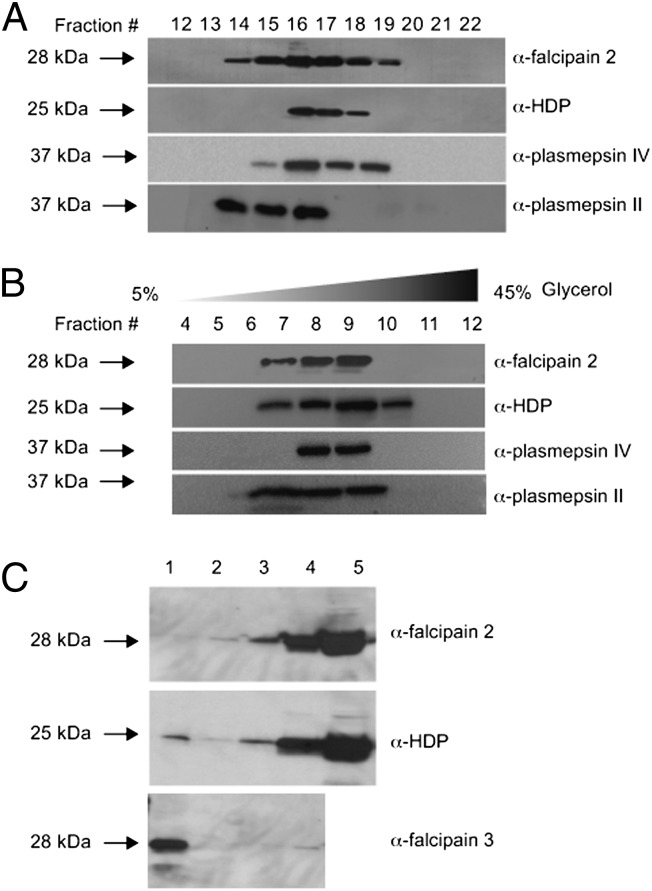
To further confirm whether cysteine protease (falcipain 2), aspartic proteases (plasmepsins II and IV), and HDP are components of a complex, food vacuole lysate was fractionated by gel filtration on a Superose-6 (Amersham) column and the presence of proteins was monitored by Western blotting, using respective antibodies. Molecular mass standards were run as a size reference in the same column. The peak of all of the proteins eluted in fraction 16 corresponded to a molecular mass of 191 kDa (Fig. 2A and Fig. S2A). The recombinant falcipain 2 was eluted at a molecular mass of ∼28 kDa on a Superdex-75 (GE Healthcare Life Sciences) column, whereas recombinant HDP eluted primarily as a monomer and as a dimer corresponding to the molecular masses of ∼25 kDa and 50 kDa, respectively, from the same column, indicating the heterogeneity of a complex composed of different proteins (Fig. S2B). The results suggest the presence of a high–molecular mass complex consisting of falcipain 2, plasmepsin II, plasmepsin IV, and HDP in the food vacuole. These results were further substantiated by cosedimentation analysis of food vacuole lysate. Western blotting of glycerol gradient fractions also revealed that the proteins falcipain 2, plasmepsin II, plasmepsin IV, and HDP cosedimented in fraction 9 at ∼198 kDa molecular mass (Fig. 2B). The sedimentation curve of molecular mass standards in different fractions is shown in Fig. S2C. The control for the sedimentation of recombinant protein is an inset in Fig S2C. HDP recombinant protein is seen in fraction 1 and 2. The presence of HDP in fraction 2 may indicate the dimeric protein (molecular mass ∼50 kDa). Falcipain 2 is in fraction 1, corresponding to its molecular mass of 28 kDa. Thus, both approaches indicate that these proteins are associated in a high–molecular mass complex of ∼200 kDa.
Fig. 2.
Hz formation complex. (A) Molecular mass determination of native falcipain 2 complex by size-exclusion chromatography of food vacuole extract on Superose-6 HR 10/300. Western blot of the eluted fractions was carried out with antibodies specific to falcipain 2, plasmepsin II, plasmepsin IV, and HDP. (B) Western blot of glycerol density gradient fractions of purified food vacuole lysate probed with antibodies specific for falcipain 2, plasmepsin II, plasmepsin IV, and HDP. Note the cosedimentation of falcipain 2, plasmepsin II, plasmepsin IV, and HDP in fraction 9 at ∼198 kDa. (C) Western blot analysis of Hz that was magnetically purified from food vacuole lysate using anti-falcipain 2, anti-HDP and anti-falcipain 3 antibodies. Lane 1, magnetic column flow-through; lane 2, wash; lane 3, first eluate; lane 4, second eluate; and lane 5, purified food vacuole.
The association of the proteins with Hz was subsequently examined by Western blot analysis of purified Hz, using anti-falcipain 2, anti-HDP, and anti-falcipain 3 antibodies. For Hz isolation, P. falciparum food vacuoles were purified from saponin-lysed trophozoites by the chemical method (21) and loaded onto the LD magnetic column (SI Text). Western analysis showed falcipain 2 and HDP in the eluates, whereas falcipain 3 was seen in the flow-through (Fig. 2C). This result suggested that falcipain 2 and HDP proteins (but not falcipain 3) were associated with Hz. Because HDP has been shown by immuno-electron microscopy to be in close proximity to Hz crystals (14), we examined this on the trophozoite-stage parasites using anti-falcipain 2 antibody. Similar to HDP localization in food vacuole, falcipain 2 was seen in proximity to Hz crystals, thereby suggesting the role of these proteins in Hz crystal formation (Fig. S2D).
HDP Interacts with Recombinant Falcipain 2.
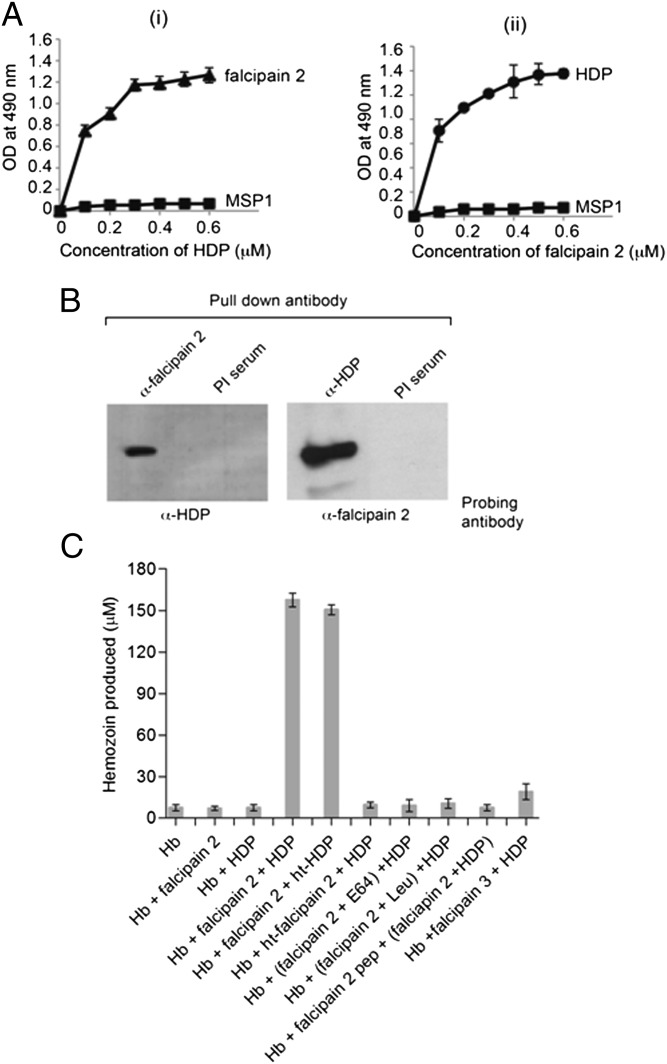
The direct interaction of falcipain 2 with HDP was analyzed using recombinant falcipain 2 (Fig. S3 A and B and ref. 24) and recombinant HDP (Fig. S1B). Reciprocal ELISA-based binding assays were performed in which either recombinant falcipain 2 or HDP was immobilized and binding of the other protein was assessed using the appropriate antibodies. The binding of falcipain 2 to immobilized HDP was concentration-dependent, as was the binding of HDP to immobilized falcipain 2 (Fig. 3A), suggesting that these two proteins can directly interact with each other. Neither of these proteins bound to rMSP-119, indicating that the interaction between falcipain 2 and HDP is specific.
Fig. 3.
Falcipain 2 binds to HDP in vitro and converts Hb to Hz. (A, i) ELISAs of HDP binding to immobilized falcipain 2. Increasing concentrations of HDP were added to wells coated with 200 ng falcipain 2 (▲). (ii) Binding of falcipain 2 to immobilized HDP. Increasing concentrations of falcipain 2 were added to wells coated with 200 ng HDP (●). MSP1 protein was used as a control (■). (B) In vitro interaction between falcipain 2 and HDP. The falcipain 2 and HDP protein mix (1 μg each) was precipitated with anti-falcipain 2 or anti-HDP antibody followed by Western blotting with the indicated antibodies. Preimmune serum was used as a control for immunoprecipitation. (C) In vitro assay for the conversion of Hb to Hz mediated by falcipain 2 and HDP. Inhibition of falcipain 2 with E-64 (5 μM), leupeptin (5 μM), or a falcipain 2 peptide (100 μM) specific for the Hb binding region added to Hb chains (300 μM) was used as a control. In the ht-falcipain 2 reaction, Hz formation was not observed; in the presence of active falcipain 2, ht-HDP formed Hz from Hb, showing its thermo stability. Recombinant active falcipain 3 (1 μM) was also used with HDP and Hb, as indicated.
The interaction between falcipain 2 and HDP was further assessed by an in vitro immunoprecipitation assay. Recombinant falcipain 2 and HDP proteins were incubated together, followed by the addition of anti-HDP or anti-falcipain 2 antibodies conjugated to Protein A/G beads. After washing the antibody-conjugated beads, proteins were eluted and subjected to Western blotting using appropriate antibodies. A preimmune serum was used as a control. As shown in Fig. 3B, eluates from both the immunoprecipitates showed an interaction between falcipain 2 and HDP. The ability of falcipain 2 and HDP to directly associate with each other and with Hz suggests that Hb degradation, Hz formation, and nucleation of Hz crystals in the food vacuoles of P. falciparum occur in association with HFCs.
In vitro Formation of Hz.
To functionally characterize HFCs, we developed the in vitro Hz formation assay using just two of the six constituents of HFC: recombinant active falcipain 2 and HDP. Recombinant falcipain 2, used in the present study, efficiently degraded the cysteine/serine protease substrate Z-Phe-Arg-7-amino-4-methyl coumarin (Z-FR-AMC) in a concentration-dependent manner (Fig. S3C) and also assessed for the percentage hydrolysis of the native Hb under conditions described for the P. falciparum food vacuole (Fig. S3D and ref. 24). Recombinant HDP converted heme to Hz in a concentration-dependent manner (Fig. S3E). On a molar basis, Hz formation from heme by recombinant HDP was ∼1,000-fold more efficient compared with neutral lipids mono palmitoyl glycerol (MPG) and mono oleoyl glycerol (MOG), which had earlier been proposed to be involved in this process (13, 25). Neutral lipids were found to be efficient in production of micromolar amount of Hz from heme, but at a concentration that was the same or in excess of heme. Histidine rich protein-2 (HRP-2) and oleic acid (OA) did not significantly produce Hz from heme (Fig. S3F). Conversion of native Hb to Hz by 0.5 μM each of recombinant falcipain 2 and HDP together was efficient, using 300 μM Hb in the assay (Fig. 3C and Fig. S4A), which was essentially linear up to 400 μM Hb (Fig. S4B). The conversion to Hb to Hz was also time dependent (Fig. S4C). Importantly, incubation of recombinant HDP and falcipain 2 together with native Hb resulted in efficient production of Hz, whereas neither of these two proteins when incubated alone with Hb converted Hb to Hz. Heat inactivation of falcipain 2 (ht-falcipain 2) or addition of falcipain 2 inhibitors [E-64 and Leupeptin (Sigma)] completely inhibited the Hz formation. The conversion of Hb to Hz in the in vitro reaction was specific because preincubation of Hb with a peptide corresponding to the Hb-binding sequence of falcipain 2 abolished the formation of Hz by falcipain 2 and HDP (26). Interestingly, the heat inactivation of HDP (ht-HDP) did not affect the Hz formation, which is consistent with the previous finding of the thermostable nature of HDP (Fig. 3C and ref. 14). Recombinant active falcipain 3 was also examined using an in vitro assay to assess the effect of a different cysteine protease on Hb degradation (Fig. S5 A–C) The addition of active falcipain 3 in place of falcipain 2 to HDP and Hb did not result in significant production of Hz. These results further suggest that falcipain 2 is an important cysteine protease for the degradation of Hb (Fig. 3C).
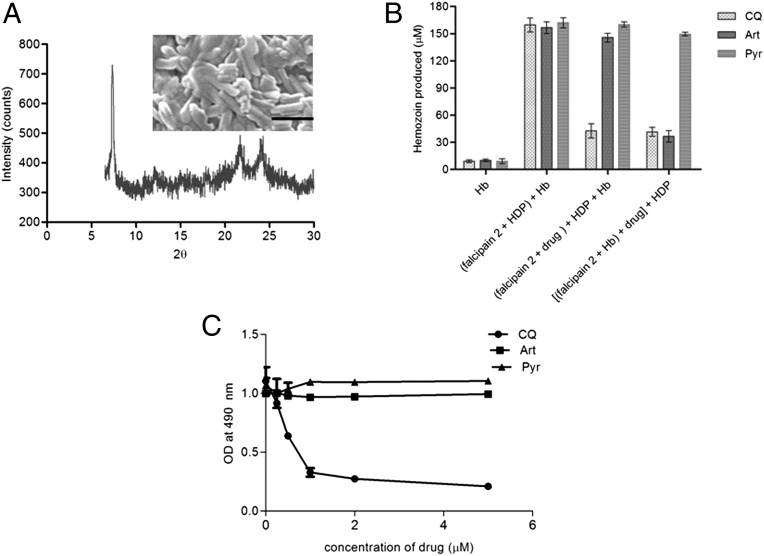
The morphology, crystal size, and homogeneity of Hz formed from Hb were assessed by a combination of physical techniques including scanning electron microscopy (SEM), X-ray diffraction powder, and infrared spectroscopy. SEM revealed ∼1-µm-long crystals that were similar in size to those of the Plasmodium native Hz crystals. X-ray diffraction pattern showed a peak at 2θ of around 7.4°, along with the other peaks that are characteristic of Hz crystals (Fig. 4A). The formation of Hz was further confirmed by infrared spectroscopy, as described previously (27). The spectra showed the characteristic peaks of hematin anhydride, including two asymmetric carboxylate stretches at 1,709 and 1,667 cm−1 and the symmetric peak at 1,210 cm−1 (Fig. S5D).
Fig. 4.
Characterization of Hz crystals and the effect of drugs on an in vitro conversion of Hb to Hz. (A) X-ray powder diffraction pattern of Hz formed from Hb in an in vitro assay. The inset shows the scanning electron micrograph of the formed Hz. (Scale bar, 1 μm.) (B) Comparisons of the effects of CQ, Art, and Pyr on the in vitro Hz formation from Hb. The reaction with only Hb as a negative control, falcipain 2 and HDP without drug, the addition of drug to falcipain 2 followed by the addition of Hb with HDP, and the addition of falcipain 2 (0.5 μM) to Hb to allow release of heme, followed by addition of drug and later addition of HDP (0.5 μM). Error bars, SD of triplicate measurements. Art, artemisinin; CQ, chloroquine; Pyr, pyrimethamine. (C) ELISA-based binding assay of falcipain 2 to immobilized Hb (200 ng) in the presence of E-64 (10 μM) and variable concentration of drugs (0–5 μM).
The formation of Hz from Hb was also examined in the presence of falcipain 2 and various other reported Hz mediators including the HDP and HRP-2 proteins; the MPG, MOG, and OA neutral lipids; and the lipids extracted from saponin-lysed parasite (Pf lipids). In the presence of falcipain 2 and HDP, more efficient conversion of Hb to Hz was seen compared with the other Hz mediators (HRP-2, MPG, MOG, and OA), indicating that HDP is the major factor required for the conversion of heme to Hz. A marginal additive effect was observed when either Pf lipids or MOG were added to falcipain 2 and HDP in the Hz formation assay (Fig. S5E).
Mode of Action of Chloroquine and Artemisinin.
Multiple lines of evidence indicate that Hb ingestion and digestion are important targets for quinoline-containing drugs and the artemisinins (17, 19). The in vitro Hz formation assay that was developed in this study was used to examine their mode of action. Incubation of falcipain 2 with different concentrations of chloroquine (0–1.5 μM) for 10 min at room temperature before the addition of HDP and Hb substantially inhibited Hz formation with an IC50 of ∼590 nM (Fig. S6A). Incubation of falcipain 2 with Hb, which allows the release of heme, followed by addition of chloroquine and then HDP, also inhibited Hz formation (Fig. 4B), suggesting the formation of a heme–chloroquine complex (28).
The incubation of falcipain 2 with chloroquine did not affect its proteolytic activity, measured using Z-FR AMC substrate (Fig. S6B). However, circular dichroism analysis of falcipain 2 indicated a change in its conformation in the presence of chloroquine (Fig. S6C). Interestingly, chloroquine reduced Hb binding to falcipain 2 in a dose-dependent manner on the ELISA, even in the presence of E-64, which blocks the catalytic activity of falcipain 2 (Fig. 4C). Taken together, these results suggest that chloroquine acts at two steps during the Hb to Hz conversion: it inhibits the binding of falcipain 2 to Hb, which in turn affects heme formation, and it also acts at the heme-to-Hz conversion step, perhaps by forming a complex with heme. Because the Hb degradation pathway in the malaria parasite also appears to be the target for the artemisinin class of endoperoxides, the effects of this drug on Hz formation were assayed in vitro. In contrast to chloroquine, incubation of recombinant falcipain 2 with artemisinin before the addition of HDP and Hb did not affect Hz formation. However, addition of different concentrations of artemisinin (0–1.5 μM) to falcipain 2 after the addition of Hb and the subsequent addition of HDP inhibited Hz formation in a dose-dependent manner with an IC50 of ∼380 nM (Fig. 4B and Fig. S6D). These results suggest that artemisinin may bind to free heme after its liberation after Hb degradation by falcipain 2, and thus its mechanism of action requires digestion of Hb (29, 30). This implies that heme bound to artemisinin is unavailable for its conversion to Hz. Pyrimethamine, a drug that binds to the active cavity of the dihydrofolate reductase–thymidylate synthase bifunctional enzyme involved in folate metabolism, did not inhibit Hz formation in vitro (Fig. 4B).
Discussion
Although a large body of evidence suggests the involvement of multiple parasite proteases, proteins, and lipids in Hb degradation and Hz formation, the sequence of events and factors leading to Hz formation are not fully understood (9, 10). The available data suggest involvement of multiple proteases in Hb degradation and explain the synergistic activities of cysteine and aspartic protease inhibitors on P. falciparum (22). A recent study also reports the collaborative roles in Hb degradation for plasmepsins and vivapains in Plasmodium vivax (31). In this study, we provide evidence for the existence in the parasite food vacuole of a multiprotein complex that contains endopeptidases, cysteine proteases (falcipain 2/2′), and aspartic proteases (plasmepsins). Coimmunoprecipitation, coelution from a gel filtration column, and cosedimentation on a density gradient showed that falcipain 2, a principal hemoglobinase, is present in a ∼200-kDa protein complex that consists of Hb-degrading parasite proteases along with HDP.
Although HRP-2 and HRP-3 have been proposed to play a role in Hz formation, we did not detect these proteins in immunoprecipitates using antibodies specific for falcipain 2 or HDP. This may be because of the low level of these proteins in the complex, though it seems more likely that HRP-2 and HRP-3 are not present in the complex because Hz formation has also been shown to take place in Plasmodium clones that lack the genes encoding either HRP-2 or HRP-3. Further, orthologs of HRP-2 are absent in P. vivax as well as in rodent parasites, which also form Hz (32). Plasmepsin I, an aspartic protease, was also not detected in the immunoprecipitates, which could be because of either the low level of this protein in the complex or its absence in HFC.
In vitro coimmunoprecipitation and ELISA-based interaction analysis support the direct interaction of falcipain 2 and HDP proteins in the complex. In addition, these proteins are associated with Hz in the food vacuole, as shown by immunoelectron microscopy and Hz isolation by the magnetic method. Together these results indicate that HDP and multiple parasite-specific hemoglobinases form complexes in the food vacuole in which Hz formation begins. The absence of another cysteine protease, falcipain 3, in the immunoprecipitates and the inability of an active recombinant falcipain 3 to produce Hz from Hb in an in vitro assay hints toward the role of falcipain 2/2′ as a major hemoglobinase involved in Hb degradation.
The existence of HFC in Plasmodium spp. possibly explains the viability of falcipain 2 knockout parasites and the redundancy among various proteases involved in Hb hydrolysis (33). Each class of proteases acts independently on Hb to enhance its degradation, and thus absence of any of these proteases may affect the efficiency of heme generation and eventual production of Hz. Because falcipain 2/2′ and the plasmepsins (II, IV, HAP) are all involved in Hb hydrolysis, the parasite may tolerate the loss of some of these proteins, including disruption of genes encoding any of these proteases (34).
Although a number of groups have demonstrated that protein- and lipid-driven processes direct heme to Hz conversion (13), there is no report for the in vitro assay showing the conversion of Hb to Hz directly using recombinant proteins. According to the results of mass spectrophotometric analysis, the Hz formation assay was developed using purified Hb and two recombinant proteins of the Hz formation complex: falcipain 2 and HDP. Importantly, these two proteins, when incubated with Hb, were able to efficiently convert Hb to Hz. This was confirmed by a number of physicochemical techniques such as infrared spectroscopy, X-ray diffraction, and SEM.
The in vitro addition of HRP-2 or oleic acid to falcipain 2 did not significantly induce Hz formation, whereas the addition of neutral lipids MOG and MPG and lipids extracted from saponin-lysed parasites (Pf lipids) to falcipain 2 converted Hb to Hz. However, the amount of Hz formed was significantly low in comparison with that produced when HDP was added to falcipain 2. A partial additive effect in Hz formation was observed when MOG or Pf lipids were added to falcipain 2 and HDP, indicating a role of HDP along with lipids in Hz crystal formation. It is possible that the presence of HDP in the complex may promote the nucleation of Hz crystals from the heme that is released by protease action. These Hz crystals may then interact with the lipid nanospheres for further extension, growth, and orientation, as is evident from the transmission electron microscopy data of the Hz crystals enclosed in lipid nanospheres (13).
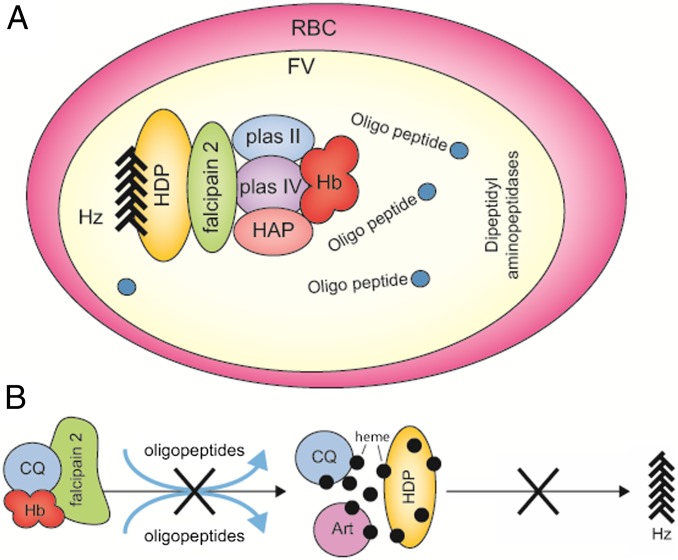
Our data suggest that a multienzyme hemoglobinase complex, along with the heme detoxification protein, exists in the Plasmodium food vacuole, referred here as HFC and schematically illustrated in the proposed model (Fig. 5A). This protein complex may enable efficient production of Hz from Hb in vivo. The efficiency of the in vitro assay developed for the conversion of Hb to Hz using two of the recombinant proteins was 40–50%. This may be the result of a single cysteine protease (falcipain 2), with around 85% activity for native Hb hydrolysis used in the reaction. The addition of other proteases, especially the aspartic proteases, may further enhance the process by making more heme available for its conversion to Hz by the associated HDP. In addition, HDP appears to be a major generator of Hz, although other reported mediators (including lipids) seem to be involved in vivo, perhaps by aiding growth and extension of the Hz and also by providing these crystals with an ambient environment to grow within the lipid nanospheres (13).
Fig. 5.
A model of the multienzyme Hz formation complex in the Plasmodium food vacuole. (A) A model for the role of the multienzyme complex in which Hb binds the HFC. Hb is degraded by endopeptidases present in the complex, and released heme is concomitantly converted to Hz by an associated HDP. Oligopeptides and dipeptides released in the food vacuole are degraded by aminopeptidases to amino acids. (B) Model to illustrate the action of CQ and Art on the in vitro conversion of Hb to Hz. CQ possibly affects Hb binding to falcipain 2 by altering its conformation and also binds to the liberated heme, thus inhibiting Hz formation. Art forms a complex with heme and inhibits Hz formation. Art, artemisinin; CQ, chloroquine.
A number of groups have reported that quinolines and artemisinin exert their actions on blood stages of Plasmodium by impairing Hz formation. Chloroquine and artemisinin have been shown to form complexes with heme in vitro and in vivo (28, 30). This study provides further insight into the modes of action of these antimalarials using the in vitro Hz formation assay. The results show that chloroquine primarily acts at two steps: first, in the Hb degradation process by inhibiting the binding of falcipain 2 to Hb, and then in Hz formation via its potential interaction with heme. However, artemisinin primarily acts after heme is liberated by the action of hemoglobinases. These actions were specific, as pyrimethamine, which acts on a different metabolic pathway, did not block Hz formation. A schematic of the mode of action of chloroquine and artemisinin is shown in Fig. 5B.
Two major conclusions can be drawn from this study: that there exists an HFC of hemoglobinases and HDP that form foci within the food vacuole and are responsible for Hz formation and that chloroquine and artemisinin act at the Hb degradation and Hz formation steps. The in vitro assay of Hb to Hz formation reported here is also likely to be useful for the screening of new antimalarials that act on this pathway.
Methods
Immunoprecipitation.
Parasites in the midtrophozoite stages were collected by saponin lysis and washed several times to remove red blood cell contamination. Purification of food vacuoles was performed as described in the SI Text, and the lysate for immunoprecipitation was prepared as described in the SI Text.
Gel Permeation Chromatography.
Food vacuoles were isolated as described in the SI Text. The lysate prepared was fractionated on a Superose-6 HR 10/300 column (Amersham), as described in the SI Text.
Glycerol Gradient Centrifugation.
Isolated food vacuoles from midtrophozoite parasite lysate were lysed in 0.5% Nonidet P-40 (Sigma)/Hepes-buffered saline (10 mM Hepes, 150 mM NaCl, 2 mM MgCl2, 10 mM KCl, 0.5 mM EDTA, and protease inhibitors) and loaded on a glycerol step gradient, as described in the SI Text.
Analysis of Protein Interaction.
In vitro interaction between HDP and falcipain 2 was examined by ELISA and coimmunoprecipitation, as described in the SI Text.
In vitro Hz Formation Assay from Hb.
In a 1-mL reaction, recombinant falcipain 2 and HDP at a final concentration of 0.5 μM each were mixed with freshly prepared solution of Hb from red blood cells (35). Falcipain 2 treated with either E-64 (Sigma) (2 μM) or leupeptin (Sigma) (2 μM) was added as a control with HDP in the reaction containing Hb. Falcipain 2 peptide (CEIVNPLTKKG) corresponding to the Hb binding domain was added to freshly prepared Hb protein from red blood cells, followed by the addition of falcipain 2 and HDP in a buffered reaction. Recombinant active falcipain 3 at a final concentration of 1µM was added with HDP to the freshly prepared Hb in an independent reaction. The reaction was buffered with 500 mM sodium acetate at pH 5.2 with 5 mM reduced glutathione and was incubated at 37°C for 3 h. Unsequestered heme was removed by repeated washing of the pellet with 2.5% SDS and 0.1 M sodium bicarbonate (pH 9.1) followed by distilled water until no soluble heme was visible in the supernatant. The Hz pellet was resuspended in 1 mL of 0.1 N NaOH, and absorbance was measured at 400 nm. A reaction containing buffered Hb alone was used as negative control. The percentage of Hz formation was calculated with molar extinction coefficient of 1 × 105 M−1⋅cm−1, as described previously (17). The assay was performed for different times (min) and also with various concentrations of Hb considering 4 Hb chains per Hb molecule. The assay was performed in the presence of drugs, as described in the SI Text.
Supplementary Material
Acknowledgments
We thank M. Labaied, K. Beck, A. Anupama, J. Carnes, Y. Ogata, C. McCormick, and the K.D.S. laboratory for their generous support; and M. Vignali and S. Mehrotra for help with figures. We also thank Prof. R. Madhubala and Advanced Instrumentation Research Facility (AIRF), Jawaharlal Nehru University for X-ray powder diffraction and SEM analysis, and R. Korde for plasmepsin IV antibody. Plasmepsin II antibody generated by D.E. Goldberg was obtained as a gift through the Malaria Research and Reference Reagent Resource center (MR4). We also thank S. Meshnick and S. Jameel for critical review of the manuscript. We thank Fogarty International Center Grant D43TW000924 for a Global Infectious Disease fellowship (to M.C.) and Grant R01AI65935 from the National Institutes of Health/National Institute of Allergy and Infectious Diseases for the proteomics facility at Seattle BioMed. We also thank the Department of Biotechnology (DBT) for supporting Projects BT/10193/MED/12/374/2007 and BT/01/CEIB/11/V/01 (supervised by P.M.); the Rotary blood bank, for providing human red blood cells; and Hamdard University. M.C. acknowledges the Council of Scientific and Industrial Research, India, for fellowship support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5283.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218412110/-/DCSupplemental.
References
- 1.Yuan J, et al. Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science. 2011;333(6043):724–729. doi: 10.1126/science.1205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krugliak M, Zhang J, Ginsburg H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol Biochem Parasitol. 2002;119(2):249–256. doi: 10.1016/s0166-6851(01)00427-3. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal PJ. Falcipains and other cysteine proteases of malaria parasites. Adv Exp Med Biol. 2011;712:30–48. doi: 10.1007/978-1-4419-8414-2_3. [DOI] [PubMed] [Google Scholar]
- 4.Klemba M, Gluzman I, Goldberg DE. A Plasmodium falciparum dipeptidyl aminopeptidase I participates in vacuolar hemoglobin degradation. J Biol Chem. 2004;279(41):43000–43007. doi: 10.1074/jbc.M408123200. [DOI] [PubMed] [Google Scholar]
- 5.Tappel AL. Unsaturated lipide oxidation catalyzed by hematin compounds. J Biol Chem. 1955;217(2):721–733. [PubMed] [Google Scholar]
- 6.Aft RL, Mueller GC. Hemin-mediated oxidative degradation of proteins. J Biol Chem. 1984;259(1):301–305. [PubMed] [Google Scholar]
- 7.Aft RL, Mueller GC. Hemin-mediated DNA strand scission. J Biol Chem. 1983;258(19):12069–12072. [PubMed] [Google Scholar]
- 8.Oliveira MF, et al. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett. 2005;579(27):6010–6016. doi: 10.1016/j.febslet.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Egan TJ. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J Inorg Biochem. 2008;102(5-6):1288–1299. doi: 10.1016/j.jinorgbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Egan TJ. Haemozoin formation. Mol Biochem Parasitol. 2008;157(2):127–136. doi: 10.1016/j.molbiopara.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Malarial haemozoin/β-haematin supports haem polymerization in the absence of protein. Nature. 1995;374(6519):269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 12.Bendrat K, Berger BJ, Cerami A. Haem polymerization in malaria. Nature. 1995;378(6553):138–139. doi: 10.1038/378138a0. [DOI] [PubMed] [Google Scholar]
- 13.Pisciotta JM, et al. The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem J. 2007;402(1):197–204. doi: 10.1042/BJ20060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jani D, et al. HDP-a novel heme detoxification protein from the malaria parasite. PLoS Pathog. 2008;4(4):e1000053. doi: 10.1371/journal.ppat.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner MJ, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray PG, Ward SA, O’Neill PM. Quinolines and artemisinin: Chemistry, biology and history. Curr Top Microbiol Immunol. 2005;295:3–38. doi: 10.1007/3-540-29088-5_1. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan DJ, Jr, Gluzman IY, Russell DG, Goldberg DE. On the molecular mechanism of chloroquine’s antimalarial action. Proc Natl Acad Sci USA. 1996;93(21):11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lelièvre J, Berry A, Benoit-Vical F. Artemisinin and chloroquine: Do mode of action and mechanism of resistance involve the same protagonists? Curr Opin Investig Drugs. 2007;8(2):117–124. [PubMed] [Google Scholar]
- 19.Meshnick SR, Jefford CW, Posner GH, Avery MA, Peters W. Second-generation antimalarial endoperoxides. Parasitol Today. 1996;12(2):79–82. doi: 10.1016/0169-4758(96)80660-0. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Sijwali PS, Pandey KC, Rosenthal PJ. Plasmodium falciparum: Biochemical characterization of the cysteine protease falcipain-2′. Exp Parasitol. 2006;112(3):187–192. doi: 10.1016/j.exppara.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Saliba KJ, Folb PI, Smith PJ. Role for the plasmodium falciparum digestive vacuole in chloroquine resistance. Biochem Pharmacol. 1998;56(3):313–320. doi: 10.1016/s0006-2952(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 22.Semenov A, Olson JE, Rosenthal PJ. Antimalarial synergy of cysteine and aspartic protease inhibitors. Antimicrob Agents Chemother. 1998;42(9):2254–2258. doi: 10.1128/aac.42.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal PJ. Cysteine proteases of malaria parasites. Int J Parasitol. 2004;34(13-14):1489–1499. doi: 10.1016/j.ijpara.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Shenai BR, Sijwali PS, Singh A, Rosenthal PJ. Characterization of native and recombinant falcipain-2, a principal trophozoite cysteine protease and essential hemoglobinase of Plasmodium falciparum. J Biol Chem. 2000;275(37):29000–29010. doi: 10.1074/jbc.M004459200. [DOI] [PubMed] [Google Scholar]
- 25.Fitch CD, Cai GZ, Chen YF, Shoemaker JD. Involvement of lipids in ferriprotoporphyrin IX polymerization in malaria. Biochim Biophys Acta. 1999;1454(1):31–37. doi: 10.1016/s0925-4439(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 26.Pandey KC, et al. The Plasmodium falciparum cysteine protease falcipain-2 captures its substrate, hemoglobin, via a unique motif. Proc Natl Acad Sci USA. 2005;102(26):9138–9143. doi: 10.1073/pnas.0502368102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaramillo M, et al. Synthetic Plasmodium-like hemozoin activates the immune response: A morphology - function study. PLoS ONE. 2009;4(9):e6957. doi: 10.1371/journal.pone.0006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey AV, et al. Mechanism of malarial haem detoxification inhibition by chloroquine. Biochem J. 2001;355(Pt 2):333–338. doi: 10.1042/0264-6021:3550333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klonis N, et al. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci USA. 2011;108(28):11405–11410. doi: 10.1073/pnas.1104063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannan R, Sahal D, Chauhan VS. Heme-artemisinin adducts are crucial mediators of the ability of artemisinin to inhibit heme polymerization. Chem Biol. 2002;9(3):321–332. doi: 10.1016/s1074-5521(02)00117-5. [DOI] [PubMed] [Google Scholar]
- 31.Moon SU, et al. Plasmodium vivax: Collaborative roles for plasmepsin 4 and vivapains in hemoglobin hydrolysis. Exp Parasitol. 2011;128(2):127–132. doi: 10.1016/j.exppara.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan DJ. Theories on malarial pigment formation and quinoline action. Int J Parasitol. 2002;32(13):1645–1653. doi: 10.1016/s0020-7519(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci USA. 2006;103(23):8840–8845. doi: 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonilla JA, Bonilla TD, Yowell CA, Fujioka H, Dame JB. Critical roles for the digestive vacuole plasmepsins of Plasmodium falciparum in vacuolar function. Mol Microbiol. 2007;65(1):64–75. doi: 10.1111/j.1365-2958.2007.05768.x. [DOI] [PubMed] [Google Scholar]
- 35.Perutz MF. Preparation of haemoglobin crystals. J Cryst Growth. 1968;2:54–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.