Abstract
The photosystem II (PSII) subunit S (PsbS) plays a key role in nonphotochemical quenching, a photoprotective mechanism for dissipation of excess excitation energy in plants. The precise function of PsbS in nonphotochemical quenching is unknown. By reconstituting PsbS together with the major light-harvesting complex of PSII (LHC-II) and the xanthophyll zeaxanthin (Zea) into proteoliposomes, we have tested the individual contributions of PSII complexes and Zea to chlorophyll (Chl) fluorescence quenching in a membrane environment. We demonstrate that PsbS is stable in the absence of pigments in vitro. Significant Chl fluorescence quenching of reconstituted LHC-II was observed in the presence of PsbS and Zea, although neither Zea nor PsbS alone was sufficient to induce the same quenching. Coreconstitution with PsbS resulted in the formation of LHC-II/PsbS heterodimers, indicating their direct interaction in the lipid bilayer. Two-photon excitation measurements on liposomes containing LHC-II, PsbS, and Zea showed an increase of electronic interactions between carotenoid S1 and Chl states, 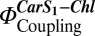 , that correlated directly with Chl fluorescence quenching. These findings are in agreement with a carotenoid-dependent Chl fluorescence quenching by direct interactions of LHCs of PSII with PsbS monomers.
, that correlated directly with Chl fluorescence quenching. These findings are in agreement with a carotenoid-dependent Chl fluorescence quenching by direct interactions of LHCs of PSII with PsbS monomers.
Frequent fluctuations in light intensity in the natural environment of green plants require a precise balance between light absorption for photosynthesis and photoprotection to avert photodamage. Under high-light conditions, plants receive more photons than they can use for photochemistry. The excess excitation energy is safely dissipated as heat by processes collectively known as nonphotochemical quenching (NPQ) (1, 2). The major component of NPQ, referred to as energy-dependent quenching (qE), is triggered by the pH gradient across the thylakoid membrane that results from the photosynthetic light reactions (2). qE is characterized by a decrease in chlorophyll (Chl) fluorescence quantum yield (1). Excessive light causes a drop in pH in the thylakoid lumen, which, in turn, activates the enzyme violaxanthin de-epoxidase (VDE) that converts violaxanthin (Vio) to zeaxanthin (Zea) in the xanthophyll cycle (2). Screening for qE-deficient Arabidopsis thaliana mutants led to the discovery that the photosystem II (PSII) subunit S (PsbS) is essential for NPQ (3). PsbS is considered a member of the light-harvesting complex (LHC) superfamily, although it is predicted to have four rather than three transmembrane helices (3). Studies with native PsbS and PsbS refolded in vitro have been inconsistent with respect to its pigment-binding ability (4–10). One interesting feature of PsbS is its reported pH-dependent dimer-to-monomer transition under high-light conditions, thought to be due to the protonation of luminal glutamates (11, 12). The dissociation of the PsbS dimer goes along with a change of location within the PSII supercomplex. Dimers appear to be mainly associated with the PSII core, whereas monomers associate with the major LHC of PSII (LHC-II) (12).
It is a matter of debate how the main qE components LHC-II, PsbS, and Zea work together to switch reversibly from an energy-transmitting state to a quenched state. Several models have been proposed to explain the mechanism of excess energy dissipation. These include simple pigment exchange of Vio for Zea (13), aggregation (14) or an internal conformational change of LHC-II (15, 16), charge transfer quenching in minor LHCs (17, 18), and quenching by carotenoid (Car) S1-Chl interactions (19). On the basis of an earlier suggestion (20), a model for qE was put forward, which defines a role for PsbS as a transient pigment-binding protein on pH-induced monomerization (21). The proposed Chl-Car heterodimer (22) could then form between a Car bound transiently to PsbS and a peripheral Chl, for example, Chl 2 in LHC-II or one of the minor LHCs. In any case, the molecular mechanism by which PsbS functions in NPQ has yet to be determined. Whereas most previous in vitro studies were conducted with LHC-II (e.g., refs. 14, 23–26) or PsbS (7–9) in detergent solution, we present here a proteoliposome system that combines LHC-II, Zea, and PsbS to study Chl fluorescence quenching directly in the membrane.
Results and Discussion
PsbS Incorporates Stably into Liposomes in the Absence of Pigments.
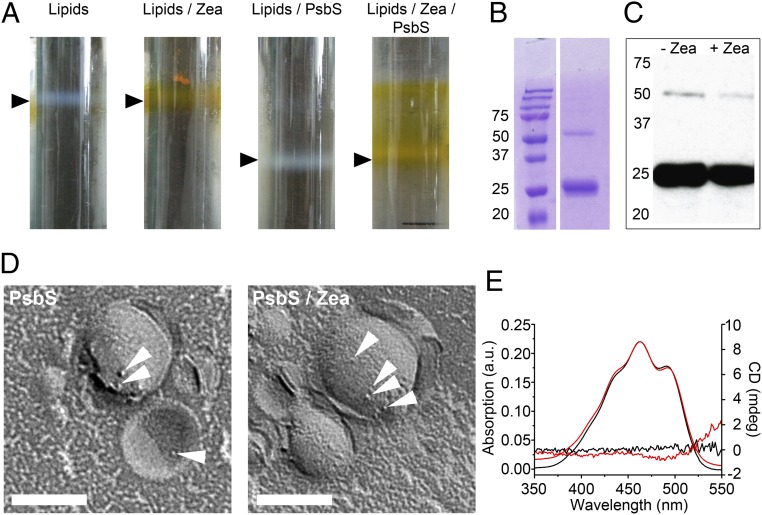
A. thaliana PsbS was expressed in Escherichia coli and refolded from inclusion bodies by detergent exchange, a method used previously for other LHCs (27). We were able to refold PsbS in the absence of pigments, as indicated by its CD spectrum (Fig. S1), and proceeded to reconstitute the protein into liposomes. To approximate the thylakoid membrane environment, we used thylakoid lipids in proportions close to those in vivo (28). Reconstitution of pigment-free PsbS into thylakoid-like liposomes was achieved by dialysis. Proteoliposomes were purified by sucrose density gradient centrifugation (Fig. 1A). Reconstitution of PsbS was confirmed by SDS/PAGE (Fig. 1B), Western blot analysis (Fig. 1C), and freeze-fracture EM (Fig. 1D). Most of the reconstituted protein was monomeric (12) (Fig. 1C). Membrane insertion of PsbS did not require pigments because at least 50% of the protein was found in proteoliposomes as judged by SDS/PAGE.
Fig. 1.
Reconstitution of PsbS into thylakoid-like liposomes. (A) Sucrose density gradients of liposomes (black arrowheads). (B) SDS/PAGE of PsbS proteoliposomes shows monomeric (∼26 kDa) and a smaller amount of dimeric (∼52 kDa) PsbS (Left, molecular mass in kilodaltons). (C) Western blot of proteoliposomes containing PsbS ± Zea in the lipid phase with a PsbS antibody. (D) Freeze-fracture electron micrographs of PsbS proteoliposomes. Protein particles are indicated by white arrowheads. (Scale bars: 100 nm.) (E) Absorption and CD difference spectra of liposomes (red, lipids with Zea minus lipids; black, PsbS proteoliposomes with Zea minus PsbS liposomes). Absorption spectra show two maxima at 463 and 491 nm. The absorption spectrum for PsbS/Zea in proteoliposomes was normalized to 463 nm. a.u., arbitrary units; mdeg, millidegrees.
Our results are in good agreement with a study of etiolated spinach leaves, which showed that PsbS incorporates stably into thylakoid membranes in the dark before the accumulation of other LHCs, which requires light and photosynthetic pigments (6). The same was seen in a Nicotiana tabacum mutant, which lacks photosynthetic pigment completely (6) but still contains PsbS, albeit at lower levels than WT plants. Evidently, PsbS can fold and insert into lipid bilayers in the absence of pigments, which may explain why refolding in the presence of pigments has failed to yield PsbS with functionally coupled pigments (8, 9).
Zea activation by PsbS has been reported to result in a red-shifted absorption spectrum and a CD spectrum indicative of excitonic coupling (7). These spectroscopic changes were interpreted to concur with the well-known absorbance increase at 535 nm, which correlates with qE. Similar in vitro studies of refolded PsbS incubated with Zea gave rise to the suggestion that the spectroscopic signatures assigned to specific pigment–protein interactions are due to pigment aggregation (9). Both experiments were performed in detergent buffer, whereas we tested the interactions between PsbS and Zea in liposomes (Fig. 1A). Western blots (Fig. 1C) and freeze-fracture EM (Fig. 1D) confirmed that PsbS incorporated into proteoliposomes equally well in the presence or absence of Zea. Absorption and CD difference spectra (Fig. 1E) of Zea-containing proteoliposome fractions revealed neither a strong red shift nor any optical activity in either the presence or absence of PsbS.
Apparently, the presence of PsbS and Zea in the proteoliposomes does not explain the absorbance increase at 535 nm and changes in the CD spectra. This could mean either that the spectroscopic properties of Zea do not change on interaction with PsbS in a membrane or that the concentrations of Zea or PsbS in the liposomes are too low for the changes to be observable.
Probing LHC-II, PsbS, and Zea in a Proteoliposome Model System.
In a next step, LHC-II was added to the proteoliposome system containing Zea and/or PsbS to investigate whether PsbS forms a Chl fluorescence quenching unit with an LHC in a Car-dependent manner. Because the interaction of LHC-II with PsbS is well documented (12, 29), we focused on LHC-II as a likely qE interaction partner.
For the purposes of our study, it was essential to work at low LHC-II concentrations so as to avoid aggregation quenching (30, 31) and, instead, to favor one-to-one interactions between LHC-II and PsbS in the membrane. High concentrations of LHC-II result in the formation of characteristic crystalline aggregates in vitro, which do not occur in chloroplast thylakoids (21).
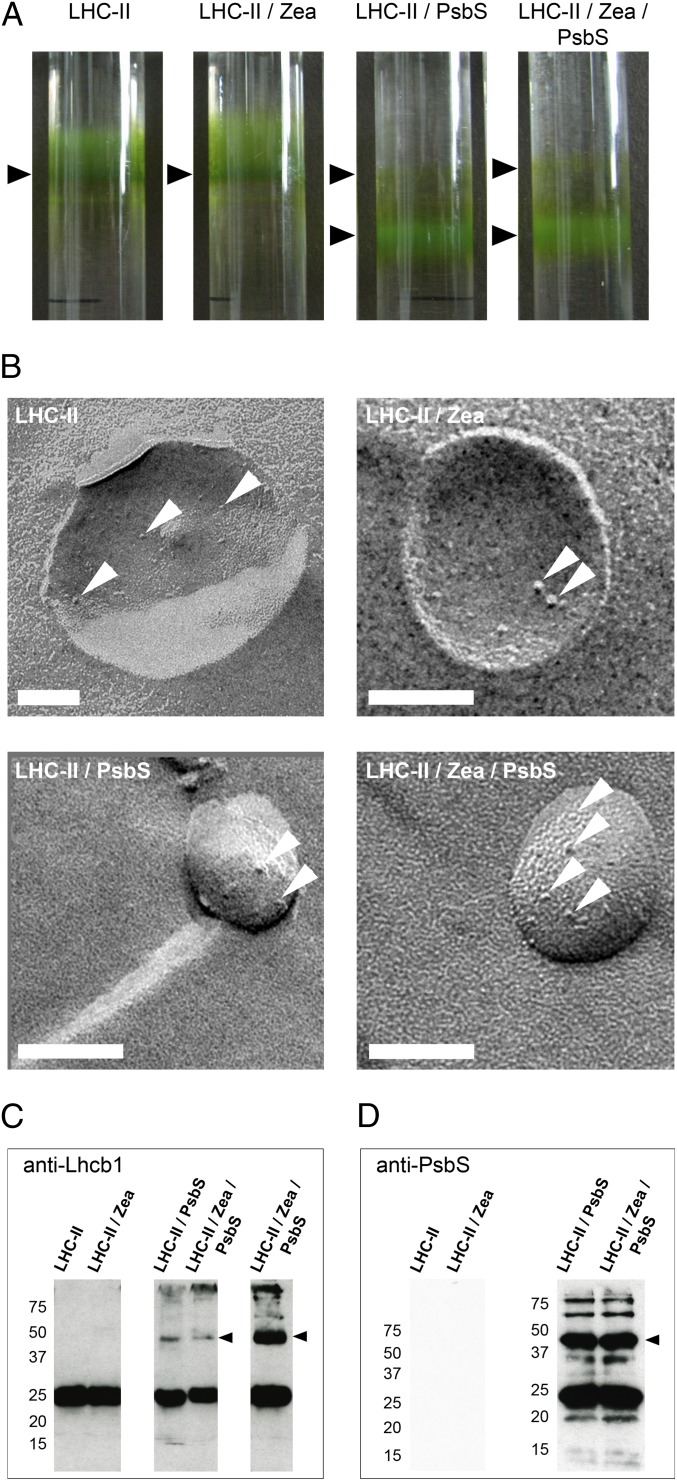
Proteoliposomes with or without Zea, containing either LHC-II alone or both LHC-II and PsbS, were produced by dialysis and purified by sucrose density gradient centrifugation as described above (Fig. 2A). The opaque proteoliposome fraction was harvested, and protein incorporation was confirmed by freeze-fracture EM (Fig. 2B) and Western blot analysis (Fig. 2 C and D). The micrographs show no crystalline arrays or LHC-II aggregates.
Fig. 2.
Proteoliposomes for in vitro investigation of qE constituents. (A) Proteoliposome bands on sucrose density gradients (arrowheads). The band above the opaque LHC-II/PsbS proteoliposome fraction contained very small liposomes with little incorporated protein and was not used further. (B) Freeze-fracture electron micrographs of proteoliposomes (protein complexes are indicated by arrowheads). (Scale bars: 100 nm.) Western blots of proteoliposomes with Lhcb1 (C) or PsbS (D) antibodies. Lanes labeled LHC-II/Zea/PsbS in C are from the same sample loaded at different concentrations. Arrowheads indicate a heterodimer consisting of LHC-II and PsbS (Left, molecular masses in kilodaltons).
Electronic Car S1-Chl Coupling in LHC-II/PsbS Heterodimers Correlates Directly with Chl Fluorescence Quenching.
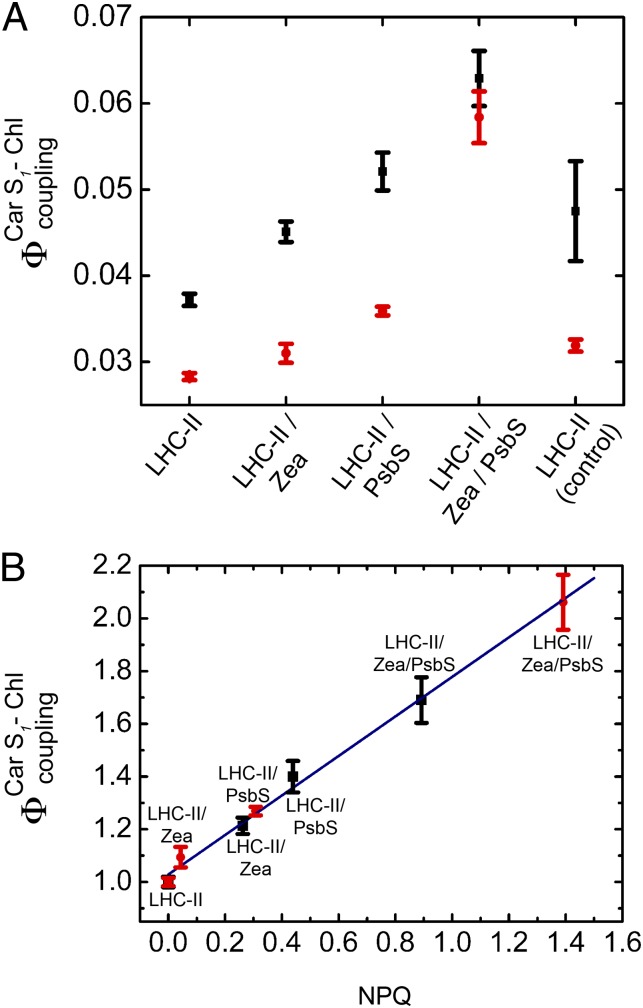
It has previously been reported (19) that the Car S1-Chl energy flow, 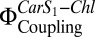 , which is closely related to electronic Car S1-Chl coupling, increases directly with NPQ. This is observed both in vitro with isolated LHC-II under conditions that promote Chl fluorescence quenching and in A. thaliana plants exposed to high light (19).
, which is closely related to electronic Car S1-Chl coupling, increases directly with NPQ. This is observed both in vitro with isolated LHC-II under conditions that promote Chl fluorescence quenching and in A. thaliana plants exposed to high light (19). 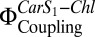 can be determined directly by comparing the Chl fluorescence observed on selective two-photon excitation (TPE) of carotenoids (Car S1) and that observed after direct Chl one-photon excitation (OPE) (19) (details are provided in Eq. 1 and SI Materials and Methods). A. thaliana mutants npq1, npq2, npq4, lut2, and wt + psbS revealed a clear dependence of
can be determined directly by comparing the Chl fluorescence observed on selective two-photon excitation (TPE) of carotenoids (Car S1) and that observed after direct Chl one-photon excitation (OPE) (19) (details are provided in Eq. 1 and SI Materials and Methods). A. thaliana mutants npq1, npq2, npq4, lut2, and wt + psbS revealed a clear dependence of 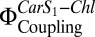 on PsbS, Zea, and lutein (Lut) (19). To investigate the correlation with Chl fluorescence quenching in a reconstituted in vitro system, we performed TPE and OPE measurements (Fig. 3 and Table 1) on proteoliposomes containing all three components: PsbS, LHC-II, and Zea. The OPE measurements provide a quantitative measure of Chl fluorescence quenching and enable us to calculate an NPQ parameter (Eq. 2) for the liposome system (Fig. 3B), in analogy to NPQ in plants. Absorption (Fig. S2) and fluorescence emission spectra (Fig. S3) recorded before and after TPE measurements confirmed that samples had not been photodamaged.
on PsbS, Zea, and lutein (Lut) (19). To investigate the correlation with Chl fluorescence quenching in a reconstituted in vitro system, we performed TPE and OPE measurements (Fig. 3 and Table 1) on proteoliposomes containing all three components: PsbS, LHC-II, and Zea. The OPE measurements provide a quantitative measure of Chl fluorescence quenching and enable us to calculate an NPQ parameter (Eq. 2) for the liposome system (Fig. 3B), in analogy to NPQ in plants. Absorption (Fig. S2) and fluorescence emission spectra (Fig. S3) recorded before and after TPE measurements confirmed that samples had not been photodamaged.
Fig. 3.
(A) Electronic interactions between Car S1 and Chl, 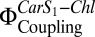 , in proteoliposomes. LHC-II in detergent solution was used as a control. All measurements were performed at pH 7.5. (B) Linear correlation between
, in proteoliposomes. LHC-II in detergent solution was used as a control. All measurements were performed at pH 7.5. (B) Linear correlation between 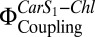 and fluorescence quenching (NPQ) in proteoliposomes. NPQ was calculated according to Eq. 2, with pulse-amplitude modulation (PAM) fluorescence corrected for scattering as detailed in Table S1. Fluorescence of LHC-II in liposomes was set as Fm, and the respective
and fluorescence quenching (NPQ) in proteoliposomes. NPQ was calculated according to Eq. 2, with pulse-amplitude modulation (PAM) fluorescence corrected for scattering as detailed in Table S1. Fluorescence of LHC-II in liposomes was set as Fm, and the respective 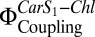 was set as 1. Two independent sets (set 1, black; set 2, red) of proteoliposomes were measured.
was set as 1. Two independent sets (set 1, black; set 2, red) of proteoliposomes were measured.
Table 1.
Electronic Car S1–Chl interactions and Chl fluorescence quenching in proteoliposomes
| Proteoliposomes | FTPE | FOPE | NPQ | 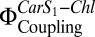 |
| Set 1 | ||||
| LHC-II | 1.00 ± 0.02 | 1.00 ± 0.01 | 0.00 | 1.00 ± 0.02 |
| LHC-II/Zea | 1.03 ± 0.03 | 0.85 ± 0.01 | 0.26 | 1.21 ± 0.03 |
| LHC-II/PsbS | 0.82 ± 0.03 | 0.58 ± 0.01 | 0.44 | 1.40 ± 0.06 |
| LHC-II/Zea/PsbS | 0.76 ± 0.04 | 0.45 ± 0.01 | 0.89 | 1.69 ± 0.09 |
| Set 2 | ||||
| LHC-II | 1.00 ± 0.01 | 1.00 ± 0.00 | 0.00 | 1.00 ± 0.02 |
| LHC-II/Zea | 1.05 ± 0.03 | 0.96 ± 0.02 | 0.04 | 1.09 ± 0.04 |
| LHC-II/PsbS | 0.81 ± 0.01 | 0.64 ± 0.00 | 0.31 | 1.27 ± 0.02 |
| LHC-II/Zea/PsbS | 0.45 ± 0.01 | 0.22 ± 0.01 | 1.39 | 2.06 ± 0.10 |
For the calculation of NPQ, the FOPE values were corrected for varying LHC-II concentrations as deduced from Chl absorption (details are provided in Table S1).
Compared with LHC-II in solution, the Car S1-Chl coupling strength in liposomes was reduced (Fig. 3). However, we found pronounced Chl fluorescence quenching and an increase in electronic interactions between Car S1 and Chl, 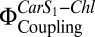 , when we coreconstituted LHC-II with PsbS. Coreconstitution of LHC-II with PsbS alone already showed an increase in
, when we coreconstituted LHC-II with PsbS. Coreconstitution of LHC-II with PsbS alone already showed an increase in 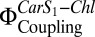 compared with coreconstitution of LHC-II plus Zea in the absence of PsbS. We attribute this to another LHC-II Car (Vio or Lut) that can substitute for Zea. When both PsbS and Zea were reconstituted with LHC-II, both
compared with coreconstitution of LHC-II plus Zea in the absence of PsbS. We attribute this to another LHC-II Car (Vio or Lut) that can substitute for Zea. When both PsbS and Zea were reconstituted with LHC-II, both 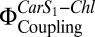 and fluorescence quenching were substantially stronger than when LHC-II was reconstituted with Zea or PsbS alone. Western blots indicated a band of ∼50 kDa in denaturing gels of proteoliposomes that contained both the LHC-II type I chlorophyll a/b-binding protein (Lhcb1) (Fig. 2C) and PsbS (Fig. 2D), indicating an LHC-II/PsbS heterodimer.
and fluorescence quenching were substantially stronger than when LHC-II was reconstituted with Zea or PsbS alone. Western blots indicated a band of ∼50 kDa in denaturing gels of proteoliposomes that contained both the LHC-II type I chlorophyll a/b-binding protein (Lhcb1) (Fig. 2C) and PsbS (Fig. 2D), indicating an LHC-II/PsbS heterodimer.
Chl fluorescence quenching by the simple addition of Zea to isolated LHC-II has been reported (25). We incorporated Zea into the lipid bilayer of proteoliposomes to mimic the in vivo system after deepoxidation of Vio by VDE. In the absence of PsbS, fluorescence quenching by Zea was almost negligible and there was no substantial increase in 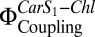 compared with controls. This was not unexpected, because the proposed simple exchange of Vio against Zea in the antenna as the sole cause of qE (13) disregards not only the proven role of PsbS in this process (3) but the initial rapid qE phase before Zea accumulates (32).
compared with controls. This was not unexpected, because the proposed simple exchange of Vio against Zea in the antenna as the sole cause of qE (13) disregards not only the proven role of PsbS in this process (3) but the initial rapid qE phase before Zea accumulates (32).
Fig. 3B shows a clear correlation between 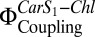 in the proteoliposomes and Chl fluorescence quenching (NPQ) calculated according to Eq. 2. The increase in Car S1-Chl coupling strength on coreconstitution of LHC-II and PsbS is not as prominent as in aggregated LHC-II or in A. thaliana plants under high light (19). It is known that LHC-II inserts into reconstituted lipid bilayers randomly in an up or down orientation (33), and the same can be assumed for the similar PsbS. Accordingly, in proteoliposomes, each PsbS can form a quenching complex with an LHC-II in the correct orientation if both are present in roughly equimolar amounts. From the freeze-fracture electron micrographs in Figs. 1 D and E and 2B, we estimate that the proteins account for only 1% of the membrane area, whereas the photosynthetic complexes are close-packed in thylakoids. The high dilution of LHC-II is necessary to ensure that the observed signal originates predominantly from one-to-one LHC-II/PsbS interactions and to avoid quenching by LHC-II aggregates (21). This results in a much-reduced probability of interaction between PsbS and LHC-II, which, in turn, accounts for the comparatively small quenching signal in the reconstituted system. However, the increase of Chl fluorescence quenching with
in the proteoliposomes and Chl fluorescence quenching (NPQ) calculated according to Eq. 2. The increase in Car S1-Chl coupling strength on coreconstitution of LHC-II and PsbS is not as prominent as in aggregated LHC-II or in A. thaliana plants under high light (19). It is known that LHC-II inserts into reconstituted lipid bilayers randomly in an up or down orientation (33), and the same can be assumed for the similar PsbS. Accordingly, in proteoliposomes, each PsbS can form a quenching complex with an LHC-II in the correct orientation if both are present in roughly equimolar amounts. From the freeze-fracture electron micrographs in Figs. 1 D and E and 2B, we estimate that the proteins account for only 1% of the membrane area, whereas the photosynthetic complexes are close-packed in thylakoids. The high dilution of LHC-II is necessary to ensure that the observed signal originates predominantly from one-to-one LHC-II/PsbS interactions and to avoid quenching by LHC-II aggregates (21). This results in a much-reduced probability of interaction between PsbS and LHC-II, which, in turn, accounts for the comparatively small quenching signal in the reconstituted system. However, the increase of Chl fluorescence quenching with 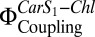 and the requirement for both PsbS and Zea are unambiguous.
and the requirement for both PsbS and Zea are unambiguous.
Our results show that (i) PsbS interacts with LHC-II in the lipid bilayer and (ii) this interaction occurs independent of the xanthophyll cycle. Although Zea is required for full qE, studies on A. thaliana mutants have also implied a role for Lut in this process (33). We suggest that this reflects the ability of the two Cars to substitute for one another in the same quenching site. This notion is consistent with our observation that coreconstitution of LHC-II with PsbS without Zea showed a moderate increase in 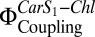 (Fig. 3A). It is also consistent with reports that overexpression of PsbS in A. thaliana plants enhances NPQ in the absence of Zea (34) and that qE is severely reduced in the npq1 mutant, which cannot convert Vio to Zea (32). We conclude that qE can proceed to some extent without Zea but that this xanthophyll is essential for maximum qE.
(Fig. 3A). It is also consistent with reports that overexpression of PsbS in A. thaliana plants enhances NPQ in the absence of Zea (34) and that qE is severely reduced in the npq1 mutant, which cannot convert Vio to Zea (32). We conclude that qE can proceed to some extent without Zea but that this xanthophyll is essential for maximum qE.
Concluding Remarks
We show that pigment-free PsbS can be reconstituted into liposomes. This has enabled us to examine the contribution of PsbS, Zea, and LHC-II to Chl fluorescence quenching in an environment that is significantly more similar to a native membrane than detergent solution. By using low-protein concentrations, we were able to dissect individual one-to-one interactions between the three key components that contribute to qE in thylakoids. Even though the qE process is obviously more than just the sum of such individual interactions, the characterization of these interactions in a well-defined membrane system is critical for a deeper understanding of the entire mechanism. The reconstituted PsbS was mostly monomeric, as also occurs after a pH-dependent dimer-to-monomer transition in high-light conditions (12). It formed a heterodimer with LHC-II on coreconstitution, resulting in substantial Chl fluorescence quenching and a concomitant increase in electronic interactions between Car S1 and Chl, 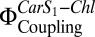 . Fluorescence quenching was most pronounced when Zea was coreconstituted with LHC-II and PsbS. These observations are consistent with a quenching model in which an LHC of PSII forms a Car-dependent quenching unit with monomeric PsbS. We cannot rule out the possibility that different antenna complexes contribute to other regulation mechanisms in the thylakoid membrane. Previous studies on charge transfer in qE have pointed to the minor LHCs as the primary quenching sites (17, 18). The mechanism investigated here could apply equally well to LHC-II or to a minor LHC. A likely candidate is CP29, which was found to interact with PsbS (29). The proteoliposome system presented here now allows for further systematic investigation of the interplay of PSII complexes and xanthophylls during qE and assessment of the minor LHCs as possible interaction partners of PsbS.
. Fluorescence quenching was most pronounced when Zea was coreconstituted with LHC-II and PsbS. These observations are consistent with a quenching model in which an LHC of PSII forms a Car-dependent quenching unit with monomeric PsbS. We cannot rule out the possibility that different antenna complexes contribute to other regulation mechanisms in the thylakoid membrane. Previous studies on charge transfer in qE have pointed to the minor LHCs as the primary quenching sites (17, 18). The mechanism investigated here could apply equally well to LHC-II or to a minor LHC. A likely candidate is CP29, which was found to interact with PsbS (29). The proteoliposome system presented here now allows for further systematic investigation of the interplay of PSII complexes and xanthophylls during qE and assessment of the minor LHCs as possible interaction partners of PsbS.
Materials and Methods
Cloning, Overexpression, and Refolding of Recombinant PsbS.
Cloning, expression in E. coli, immobilized metal ion affinity chromatography (IMAC) purification of solubilized A. thaliana PsbS, and refolding by detergent exchange are described in detail in SI Materials and Methods.
Gel Electrophoresis and Western Blotting.
SDS/PAGE was performed according to the method of Laemmli (35) under reducing conditions. Anti-PsbS (provided by C. Büchel, Goethe University, Frankfurt, Germany) or anti-Lhcb1 (Agrisera) antibody was used for Western blotting. Details are provided in SI Materials and Methods.
Isolation of Pea LHC-II and Pigment Analysis.
LHC-II from Pisum sativum was purified as described (36). Pigments were extracted from LHC-II with 2-butanol and analyzed by reverse-phase HPLC using a Synergi HydroRP column (Phenomenex) and a Shimadzu LC-20A Prominence System by means of a method adapted from Gilmore and Yamamoto (37) as detailed in SI Materials and Methods.
Reconsitution of PsbS and LHC-II into Thylakoid-Like Liposomes.
Monogalactosyl diacylglycerol (MGDG), digalactosyl diacylglycerol (DGDG), phosphatidyl gycerol (PG), and sulphoquinovosyl diacylglycerol (SQDG) from spinach thylakoids were purchased from Lipid Products. Lipids were mixed in chloroform [50% (wt/vol) MGDG, 31% (wt/vol) DGDG, 10.7% (wt/vol) PG, and 8.3% (wt/vol) SQDG] (28) and dried under nitrogen to a thin film. Lipids were solubilized for 2 h at room temperature in 50 mM Hepes buffer (pH 7.5) and n-octyl-β-D-glucopyranoside (OG; Glycon Biochemicals GmbH) at a detergent-to-lipid ratio of 1.5 (wt/wt). Zea was added to the lipids at 0.05 mol% (relative to 1 mol of total lipids) for experiments with LHC-II/PsbS containing proteoliposomes and at 1.9 mol% for experiments with PsbS liposomes. Purified pea LHC-II in 1% n-nonyl-β-D-glucoside (Calbiochem) was centrifuged at 20,000 × g and 4 °C for 15 min and added at a lipid-to-Chl ratio of 100 (mol/mol). Refolded PsbS in 50 mM Hepes (pH 7.5) and 1% OG was centrifuged at 100,000 × g and 4 °C for 20 min and added at a lipid-to-protein ratio of 10 (wt/wt). The lipid/protein solution was agitated for 1 h at room temperature and transferred to Spectra/Por dialysis bags with a 12- to 14-kDa molecular mass cutoff (Spectrum Laboratories). Dialysis was performed for 48 h at 4 °C and for 3 h at room temperature with five changes of dialysis buffer [50 mM Hepes (pH 7.5)]. For further purification, proteoliposomes were layered onto sucrose density gradients [10–45% (wt/vol) sucrose in 50 mM Hepes (pH 7.5)] and centrifuged at 200,000 × g and 4 °C for 18–20 h. Proteoliposomes were harvested from sucrose gradients and concentrated by centrifugation at 300,000 × g and 4 °C for 20 min as required.
Absorption, CD, and Fluorescence Spectroscopy.
Absorption spectra were recorded on a Lambda 25 UV/VIS spectrometer or a Lambda Bio 40 UV/VIS spectrometer (PerkinElmer). CD spectra were recorded on a Jasco J-810 spectropolarimeter with a scan speed of 100 nm/min. Fluorescence emission spectra were recorded between 640 and 740 nm on a Cary Eclipse fluorescence spectrophotometer (Varian) with the excitation wavelength set to 441 nm.
Freeze-Fracture EM.
Samples were frozen and fractured essentially as reported by Lehner et al. (38), as described in detail in SI Materials and Methods.
Fluorescence Correlation Spectroscopy, Lifetime Measurements, and Dynamic Light Scattering.
To test the successful incorporation of LHC-II into the proteoliposomes, we performed fluorescence lifetime and fluorescence correlation spectroscopy (FCS) measurements (SI Materials and Methods) as well as dynamic light scattering (DLS). In agreement with previous studies (23, 24), LHC-II in detergent solution showed the expected average lifetime of ∼4 ns at neutral pH. Dilution into acidic, detergent-free buffer resulted in the characteristic shortening of the lifetime to ∼0.7 ns (24) due to the formation of quenching aggregates. Incorporation of LHC-II into thylakoid-like liposomes caused only a minor decrease in lifetime to ∼3.8 ns, consistent with other reports (35). Compared with the average Chl fluorescence lifetimes recorded at neutral pH, no changes were observed on acidification of the buffer, indicating that LHC-II had been fully reconstituted; otherwise dilution into detergent-free acidic buffer would have resulted in aggregation quenching (24). FCS to monitor the respective diffusion times (Table S2) and DLS of proteoliposomes or control samples (Table S3) confirmed that there were no detectable changes in the proteoliposome samples on dilution into buffers of different pH. Particle size distribution measurements were performed in a DynaPro DLS instrument (Dynamics Version 6.7.7.6; Wyatt Technology). Each sample was measured 10 times with an acquisition time of 5 s at 25 °C.
TPE Spectroscopy.
The theory and method for the quantitative measurement of the electronic coupling between Car S1 and Chl, 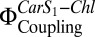 , have been described previously (19) and are detailed in SI Materials and Methods. The confocal setup enabled measurement of fluorescence after TPE (FTPE) and fluorescence after OPE (FOPE) at the same spot. The coupling parameter,
, have been described previously (19) and are detailed in SI Materials and Methods. The confocal setup enabled measurement of fluorescence after TPE (FTPE) and fluorescence after OPE (FOPE) at the same spot. The coupling parameter, 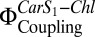 , which is closely related to the electronic interaction between Car S1 and Chl, is obtained according to Eq. 1:
, which is closely related to the electronic interaction between Car S1 and Chl, is obtained according to Eq. 1:
 |
NPQ was calculated from the fluorescence measured with the PAM fluorometer, where Fm and Fm′ are fluorescence intensities of unquenched and quenched samples, respectively:
 |
Supplementary Material
Acknowledgments
We thank Tiago Barros for valuable discussions and help during the initial stage of the project, Friederike Joos for excellent technical assistance in freeze-fracture EM, Claudia Büchel for providing the PsbS antibody, and Stephen Marino for critically reading the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205561110/-/DCSupplemental.
References
- 1.Holt NE, Fleming GR, Niyogi KK. Toward an understanding of the mechanism of nonphotochemical quenching in green plants. Biochemistry. 2004;43(26):8281–8289. doi: 10.1021/bi0494020. [DOI] [PubMed] [Google Scholar]
- 2.de Bianchi S, Ballottari M, Dall’osto L, Bassi R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans. 2010;38(2):651–660. doi: 10.1042/BST0380651. [DOI] [PubMed] [Google Scholar]
- 3.Li XP, et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403(6768):391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 4.Funk C, Schröder WP, Green BR, Renger G, Andersson B. The intrinsic 22 kDa protein is a chlorophyll-binding subunit of photosystem II. FEBS Lett. 1994;342(3):261–266. doi: 10.1016/0014-5793(94)80513-x. [DOI] [PubMed] [Google Scholar]
- 5.Funk C, et al. The PSII-S protein of higher plants: A new type of pigment-binding protein. Biochemistry. 1995;34(35):11133–11141. doi: 10.1021/bi00035a019. [DOI] [PubMed] [Google Scholar]
- 6.Funk C, Adamska I, Green BR, Andersson B, Renger G. The nuclear-encoded chlorophyll-binding photosystem II-S protein is stable in the absence of pigments. J Biol Chem. 1995;270(50):30141–30147. doi: 10.1074/jbc.270.50.30141. [DOI] [PubMed] [Google Scholar]
- 7.Aspinall-O’Dea M, et al. In vitro reconstitution of the activated zeaxanthin state associated with energy dissipation in plants. Proc Natl Acad Sci USA. 2002;99(25):16331–16335. doi: 10.1073/pnas.252500999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici P, et al. Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J Biol Chem. 2002;277(25):22750–22758. doi: 10.1074/jbc.M200604200. [DOI] [PubMed] [Google Scholar]
- 9.Bonente G, Howes BD, Caffarri S, Smulevich G, Bassi R. Interactions between the photosystem II subunit PsbS and xanthophylls studied in vivo and in vitro. J Biol Chem. 2008;283(13):8434–8445. doi: 10.1074/jbc.M708291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XP, Phippard A, Pasari J, Niyogi KK. Structure-function analysis of photosystem II subunit S (PsbS) in vivo. Funct Plant Biol. 2002;29(10):1131–1139. doi: 10.1071/FP02065. [DOI] [PubMed] [Google Scholar]
- 11.Li XP, et al. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem. 2004;279(22):22866–22874. doi: 10.1074/jbc.M402461200. [DOI] [PubMed] [Google Scholar]
- 12.Bergantino E, et al. Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc Natl Acad Sci USA. 2003;100(25):15265–15270. doi: 10.1073/pnas.2533072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank HA, et al. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth Res. 1994;41(3):389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- 14.Horton P, et al. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll-protein complex. FEBS Lett. 1991;292(1-2):1–4. doi: 10.1016/0014-5793(91)80819-o. [DOI] [PubMed] [Google Scholar]
- 15.Pascal AA, et al. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature. 2005;436(7047):134–137. doi: 10.1038/nature03795. [DOI] [PubMed] [Google Scholar]
- 16.Ruban AV, et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450(7169):575–578. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- 17.Avenson TJ, et al. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem. 2008;283(6):3550–3558. doi: 10.1074/jbc.M705645200. [DOI] [PubMed] [Google Scholar]
- 18.Ahn TK, et al. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science. 2008;320(5877):794–797. doi: 10.1126/science.1154800. [DOI] [PubMed] [Google Scholar]
- 19.Bode S, et al. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc Natl Acad Sci USA. 2009;106(30):12311–12316. doi: 10.1073/pnas.0903536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabó I, Bergantino E, Giacometti GM. Light and oxygenic photosynthesis: Energy dissipation as a protection mechanism against photo-oxidation. EMBO Rep. 2005;6(7):629–634. doi: 10.1038/sj.embor.7400460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros T, Royant A, Standfuss J, Dreuw A, Kühlbrandt W. Crystal structure of plant light-harvesting complex shows the active, energy-transmitting state. EMBO J. 2009;28(3):298–306. doi: 10.1038/emboj.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt NE, et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307(5708):433–436. doi: 10.1126/science.1105833. [DOI] [PubMed] [Google Scholar]
- 23.Palacios MA, de Weerd FL, Ihalainen JA, van Grondelle R, van Amerongen H. Superradiance and exciton (de)localization in light-harvesting complex II from green plants? J Phys Chem B. 2002;106(22):5782–5787. [Google Scholar]
- 24.Barzda V, et al. Singlet-singlet annihilation kinetics in aggregates and trimers of LHCII. Biophys J. 2001;80(5):2409–2421. doi: 10.1016/S0006-3495(01)76210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wentworth M, Ruban AV, Horton P. Chlorophyll fluorescence quenching in isolated light harvesting complexes induced by zeaxanthin. FEBS Lett. 2000;471(1):71–74. doi: 10.1016/s0014-5793(00)01369-7. [DOI] [PubMed] [Google Scholar]
- 26.Ruban AV, Young A, Horton P. Modulation of chlorophyll fluorescence quenching in isolated light-harvesting complex of photosystem II. Biochim Biophys Acta. 1994;1186(1-2):123–127. [Google Scholar]
- 27.Standfuss J, Kühlbrandt W. The three isoforms of the light-harvesting complex II: Spectroscopic features, trimer formation, and functional roles. J Biol Chem. 2004;279(35):36884–36891. doi: 10.1074/jbc.M402348200. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Boggasch S, Haase W, Paulsen H. Thermal stability of trimeric light-harvesting chlorophyll a/b complex (LHCIIb) in liposomes of thylakoid lipids. Biochim Biophys Acta. 2006;1757(12):1642–1648. doi: 10.1016/j.bbabio.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Teardo E, et al. Evidences for interaction of PsbS with photosynthetic complexes in maize thylakoids. Biochim Biophys Acta. 2007;1767(6):703–711. doi: 10.1016/j.bbabio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Mullineaux CW, Pascal AA, Horton P, Holzwarth AR. Excitation-energy quenching in aggregates of the LHC-II chlorophyll-protein complex—A time-resolved fluorescence study. Biochim Biophys Acta. 1993;1141(1):23–28. [Google Scholar]
- 31.Ide JP, Klug DR, Kühlbrandt W, Giorgi LB, Porter G. The state of detergent solubilised light-harvesting chlorophyll-a/b protein complex as monitored by picosecond time-resolved fluorescence and circular dichroism. Biochim Biophys Acta. 1987;893(2):349–364. [Google Scholar]
- 32.Niyogi KK, Grossman AR, Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998;10(7):1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogson BJ, Niyogi KK, Björkman O, DellaPenna D. Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci USA. 1998;95(22):13324–13329. doi: 10.1073/pnas.95.22.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crouchman S, Ruban A, Horton P. PsbS enhances nonphotochemical fluorescence quenching in the absence of zeaxanthin. FEBS Lett. 2006;580(8):2053–2058. doi: 10.1016/j.febslet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kühlbrandt W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005;24(5):919–928. doi: 10.1038/sj.emboj.7600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilmore AM, Yamamoto HY. Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C-18 high-performance liquid-chromatographic column. J Chromatogr A. 1991;543:137–145. [Google Scholar]
- 38.Lehner J, et al. The morphogene AmiC2 is pivotal for multicellular development in the cyanobacterium Nostoc punctiforme. Mol Microbiol. 2011;79(6):1655–1669. doi: 10.1111/j.1365-2958.2011.07554.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.